Studies on the Thermochemical Conversion of Waste Tyre Rubber—A Review
Abstract
:1. Introduction
2. Quantities and Properties of Waste Tyres
2.1. Vehicles, Tyres, and Tyre Waste
2.2. Tyre Characteristics
3. Disposal of Used Tyres
- Material recovery is a process in which particular groups of materials from which the tyre was made are obtained so that they can be reused [16].
- Energy recovery is a process in which used tyre material undergoes thermochemical conversion, resulting in the extraction of fractions with applications aimed at energy production [17].
3.1. Material Recovery
3.2. Energy Recovery
3.3. Combustion of Waste Tyres
4. Pyrolysis of Waste Tyres
4.1. Effect of Temperature and Heating Rate
4.2. Effect of Particle Size
4.3. Effect of Pressure
4.4. Effect of Catalysts
4.5. Characteristics of Pyrolytic Gas
4.6. Characteristics of Pyrolytic Oil
4.7. Characteristics of Tyre Char
5. Waste Tyre Rubber Gasification
5.1. The Course of the Used Tyre Gasification Process
- Stage I, which occurs in the temperature range of about 250 to 550 °C, the accompanying mass loss is about 60%. This loss is associated with pyrolysis, resulting from the degradation of natural and synthetic rubber, with the total content in tyres ranging from 40 to 50%, along with the release of volatile compounds, i.e., carbon monoxide and carbon dioxide, methane, hydrogen, and sulphur oxide IV [30,128].
- Stage II is associated with the gasification reactions between the high elemental carbon tyre char formed in the first stage and the gasification agent. This stage begins at a temperature of about 800–850 °C and continues until the elemental carbon is fully reacted. During this stage, a significant intensification of the formation of gaseous components is observed, the composition of which depends primarily on the gasification agent used [30,128].
5.2. Effects of Temperature
5.3. Effects of Heating Rate
5.4. Effects of Total Pressure
5.5. Effects of the Amount of Gasification Agent
5.6. Effect of Particle Size
5.7. Effect of Surface Morphology and Mineral Matter
5.8. Char Activation
5.9. Effect of Catalysts
5.10. Gasification of Oil from Waste Tyre Pyrolysis
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chakraborty, S.; Dadashpoor, H.; Novotný, J.; Maity, I.; Follmann, A.; Patel, P.P.; Roy, U.; Pramanik, S. In Pursuit of Sustainability—Spatio-Temporal Pathways of Urban Growth Patterns in the World’s Largest Megacities. Cities 2022, 131, 103919. [Google Scholar] [CrossRef]
- Leeson, G.W. The Growth, Ageing and Urbanisation of Our World. J. Popul. Ageing 2018, 11, 107–115. [Google Scholar] [CrossRef]
- Burger, M.J.; Morrison, P.S.; Hendriks, M.; Hoogerbrugge, M.M. Urban-Rural Happiness Differentials across the World. In World Happiness Report 2020; Chapter 4; Sustainable Development Solutions Network: New York, NY, USA, 2020. [Google Scholar]
- Bandyopadhyay, A.; Rej, S.; Villanthenkodath, M.A.; Mahalik, M.K. The Role of Nuclear Energy Consumption in Abatement of Ecological Footprint: Novel Insights from Quantile-on-Quantile Regression. J. Clean. Prod. 2022, 358, 132052. [Google Scholar] [CrossRef]
- Razzaq, A.; Wang, S.; Adebayo, T.S.; Saleh Al-Faryan, M.A. The Potency of Natural Resources on Ecological Sustainability in PIIGS Economies. Resour. Policy 2022, 79, 102941. [Google Scholar] [CrossRef]
- Zhang, A.; Venkatesh, V.G.; Liu, Y.; Wan, M.; Qu, T.; Huisingh, D. Barriers to Smart Waste Management for a Circular Economy in China. J. Clean. Prod. 2019, 240, 118198. [Google Scholar] [CrossRef]
- Alaedini, A.H.; Tourani, H.K.; Saidi, M. A Review of Waste-to-Hydrogen Conversion Technologies for Solid Oxide Fuel Cell (SOFC) Applications: Aspect of Gasification Process and Catalyst Development. J. Environ. Manag. 2023, 329, 117077. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, W.; Manalo, A.; Siddique, R.; Mendis, P.; Zhuge, Y.; Wong, H.S.; Lokuge, W.; Aravinthan, T.; Schubel, P. Recycling of Landfill Wastes (Tyres, Plastics and Glass) in Construction—A Review on Global Waste Generation, Performance, Application and Future Opportunities. Resour. Conserv. Recycl. 2021, 173, 105745. [Google Scholar] [CrossRef]
- Chen, B.; Zheng, D.; Xu, R.; Leng, S.; Han, L.; Zhang, Q.; Liu, N.; Dai, C.; Wu, B.; Yu, G.; et al. Disposal Methods for Used Passenger Car Tires: One of the Fastest Growing Solid Wastes in China. Green. Energy Environ. 2022, 7, 1298–1309. [Google Scholar] [CrossRef]
- Ramarad, S.; Khalid, M.; Ratnam, C.T.; Chuah, A.L.; Rashmi, W. Waste Tire Rubber in Polymer Blends: A Review on the Evolution, Properties and Future. Prog. Mater. Sci. 2015, 72, 100–140. [Google Scholar] [CrossRef]
- Mohammad, A.; Paleologos, E.K.; Ogrodnik, P.; Koda, E.; Osiński, P.; Podlasek, A.; Vaverková, M.D.; Goli, V.S.N.S.; Singh, P.; Wang, K.; et al. Occurrence and Ecotoxicological Effects of Fires at Municipal Solid Waste Landfills. Environ. Geotech. 2023, 1–14. [Google Scholar] [CrossRef]
- Hwang, J.G.; Lee, B.K.; Choi, M.K.; Park, H.C.; Choi, H.S. Optimal Production of Waste Tire Pyrolysis Oil and Recovery of High Value-Added D-Limonene in a Conical Spouted Bed Reactor. Energy 2023, 262, 125519. [Google Scholar] [CrossRef]
- Page, T.S.; Almeda, R.; Koski, M.; Bournaka, E.; Nielsen, T.G. Toxicity of Tyre Wear Particle Leachates to Marine Phytoplankton. Aquat. Toxicol. 2022, 252, 106299. [Google Scholar] [CrossRef] [PubMed]
- Trudsø, L.L.; Nielsen, M.B.; Hansen, S.F.; Syberg, K.; Kampmann, K.; Khan, F.R.; Palmqvist, A. The Need for Environmental Regulation of Tires: Challenges and Recommendations. Environ. Pollut. 2022, 311, 119974. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, N.; Zabaniotou, A. Features of an Efficient and Environmentally Attractive Used Tyres Pyrolysis with Energy and Material Recovery. Renew. Sustain. Energy Rev. 2013, 20, 539–558. [Google Scholar] [CrossRef]
- Bulei, C.; Todor, M.P.; Heput, T.; Kiss, I. Directions for Material Recovery of Used Tires and Their Use in the Production of New Products Intended for the Industry of Civil Construction and Pavements. IOP Conf. Ser. Mater. Sci. Eng. 2018, 294, 012064. [Google Scholar] [CrossRef]
- Abdallah, R.; Juaidi, A.; Assad, M.; Salameh, T.; Manzano-Agugliaro, F. Energy Recovery from Waste Tires Using Pyrolysis: Palestine as Case of Study. Energies 2020, 13, 1817. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Kucinska-Lipka, J.; Janik, H.; Balas, A. Progress in Used Tyres Management in the European Union: A Review. Waste Manag. 2012, 32, 1742–1751. [Google Scholar] [CrossRef]
- Hita, I.; Arabiourrutia, M.; Olazar, M.; Bilbao, J.; Arandes, J.M.; Castaño, P. Opportunities and Barriers for Producing High Quality Fuels from the Pyrolysis of Scrap Tires. Renew. Sustain. Energy Rev. 2016, 56, 745–759. [Google Scholar] [CrossRef]
- Czerski, G.; Śpiewak, K.; Makowska, D.; Grycova, B. Study on Steam Co-Gasification of Waste Tire Char and Sewage Sludge. Energies 2023, 16, 2156. [Google Scholar] [CrossRef]
- International Organization of Motor Vehicle Manufacturers. Vehicle in Use. Available online: https://www.oica.net/wp-content/uploads/Total-World-vehicles-in-use-2020.pdf (accessed on 25 July 2023).
- Eldem, B.; Kluczek, A.; Bagiński, J. The COVID-19 Impact on Supply Chain Operations of Automotive Industry: A Case Study of Sustainability 4.0 Based on Sense–Adapt–Transform Framework. Sustainability 2022, 14, 5855. [Google Scholar] [CrossRef]
- Stojczew, K. Ocena Wpływu Pandemii COVID-19 Na Sytuację w Branży Motoryzacyjnej w Polsce. Stud. Ind. Geogr. Comm. Pol. Geogr. Soc. 2021, 35, 64–84. [Google Scholar] [CrossRef]
- Markl, E.; Lackner, M. Devulcanization Technologies for Recycling of Tire-Derived Rubber: A Review. Materials 2020, 13, 1246. [Google Scholar] [CrossRef] [PubMed]
- International Organization of Motor Vehicle Manufacturers. Production Statistics. Available online: https://www.oica.net/wp-content/uploads/By-country-region-2021.pdf (accessed on 25 July 2023).
- European Tyre & Rubber Industry—Statistics. Available online: https://www.etrma.org/wp-content/uploads/2021/12/20211215-Statistics-booklet-2021VF.pdf (accessed on 25 July 2023).
- Thomas, B.S.; Gupta, R.C. A Comprehensive Review on the Applications of Waste Tire Rubber in Cement Concrete. Renew. Sustain. Energy Rev. 2016, 54, 1323–1333. [Google Scholar] [CrossRef]
- Mohammed, B.; Adamu, M.; Mohammed, B.S.; Shafiq, N. A Review on The Effect of Crumb Rubber on The Properties of Rubbercrete. Int. J. Civ. Eng. Technol. (IJCIET) 2017, 8, 599–615. [Google Scholar]
- Czajczyńska, D.; Krzyżyńska, R.; Jouhara, H.; Spencer, N. Use of Pyrolytic Gas from Waste Tire as a Fuel: A Review. Energy 2017, 134, 1121–1131. [Google Scholar] [CrossRef]
- Kandasamy, J.; Gökalp, I. Pyrolysis, Combustion, and Steam Gasification of Various Types of Scrap Tires for Energy Recovery. Energy Fuels 2015, 29, 346–354. [Google Scholar] [CrossRef]
- End-of-Life Tyre Report. 2015. European Tyre & Rubber Manufactures’ Association. Available online: https://www.etrma.org/wp-content/uploads/2019/09/elt-report-v9a-final.pdf (accessed on 18 November 2023).
- End of Life Tyres Management—Europe. 2019. European Tyre & Rubber Manufactures’ Association. Available online: https://www.etrma.org/wp-content/uploads/2021/05/20210520_ETRMA_PRESS-RELEASE_ELT-2019.pdf (accessed on 18 November 2023).
- End of Life Tyres Management—Europe. 2017. European Tyre & Rubber Manufactures’ Association. Available online: https://www.etrma.org/wp-content/uploads/2019/11/20191119-Europe-92-of-all-End-of-Life-Tyres-collected-and-treated-in-2017.pdf (accessed on 18 November 2023).
- Fu, H.; Liang, X.; Chen, K.; Wang, Y.; Xiao, Z. Study on Key Mechanical Properties of the Flexible Spoke Non-Pneumatic Tire Considering Thermo-Mechanical Coupling. Adv. Eng. Softw. 2022, 173, 103281. [Google Scholar] [CrossRef]
- Katarzyna, P.; Izabela, P.; Patrycja, B.-W.; Weronika, K.; Andrzej, T. LCA as a Tool for the Environmental Management of Car Tire Manufacturing. Appl. Sci. 2020, 10, 7015. [Google Scholar] [CrossRef]
- Siddika, A.; Al Mamun, M.A.; Alyousef, R.; Amran, Y.H.M.; Aslani, F.; Alabduljabbar, H. Properties and Utilizations of Waste Tire Rubber in Concrete: A Review. Constr. Build. Mater. 2019, 224, 711–731. [Google Scholar] [CrossRef]
- Valentini, F.; Pegoretti, A. End-of-Life Options of Tyres. Review. Adv. Ind. Eng. Polym. Res. 2022, 5, 203–213. [Google Scholar] [CrossRef]
- Xiao, Z.; Pramanik, A.; Basak, A.K.; Prakash, C.; Shankar, S. Material Recovery and Recycling of Waste Tyres—A Review. Clean. Mater. 2022, 5, 100115. [Google Scholar] [CrossRef]
- Torretta, V.; Rada, E.C.; Ragazzi, M.; Trulli, E.; Istrate, I.A.; Cioca, L.I. Treatment and Disposal of Tyres: Two EU Approaches. A Review. Waste Manag. 2015, 45, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Roychand, R.; Gravina, R.J.; Zhuge, Y.; Ma, X.; Youssf, O.; Mills, J.E. A Comprehensive Review on the Mechanical Properties of Waste Tire Rubber Concrete. Constr. Build. Mater. 2020, 237, 117651. [Google Scholar] [CrossRef]
- Johannessen, C.; Liggio, J.; Zhang, X.; Saini, A.; Harner, T. Composition and Transformation Chemistry of Tire-Wear Derived Organic Chemicals and Implications for Air Pollution. Atmos. Pollut. Res. 2022, 13, 101533. [Google Scholar] [CrossRef]
- De, S.K.; White, J.R. Rubber Technologist’s Handbook; Rapra Technology Limited: Shrewsbury, UK, 2001; Volume 1. [Google Scholar]
- Bockstal, L.; Berchem, T.; Schmetz, Q.; Richel, A. Devulcanisation and Reclaiming of Tires and Rubber by Physical and Chemical Processes: A Review. J. Clean. Prod. 2019, 236, 117574. [Google Scholar] [CrossRef]
- Gent, A.N.; Walter, J.D. Pneumatic Tire; 2006; Mechanical Engineering Faculty Research, 854. Available online: https://ideaexchange.uakron.edu/mechanical_ideas/854 (accessed on 18 November 2023).
- Rodgers, B.; Waddell, W. Tire Engineering. In Science and Technology of Rubber; Elsevier: Amsterdam, The Netherlands, 2005; pp. 619–661. [Google Scholar]
- Shmurak, I.L. Cord for Tyres and Rubber–Cord Casings. Review. Int. Polym. Sci. Technol. 2014, 41, 23–26. [Google Scholar] [CrossRef]
- Trautner, S.; Lackner, J.; Spendelhofer, W.; Huber, N.; Pedarnig, J.D. Quantification of the Vulcanizing System of Rubber in Industrial Tire Rubber Production by Laser-Induced Breakdown Spectroscopy (LIBS). Anal. Chem. 2019, 91, 5200–5206. [Google Scholar] [CrossRef]
- Ayar, M.; Dalkiran, A.; Kale, U.; Nagy, A.; Karakoc, T.H. Investigation of the Substitutability of Rubber Compounds with Environmentally Friendly Materials. Sustainability 2021, 13, 5251. [Google Scholar] [CrossRef]
- Kim, H.H.; Lee, S.-J. Effect of Crumb Rubber on Viscosity of Rubberized Asphalt Binders Containing Wax Additives. Constr. Build. Mater. 2015, 95, 65–73. [Google Scholar] [CrossRef]
- Kim, H.H.; Mazumder, M.; Lee, S.-J. Recycling of Aged Asphalt Binders with Wax Warm Additives. Road Mater. Pavement Des. 2018, 19, 1203–1215. [Google Scholar] [CrossRef]
- Yan, Y.; Roque, R.; Hernando, D.; Chun, S. Cracking Performance Characterisation of Asphalt Mixtures Containing Reclaimed Asphalt Pavement with Hybrid Binder. Road Mater. Pavement Des. 2019, 20, 347–366. [Google Scholar] [CrossRef]
- Khern, Y.C.; Paul, S.C.; Kong, S.Y.; Babafemi, A.J.; Anggraini, V.; Miah, M.J.; Šavija, B. Impact of Chemically Treated Waste Rubber Tire Aggregates on Mechanical, Durability and Thermal Properties of Concrete. Front. Mater. 2020, 7, 90. [Google Scholar] [CrossRef]
- Karimi, H.R.; Aliha, M.R.M.; Ebneabbasi, P.; Salehi, S.M.; Khedri, E.; Haghighatpour, P.J. Mode I and Mode II Fracture Toughness and Fracture Energy of Cement Concrete Containing Different Percentages of Coarse and Fine Recycled Tire Rubber Granules. Theor. Appl. Fract. Mech. 2023, 123, 103722. [Google Scholar] [CrossRef]
- Pacheco-Torres, R.; Cerro-Prada, E.; Escolano, F.; Varela, F. Fatigue Performance of Waste Rubber Concrete for Rigid Road Pavements. Constr. Build. Mater. 2018, 176, 539–548. [Google Scholar] [CrossRef]
- Fazli, A.; Rodrigue, D. Recycling Waste Tires into Ground Tire Rubber (GTR)/Rubber Compounds: A Review. J. Compos. Sci. 2020, 4, 103. [Google Scholar] [CrossRef]
- Celeiro, M.; Armada, D.; Ratola, N.; Dagnac, T.; de Boer, J.; Llompart, M. Evaluation of Chemicals of Environmental Concern in Crumb Rubber and Water Leachates from Several Types of Synthetic Turf Football Pitches. Chemosphere 2021, 270, 128610. [Google Scholar] [CrossRef] [PubMed]
- Jastifer, J.R.; McNitt, A.S.; Mack, C.D.; Kent, R.W.; McCullough, K.A.; Coughlin, M.J.; Anderson, R.B. Synthetic Turf: History, Design, Maintenance, and Athlete Safety. Sports Health Multidiscip. Approach 2019, 11, 84–90. [Google Scholar] [CrossRef]
- Reschner, K. Scrap Tire Recycling: A Summary of Prevalent Disposal and Recycling Methods; EnTire Engineering: Berlin, Germany, 2008. [Google Scholar]
- Euroshield Rubber, Trusted Euroshield Rubber Roofing Installation. Available online: https://www.euroshieldroofing.com/ (accessed on 18 November 2023).
- Chudy, J.; Kasperowicz, M.; Oleśkiewicz, M. Technological Aspects of Car Tire Recycling. Autobusy Tech. Eksploat. Syst. Transp. 2013, 14, 82–85. [Google Scholar]
- Hazarika, H.; Fukumoto, Y. Sustainable Solution for Seawall Protection against Tsunami-Induced Damage. Int. J. Geomech. 2016, 16, C4016005. [Google Scholar] [CrossRef]
- Leng, S.; Wang, X.; Wang, J.; Liu, Y.; Ma, F.; Zhong, X. Waste Tire Pyrolysis for the Production of Light Hydrocarbons over Layered Catalysts. Energy Technol. 2015, 3, 851–855. [Google Scholar] [CrossRef]
- Luo, S.; Feng, Y. The Production of Fuel Oil and Combustible Gas by Catalytic Pyrolysis of Waste Tire Using Waste Heat of Blast-Furnace Slag. Energy Convers. Manag. 2017, 136, 27–35. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.A.; Rehan, M.; Aburiazaiza, A.S.; Gardy, J.; Nizami, A.S. Effect of Advanced Catalysts on Tire Waste Pyrolysis Oil. Process Saf. Environ. Prot. 2018, 116, 542–552. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Ma, L.; Chang, J. Vacuum Pyrolysis of Waste Tires with Basic Additives. Waste Manag. 2008, 28, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.; Horvat, A.; Batuecas, E.; Abelha, P. Waste Tyres Valorisation through Gasification in a Bubbling Fluidised Bed: An Exhaustive Gas Composition Analysis. Renew. Energy 2022, 200, 1438–1446. [Google Scholar] [CrossRef]
- Czajczyńska, D.; Czajka, K.; Krzyżyńska, R.; Jouhara, H. Waste Tyre Pyrolysis—Impact of the Process and Its Products on the Environment. Therm. Sci. Eng. Prog. 2020, 20, 100690. [Google Scholar] [CrossRef]
- Aylón, E.; Fernández-Colino, A.; Murillo, R.; Navarro, M.V.; García, T.; Mastral, A.M. Valorisation of Waste Tyre by Pyrolysis in a Moving Bed Reactor. Waste Manag. 2010, 30, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Haniu, H.; Rafiqul Alam Beg, M. Liquid Fuels and Chemicals from Pyrolysis of Motorcycle Tire Waste: Product Yields, Compositions and Related Properties. Fuel 2008, 87, 3112–3122. [Google Scholar] [CrossRef]
- Bing, W.; Hongbin, Z.; Zeng, D.; Yuefeng, F.; Yu, Q.; Rui, X. Microwave Fast Pyrolysis of Waste Tires: Effect of Microwave Power on Product Composition and Quality. J. Anal. Appl. Pyrolysis 2021, 155, 104979. [Google Scholar] [CrossRef]
- Janajreh, I.; Raza, S.S. Numerical Simulation of Waste Tyres Gasification. Waste Manag. Res. J. Sustain. Circ. Econ. 2015, 33, 460–468. [Google Scholar] [CrossRef]
- Rahman, M.A.; Aziz, M.A. Solar Pyrolysis of Scrap Tire: Optimization of Operating Parameters. J. Mater. Cycles Waste Manag. 2018, 20, 1207–1215. [Google Scholar] [CrossRef]
- Wang, L.; Chai, M.; Liu, R.; Cai, J. Synergetic Effects during Co-Pyrolysis of Biomass and Waste Tire: A Study on Product Distribution and Reaction Kinetics. Bioresour. Technol. 2018, 268, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Ongen, A.; Ozcan, H.K.; Elmaslar Ozbas, E.; Pangaliyev, Y. Gasification of Waste Tires in a Circulating Fixed-Bed Reactor within the Scope of Waste to Energy. Clean. Technol. Environ. Policy 2019, 21, 1281–1291. [Google Scholar] [CrossRef]
- Molino, A.; Donatelli, A.; Marino, T.; Aloise, A.; Rimauro, J.; Iovane, P. Waste Tire Recycling Process for Production of Steam Activated Carbon in a Pilot Plant. Resour. Conserv. Recycl. 2018, 129, 102–111. [Google Scholar] [CrossRef]
- Czerski, G.; Grzywacz, P.; Dziok, T.; Śpiewak, K.; Makowska, D. Experimental Evaluation of the Dry Coal Deshaling by Pneumatic Vibrating FGX Separator on the CO2 Gasification Process. Int. J. Energy Res. 2021, 45, 5412–5422. [Google Scholar] [CrossRef]
- Li, X.; Ma, B.; Xu, L.; Hu, Z.; Wang, X. Thermogravimetric Analysis of the Co-Combustion of the Blends with High Ash Coal and Waste Tyres. Thermochim. Acta 2006, 441, 79–83. [Google Scholar] [CrossRef]
- Pan, D.; Jiang, W.; Guo, R.; Huang, Y.; Pan, W. Thermogravimetric and Kinetic Analysis of Co-Combustion of Waste Tires and Coal Blends. ACS Omega 2021, 6, 5479–5484. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Gong, M.; Lester, E.; Hall, P. Characteristics and Synergistic Effects of Co-Firing of Coal and Carbonaceous Wastes. Fuel 2013, 104, 194–200. [Google Scholar] [CrossRef]
- Singh, S.; Nimmo, W.; Gibbs, B.M.; Williams, P.T. Waste Tyre Rubber as a Secondary Fuel for Power Plants. Fuel 2009, 88, 2473–2480. [Google Scholar] [CrossRef]
- Carmo-Calado, L.; Hermoso-Orzáez, M.J.; Mota-Panizio, R.; Guilherme-Garcia, B.; Brito, P. Co-Combustion of Waste Tires and Plastic-Rubber Wastes with Biomass Technical and Environmental Analysis. Sustainability 2020, 12, 1036. [Google Scholar] [CrossRef]
- Giere, R.; Smith, K.; Blackford, M. Chemical Composition of Fuels and Emissions from a Coal+tire Combustion Experiment in a Power Station. Fuel 2006, 85, 2278–2285. [Google Scholar] [CrossRef]
- Singh, S.; Nimmo, W.; Williams, P.T. An Experimental Study of Ash Behaviour and the Potential Fate of ZnO/Zn in the Co-Combustion of Pulverised South African Coal and Waste Tyre Rubber. Fuel 2013, 111, 269–279. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Y.; Wang, R.; Gu, L.; Zhu, Z.; Yu, M.; Yang, X.; Liu, Y. Analysis on Zn-Rich Waste Tire Treatment via Co-Combustion with Coal: Thermal Property and Migration/Leaching of Heavy Metals. J. Environ. Chem. Eng. 2021, 9, 106365. [Google Scholar] [CrossRef]
- Onenc, S.; Retschitzegger, S.; Evic, N.; Kienzl, N.; Yanik, J. Characteristics and Synergistic Effects of Co-Combustion of Carbonaceous Wastes with Coal. Waste Manag. 2018, 71, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Mentes, D.; Tóth, C.E.; Nagy, G.; Muránszky, G.; Póliska, C. Investigation of Gaseous and Solid Pollutants Emitted from Waste Tire Combustion at Different Temperatures. Waste Manag. 2022, 149, 302–312. [Google Scholar] [CrossRef]
- Conesa, J.A.; Font, R.; Fullana, A.; Martín-Gullón, I.; Aracil, I.; Gálvez, A.; Moltó, J.; Gómez-Rico, M.F. Comparison between Emissions from the Pyrolysis and Combustion of Different Wastes. J. Anal. Appl. Pyrolysis 2009, 84, 95–102. [Google Scholar] [CrossRef]
- Choi, G.-G.; Jung, S.-H.; Oh, S.-J.; Kim, J.-S. Total Utilization of Waste Tire Rubber through Pyrolysis to Obtain Oils and CO2 Activation of Pyrolysis Char. Fuel Process. Technol. 2014, 123, 57–64. [Google Scholar] [CrossRef]
- Kumar Singh, R.; Ruj, B.; Jana, A.; Mondal, S.; Jana, B.; Kumar Sadhukhan, A.; Gupta, P. Pyrolysis of Three Different Categories of Automotive Tyre Wastes: Product Yield Analysis and Characterization. J. Anal. Appl. Pyrolysis 2018, 135, 379–389. [Google Scholar] [CrossRef]
- Niu, M.; Sun, R.; Ding, K.; Gu, H.; Cui, X.; Wang, L.; Hu, J. Synergistic Effect on Thermal Behavior and Product Characteristics during Co-Pyrolysis of Biomass and Waste Tire: Influence of Biomass Species and Waste Blending Ratios. Energy 2022, 240, 122808. [Google Scholar] [CrossRef]
- Kordoghli, S.; Paraschiv, M.; Kuncser, R.; Tazerout, M.; Zagrouba, F. Catalysts’ Influence on Thermochemical Decomposition of Waste Tires. Environ. Prog. Sustain. Energy 2017, 36, 1560–1567. [Google Scholar] [CrossRef]
- Chao, L.; Zhang, C.; Zhang, L.; Gholizadeh, M.; Hu, X. Catalytic Pyrolysis of Tire Waste: Impacts of Biochar Catalyst on Product Evolution. Waste Manag. 2020, 116, 9–21. [Google Scholar] [CrossRef]
- Li, D.; Lei, S.; Lin, F.; Zhong, L.; Ma, W.; Chen, G. Study of Scrap Tires Pyrolysis—Products Distribution and Mechanism. Energy 2020, 213, 119038. [Google Scholar] [CrossRef]
- Arabiourrutia, M.; Lopez, G.; Artetxe, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Waste Tyre Valorization by Catalytic Pyrolysis—A Review. Renew. Sustain. Energy Rev. 2020, 129, 109932. [Google Scholar] [CrossRef]
- Mkhize, N.M.; van der Gryp, P.; Danon, B.; Görgens, J.F. Effect of Temperature and Heating Rate on Limonene Production from Waste Tyre Pyrolysis. J. Anal. Appl. Pyrolysis 2016, 120, 314–320. [Google Scholar] [CrossRef]
- Martínez, J.D.; Puy, N.; Murillo, R.; García, T.; Navarro, M.V.; Mastral, A.M. Waste Tyre Pyrolysis—A Review. Renew. Sustain. Energy Rev. 2013, 23, 179–213. [Google Scholar] [CrossRef]
- Oyedun, A.; Lam, K.-L.; Fittkau, M.; Hui, C.-W. Optimisation of Particle Size in Waste Tyre Pyrolysis. Fuel 2012, 95, 417–424. [Google Scholar] [CrossRef]
- Gao, N.; Li, A.; Li, W. Research into Fine Powder and Large Particle Tyre Pyrolysis. Waste Manag. Res. J. Sustain. Circ. Econ. 2009, 27, 242–250. [Google Scholar] [CrossRef]
- Ma, S.; Leong, H.; He, L.; Xiong, Z.; Han, H.; Jiang, L.; Wang, Y.; Hu, S.; Su, S.; Xiang, J. Effects of Pressure and Residence Time on Limonene Production in Waste Tires Pyrolysis Process. J. Anal. Appl. Pyrolysis 2020, 151, 104899. [Google Scholar] [CrossRef]
- Czajczyńska, D.; Anguilano, L.; Ghazal, H.; Krzyżyńska, R.; Reynolds, A.J.; Spencer, N.; Jouhara, H. Potential of Pyrolysis Processes in the Waste Management Sector. Therm. Sci. Eng. Prog. 2017, 3, 171–197. [Google Scholar] [CrossRef]
- Kumar, A.; Yan, B.; Tao, J.; Li, J.; Kumari, L.; Tafa Oba, B.; Akintayo Aborisade, M.; Ali Jamro, I.; Chen, G. Co-Pyrolysis of de-Oiled Microalgal Biomass Residue and Waste Tires: Deeper Insights from Thermal Kinetics, Behaviors, Drivers, Bio-Oils, Bio-Chars, and in-Situ Evolved Gases Analyses. Chem. Eng. J. 2022, 446, 137160. [Google Scholar] [CrossRef]
- Kumar, A.; Yan, B.; Cheng, Z.; Tao, J.; Hassan, M.; Li, J.; Kumari, L.; Oba, B.T.; Aborisade, M.A.; Jamro, I.A.; et al. Co-Pyrolysis of Hydrothermally Pre-Treated Microalgae Residue and Polymeric Waste (Plastic/Tires): Comparative and Dynamic Analyses of Pyrolytic Behaviors, Kinetics, Chars, Oils, and in-Situ Gas Emissions. Fuel 2023, 331, 125814. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, M.; Chen, G.; Zhang, M.; Sun, T.; Burra, K.G.; Guo, S.; Chen, Y.; Yang, S.; Li, Z.; et al. Co-Pyrolysis Characteristics of Waste Tire and Maize Stalk Using TGA, FTIR and Py-GC/MS Analysis. Fuel 2023, 337, 127206. [Google Scholar] [CrossRef]
- Mikulski, M.; Ambrosewicz-Walacik, M.; Hunicz, J.; Nitkiewicz, S. Combustion Engine Applications of Waste Tyre Pyrolytic Oil. Prog. Energy Combust. Sci. 2021, 85, 100915. [Google Scholar] [CrossRef]
- Nkosi, N.; Edison, M. A Review and Discussion of Waste Tyre Pyrolysis and Derived Products. In Proceedings of the World Congress on Engineering Volume 2, WCE 2014, London, UK, 2–4 July 2014. [Google Scholar]
- Ghugare, S.B.; Tambe, S.S. Genetic Programming Based High Performing Correlations for Prediction of Higher Heating Value of Coals of Different Ranks and from Diverse Geographies. J. Energy Inst. 2017, 90, 476–484. [Google Scholar] [CrossRef]
- Mui, E.L.K.; Cheung, W.H.; McKay, G. Tyre Char Preparation from Waste Tyre Rubber for Dye Removal from Effluents. J. Hazard. Mater. 2010, 175, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Yang, Y.; Sun, J.; Zhao, X.; Wang, W.; Mao, Y.; Ma, C. Effect of Power Level on the Microwave Pyrolysis of Tire Powder. Energy 2017, 127, 571–580. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Li, A.; Irfan, M.; Du, Y.; Di, W. Comparative Pyrolysis Behaviors of Tire Tread and Side Wall from Waste Tire and Characterization of the Resulting Chars. J. Environ. Manag. 2019, 232, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.D.; Murillo, R.; García, T.; Veses, A. Demonstration of the Waste Tire Pyrolysis Process on Pilot Scale in a Continuous Auger Reactor. J. Hazard. Mater. 2013, 261, 637–645. [Google Scholar] [CrossRef]
- Seng-eiad, S.; Jitkarnka, S. Untreated and HNO3-Treated Pyrolysis Char as Catalysts for Pyrolysis of Waste Tire: In-Depth Analysis of Tire-Derived Products and Char Characterization. J. Anal. Appl. Pyrolysis 2016, 122, 151–159. [Google Scholar] [CrossRef]
- Acevedo, B.; Barriocanal, C.; Alvarez, R. Pyrolysis of Blends of Coal and Tyre Wastes in a Fixed Bed Reactor and a Rotary Oven. Fuel 2013, 113, 817–825. [Google Scholar] [CrossRef]
- Wang, Z.; Burra, K.G.; Zhang, M.; Li, X.; Policella, M.; Lei, T.; Gupta, A.K. Co-Pyrolysis of Waste Tire and Pine Bark for Syngas and Char Production. Fuel 2020, 274, 117878. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Li, H.; Han, X.; Zhang, M.; Sun, Y.; Fan, X.; Tu, R.; Zeng, Y.; Xu, C.C.; et al. Applications of Catalysts in Thermochemical Conversion of Biomass (Pyrolysis, Hydrothermal Liquefaction and Gasification): A Critical Review. Renew. Energy 2022, 196, 462–481. [Google Scholar] [CrossRef]
- Śpiewak, K.; Czerski, G.; Porada, S. Effect of K, Na and Ca-Based Catalysts on the Steam Gasification Reactions of Coal. Part I: Type and Amount of One-Component Catalysts. Chem. Eng. Sci. 2021, 229, 116024. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Wang, C.L. Fluidized-Bed Gasification of Waste Tire Powders. Fuel Process. Technol. 2003, 84, 175–196. [Google Scholar] [CrossRef]
- San Miguel, G.; Fowler, G.D.; Sollars, C.J. A Study of the Characteristics of Activated Carbons Produced by Steam and Carbon Dioxide Activation of Waste Tyre Rubber. Carbon 2003, 41, 1009–1016. [Google Scholar] [CrossRef]
- Scott, S.A.; Davidson, J.F.; Dennis, J.S.; Fennell, P.S.; Hayhurst, A.N. The Rate of Gasification by CO2 of Chars from Waste. Proc. Combust. Inst. 2005, 30, 2151–2159. [Google Scholar] [CrossRef]
- Song, B.-H. Gasification Kinetics of Waste Tire Char and Sewage Sludge Char with Steam in a Thermobalance Reactor. J. Ind. Eng. Chem. 2005, 11, 361–367. [Google Scholar]
- González, J.F.; Encinar, J.M.; González-García, C.M.; Sabio, E.; Ramiro, A.; Canito, J.L.; Gañán, J. Preparation of Activated Carbons from Used Tyres by Gasification with Steam and Carbon Dioxide. Appl. Surf. Sci. 2006, 252, 5999–6004. [Google Scholar] [CrossRef]
- Song, B.H.; Kim, S.D. Gasification of Tire Scrap and Sewage Sludge in a Circulating Fluidized Bed with a Draft Tube. Stud. Surf. Sci. Catal. 2006, 159, 565–568. [Google Scholar]
- Xiao, G.; Ni, M.-J.; Chi, Y.; Cen, K.-F. Low-Temperature Gasification of Waste Tire in a Fluidized Bed. Energy Convers. Manag. 2008, 49, 2078–2082. [Google Scholar] [CrossRef]
- Betancur, M.; Martínez, J.D.; Murillo, R. Production of Activated Carbon by Waste Tire Thermochemical Degradation with CO2. J. Hazard. Mater. 2009, 168, 882–887. [Google Scholar] [CrossRef]
- Galvagno, S.; Casciaro, G.; Casu, S.; Martino, M.; Mingazzini, C.; Russo, A.; Portofino, S. Steam Gasification of Tyre Waste, Poplar, and Refuse-Derived Fuel: A Comparative Analysis. Waste Manag. 2009, 29, 678–689. [Google Scholar] [CrossRef] [PubMed]
- López, G.; Olazar, M.; Artetxe, M.; Amutio, M.; Elordi, G.; Bilbao, J. Steam Activation of Pyrolytic Tyre Char at Different Temperatures. J. Anal. Appl. Pyrolysis 2009, 85, 539–543. [Google Scholar] [CrossRef]
- Straka, P.; Bučko, Z. Co-Gasification of a Lignite/Waste-Tyre Mixture in a Moving Bed. Fuel Process. Technol. 2009, 90, 1202–1206. [Google Scholar] [CrossRef]
- Donatelli, A.; Iovane, P.; Molino, A. High Energy Syngas Production by Waste Tyres Steams Gasification in a Rotary Kiln Pilot Plant. Experimental and Numerical Investigations. Fuel 2010, 89, 2721–2728. [Google Scholar] [CrossRef]
- Piatkowski, N.; Steinfeld, A. Reaction Kinetics of the Combined Pyrolysis and Steam-Gasification of Carbonaceous Waste Materials. Fuel 2010, 89, 1133–1140. [Google Scholar] [CrossRef]
- Karatas, H.; Olgun, H.; Akgun, F. Experimental Results of Gasification of Waste Tire with Air&CO2, Air&Steam and Steam in a Bubbling Fluidized Bed Gasifier. Fuel Process. Technol. 2012, 102, 166–174. [Google Scholar] [CrossRef]
- López, F.A.; Centeno, T.A.; Alguacil, F.J.; Lobato, B.; López-Delgado, A.; Fermoso, J. Gasification of the Char Derived from Distillation of Granulated Scrap Tyres. Waste Manag. 2012, 32, 743–752. [Google Scholar] [CrossRef]
- Portofino, S.; Donatelli, A.; Iovane, P.; Innella, C.; Civita, R.; Martino, M.; Matera, D.A.; Russo, A.; Cornacchia, G.; Galvagno, S. Steam Gasification of Waste Tyre: Influence of Process Temperature on Yield and Product Composition. Waste Manag. 2013, 33, 672–678. [Google Scholar] [CrossRef]
- Elbaba, I.F.; Wu, C.; Williams, P.T. Catalytic Pyrolysis-Gasification of Waste Tire and Tire Elastomers for Hydrogen Production. Energy Fuels 2010, 24, 3928–3935. [Google Scholar] [CrossRef]
- Elbaba, I.F.; Wu, C.; Williams, P.T. Hydrogen Production from the Pyrolysis–Gasification of Waste Tyres with a Nickel/Cerium Catalyst. Int. J. Hydrogen Energy 2011, 36, 6628–6637. [Google Scholar] [CrossRef]
- Portofino, S.; Casu, S.; Iovane, P.; Russo, A.; Martino, M.; Donatelli, A.; Galvagno, S. Optimizing H2 Production from Waste Tires via Combined Steam Gasification and Catalytic Reforming. Energy Fuels 2011, 25, 2232–2241. [Google Scholar] [CrossRef]
- Elbaba, I.F.; Williams, P.T. Two Stage Pyrolysis-Catalytic Gasification of Waste Tyres: Influence of Process Parameters. Appl. Catal. B 2012, 125, 136–143. [Google Scholar] [CrossRef]
- Lahijani, P.; Zainal, Z.A.; Mohamed, A.R.; Mohammadi, M. Co-Gasification of Tire and Biomass for Enhancement of Tire-Char Reactivity in CO2 Gasification Process. Bioresour. Technol. 2013, 138, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Elbaba, I.F.; Williams, P.T. Deactivation of Nickel Catalysts by Sulfur and Carbon for the Pyrolysis–Catalytic Gasification/Reforming of Waste Tires for Hydrogen Production. Energy Fuels 2014, 28, 2104–2113. [Google Scholar] [CrossRef]
- Gurai, C. Gasification Kinetics of Blends of Waste Tyre and Typical South African Coals. Master’s Thesis, North-West University, Potchefstroom, South Africa, 2015. [Google Scholar]
- Al-Rahbi, A.S.; Williams, P.T. Hydrogen-Rich Syngas Production and Tar Removal from Biomass Gasification Using Sacrificial Tyre Pyrolysis Char. Appl. Energy 2017, 190, 501–509. [Google Scholar] [CrossRef]
- Issac, M.; Dai, B.; Zhang, L. Kinetics Underpinning the C-CO2 Gasification of Waste Tyre Char and Its Interaction with Coal Char upon Co-Gasification. Fuel 2019, 256, 115991. [Google Scholar] [CrossRef]
- Lahijani, P.; Mohammadi, M.; Mohamed, A.R. Investigation of Synergism and Kinetic Analysis during CO2 Co-Gasification of Scrap Tire Char and Agro-Wastes. Renew. Energy 2019, 142, 147–157. [Google Scholar] [CrossRef]
- Nanda, S.; Reddy, S.N.; Hunter, H.N.; Vo, D.-V.N.; Kozinski, J.A.; Gökalp, I. Catalytic Subcritical and Supercritical Water Gasification as a Resource Recovery Approach from Waste Tires for Hydrogen-Rich Syngas Production. J. Supercrit. Fluids 2019, 154, 104627. [Google Scholar] [CrossRef]
- Policella, M.; Wang, Z.; Burra, K.G.; Gupta, A.K. Characteristics of Syngas from Pyrolysis and CO2-Assisted Gasification of Waste Tires. Appl. Energy 2019, 254, 113678. [Google Scholar] [CrossRef]
- Preciado-Hernandez, J.; Zhang, J.; Zhu, M.; Zhang, Z.; Zhang, D. An Experimental Study of CO2 Gasification Kinetics during Activation of a Spent Tyre Pyrolysis Char. Chem. Eng. Res. Des. 2019, 149, 129–137. [Google Scholar] [CrossRef]
- Salavati, S.; Zhang, C.; Zhang, S.; Liu, Q.; Gholizadeh, M.; Hu, X. Cross-Interaction during Co-Gasification of Wood, Weed, Plastic, Tire and Carton. J. Environ. Manag. 2019, 250, 109467. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Burra, K.G.; Lei, T.; Gupta, A.K. Co-Gasification Characteristics of Waste Tire and Pine Bark Mixtures in CO2 Atmosphere. Fuel 2019, 257, 116025. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, J.; Yang, L.; Zhu, Y. Co-Gasification Characteristics of Scrap Tyre with Pine Sawdust Using Thermogravimetric and a Whole-Tyre Gasifier Reactor. Energy Procedia 2019, 158, 37–42. [Google Scholar] [CrossRef]
- Betancur, M.; Natalia Arenas, C.; Daniel Martínez, J.; Victoria Navarro, M.; Murillo, R. CO2 Gasification of Char Derived from Waste Tire Pyrolysis: Kinetic Models Comparison. Fuel 2020, 273, 117745. [Google Scholar] [CrossRef]
- Hungwe, D.; Ding, L.; Khoshbouy, R.; Yoshikawa, K.; Takahashi, F. Kinetics and Physicochemical Morphology Evolution of Low and High-Ash Pyrolytic Tire Char during CO2 Gasification. Energy Fuels 2020, 34, 118–129. [Google Scholar] [CrossRef]
- Lahijani, P.; Mohammadi, M.; Mohamed, A.R. Investigation of Synergy and Inhibition Effects during Co-Gasification of Tire Char and Biomass in CO2 Environment. Biomass Convers. Biorefin. 2022, 12, 2229–2241. [Google Scholar] [CrossRef]
- Šuhaj, P.; Husár, J.; Haydary, J. Gasification of RDF and Its Components with Tire Pyrolysis Char as Tar-Cracking Catalyst. Sustainability 2020, 12, 6647. [Google Scholar] [CrossRef]
- Czerski, G.; Śpiewak, K.; Grzywacz, P.; Wierońska-Wiśniewska, F. Assessment of the Catalytic Effect of Various Biomass Ashes on CO2 Gasification of Tire Char. J. Energy Inst. 2021, 99, 170–177. [Google Scholar] [CrossRef]
- Paulauskas, R.; Zakarauskas, K.; Striūgas, N. An Intensification of Biomass and Waste Char Gasification in a Gasifier. Energies 2021, 14, 1983. [Google Scholar] [CrossRef]
- Preciado-Hernandez, J.; Zhang, J.; Jones, I.; Zhu, M.; Zhang, Z.; Zhang, D. An Experimental Study of Gasification Kinetics during Steam Activation of a Spent Tyre Pyrolysis Char. J. Environ. Chem. Eng. 2021, 9, 105306. [Google Scholar] [CrossRef]
- Song, W.; Zhou, J.; Li, Y.; Li, S.; Yang, J. Utilization of Waste Tire Powder for Gaseous Fuel Generation via CO2 Gasification Using Waste Heat in Converter Vaporization Cooling Flue. Renew. Energy 2021, 173, 283–296. [Google Scholar] [CrossRef]
- Zhang, J.; Jones, I.; Zhu, M.; Zhang, Z.; Preciado-Hernandez, J.; Zhang, D. Pore Development during CO2 and Steam Activation of a Spent Tyre Pyrolysis Char. Waste Biomass Valorization 2021, 12, 2097–2108. [Google Scholar] [CrossRef]
- Grzywacz, P.; Czerski, G.; Gańczarczyk, W. Effect of Pyrolysis Atmosphere on the Gasification of Waste Tire Char. Energies 2021, 15, 34. [Google Scholar] [CrossRef]
- Irfan, M.; Li, A.; Zhang, L.; Liu, J.; Farooqi, T.J.A.; Javid, M.; Rauf, A. Waste Tire Derived Char Supported Ni-Fe Catalyst for Catalytic Thermochemical Conversion of Wet Municipal Solid Waste. Int. J. Energy Res. 2022, 46, 3634–3646. [Google Scholar] [CrossRef]
- Śpiewak, K.; Soprych, P.; Czerski, G. Influence of Pressure and Sunflower Husks Ash as Catalyst on Tire-Char Steam Gasification. Energy Rep. 2023, 9, 1–15. [Google Scholar] [CrossRef]
- Śpiewak, K.; Czerski, G.; Soprych, P. Steam Gasification of Tire Char Supported by Catalysts Based on Biomass Ashes. Energy 2023, 285, 129378. [Google Scholar] [CrossRef]
- Mastral, A.M.; Murillo, R.; García, T.; Navarro, M.V.; Callen, M.S.; López, J.M. Study of the Viability of the Process for Hydrogen Recovery from Old Tyre Oils. Fuel Process. Technol. 2002, 75, 185–199. [Google Scholar] [CrossRef]
- Okoye, C.O.; Jones, I.; Zhu, M.; Zhang, Z.; Zhang, D. Manufacturing of Carbon Black from Spent Tyre Pyrolysis Oil—A Literature Review. J. Clean. Prod. 2021, 279, 123336. [Google Scholar] [CrossRef]
- Gašparovič, L.; Šugár, L.; Jelemenský, Ľ.; Markoš, J. Catalytic Gasification of Pyrolytic Oil from Tire Pyrolysis Process. Chem. Pap. 2013, 67, 1504–1513. [Google Scholar] [CrossRef]
- Hrabovsky, M.; Konrad, M.; Hlina, M.; Kavka, T.; Chumak, O.M.; Maslani, A. Steam Plasma Gasification of Pyrolytic Oil from Used Tires. In Proceedings of the 20th International Symposium on Plasma Chemistry, Philadelphia, PA, USA, 24–29 July 2011; pp. 24–29. [Google Scholar]
- Cuadrat, A.; Abad, A.; García-Labiano, F.; Gayán, P.; de Diego, L.F.; Adánez, J. Relevance of the Coal Rank on the Performance of the in Situ Gasification Chemical-Looping Combustion. Chem. Eng. J. 2012, 195–196, 91–102. [Google Scholar] [CrossRef]
- Yan, Q.; Huang, J.; Zhao, J.; Li, C.; Xia, L.; Fang, Y. Investigation into the Kinetics of Pressurized Steam Gasification of Chars with Different Coal Ranks. J. Therm. Anal. Calorim. 2014, 116, 519–527. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, C.; Bai, J.; Wang, X.; Xing, L.; Li, X.; Han, B.; Kong, L.; Bai, Z.; Li, H.; et al. Comparative Study on the Effects of Heating Rate on Char Gasification Behaviors by Thermogravimetric Analyzer and High-Temperature Stage Microscope under Non-Isothermal Condition. Fuel 2023, 343, 127972. [Google Scholar] [CrossRef]
- Coetzee, G.H.; Sakurovs, R.; Neomagus, H.W.J.P.; Everson, R.C.; Mathews, J.P.; Bunt, J.R. Particle Size Influence on the Pore Development of Nanopores in Coal Gasification Chars: From Micron to Millimeter Particles. Carbon 2017, 112, 37–46. [Google Scholar] [CrossRef]
- Guizani, C.; Escudero Sanz, F.J.; Salvador, S. Influence of Temperature and Particle Size on the Single and Mixed Atmosphere Gasification of Biomass Char with H2O and CO2. Fuel Process. Technol. 2015, 134, 175–188. [Google Scholar] [CrossRef]
- Aranda, A.; Murillo, R.; García, T.; Callén, M.S.; Mastral, A.M. Steam Activation of Tyre Pyrolytic Carbon Black: Kinetic Study in a Thermobalance. Chem. Eng. J. 2007, 126, 79–85. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; He, Y.; Kong, D.; Klein, B.; Yin, S.; Zhao, H. Co-Pyrolysis Characteristics of Lignite and Biomass and Efficient Adsorption of Magnetic Activated Carbon Prepared by Co-Pyrolysis Char Activation and Modification for Coking Wastewater. Fuel 2022, 324, 124816. [Google Scholar] [CrossRef]
- Liou, T.-H.; Tseng, Y.-K.; Zhang, T.-Y.; Liu, Z.-S.; Chen, J.-Y. Rice Husk Char as a Sustainable Material for the Preparation of Graphene Oxide-Supported Biocarbons with Mesoporous Structure: A Characterization and Adsorption Study. Fuel 2023, 344, 128042. [Google Scholar] [CrossRef]
- Kumaravel, S.T.; Murugesan, A.; Kumaravel, A. Tyre Pyrolysis Oil as an Alternative Fuel for Diesel Engines—A Review. Renew. Sustain. Energy Rev. 2016, 60, 1678–1685. [Google Scholar] [CrossRef]
- Williams, P.T.; Taylor, D.T. Aromatization of Tyre Pyrolysis Oil to Yield Polycyclic Aromatic Hydrocarbons. Fuel 1993, 72, 1469–1474. [Google Scholar] [CrossRef]



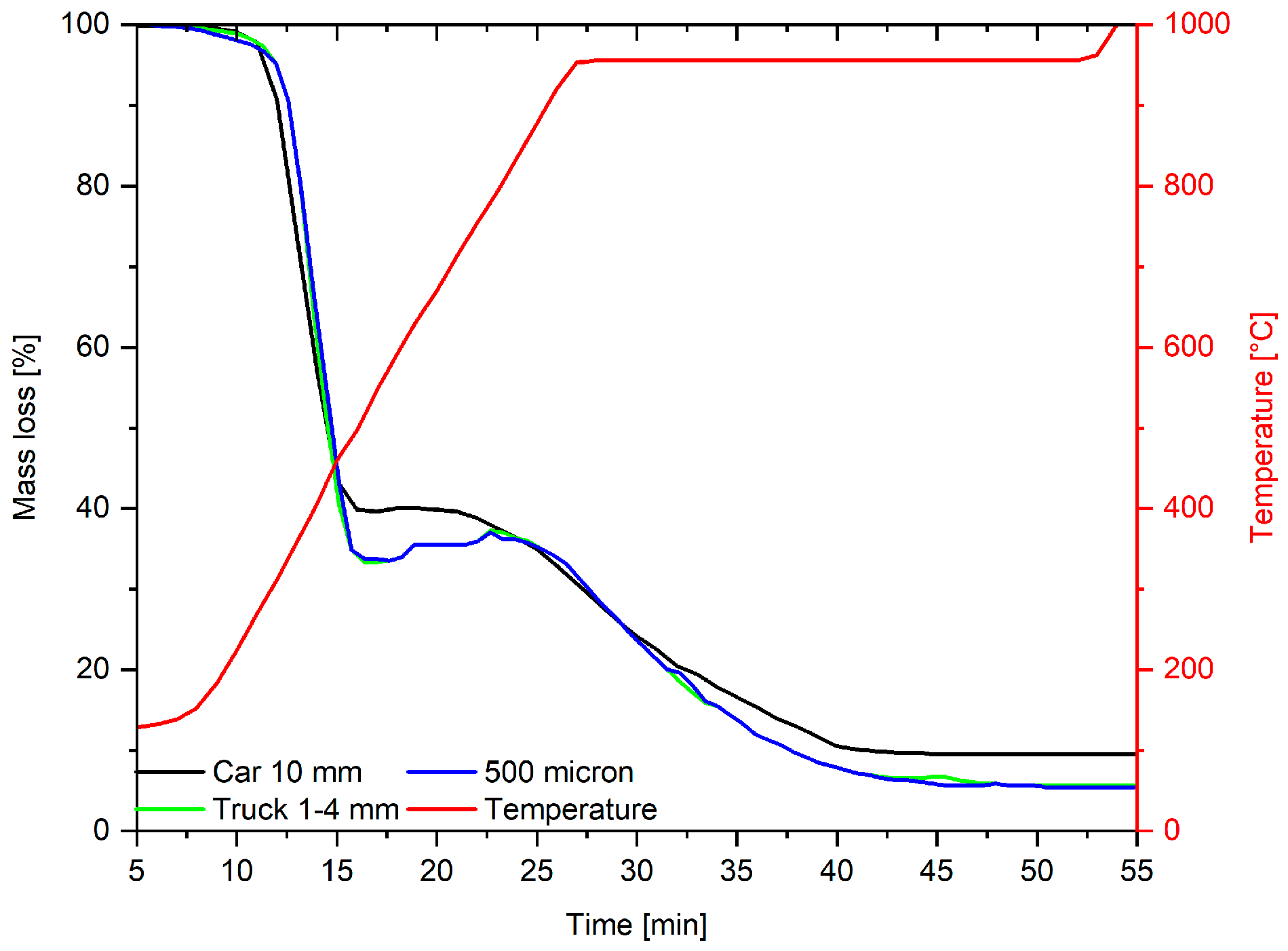
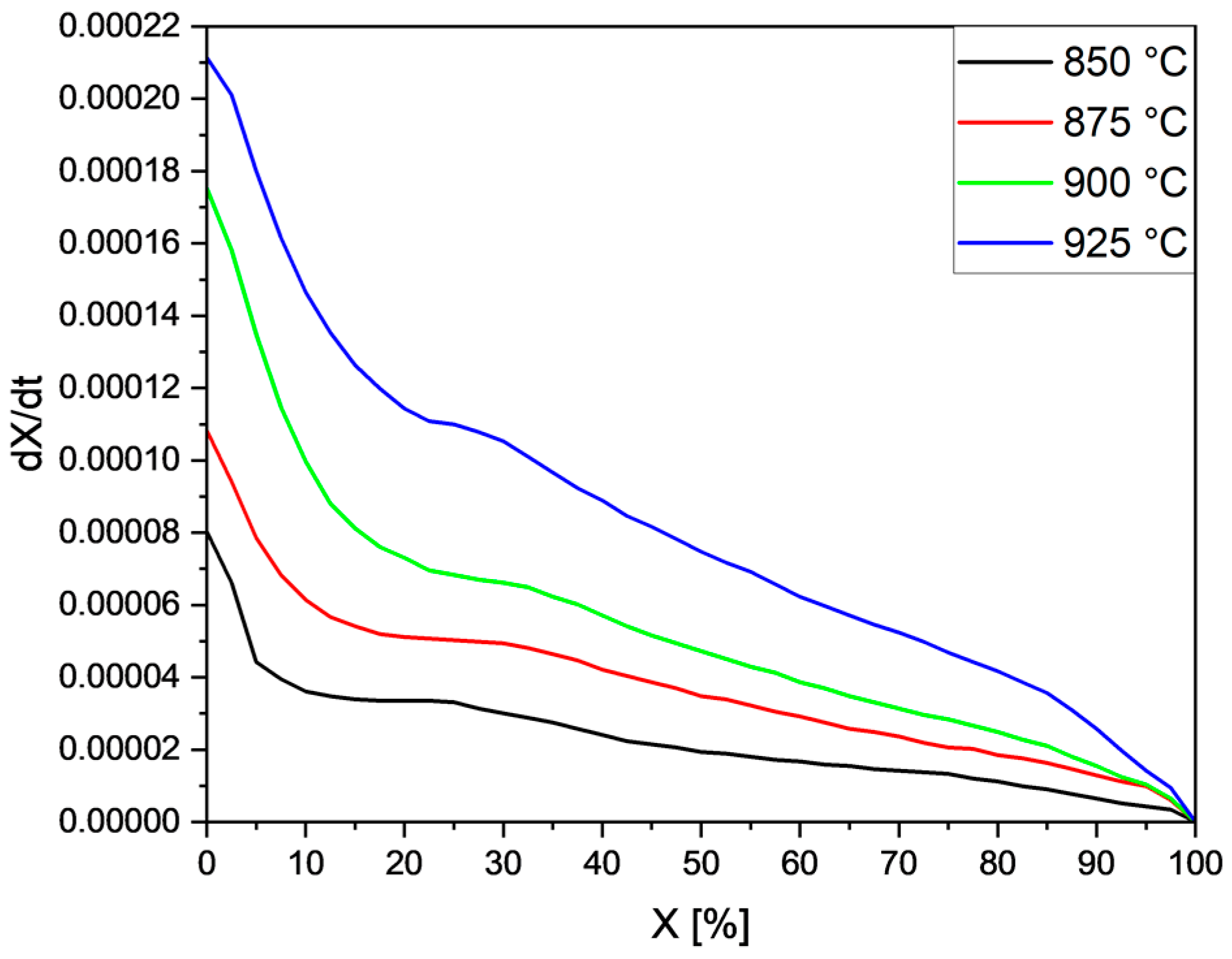
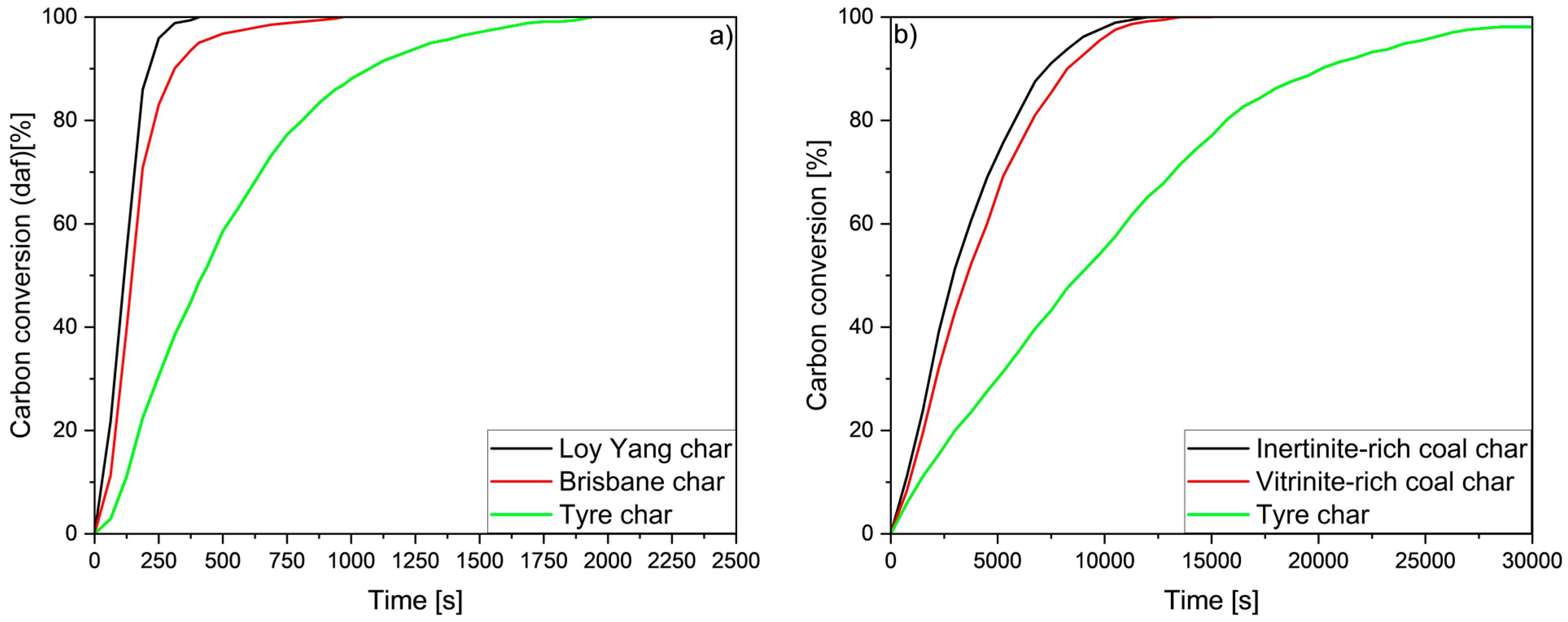

| Type of Tyres | Rubber | Carbon Black | Silica | Metals | Textile | Others * | |
|---|---|---|---|---|---|---|---|
| Natural | Synthetic | ||||||
| Car tyres [wt.%] | |||||||
| [19] | 14.0 | 27.0 | 28.0 | - | 14.0–15.0 | - | - |
| [29] | 22.0 | 23.0 | 28.0 | - | 13.0 | - | 14.0 |
| [36] a | 21.0–42.0 | 40.0–55.0 | 30.0–38.0 | - | - | - | 3.0–7.0 |
| [36] b | 41.0–48.0 | - | 22.0–28.0 | - | 13.0–16.0 | - | 4.0–6.0 |
| [37] | 21.2 | 24.5 | 18.9 | 7.7 | 10.8 | 3.7 | 13.1 |
| [38] | 47 | 22.5 | 14.0 | 5.5 | 11.0 | ||
| Truck tyres [wt.%] | |||||||
| [27] | 27.0 | 14.0 | 28.0 | - | 14.0–15.0 | - | 16.0–17.0 |
| [29] | 30.0 | 15.0 | 20.0 | - | 25.0 | - | 10.0 |
| [36] | 41.0–45.0 | 20.0–28.0 | - | 20.0–27.0 | - | 0.0–10.0 | |
| [37] | 37.1 | 10.0 | 22.3 | 1.3 | 21.1 | 0.2 | 8.0 |
| [38] | 45.0 | 21.0 | 23.5 | 1.0 | 9.5 | ||
| Other tyres [wt.%] | |||||||
| [27] | 47.0 | 22.0 | 12.0 | 10.0 | 9.0 | ||
| [39] | 48.0 | 22.0 | 15.0 | 5.0 | 10.0 | ||
| [40] | 51.0 | 25.0 | - | - | 4.5 | ||
| Material | Ultimate Analysis [wt.%] | Proximate Analysis [wt.%] | Calorific Value [MJ/kg] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O | FC | M | A | VM | HHV | |
| Waste tyre [66] | 74.8 1 | 5.5 1 | 0.7 1 | 1.1 1 | 9.0 2 | 25.2 2 | 1.2 3 | 8.9 3 | 64.8 3 | 35.1 3 |
| Waste tyre [67] | 85.9 4 | 6.8 4 | 0.7 4 | 2.2 4 | 4.5 2 | 24.1 2 | 2.3 3 | 7.3 3 | 66.3 3 | 33.3 3 |
| Mixture of trucks, tractors, and cars tyres [68] | 81.7 3 | 6.5 3 | 0.6 3 | 1.9 3 | - | - | 0.7 3 | 6.6 3 | 64.6 3 | - |
| Motorcycle tyre [69] | 75.5 1 | 6.8 1 | 0.8 1 | 1.4 1 | 15.5 2 | 20.9 3 | 1.5 3 | 20.1 3 | 57.5 3 | 29.1 1 |
| Waste tyre [70] | 82.1 1 | 7.6 1 | 0.6 1 | 2.1 1 | 7.5 1 | 33.4 1 | 0.9 1 | 5.2 1 | 60.6 1 | - |
| Waste tyre [71] | 73.8 1 | 6.8 1 | 0.3 1 | 1.3 1 | 9.0 1 | 23.2 | 1.0 | 8.8 1 | 68.0 | 36.0 1 |
| Car tyre [72] | 78.3 | 7.1 | 0.8 | - | 13.8 2 | 30.5 1 | 1.5 | 4.3 1 | 61.6 1 | 30.2 1 |
| Waste tyre [73] | 79.5 1 | 9.2 1 | 0.6 1 | 2.0 1 | 8.7 1 | 33.1 1 | 1.2 1 | 3.2 1 | 62.5 1 | - |
| Waste tyre [74] | 80.4 | 8.7 | 0.3 | 1.6 | 9.0 2 | 23.5 1 | 1.0 1 | 5.5 1 | 69.1 1 | 38.3 1 |
| Waste tyre [75] | 84.1 1 | 7.3 1 | 0.3 1 | 2.3 1 | 0.8 2 | 33.5 1 | 0.8 | 5.2 1 | 61.3 1 | 38.6 |
| Material [Reference] | Reactor | Catalyst | Particle Size [mm] | Measurement Conditions | Yields [%] | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature [°C] | Pressure | Heat. Rate [°C/min] | Gas | Liquid | Solid | |||||
| Waste tyre [88] | Fixed bed | - | 1–2 | 500 600 700 800 | atmospheric | 10 | 22.6 28.7 29.5 30.1 | 38.3 30.9 30.5 29.8 | 36.7 36.6 37.1 36.9 | |
| Light vehicle tyre Medium vehicle tyre Heavy vehicle tyre [89] | Batch reactor | - | 10–30 | 550 600 650 700 650 700 750 800 650 700 750 800 | 3 bars | 20 | 12.5 12.0 10.0 12.5 17.5 16.5 12.5 11.0 12.5 9.5 10.0 9.5 | 43.0 45.0 51.0 48.5 44.0 41.0 45.0 45.0 54.0 58.0 64.0 58.0 | 44.5 43.0 39.0 39.0 38.5 42.5 42.5 44.0 33.5 32.5 26.0 32.5 | |
| Waste tyre and blast-furnace slag [63] | B-W 1–4 B-W 3–2 B-W 2–3 B-W 4–1 B-W 1–4 B-W 3–2 B-W 2–3 B-W 4–1 B-W 1–4 B-W 3–2 B-W 2–3 B-W 4–1 | Rotary pyrolysis reactor | - | 0–10 | 600 800 1000 | atmospheric | - | 7.1 8.6 8.1 8.0 9.3 8.8 8.2 8.8 8.3 10.1 10.5 11.1 | 30.2 32.7 39.1 40.7 31.1 34.9 40.0 45.7 47.2 53.7 55.8 57.3 | 62.7 58.7 52.8 51.3 59.6 56.3 51.8 45.5 44.5 36.2 33.7 31.6 |
| [90] | Waste tyre Rice husk WT-RH 1–4 WT-RH 1–1 WT-RH 4–1 Wheat straw WT-WS 1–4 WT-WS 1–1 WT-WS 4–1 Moso bamboo WT-MB 1–4 WT-MB 1–1 WT-MB 4–1 | Fixed bed | - | 0.15–0.3 | 800 | atmospheric | 20 | 17.0 14.0 16.0 18.0 17.5 28.5 22.5 21.0 18.0 27.5 22.0 20.0 19.5 | 36.0 47.5 44.0 39.5 38.0 43.0 45.0 41.5 38.5 51.0 51.5 41.5 39.0 | 47.0 38.5 40.0 42.5 44.5 28.5 32.5 37.5 43.5 21.5 26.5 38.5 41.5 |
| Light vehicle tyre Case I Light vehicle tyre Case II [91] | Batch reactor | - CaCO3 Al2O3 ZSM-5 MgO - CaCO3 Al2O3 ZSM-5 MgO | 2 × 3 | 550 | atmospheric | 8–11 | 19.0 23.0 30.0 25.5 17.0 19.0 37.0 33.0 25.0 20.0 | 36.0 39.5 32.0 30.0 42.5 36.0 22.5 26.0 34.5 38.5 | 45.0 37.5 38.0 44.5 40.5 45.0 40.5 41.0 40.5 41.5 | |
| Waste tyre [92] | Semi-batch reactor | - Biochar | 2–3 | 500 | - | - | 41.0 40.0 | 25.5 24.5 | 33.5 35.5 | |
| Waste tyre [65] | - | - Na2CO3 NaOH | - | 450 500 550 600 450 500 550 600 450 480 500 520 550 | Vacuum (3.5–4.0 kPa) | 20 | 15.4 21.8 16.0 16.3 14.8 15.3 14.6 16.2 14.2 13.7 13.3 16.8 20.8 | 32.9 42.1 47.1 48.8 36.5 42.0 47.8 48.5 38.5 49.7 48.1 46.9 39.0 | 51.7 36.1 36.9 34.8 48.7 42.7 37.6 35.2 47.0 36.7 38.6 36.4 40.2 | |
| Reference | Conditions | Ultimate Analysis [wt.%] | Proximate Analysis [wt.%] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O | FC | M | A | VM | ||
| [108] | 9 W/g 4 | 82.3 | 0.5 | 0.5 | 3.0 | - | 82.1 | 0.1 | 16.3 | 1.5 |
| 15 W/g 4 | 82.7 | 0.4 | 0.4 | 2.9 | - | 82.7 | 0.1 | 16.1 | 1.1 | |
| 24 W/g 4 | 83.2 | 0.4 | 0.4 | 3.1 | - | 82.6 | 0.1 | 16.3 | 1.1 | |
| [88] | 500 °C | 82.8 | 0.6 | 0.4 | 4.0 | - | - | - | 12.2 | - |
| 600 °C | 83.0 | 0.4 | 0.4 | 4.5 | - | - | - | 11.7 | - | |
| 700 °C | 86.2 | 0.4 | 0.2 | 1.7 | - | - | - | 11.5 | - | |
| 800 °C | 85.6 | 0.3 | 0.2 | 2.0 | - | - | - | 11.9 | - | |
| [107] | 400 °C | 80.3 | 2.1 | 0.3 | 2.4 | 7.7 1 | - | - | 7.3 | - |
| 500 °C | 81.2 | 2.0 | 0.2 | 1.7 | 5.9 1 | - | - | 9.0 | - | |
| 600 °C | 81.3 | 1.8 | 0.2 | 2.3 | 4.2 1 | - | - | 10.3 | - | |
| 700 °C | 83.5 | 1.6 | 0.3 | 2.5 | 1.8 1 | - | - | 10.3 | - | |
| 800 °C | 83.3 | 1.0 | 0.2 | 2.5 | 0.1 1 | - | - | 13.0 | - | |
| 900 °C | 88.0 | 1.0 | 0.2 | 2.6 | 0.1 1 | - | - | 8.0 | - | |
| [111] | 500 °C | 74.6 | 1.1 | 0.5 | 2.5 | - | - | - | - | - |
| [112] | 850 °C Fixed bed | 77.4 2 | 0.4 2 | 0.4 2 | 2.3 2 | 3.1 2 | - | - | 17.2 2 | - |
| 850 °C Rotary oven | 73.9 2 | 0.4 2 | 0.4 2 | 2.5 2 | 3.5 2 | - | - | 20.4 2 | - | |
| [110] | 700 °C | 84.4 3 | 1.3 3 | 0.5 3 | 2.3 3 | - | 79.3 3 | 3.6 3 | 12.4 3 | 4.7 3 |
| [109] | 400 °C | 85.3 | 0.3 | 4.3 | - | - | 83.2 | 0.7 | 7.7 | 7.8 |
| 450 °C | 90.6 | 0.6 | 0.4 | - | - | 88.8 | 0.5 | 7.0 | 3.8 | |
| 500 °C | 88.8 | 0.6 | 0.5 | - | - | 87.6 | 1.0 | 6.5 | 4.9 | |
| 550 °C | 89.2 | 0.5 | 0.4 | - | - | 88.2 | 0.7 | 6.7 | 4.4 | |
| 600 °C | 89.1 | 0.5 | 0.4 | - | - | 88.9 | 0.5 | 6.8 | 3.7 | |
| Reference | Conditions | SBET [m2/g] | Vmicropore [cm3/g] | Vmesopore [cm3/g] | Vmacropore [cm3/g] | Vtotal [cm3/g] | Average Pore Diameter [nm] |
|---|---|---|---|---|---|---|---|
| [88] | 500 °C | 73.47 | 0.0022 | 0.1829 | 0.0110 | 0.1961 | 10.67 |
| 600 °C | 77.63 | 0.0047 | 0.1806 | 0.0092 | 0.1945 | 10.02 | |
| 700 °C | 71.55 | - | 0.3505 | 0.0167 | 0.3672 | 20.53 | |
| 800 °C | 70.87 | - | 0.3645 | 0.0776 | 0.4421 | 24.95 | |
| [107] | 400 °C | 10.0 | 0.000 | 0.023 | - | 0.023 | - |
| 500 °C | 156.0 | 0.024 | 0.099 | - | 0.123 | - | |
| 600 °C | 136.0 | 0.015 | 0.178 | - | 0.193 | - | |
| 700 °C | 96.0 | 0.013 | 0.174 | - | 0.187 | - | |
| 800 °C | 85.0 | 0.008 | 0.192 | - | 0.200 | - | |
| 900 °C | 87.0 | 0.008 | 0.104 | - | 0.112 | - | |
| [109] | 500 °C | 44.7 | - | - | - | 0.17 | 14.96 |
| [111] | 500 °C | 70.0 | - | - | - | 0.39 | 22.97 |
| [112] | 850 °C Fixed bed | 57.0 | 0.02 | 0.23 | - | 0.25 | |
| 850 °C Rotary oven | 68.0 | 0.02 | 0.25 | - | 0.27 | - | |
| [113] | 900 °C | 38.0 | - | - | - | - | - |
| Reference | Reactor | Material /Catalyst | Particle Size [mm] | Temperature [°C] | Pressure [MPa] | Gasifying Agent | Description |
|---|---|---|---|---|---|---|---|
| [116] | Fluidized bed | Tyre powder | 0.4–2.1 | 350–900 | - | Air | Evaluation of the effects of different process conditions (air amount and temperature) and raw materials (feeding and particle size) on the parameters characterising the gasification process and the obtained products. |
| [117] | Rotary furnace | Tyre char | ≤0.15 | 925–1100 | CO2, steam | Effects of temperature and type of gasification agent on the structure of pyrolysis tyre char during its activation. | |
| [118] | Fluidized bed | Chars of tyre, sewage sludge, rietspruit, activated carbon | 0.355–0.710 | 800–1000 | - | CO2 | Comparison of reactivity of tyre char and char from other materials. |
| [119] | Thermobalance | Chars of tyre, sewage sludge, and coal | – | 550–850 | - | Steam | Analysis of the kinetics of the gasification process of various chars, including char from tyre pyrolysis. |
| [120] | - | Tyre char | – | 750–900 | - | CO2, steam | Effects of temperature, activation time, and type of activation agent on the morphological properties of tyre char. |
| [121] | Fluidized bed | Waste tyre scrap, sewage sludge | 0.6–1.2 | 650–850 | - | Steam | Analysis of the possibility of co-gasification of used tyres with sewage sludge. |
| [122] | Fluidized bed | Waste tyre | – | 400–800 | - | Air | Analysis of the effect of temperature and air amount on the yields of gaseous and solid products. |
| [123] | Fixed bed | Tyre char | – | 800–900 | - | CO2 | Analysis of the effect of temperature, flow rate of the activating agent, and reaction time on the properties characterising the surface of the obtained solid product. |
| [124] | Rotary kiln reactor | Scrap tyre, RDF, poplar | – | 850 | 0.1 | Steam | Evaluation of steam gasification of various raw materials, including used tyres, along with a characterisation of the resulting gas products. |
| [125] | Fixed bed | Tyre char | ≤1 | 850–900 | 0.12 | Steam | Effect of temperature during steam activation of low-reactive tyre char. |
| [126] | Moving bed | Waste tyre char, lignite char | 0.2 | 850 | 2.7 | Steam–oxygen | Evaluation of the possibility of co-gasification of lignite and used tyres—qualitative analysis of the gas obtained and the parameters determining the course of the process. |
| [127] | Rotary kiln gasifier | Rubber tyre | – | 850 | - | Steam | Analysis of the effect of gasification agent concentration on gas fraction yield. |
| [128] | TGA | Scrap tyre, sewage sludge, industrial sludge, fluff | 0.1–30 | 1250 | 0.1 | Steam | Thermogravimetric gasification of various materials, including rubber from used tyres. |
| [129] | Fluidized bed | Scrap tyre | 0.6–1.0 | 720–820 | - | Steam, air–steam, air–CO2 | Effect of the type of gasification agent on the calorific value and composition of the gas obtained. |
| [130] | Fixed bed | Distillation scrap tyre char, semianthracite, medium and high-volatile bituminous coal | 0.1–0.2 | 1000 | 0.1–1.5 | Steam-oxygen | Analysis of the effect of pressure on the gasification of various materials and the composition of the resulting gas products. |
| [131] | Rotary kiln reactor | Waste tyre | ≤6 | 850–1000 | 0.1 | Steam | Analysis of the effect of the temperature of the tyre rubber gasification process on the characteristics of the obtained products. |
| [132] | Fixed bed | Tread rubber–Ni-Mg-Al | – | 800 | - | Steam | Gas product yields during a two-step pyrolysis/gasification reaction of tyre rubber tread in the presence of a Ni-Mg-Al catalyst. |
| [133] | Fixed bed | Rubber tread-Ni/CeO2/Al2O3 | ≤6 | 800 | - | Steam | Effect of the presence and amount of catalyst on yields and composition of gas products during two-stage pyrolysis–gasification of tyre rubber. |
| [134] | Rotary kiln reactor | Scrap tyre–olivine, dolomite | ≤2 | 0.1 | Steam | Effects of the presence of two types of catalysts on gas product yields and gas composition from the gasification/reforming of used tyres. | |
| [135] | Fixed bed | Rubber tread-Ni/Al2O3 | ≤6 | 600–900 | Steam | Effects of the presence and amount of catalytic additive, process temperature, and amount of steam on product yields and gas composition during a two-step pyrolysis/gasification reaction of tyre tread. | |
| [136] | - | Tyre char, palm empty fruit bunch, almond shell | ≤0.075 | 850–1000 | - | CO2 | Analysis of the gasification reaction course of tyre char with the addition of two types of biomass chars as potential catalysts. |
| [137] | Fixed bed | Tread rubber- Ni/Al2O3, Ni/dolomite | 800 | Steam | Effect of the addition of two catalysts on the yield of gaseous products during the two-stage pyrolysis–gasification process of tyre rubber. | ||
| [138] | - | Tyre char, coal chars | ≤0.075 | 900–975 | 0.1 | CO2 | Comparison of the gasification course, reactivity, and kinetic parameters of chars from tyre and coal pyrolysis, as well as their blends. |
| [30] | TGA | Scrap tyre | 0.5–10 | 1000 | - | Steam | Thermogravimetric study of the gasification process of rubber from car and truck tyres of different particle sizes. |
| [139] | Fixed bed | Tyre char, wood pellets | ≤1 | 700–900 | - | Steam | Possibilities of using tyre char to remove tar from biomass pyrolysis during steam gasification. |
| [140] | - | Tyre char, coal chars | 0.063–0.105 | 1000–1300 | - | CO2 | Comparison of reactivity and gasification kinetics of chars from pyrolysis of tyre and coal. |
| [141] | - | Tyre char, cattle manure, palm empty fruit bunch, almond shell, rubber seed shell | 0.150–0.212 | 1127 | - | CO2 | Effect of heating rate and addition of biomass as potential sources of catalytic compounds on the characteristics and kinetics of the gasification process of tyre char. |
| [142] | Tubular batch reactor | Scrap tyre-Ba(OH)2, Ca(OH)2, Mg(OH)2, Ni/SiO2-Al2O3, Ru/Al2O3 | – | 325–625 | 21–23 | Subcritical and supercritical steam | Analysis of the effect of process parameters and the presence of different types of catalysts on the gasification of used tyres under subcritical and supercritical conditions. |
| [74] | Fixed bed | Waste tyre | 0.5–1.0 | 600–800 | Atmospheric | Air, oxygen | Analysis of the effect of flow rate and type of gasification agent on product yields, when gasifying rubber from tyres. |
| [143] | Fixed bed | Rubber tread | 20 × 20 | 700–1000 | Atmospheric | CO2 | Effect of temperature on the yield of gas products and the quality of the obtained syngas. |
| [144] | Fixed bed | Tyre char | – | 750–1050 | Atmospheric | CO2 | Effect of gasification agent concentration, reaction time on conversion rate and kinetic parameters using several models during gasification/activation of tyre char. |
| [145] | - | Wood, weed, plastic, carton, waste tyre-zeolite catalyst A4 | 2–3 | 1100 | - | Oxygen | Analysis of the composition of the products obtained, during catalytic gasification of tyres and a mixture of various wastes. |
| [146] | Fixed bed | Waste tyre, pine bark | 15 × 15 | 800–900 | - | CO2 | Evaluating the effect of gasification temperature and the blend ratio of two feedstocks on the yield of gas components. |
| [147] | Fixed bed | Scrap tyre, pine sawdust | 20–30 | – | - | Oxygen | Effect of co-gasification of different mixtures of tyre rubber and pine sawdust on the characteristics of the resulting gas products. |
| [148] | - | Tyre char | 0.2–0.4 | 825–925 | - | CO2 | Evaluation of the kinetics of the gasification process of char from tyre pyrolysis using different kinetic models. |
| [149] | - | Tyre tread, sidewall tyre | ~0.2 | 850–925 | Atmospheric | CO2 | Analyses of the effect of the presence of minerals contained in various tyre fragments on the gasification process. |
| [150] | - | Tyre char, rambutan peel | 0.150–0.212 | 1127 | - | CO2 | Effect of different amounts of biomass additive on the kinetics of tyre char gasification process. |
| [151] | - | Plastic, paper, textiles, wood chips, RDF- tyre char | 2–5 | 700–900 | - | Air | The possibility of using tyre char as a potential catalyst for removing tar produced during the gasification of various materials and their mixtures. |
| [152] | TGA | Tyre char and ashes from corn cobs, beet pulp, sunflower husks, beech chips and coal | ≤0.2 | 1100 | 0.1 | CO2 | Analysis of the effect of the presence of different amounts of fly ash and biomass ashes as potential sources of compounds showing catalytic activity in the gasification process, on the reactivity of tyre char. |
| [153] | Downdraft gasifier | Wood char, sewage sludge char, tyre char | – | 1400 | - | Air | Comparison of the gasification capabilities of three chars obtained from different raw materials. |
| [154] | Fixed bed | Tyre char | ≤0.15 | 750–1050 | Atmospheric | Steam | The effect of the concentration of the gasification agent, as well as the reaction time, on the gasification/activation process, including the conversion rates achieved and the kinetic parameters determined using various models. |
| [155] | - | Waste tyre | ≤0.149 | 900–1100 | - | CO2 | Effect of temperature and particle size on the yield of the gas fraction obtained during gasification of used tyres. |
| [156] | Fixed bed | Tyre char | ≤0.15 | 750–1050 | - | CO2, steam | Evaluation of the effects of temperature, activation duration and activation agent concentration on the structural properties of the tyre char. |
| [157] | - | Tyre char | ≤0.2 | 1100 | 0.1 | CO2 | Influence of the type of atmosphere used during pyrolysis of waste tyres on the gasification process of the resulting tyre char. |
| [158] | - | MSW- Ni-tyre char, Fe-tyre char, Ni-Fe-tyre char | 1–2 | 700–850 | Atmospheric | Water form MSW | Evaluation of the feasibility of using nickel-iron catalysts applied to char from tyre pyrolysis by impregnation, during municipal waste gasification. |
| [66] | Fluidized bed | Waste tyre | – | 700–850 | - | Air, air + steam | Analysis of the effect of the type of atmosphere, the process temperature, and the ER equivalence ratio on the production of gaseous components during the gasification of used tyres. |
| [159] | Fixed bed | Tyre char, sunflower husk ash | <0.2 | 800–1000 | 0.5–1.0 | Steam | Evaluation of the effect of conditions (pressure and temperature) on the process and kinetic parameters during steam gasification of tyre char in the presence of 5–15% sunflower husk ash as a catalyst. |
| [160] | Fixed bed | Tyre char, ashes from corn cobs, beet pulp, sunflower husks, beech chips | <0.2 | 800–1000 | 1.0 | Steam | Evaluation of the effect of different biomass ashes addition as catalyst on the process and kinetic parameters during steam gasification of tyre char. |
| [161] | - | Pyrolysis oil from tyre | – | 1000–1500 | 0.1–5.0 | Oxygen/steam | Mass and energy balances for thermodynamic approach during the gasification of pyrolysis oil from tyre. |
| [162] | - | Tyre oil | – | – | Nitrogen + oxygen | The addition of excess oxygen (25 vol) to the mixture lowers the solid fraction yield by converting some of the feedstock into gas. | |
| [163] | Tubular reactor | Pyrolysis oil from a scrap tyre, dolomite, regenerated dolomite, | – | 600–800 | Steam | Evaluation of the feasibility of steam gasification of pyrolysis oil from a scrap tyres in the presence of a dolomite catalyst. | |
| [164] | Plasma rector | Pyrolytic oil from used tyres | 0.5 | 1700 | Steam/ CO2/O2 | Effect of steam/plasma gasification of pyrolysis oil from tyre on gas composition. |
| Reference | Gasifying Agent | Variable | Fraction [wt.%] | Gas Composition [vol.%] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Solid | Liquid | Gas | H2 | CO | CO2 | CH4 | C2+ | ||||
| [131] | Steam | Temperature [°C] | 850 | 43.4 | 27.0 | 34.7 | 51.5 * | 6.1 * | 3.0 * | 30.3 * | 9.1 * |
| 925 | 38.5 | 21.8 | 64.5 | 55.7 * | 19.1 * | 4.3 * | 15.7 * | 5.2 * | |||
| 1000 | 33.3 | 5.3 | 85.9 | 65.1 * | 17.4 * | 7.6 * | 8.7 * | 1.2 * | |||
| [132] | Steam | Catalyst addition | - | 32.0 | 41.5 | 25.9 | 24.7 | 3.2 | 3.8 | 37.3 | 31.1 |
| Ni-Mg-Al | 41.6 | 17.1 | 36.2 | 66.7 | 16.0 | 5.3 | 8.7 | 3.3 | |||
| [137] | Steam | Ni/Al2O3 catalyst addition | 1 cycle | 41.7 | 31.9 | 39.8 | 51.5 * | 9.8 * | 9.6 * | 16.6 * | 12.5 * |
| 2 cycles | 41.5 * | 9.2 * | 10.9 * | 22.1 * | 16.3 * | ||||||
| 3 cycles | 39.1 * | 9.2 * | 10.2 * | 23.8 * | 17.7 * | ||||||
| 4 cycles | 39.9 * | 9.5 * | 11.5 * | 21.4 * | 17.7 * | ||||||
| Ni/dolomite catalyst addition | 1 cycle | 38.0 | 32.7 | 50.1 | 60.8 * | 9.8 * | 15.1 * | 8.4 * | 5.9 * | ||
| 2 cycles | 52.7 * | 8.2 * | 13.3 * | 15.0 * | 10.8 * | ||||||
| 3 cycles | 52.9 * | 8.5 * | 13.2 * | 15.1 * | 10.3 * | ||||||
| 4 cycles | 53.9 * | 9.2 * | 13.5 * | 14.5 * | 8.9 * | ||||||
| [127] | Steam | Steam-tyres ratio [kgsteam/kgtyre] | 0.33 | - | - | - | 52.4 | 13.5 | 4.2 | 29.9 | - |
| 0.50 | - | - | - | 55.8 | 16.0 | 5.9 | 22.3 | - | |||
| 0.67 | - | - | - | 54.1 | 15.1 | 7.3 | 23.5 | - | |||
| 1.00 | - | - | - | 56.3 | 14.9 | 8.5 | 20.3 | - | |||
| 1.30 | - | - | - | 57.0 | 17.7 | 12.6 | 12.7 | - | |||
| [133] | Steam | CeO catalyst addition at 500 °C | 0 | 36.6 | 28.0 | 35.0 | 51.4 * | 10.0 * | 9.6 * | 16.6 * | 12.4 * |
| 5 | 32.8 | 28.2 | 34.3 | 49.6 * | 9.2 * | 11.1 * | 17.2 * | 12.9 * | |||
| 15 | 34.6 | 25.9 | 37.8 | 52.8 * | 10.4 * | 11.2 * | 14.5 * | 11.1 * | |||
| 30 | 35.3 | 27.6 | 34.9 | 57.2 * | 9.2 * | 10.6 * | 14.2 * | 8.8 * | |||
| CeO catalyst addition at 750 °C | 0 | 22.4 | 22.4 | 32.5 | - | - | - | - | - | ||
| 5 | 22.5 | 22.5 | 34.1 | - | - | - | - | - | |||
| 15 | 18.5 | 18.5 | 37.9 | - | - | - | - | - | |||
| 30 | 19.9 | 19.9 | 35.7 | - | - | - | - | - | |||
| Ni catalyst addition at 500 °C | 5 | 35.0 | 28.4 | 33.4 | 49.4 * | 8.4 * | 11.3 * | 16.4 * | 14.5 * | ||
| 10 | 34.6 | 25.9 | 37.8 | 53.0 * | 10.1 * | 11.1 * | 14.6 * | 11.2 * | |||
| 20 | 36.1 | 23.4 | 38.1 | 55.8 * | 11.1 * | 9.6 * | 14.2 * | 9.3 * | |||
| Ni catalyst addition at 750 °C | 5 | 34.7 | 24.2 | 35.4 | - | - | - | - | - | ||
| 10 | 34.4 | 18.5 | 37.9 | - | - | - | - | - | |||
| 20 | 35.7 | 17.7 | 37.8 | - | - | - | - | - | |||
| [135] | Steam | Catalyst: tyre ration [g/g] | 0.5 | 41.7 | 31.9 | 39.8 | 51.5 * | 9.8 * | 10.5 * | 17.0 * | 11.2 * |
| 1.0 | 45.3 | 16.9 | 44.4 | 62.5 * | 12.1 * | 7.2 * | 12.1 * | 6.1 * | |||
| 1.5 | 50.3 | 15.2 | 58.2 | 63.4 * | 17.6 * | 7.7 * | 8.5 * | 2.8 * | |||
| 2.0 | 52.3 | 8.7 | 51.2 | 68.2 * | 17.6 * | 7.9 * | 5.6 * | 0.7 * | |||
| Temperature [°C] | 600 | 43.3 | 38.6 | 14.3 | 62.1 * | 5.2 * | 7.3 * | 8.6 * | 16.8 * | ||
| 700 | 44.5 | 33.1 | 30.6 | 53.0 * | 8.8 * | 11.8 * | 13.3 * | 13.1 * | |||
| 800 | 41.7 | 31.9 | 39.8 | 51.5 * | 9.7 * | 9.7 * | 16.7 * | 12.5 * | |||
| 900 | 39.6 | 19.5 | 56.8 | 56.7 * | 15.2 * | 8.4 * | 15.0 * | 4.7 * | |||
| Water injected rate [g/h] | 2.85 | 43.5 | 31.8 | 30.4 | 50.6 * | 9.6 * | 6.6 * | 20.4 * | 12.8 * | ||
| 4.74 | 41.7 | 31.9 | 39.8 | 51.4 * | 9.8 * | 9.7 * | 16.6 * | 12.5 * | |||
| 10.4 | 37.5 | 29.2 | 48.4 | 52.1 * | 11.6 * | 11.4 * | 13.0 * | 11.9 * | |||
| 15.2 | 35.1 | 30.0 | 47.1 | 50.0 * | 11.0 * | 11.8 * | 13.1 * | 14.1 * | |||
| [151] | Steam | Wood chips/tyre char ratio | 0.0 | 2.7 | 13.1 | 84.3 | - | - | - | - | - |
| 0.2 | 3.1 | 6.2 | 90.7 | - | - | - | - | - | |||
| 0.5 | 1.2 | 7.7 | 91.1 | - | - | - | - | - | |||
| 1.0 | 2.4 | 4.7 | 93.0 | - | - | - | - | - | |||
| Paper/tyre char ratio | 0.0 | 17.9 | 4.8 | 77.3 | - | - | - | - | - | ||
| 0.2 | 15.7 | 7.1 | 77.2 | - | - | - | - | - | |||
| 0.5 | 15.4 | 4.5 | 80.2 | - | - | - | - | - | |||
| 1.0 | 16.2 | 5.0 | 78.8 | - | - | - | - | - | |||
| Textile/tyre char ratio | 0.0 | 2.7 | 4.6 | 92.7 | - | - | - | - | - | ||
| 0.2 | 2.3 | 5.9 | 91.7 | - | - | - | - | - | |||
| 0.5 | 2.6 | 3.7 | 93.7 | - | - | - | - | - | |||
| 1.0 | 2.6 | 3.7 | 93.7 | - | - | - | - | - | |||
| Plastics/tyretyre char ratio | 0.0 | 6.9 | 18.8 | 74.3 | - | - | - | - | - | ||
| 0.2 | 6.0 | 10.0 | 84.0 | - | - | - | - | - | |||
| 0.5 | 5.6 | 11.0 | 83.5 | - | - | - | - | - | |||
| 1.0 | 5.9 | 11.2 | 83.0 | - | - | - | - | - | |||
| RDF/tyre char ratio | 0.0 | 26.3 | 6.7 | 67.0 | - | - | - | - | - | ||
| 0.2 | 25.8 | 7.4 | 66.8 | - | - | - | - | - | |||
| 0.5 | 25.8 | 7.3 | 67.0 | - | - | - | - | - | |||
| 1.0 | 25.8 | 8.0 | 66.2 | - | - | - | - | - | |||
| [129] | Steam | - | - | - | - | 48.8 | 3.9 | 3.3 | 26.4 | - | |
| Air-steam | - | - | - | 22.6 | 4.9 | 9.6 | 11.9 | - | |||
| Air-CO2 | - | - | - | 30.7 | 5.5 | 12.8 | 15.6 | - | |||
| [145] | Oxygen | Zeolite A4 catalyst | 8.9 | 24.7 | 64.3 | 9.8 | 0.1 | 0.3 | 8.0 | 53.8 | |
| [158] | Water from MSW | Catalyst-Tyre Char | - | 14.2 * | 9.1 * | 70.5 * | 29.3 * | 23.4 * | 26.5 * | 13.9 * | 6.9 * |
| Ni | 12.4 * | 2.2 * | 94.2 * | 40.0 * | 24.5 * | 23.0 * | 7.8 * | 4.7 * | |||
| Fe | 12.9 * | 2.8 * | 91.8 * | 38.2 * | 22.0 * | 25.5 * | 9.4 * | 4.9 * | |||
| Ni-Fe | 12.6 * | 2.5 * | 96.6 * | 42.2 * | 21.1 * | 23.5 * | 9.0 * | 4.2 * | |||
| [74] | Air | Air flow rate 0.05 L/min | 600 °C | 51.2 | 11.1 | 37.7 | - | - | - | - | - |
| 700 °C | 49.2 | 11.8 | 39.2 | - | - | - | - | - | |||
| 800 °C | 51.1 | 12.4 | 36.5 | - | - | - | - | - | |||
| Air flow rate 0.10 L/min | 600 °C | 50.6 | 15.8 | 33.6 | - | - | - | - | - | ||
| 700 °C | 48.0 | 15.8 | 36.2 | - | - | - | - | - | |||
| 800 °C | 53.5 | 13.5 | 33.0 | - | - | - | - | - | |||
| Air flow rate 0.20 L/min | 600 °C | 48.8 | 19.1 | 32.1 | - | - | - | - | - | ||
| 700 °C | 50.6 | 17.1 | 32.3 | - | - | - | - | - | |||
| 800 °C | 49.7 | 10.7 | 39.6 | - | - | - | - | - | |||
| Air flow rate 0.30 L/min | 600 °C | 47.4 | 15.8 | 36.8 | - | - | - | - | - | ||
| 700 °C | 46.1 | 19.2 | 34.7 | - | - | - | - | - | |||
| 800 °C | 43.1 | 15.8 | 41.1 | - | - | - | - | - | |||
| Air flow rate 0.40 L/min | 600 °C | 50.9 | 14.5 | 34.6 | - | - | - | - | - | ||
| 700 °C | 47.3 | 19.0 | 33.7 | - | - | - | - | - | |||
| 800 °C | 48.8 | 16.0 | 35.2 | - | - | - | - | - | |||
| Air flow rate 0.50 L/min | 600 °C | 50.1 | 18.3 | 31.6 | - | - | - | - | - | ||
| 700 °C | 47.2 | 20.8 | 32.0 | - | - | - | - | - | |||
| 800 °C | 47.8 | 17.8 | 34.4 | - | - | - | - | - | |||
| Oxygen | Oxygen flow rate 0.01 L/min | 600 °C | 53.0 | 8.2 | 38.8 | - | - | - | - | - | |
| 700 °C | 50.4 | 12.7 | 36.9 | - | - | - | - | - | |||
| 800 °C | 52.1 | 10.7 | 37.2 | - | - | - | - | - | |||
| [122] | Air | Equivalence ratio 0.2 | 560 °C | - | - | - | 16.9 * | 41.7 * | 14.8 * | 9.8 * | 16.8 * |
| 650 °C | - | - | - | 17.4 * | 25.4 * | 15.5 * | 13.0 * | 28.7 * | |||
| 720 °C | - | - | - | 11.9 * | 18.0 * | 15.9 * | 15.1 * | 39.1 * | |||
| 800 °C | - | - | - | 11.4 * | 18.2 * | 17.0 * | 14.9 * | 38.5 * | |||
| Equivalence ratio 0.4 | 470 °C | - | - | - | 4.8 * | 53.6 * | 31.4 * | 2.4 * | 7.8 * | ||
| 550 °C | - | - | - | 13.8 * | 32.7 * | 30.5 * | 7.9 * | 15.1 * | |||
| 630 °C | - | - | - | 17.0 * | 22.3 * | 30.0 * | 4.9 * | 25.8 * | |||
| 710 °C | - | - | - | 10.8 * | 16.1 * | 27.3 * | 11.8 * | 34.0 * | |||
| Equivalence ratio 0.6 | 430 °C | - | - | - | 1.6 * | 36.5 * | 54.1 * | 2.3 * | 5.5 * | ||
| 550 °C | - | - | - | 7.2 * | 29.1 * | 47.8 * | 6.9 * | 9.0 * | |||
| 600 °C | - | - | - | 7.4 * | 22.4 * | 41.9 * | 5.1 * | 23.2 * | |||
| 700 °C | - | - | - | 4.4 * | 17.3 * | 38.5 * | 10.1 * | 29.7 * | |||
| [163] | Steam | Non-catalytic | 800 °C | - | - | - | 24.7 | 4.4 | 3.1 | 24.0 | - |
| 600 °C | - | - | - | 44.0 | 0.2 | 0.0 | 8.8 | - | |||
| Dolomite | 700 °C | - | - | - | 56.5 | 0.8 | 0.0 | 9.7 | - | ||
| 800 °C | - | - | - | 60.6 | 5.2 | 9.2 | 13.0 | - | |||
| Regenerated dolomite | 800 °C | - | - | - | 61.5 | 5.6 | 9.2 | 11.7 | - | ||
| 800 °C | - | - | - | 54.5 | 5.7 | 9.8 | 11.9 | - | |||
| [164] | Plasma/Steam | - | 1700 °C | 58.0 | 32.0 | 4.0 | 5.0 | - | |||
| Plasma/CO2 | 27.0 | 53.0 | 16.0 | 3.0 | - | ||||||
| Plasma/O2 | 48.0 | 47.0 | 3.0 | 1.5 | - | ||||||
| Plasma/CO2 + O2 | 19.0 | 54.0 | 25.0 | 1.5 | - | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soprych, P.; Czerski, G.; Grzywacz, P. Studies on the Thermochemical Conversion of Waste Tyre Rubber—A Review. Energies 2024, 17, 14. https://doi.org/10.3390/en17010014
Soprych P, Czerski G, Grzywacz P. Studies on the Thermochemical Conversion of Waste Tyre Rubber—A Review. Energies. 2024; 17(1):14. https://doi.org/10.3390/en17010014
Chicago/Turabian StyleSoprych, Piotr, Grzegorz Czerski, and Przemysław Grzywacz. 2024. "Studies on the Thermochemical Conversion of Waste Tyre Rubber—A Review" Energies 17, no. 1: 14. https://doi.org/10.3390/en17010014
APA StyleSoprych, P., Czerski, G., & Grzywacz, P. (2024). Studies on the Thermochemical Conversion of Waste Tyre Rubber—A Review. Energies, 17(1), 14. https://doi.org/10.3390/en17010014








