Tuning Hydrogen Storage Properties in La1−xYxNi4.5Cu0.5 (x = 0.1; 0.2; 0.3; 0.4, 0.5) Alloys
Abstract
:1. Introduction
2. Experimental Section
3. Results
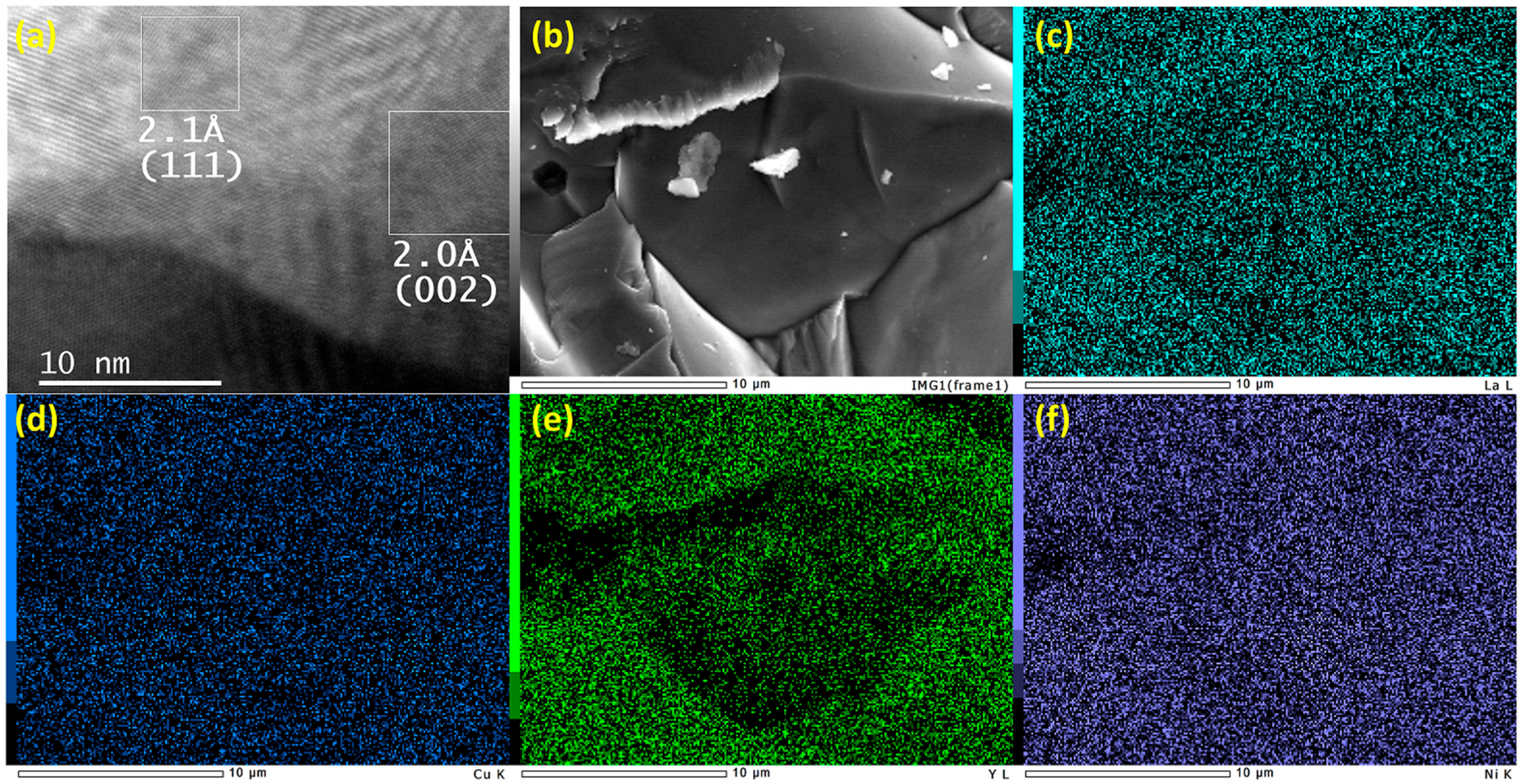
3.1. Structural Properties
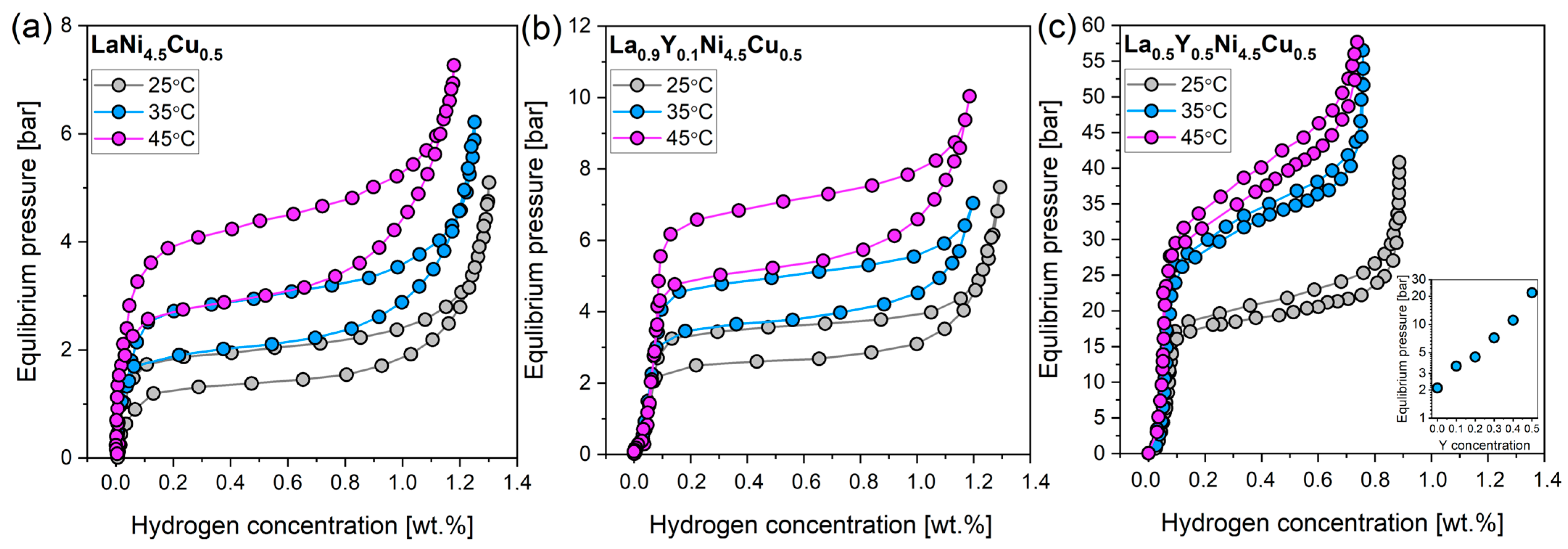
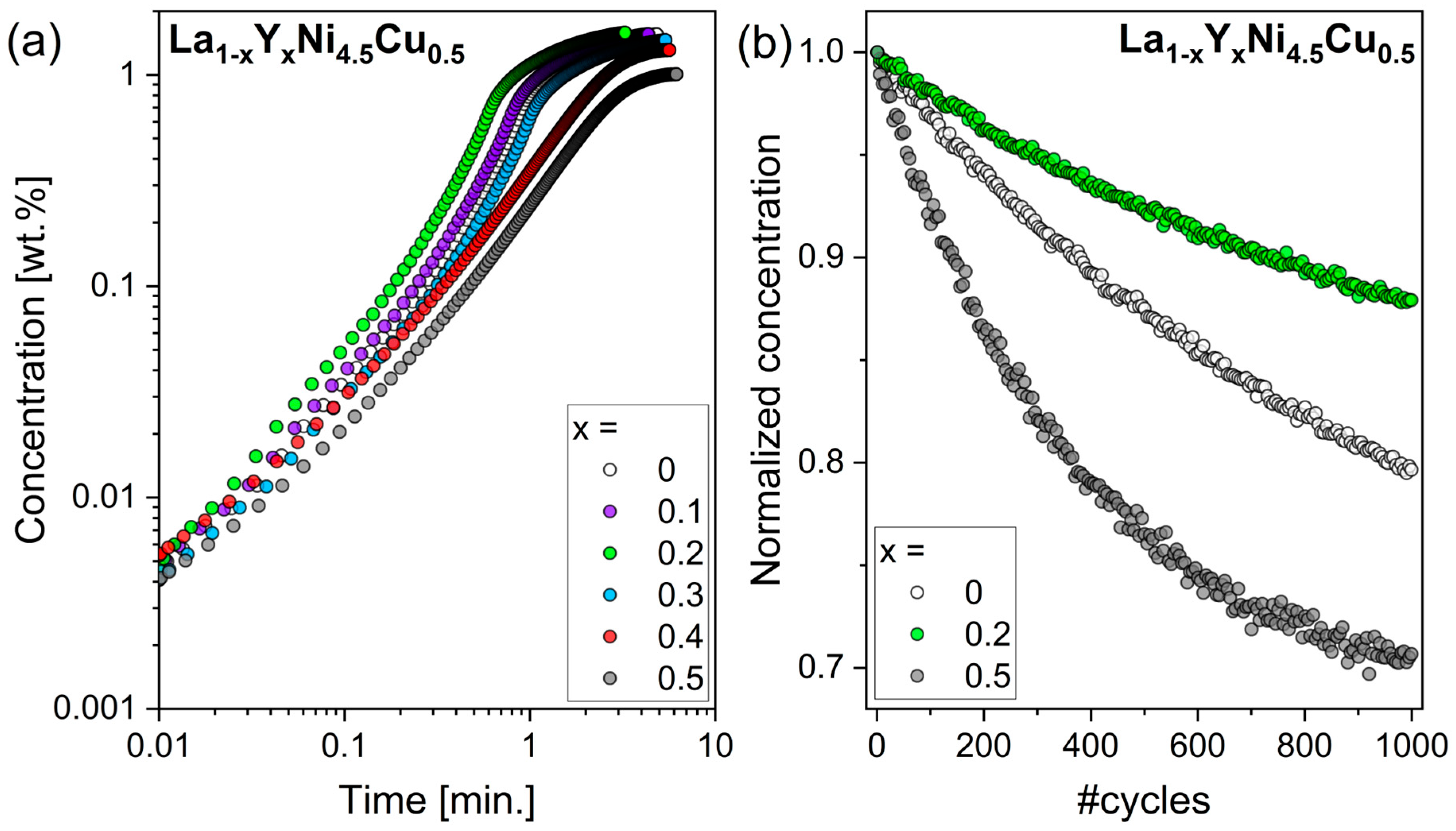
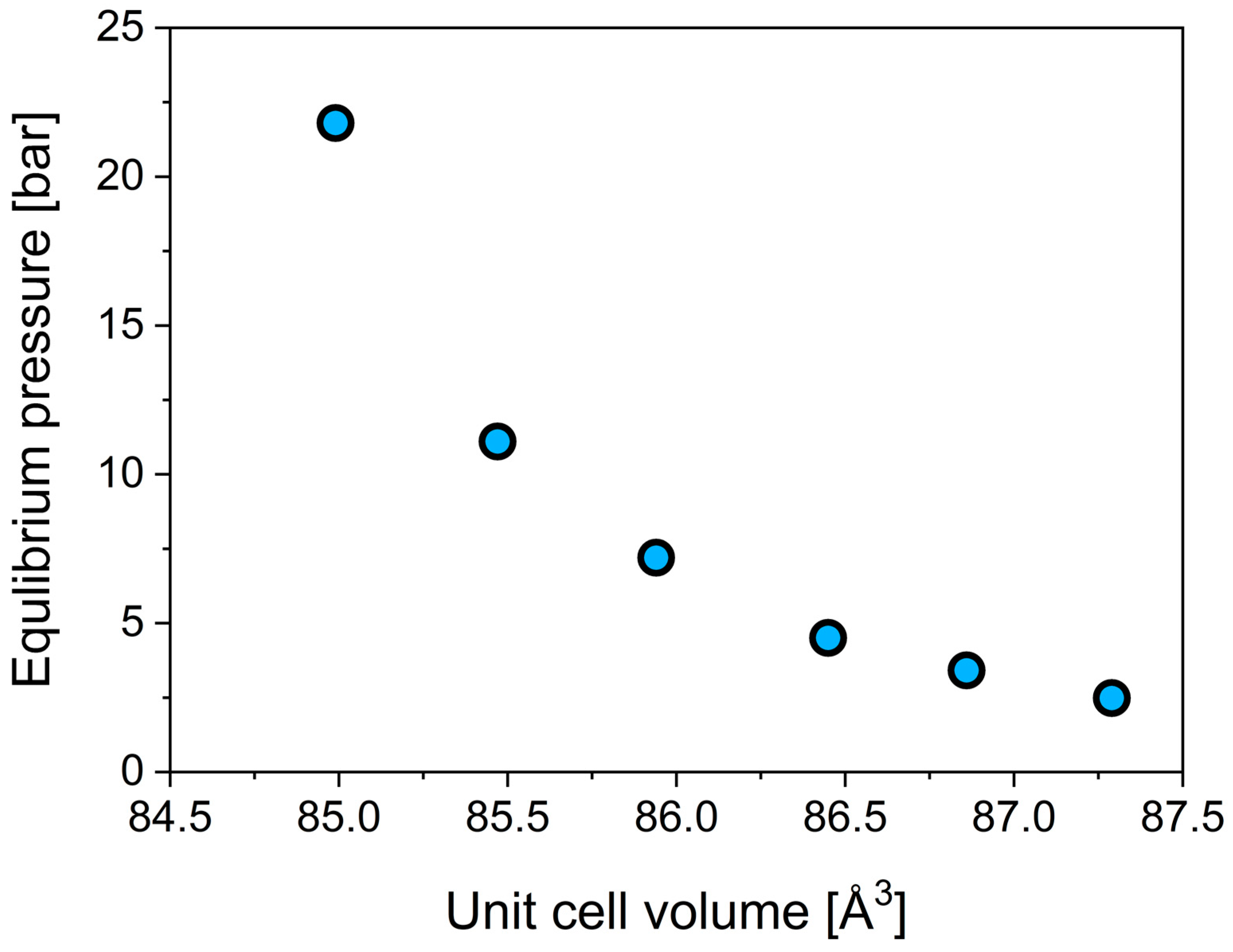
3.2. Hydrogen Sorption Properties
3.3. Structural Properties after Cycle Life Studies
4. Concluding Remarks and Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ivey, D.G.; Northwood, D.O. Storing energy in metal hydrides: A review of the physical metallurgy. J. Mater. Sci. 1983, 18, 321–347. [Google Scholar] [CrossRef]
- Tarasov, B.P.; Fursikov, P.V.; Volodin, A.A.; Bocharnikov, M.S.; Shimkus, Y.Y.; Kashin, A.M.; Yartys, V.A.; Chidziva, S.; Pasupathi, S.; Lototskyy, M.V. Metal hydride hydrogen storage and compression systems for energy storage technologies. Int. J. Hydrogen Energy 2021, 46, 13647–13657. [Google Scholar] [CrossRef]
- Ouyang, L.; Huang, J.; Wang, H.; Liu, J.; Zhu, M. Progress of hydrogen storage alloys for Ni-MH rechargeable power batteries in electric vehicles: A review. Mater. Chem. Phys. 2017, 200, 164–178. [Google Scholar] [CrossRef]
- Notten, P.H.L.; Hokkeling, P. Double-Phase Hydride Forming Compounds: A New Class of Highly Electrocatalytic Materials. J. Electrochem. Soc. 1991, 138, 1877–1885. [Google Scholar] [CrossRef]
- Lototskyy, M.; Nyamsi, S.N.; Pasupathi, S.; Wærnhus, I.; Vik, A.; Ilea, C.; Yartys, V. A concept of combined cooling, heating and power system utilising solar power and based on reversible solid oxide fuel cell and metal hydrides. Int. J. Hydrogen Energy 2018, 43, 18650–18663. [Google Scholar] [CrossRef]
- Wang, X.; Chen, R.; Zhang, Y.; Chen, C.; Wang, Q. Hydrogen storage alloys for high-pressure suprapure hydrogen compressor. J. Alloys Compd. 2006, 420, 322–325. [Google Scholar] [CrossRef]
- Modibane, K.D.; Williams, M.; Lototskyy, M.; Davids, M.W.; Klochko, Y.; Pollet, B.G. Poisoning-tolerant metal hydride materials and their application for hydrogen separation from CO2/CO containing gas mixtures. Int. J. Hydrogen Energy 2013, 38, 9800–9810. [Google Scholar] [CrossRef]
- Joubert, J.-M.; Paul-Boncour, V.; Cuevas, F.; Zhang, J.; Latroche, M. LaNi5 related AB5 compounds: Structure, properties and applications. J. Alloys Compd. 2021, 862, 158163. [Google Scholar] [CrossRef]
- Mishra, B.; Olson, D.L.; Termsuksawad, P. Sensing of hydrogen in advanced Ni-MH battery materials. Adv. Mater. Energy Convers. II 2004, II, 111–118. [Google Scholar]
- Ino, S.; Sato, M.; Hosono, M.; Izumi, T. Development of a soft metal hydride actuator using a laminate bellows for rehabilitation systems. Sens. Actuators B Chem. 2009, 136, 86–91. [Google Scholar] [CrossRef]
- Li, K.; Nuchkrua, T.; Zhao, H.; Yuan, Y.; Boonto, S. Learning-based Adaptive Robust Control of Manipulated Pneumatic Artificial Muscle Driven by H2-based Metal Hydride. In Proceedings of the 2018 IEEE 14th International Conference on Automation Science and Engineering (CASE), Munich, Germany, 20–24 August 2018; p. 8560584. [Google Scholar]
- Kuijpers, F.A.; van Mal, H.H. Sorption hysteresis in the LaNi5-H and SmCo5-H systems. J. Less Common Met. 1971, 23, 395–398. [Google Scholar] [CrossRef]
- Sakai, T.; Oguro, K.; Miyamura, H.; Kuriyama, N.; Kato, A.; Ishikawa, H.; Iwakura, C. Some factors affecting the cycle lives of LaNi5-based alloy electrodes of hydrogen batteries. J. Less Common Met. 1990, 161, 193–202. [Google Scholar] [CrossRef]
- Liu, J.; Li, K.; Cheng, H.; Yan, K.; Wang, Y.; Liu, Y.; Jin, H.; Zheng, Z. New insights into the hydrogen storage performance degradation and Al functioning mechanism of LaNi5−xAlx alloys. Int. J. Hydrogen Energy 2017, 42, 24904–24914. [Google Scholar] [CrossRef]
- Takeshita, T.; Gschneidner, K.A., Jr.; Lakner, J.F. High pressure hydrogen absorption study on YNi5, LaPt5 and ThNi5. J. Less Common Met. 1981, 78, P43–P47. [Google Scholar] [CrossRef]
- Takeshita, T.; Gschneidner, K.A., Jr.; Thome, D.K.; McMasters, O.D. Low-temperature heat-capacity study of Haucke compounds CaNi5, YNi5, LaNi5, and ThNi5. Phys. Rev. B 1980, 21, 5636–5641. [Google Scholar] [CrossRef]
- Mitrokhin, S.; Zotov, T.; Movlaev, E.; Verbetsky, V. Synthesis and properties of AB5-type hydrides at elevated pressures. J. Alloys Compd. 2007, 446–447, 603–605. [Google Scholar] [CrossRef]
- Spada, F.; Bowman, R.; Cantrell, J. Hydrogen absorption by LaCu5 and nuclear magnetic resonance (NMR) studies of hydrogen diffusion in β-LaCu5 hydride. J. Less Common Met. 1987, 129, 261–270. [Google Scholar] [CrossRef]
- Spodaryk, M.; Gasilova, N.; Züttel, A. Hydrogen storage and electrochemical properties of LaNi5−xCux hydride-forming alloys. J. Alloys Compd. 2019, 775, 175–180. [Google Scholar] [CrossRef]
- Endrzheevskaya, S.N.; Luk’Yanchikov, V.S.; Shablina, A.G.; Skorokhod, V.V.; Denbnovetskaya, E.N. Reactions of intermetallic compounds of the La-Ni-Cu system with hydrogen and hydrogen-containing gas mixtures. Powder Metall. Met. Ceram. 1984, 23, 710–713. [Google Scholar] [CrossRef]
- Kadir, K.; Sakai, T.; Uehara, I.; Eriksson, L. YCu3Al2, an example of an AB5 structure type. Acta Crystallogr. C 2001, 57, 999–1000. [Google Scholar] [CrossRef]
- CColinet, C.; Pasturel, A.; Percheron-Guégan, A.; Achard, J. Enthalpies of formation and hydrogenation of La(Ni(1−x)Cox)5 compounds. J. Less Common Met. 1987, 134, 109–122. [Google Scholar] [CrossRef]
- Pasturel, A.; Liautaud, F.; Colinet, C.; Allibert, C.; Percheron-Guégan, A.; Achard, J.C. Thermodynamic study of the LaNi5−xCux system. J. Less Common Met. 1984, 96, 93–97. [Google Scholar] [CrossRef]
- De Negri, S.; Solokha, P.; Saccone, A.; Pavlyuk, V. Isothermal section of the La-Ni-Zn system from 16.7 to 100 at% La at 400 °C. Intermetallics 2008, 16, 168–178. [Google Scholar] [CrossRef]
- Joubert, J.-M.; Charton, J.; Percheron-Guégan, A. Investigation of structural and hydrogen absorption properties in the LaNi5−xPtx-H2 system. J. Solid State Chem. 2003, 173, 379–386. [Google Scholar] [CrossRef]
- Prigent, J.; Joubert, J.-M.; Gupta, M. The phase diagrams of the ternary systems La-Ni-M (M = Re, Ru, Os, Rh, Ir, Pd, Ag, Au) in the La-poor region. Intermetallics 2011, 19, 295–301. [Google Scholar] [CrossRef]
- Diaz, H.; Percheronguegan, A.; Achard, J.; Chatillon, C.; Mathieu, J. Thermodynamic and structural properties of LaNi5−yAly compounds and their related hydrides. Int. J. Hydrogen Energy 1979, 4, 445–454. [Google Scholar] [CrossRef]
- Laurencelle, F.; Dehouche, Z.; Goyette, J. Hydrogen sorption cycling performance of LaNi4.8Sn0.2. J. Alloys Compd. 2006, 424, 266–271. [Google Scholar] [CrossRef]
- Yartys, V.A.; Denys, R.V.; Webb, C.J.; Mæhlen, J.P.; Gray, E.M.; Blach, T.; Isnard, O.; Barnsley, L.C. High pressure in situ diffraction studies of metal–hydrogen systems. J. Alloys Compd. 2011, 509S, S817–S822. [Google Scholar] [CrossRef]
- Sastri, M.V.C.; Viswanathan, B.; Murthy, S.S. Metal hydrides; Narosa Publishing House: New Delhi, India; Springer: Berlin, Germany, 1998. [Google Scholar]
- Kumar, E.A.; Maiya, M.P.; Murthy, S.S.; Viswanathan, B. Structural, hydrogen storage and thermodynamic properties of some mischmetal-nickel alloys with partial substitutions for nickel. J. Alloys Compd. 2009, 476, 92–97. [Google Scholar] [CrossRef]
- Kumar, S.V.; Kumar, E.A.; Maiya, M.P.; Murthy, S.S. Experimental and theoretical studies on static and dynamic pressure-concentration isotherms of MmNi5−xAlx (x = 0, 0.3, 0.5 and 0.8) hydrides. Int. J. Hydrogen Energy 2014, 39, 18940–18951. [Google Scholar]
- Shinar, J.; Shaltiel, D.; Davidov, D.; Grayevsky, A. Hydrogen sorption properties of the La1−xCaxNi5 and La(Ni1−xCux)5 systems. J. Less Common Met. 1978, 60, 209–219. [Google Scholar] [CrossRef]
- Herbrig, K.; Pohlmann, C.; Gondek, Ł.; Figiel, H.; Kardjilov, N.; Hilger, A.; Manke, I.; Banhart, J.; Kieback, B.; Röntzsch, L. Investigations of the structural stability of metal hydride composites by in-situ neutron imaging. J. Power Source 2015, 293, 109–118. [Google Scholar] [CrossRef]
- Heubner, F.; Hilger, A.; Kardjilov, N.; Manke, I.; Kieback, B.; Gondek, Ł.; Banhart, J.; Röntzsch, L. In-operando stress measurement and neutron imaging of metal hydride composites for solid-state hydrogen storage. J. Power Source 2018, 397, 262–270. [Google Scholar] [CrossRef]
- Available online: https://www.energy.gov/eere/vehicles/articles/us-drive-hydrogen-storage-technical-team-roadmap (accessed on 12 July 2023).
- von Colbe, J.B.; Ares, J.-R.; Barale, J.; Baricco, M.; Buckley, C.; Capurso, G.; Gallandat, N.; Grant, D.M.; Guzik, M.N.; Jacob, I.; et al. Application of hydrides in hydrogen storage and compression: Achievements, outlook and perspectives. Int. J. Hydrogen Energy 2019, 44, 7780–7808. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Czub, J.; Jamka, W.; Przewoźnik, J.; Zarzecka, A.; Hoser, A.; Wallacher, D.; Grimm, N.; Gondek, Ł. Structural peculiarities in the β phase of the La0.75Ce0.25Ni4.8Al0.2 deuterides. J. Alloys Compd. 2019, 788, 533–540. [Google Scholar] [CrossRef]

| Sample | a (Å) | c (Å) | V (Å3) | Strain (%) |
|---|---|---|---|---|
| LaNi4.5Cu0.5 | 5.0203(1) | 3.9991(1) | 87.29(1) | 0.211(6) |
| La0.9Y0.1Ni4.5Cu0.5 | 5.0102(1) | 3.9957(2) | 86.86(1) | 0.216(8) |
| La0.8Y0.2Ni4.5Cu0.5 | 5.0001(1) | 3.9930(1) | 86.45(1) | 0.206(7) |
| La0.7Y0.3Ni4.5Cu0.5 | 4.9867(1) | 3.9909(1) | 85.94(1) | 0.196(7) |
| La0.6Y0.4Ni4.5Cu0.5 | 4.9732(1) | 3.9904(1) | 85.47(1) | 0.209(7) |
| La0.5Y0.5Ni4.5Cu0.5 | 4.9605(1) | 3.9884(1) | 84.99(1) | 0.214(6) |
| Sample | #Cycles to Full Activation | ΔHABS ΔHDES (kJ/moleH2) | ΔSABS ΔSDES (kJ/moleH2K) | Max. Uptake (wt.%) | Pabs (bar) | Pdes (bar) |
|---|---|---|---|---|---|---|
| LaNi4.5Cu0.5 | 3 | −30.1(1) 30.9(1) | −107.3(3) 106.8(2) | 1.56(1) | 2.1 | 1.4 |
| La0.9Y0.1Ni4.5Cu0.5 | 7 | −27.2(1) 27.5(1) | −99.8(4) 100.4(3) | 1.56(1) | 3.6 | 2.7 |
| La0.8Y0.2Ni4.5Cu0.5 | 6 | −26.9(1) 29.0(1) | −104.2(3) 107.7(3) | 1.58(1) | 4.5 | 3.5 |
| La0.7Y0.3Ni4.5Cu0.5 | 6 | −26.5(1) 28.7(1) | −105.9(3) 109.0(3) | 1.46(1) | 7.2 | 5.8 |
| La0.6Y0.4Ni4.5Cu0.5 | 31 | −26.0(1) 29.2(1) | −107.2(3) 116.2(4) | 1.31(1) | 11.1 | 11.7 |
| La0.5Y0.5Ni4.5Cu0.5 | 46 | −23.6(1) 26.5(1) | −105.1(2) 114.1(2) | 1.01(1) | 21.8 | 19.6 |
| Sample | a (Å) | c (Å) | V (Å3) | Strain (%) |
|---|---|---|---|---|
| LaNi4.5Cu0.5 | 5.0211(1) | 3.9998(1) | 87.33(1) | 0.523(9) |
| La0.9Y0.1Ni4.5Cu0.5 | 5.0110(1) | 3.9963(1) | 86.90(1) | 0.482(9) |
| La0.8Y0.2Ni4.5Cu0.5 | 5.0021(1) | 3.9941(1) | 86.55(1) | 0.412(8) |
| La0.7Y0.3Ni4.5Cu0.5 | 4.9892(1) | 3.9982(1) | 86.19(1) | 0.487(8) |
| La0.6Y0.4Ni4.5Cu0.5 | 4.9796(1) | 3.9978(1) | 85.85(1) | 0.591(8) |
| La0.5Y0.5Ni4.5Cu0.5 + AMO | 4.9674(1) | 3.9899(1) | 85.26(1) | 0.638(8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mańka, M.; Kinoshita, T.; Nowak, A.; Ludwik, A.; Klimkowicz, A.; Takasaki, A.; Gondek, Ł. Tuning Hydrogen Storage Properties in La1−xYxNi4.5Cu0.5 (x = 0.1; 0.2; 0.3; 0.4, 0.5) Alloys. Energies 2024, 17, 71. https://doi.org/10.3390/en17010071
Mańka M, Kinoshita T, Nowak A, Ludwik A, Klimkowicz A, Takasaki A, Gondek Ł. Tuning Hydrogen Storage Properties in La1−xYxNi4.5Cu0.5 (x = 0.1; 0.2; 0.3; 0.4, 0.5) Alloys. Energies. 2024; 17(1):71. https://doi.org/10.3390/en17010071
Chicago/Turabian StyleMańka, Mateusz, Tomohiro Kinoshita, Anita Nowak, Aleksandra Ludwik, Alicja Klimkowicz, Akito Takasaki, and Łukasz Gondek. 2024. "Tuning Hydrogen Storage Properties in La1−xYxNi4.5Cu0.5 (x = 0.1; 0.2; 0.3; 0.4, 0.5) Alloys" Energies 17, no. 1: 71. https://doi.org/10.3390/en17010071
APA StyleMańka, M., Kinoshita, T., Nowak, A., Ludwik, A., Klimkowicz, A., Takasaki, A., & Gondek, Ł. (2024). Tuning Hydrogen Storage Properties in La1−xYxNi4.5Cu0.5 (x = 0.1; 0.2; 0.3; 0.4, 0.5) Alloys. Energies, 17(1), 71. https://doi.org/10.3390/en17010071








