Automotive e-Fuels via Hydrocracking of FT-Wax: e-Gasoline and e-Diesel Production
Highlights
- Hydrocracking activity of Ni-W was better over zeolite-supported Ni-W considering e-gasoline selectivity and hydrogen consumption
- Optimal hydrocracking operation achieved at 8.3 MPa pressure, 603 K temperature, and 2500 scfb H2/oil
- E-gasoline and e-diesel were produced via solar hydrogen fed hydrocracking of Fischer–Tropsch wax
- E-gasoline and e-diesel abide by key EN228 and EN590 specs respectively
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock
2.2. Testing Infrastructure
2.3. Experimental Procedure
2.4. Analysis
2.5. Storage Stability Study
3. Results
3.1. Evaluation of Ni-W Catalyst
3.2. Evaluation of NiW Zeolite-Supported Catalyst
3.3. Evaluation of NiW Al2O3—SiO2 Supported Catalyst
3.4. Product Evaluation
3.5. Storage Stability Study
4. Discussion
5. Conclusions
- −
- The optimum conditions for the NiW catalyst include a 8.3 MPa pressure, 603 K temperature, 1 hr−1 LHSV, and 2500 scfb H2/oil ratio.
- −
- The optimum conditions for the NiW zeolite-supported catalyst include a 8.3 MPa pressure, 588 K temperature, 1 hr−1 LHSV, and 3000 scfb H2/oil ratio.
- −
- Crushing the NiW Al2O3—SiO2-supported catalyst into smaller-diameter particles to fit into the reactor destroyed its activity, leading to no hydrocracking reactions.
- −
- The optimum Ni-W catalyst product rendered a higher e-gasoline content and lower residue content compared to that of the Ni-W zeolite-supported catalyst, while the hydrogen consumption was about 40% lower compared to that achieved by the NiW zeolite-supported catalyst.
- −
- High-quality e-gasoline, e-diesel, and heavy e-fuel were produced via the hydrocracking of wax from Fischer–Tropsch synthesis, also exhibiting significant stability over a maximum storage period of 4 months in the examined conditions.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations—Symbols
| CERTH | Centre for Research & Technology Hellas |
| CLG | Chemical Looping Gasification |
| CPERI | Chemical Process & Energy Resources Institute |
| DHD | Dehydrogenation |
| DMDS | Di-methyl-di-sulfide |
| DOS | Days On Stream |
| FT | Fischer–Tropsch |
| GHG | Green House Gas emissions |
| HAGO | Heavy Atmospheric Gas Oil |
| HD | Hydrogenation |
| HHV | High Heating Value |
| I.D. | Inlet diameter (referred to hydrotreating reactor) |
| KIST | Korea Institute of Science and Technology |
| LAGO | Light Atmospheric Gas Oil |
| LEFH | Laboratory of Environmental Fuels and Hydrocarbons |
| LHSV | Liquid Hourly Space Velocity |
| LPG | Liquified Petroleum Gas |
| NA | Not Available |
| RON | Research Octane Number |
| RVP | Reid vapor pressure |
| TAN | Total Acid Number |
| TRL | Technology Readiness Level |
| VGO | Vacuum Gas Oil |
| WC | Water Content |
| WHSV | Weight Hourly Space Velocity |
References
- Concawe, Environmental Science for European Refining, Aramco. In E-Fuels: A Technoeconomic Assessment of European Domestic Production and Imports Towards 2050; Report No. 17/22; Concawe: Brussels, Belgium, 2022.
- Chemical Looping Gasification for Sustainable Production of Biofuels—CLARA. Available online: www.clara-h2020.eu (accessed on 18 November 2023).
- Suo, Y.; Yao, Y.; Zhang, Y.; Xing, S.; Yuan, Z.Y. Recent advances in cobalt-based Fischer-Tropsch synthesis catalysts. J. Ind. Eng. Chem. 2022, 115, 92–119. [Google Scholar] [CrossRef]
- Sage, V.; Sun, Y.; Hazewinkel, P.; Bhatelia, T.; Braconnier, L.; Tang, L.; Chiang, K.; Batten, M.; Burke, N. Modified product selectivity in Fischer-Tropsch synthesis by catalyst pre-treatment. Fuel Process. Technol. 2017, 167, 183–192. [Google Scholar] [CrossRef]
- Leckel, D.; Liwanga-Ehumbu, M. Diesel-selective hydrocracking of an iron-based-Fischer-Tropsch wax fraction (C15-C45) using a MoO3-modified noble metal catalyst. Energy Fuels 2006, 20, 2330–2336. [Google Scholar] [CrossRef]
- Tomasek, S.; Lonyi, F.; Valyon, J.; Wollmann, A.; Hancsók, J. Hydrocracking of Fischer–Tropsch Paraffin Mixtures over Strong Acid Bifunctional Catalysts to Engine Fuels. ACS Omega 2020, 5, 26413–26420. [Google Scholar] [CrossRef]
- Bouchy, C.; Hastoy, G.; Guillon, E.; Martens, J.A. Fischer-Tropsch Waxes Upgrading via Hydrocracking and Selective Hydroisomerization. Oil Gas Sci. Technol.-Rev. IFP 2009, 64, 91–112. [Google Scholar] [CrossRef]
- Pleyer, O.; Petr, S.; Dan, V.; Jiří, H.; Radek, Č. Hydrocracking of fischer-tropsch wax. PALIVA 2020, 12, 26–33. [Google Scholar] [CrossRef]
- Hodala, J.L.; Jung, J.S.; Yang, E.H.; Hong, G.H.; Noh, Y.S.; Moon, D.J. Hydrocracking of FT-wax to fuels over non-noble metal catalysts. Fuel 2016, 185, 339–347. [Google Scholar] [CrossRef]
- Li, T.; Tao, Z.; Hu, C.; Zhao, C.; Yi, F.; Zhao, G.; Zhang, L.; Yang, Y. Brønsted acidity of amorphous silica-aluminas for hydrocracking of Fischer-Tropsch wax into diesel fractions. Appl. Catal. A Gen. 2022, 630, 118439. [Google Scholar] [CrossRef]
- Fratczak, J.; Carmona, H.P.; Tisler, Z.; Herrador, J.M.H.; Gholami, Z. Hydrocracking of heavy fischer-tropsch wax distillation residues and its blends with vacuum gas oil using phonolite-based catalysts. Molecules 2021, 26, 7172. [Google Scholar] [CrossRef]
- Gregor, J.H. Fischer-Tropsch products as liquid fuels or chemicals. Catal. Lett. 1990, 7, 317–331. [Google Scholar] [CrossRef]
- Gamba, S.; Pellegrini, L.A.; Calemma, V.; Gambaro, C. Liquid fuels from Fischer–Tropsch wax hydrocracking: Isomer distribution. Catal. Today 2010, 156, 58–64. [Google Scholar] [CrossRef]
- Calemma, V.; Gambaro, C.; Parker, W.O., Jr.; Carbone, R.; Giardino, R.; Scorletti, P. Middle distillates from hydrocracking of FT waxes: Composition, characteristics and emission properties. Catal. Today 2010, 149, 40–46. [Google Scholar] [CrossRef]
- Yan, P.-H.; Tao, Z.-C.; Hao, K.; Wang, Y.-D.; Yang, Y.; Li, Y.-W. Effect of impregnation methods on nickel-tungsten catalysts and its performance on hydrocracking Fischer-Tropsch wax. J. Fuel Chem. Technol. 2013, 41, 691–697. [Google Scholar] [CrossRef]
- O’Connell, A.; Georgiadou, M.; Schleker, T. Advanced Alternative Fuels. Technolgy Development Report; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar] [CrossRef]
- Standards EN 590; Automotive Fuels—Diesel—Requirements and Test Methods. European Committee: Brussels, Belgium, 2009.
- Standard ISO/IEC 19540-1; Information Technology—Object Management Group Unified Architecture Framework (OMG UAF)—Part 1: Domain Metamodel (DMM). ISO and IEC Joint Technical Committee: Washington, DC, USA, 2022.
- Dimitriadis, A.; Seljak, T.; Vihar, R.; Baškovič, U.Z.; Dimaratos, A.; Bezergianni, S.; Samaras, Z.; Katrašnik, T. Improving PM-NOx trade-off with paraffinic fuels: A study towards diesel engine optimization with HVO. Fuel 2020, 265, 116921. [Google Scholar] [CrossRef]
- Kirsch, H.; Sommer, U.; Pfeifer, P.; Dittmeyer, R. Power-to-fuel conversion based on reverse water-gas-shift, Fischer-Tropsch Synthesis and hydrocracking: Mathematical modeling and simulation in Matlab/Simulink. Chem. Eng. Sci. 2020, 227, 115930. [Google Scholar] [CrossRef]
- Kirsch, H.; Lochmahr, N.; Staudt, C.; Pfeifer, P.; Dittmeyer, R. Production of CO2-neutral liquid fuels by integrating Fischer-Tropsch synthesis and hydrocracking in a single micro-structured reactor: Performance evaluation of different configurations by factorial design experiments. Chem. Eng. J. 2020, 393, 124553. [Google Scholar] [CrossRef]
- Vedachalam, S.; Boahene, P.; Dalai, A.K. Production of jet fuel by hydrorefining of Fischer-Tropsch wax over Pt/Al-TUD-1 bifunctional catalyst. Fuel 2021, 300, 121008. [Google Scholar] [CrossRef]
- Dimitriadis, A.; Chrysikou, L.P.; Meletidis, G.; Terzis, G.; Auersvald, M.; Kubicka, D.; Bezergianni, S. Bio-based refinery intermediate production via hydrodeoxygenation of fast pyrolysis bio-oil. Renew. Energy 2021, 168, 593–605. [Google Scholar] [CrossRef]
- Ziogou, C.; Ipsakis, D.; Seferlis, P.; Bezergianni, S.; Papadopoulou, S.; Voutetakis, S. Optimal production of renewable hydrogen based on an efficient energy management strategy. Energy 2013, 55, 58–67. [Google Scholar] [CrossRef]
- Halmenschlager, G.M.; Brar, M.; Apan, I.T.; Klerk, A. Hydrocracking vacuum gas oil with wax. Catal. Today 2020, 353, 187–196. [Google Scholar] [CrossRef]
- D7169-23; Standard Test Method for Boiling Point Distribution of Samples with Residues Such as Crude Oils and Atmospheric and Vacuum Residues by High Temperature Gas Chromatography. ASTM International: West Conshohocken, PA, USA, 2023.
- D4294-21; Standard Test Method for Sulfur in Petroleum and Petroleum Products by Energy Dispersive X-ray Fluorescence Spectrometry. ASTM International: West Conshohocken, PA, USA, 2021.
- D6304-20; Standard Test Method for Determination of Water in Petroleum Products, Lubricating Oils, and Additives by Coulometric Karl Fischer Titration. ASTM International: West Conshohocken, PA, USA, 2020.
- E203-16; Standard Test Method for Water Using Volumetric Karl Fischer Titration. ASTM International: West Conshohocken, PA, USA, 2016.
- D445-21; Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity). ASTM International: West Conshohocken, PA, USA, 2021.
- D976-06; Standard Test Method for Calculated Cetane Index of Distillate Fuels. ASTM International: West Conshohocken, PA, USA, 2006.
- Standard EN 15751:2014; Automotive Fuels—Fatty Acid Methyl Ester (FAME) Fuel and Blends with Diesel Fuel—Determination of Oxidation Stability by Accelerated Oxidation Method. European Committee: Brussels, Belgium, 2014.
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Chrysikou, L.P.; Litinas, A.; Bezergianni, S. Assessment of biodiesel stability under long-term storage and dynamic accelerated oxidation: A comparison approach. Clean Technol. Environ. Policy 2022, 24, 2583–2593. [Google Scholar] [CrossRef]
- Sie, S.T. Acid-catalyzed cracking of paraffinic hydrocarbons. 1. Discussion of existing mechanism and proposal of a new mechanism. Ind. Eng. Chem. Res. 1992, 31, 1881–1889. [Google Scholar] [CrossRef]
- Sie, S.T. Acid-catalyzed cracking of paraffinic hydrocarbons. 2. Evidence for the protonated cyclopropane mechanism from catalytic cracking experiments. Ind. Eng. Chem. Res. 1993, 32, 397–402. [Google Scholar] [CrossRef]
- Sie, S.T. Acid-catalyzed cracking of paraffinic hydrocarbons. 3. Evidence for the protonated cyclopropane mechanism from hydrocracking/hydroisomerization experiments. Ind. Eng. Chem. Res. 1993, 32, 403–408. [Google Scholar] [CrossRef]
- Khuong, L.S.; Zulkifli, N.W.M.; Masjuki, H.H.; Niza Mohamad, E.; Arslan, A.; Mosarof, M.H.; Azham, A. A review on the effect of bioethanol dilution on the properties and performance of automotive lubricants in gasoline engines. RSC Adv. 2016, 6, 66847. [Google Scholar] [CrossRef]
- Gómez, N.; Banks, S.W.; Nowakowski, D.J.; Rosas, J.G.; Cara, J.; Sánchez, M.E.; Bridgwater, A.V. Effect of temperature on product performance of a high ash biomass during fast pyrolysis and its bio-oil storage evaluation. Fuel Process. Technol. 2018, 172, 97–105. [Google Scholar] [CrossRef]



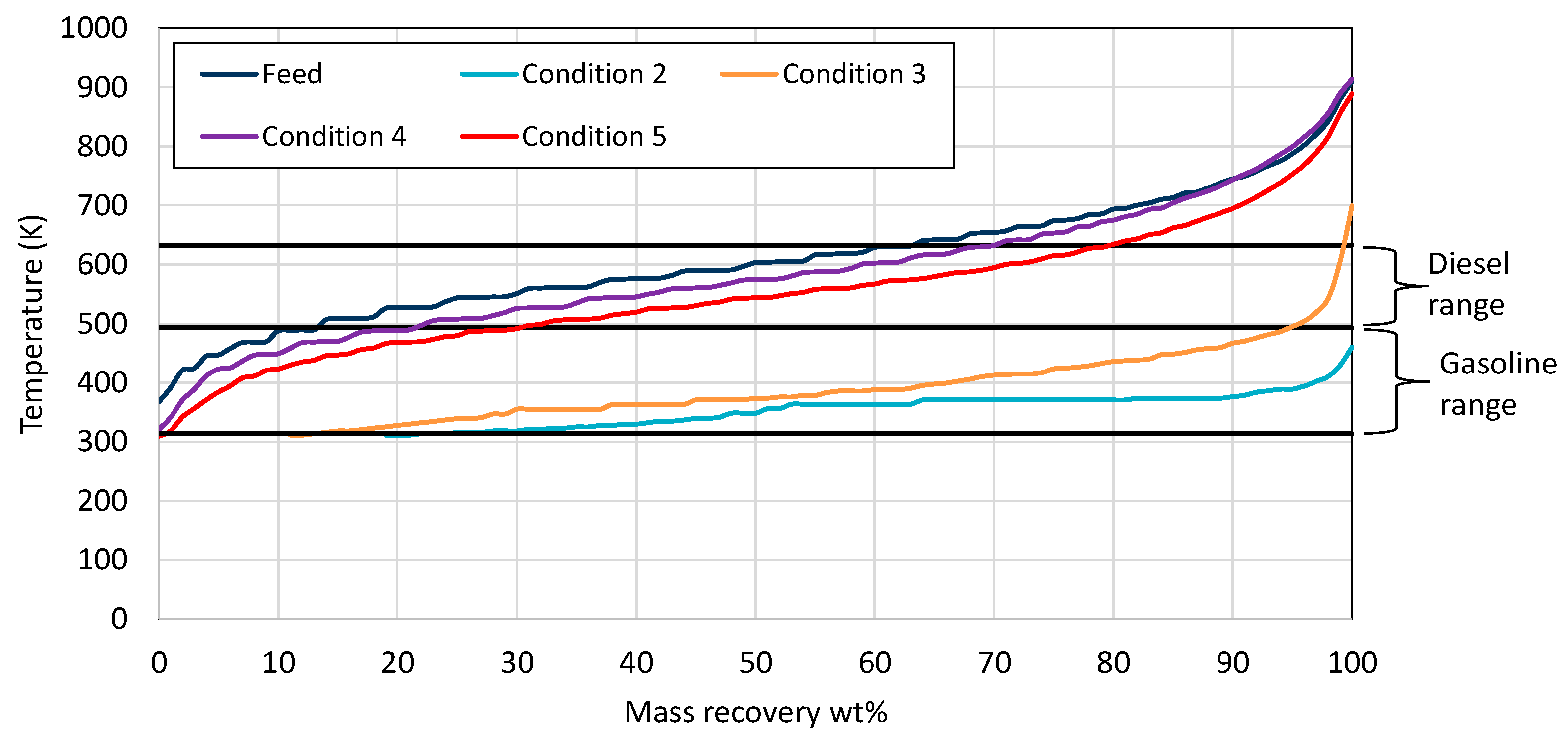






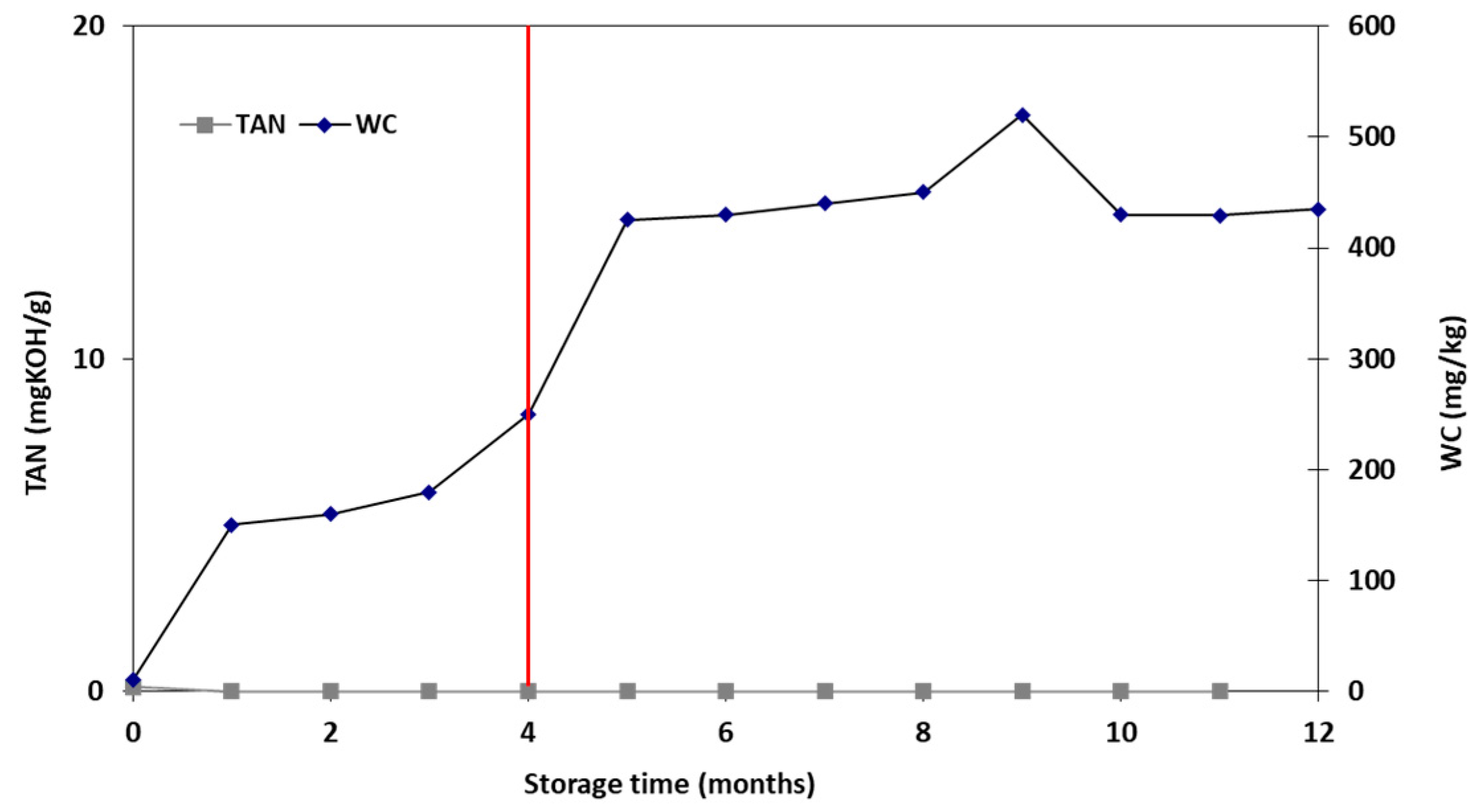
| Properties | Units | FT-Wax | Analysis Method |
|---|---|---|---|
| Density at 288 K | g/mL | 0.7897 | ASTM D-4052 |
| Sulfur dry basis | wppm | 2.5 | ASTM D-5453 |
| Viscosity at 313 K | cSts | 2.339 | ASTM D445 |
| TAN | mgKOH/g | 0.2 | ASTM D-664 |
| Hydrogen dry basis | wt% | 14.56 | ASTM D-5291 |
| Carbon dry basis | wt% | 84.4 | ASTM D-5291 |
| Oxygen dry basis | wt% | 1.04 | Calculated by difference |
| Nitrogen dry basis | wt% | 0.5 | ASTM D-4629 |
| Gasoline | wt% | 13 | ASTM D-7169 |
| Diesel | wt% | 50 | ASTM D-7169 |
| Residue | wt% | 37 | ASTM D-7169 |
| Unit | Catalyst Conditioning | Cond. 1 | Cond. 2 | Cond. 3 | Cond. 4 | Cond. 5 | Cond. 6 | Cond. 7 | |
|---|---|---|---|---|---|---|---|---|---|
| DOS | - | 1–3 | 4–7 | 8–10 | 11 | 12–15 | 16–21 | 22–23 | 24–25 |
| Pres. | MPa | 14.8 | 10.3 | 13.8 | 13.8 | 12.4 | 8.3 | 8.3 | 8.3 |
| Temp. | K | 613 | 623 | 653 | 633 | 623 | 603 | 573 | 603 |
| H2/Oil | scfb | 3640 | 3000 | 3000 | 3000 | 3000 | 3000 | 3000 | 2500 |
| LHSV | hr−1 | 2.18 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Feed | - | HAGO | wax | wax | wax | wax | wax | wax | wax |
| Units | Cond. 1 | Cond. 2 | Cond. 3 | Cond. 4 | Cond. 5 | Cond. 6 | Cond. 7 | ||
|---|---|---|---|---|---|---|---|---|---|
| Hydrogen | H2 | v/v % | 99.89 | 92.85 | - | 97.52 | 98.71 | 99.86 | 98.54 |
| Methane | CH4 | v/v % | 0.02 | 0.68 | - | 0.16 | 0.04 | 0.01 | 0.04 |
| Propane | C3H8 | v/v % | 0.01 | 2.05 | - | 0.01 | 0.21 | 0.00 | 0.20 |
| Isobutane | i-C4H10 | v/v % | 0.00 | 1.88 | - | 0.66 | 0.36 | 0.00 | 0.29 |
| N-Butane | n-C4H10 | v/v % | 0.01 | 1.05 | - | 0.33 | 0.18 | 0.01 | 0.18 |
| Isopentane | i-C5H12 | v/v % | 0.00 | 0.76 | - | 0.34 | 0.21 | 0.00 | 0.24 |
| N-Pentane | n-C5H12 | v/v % | 0.00 | 0.32 | - | 0.13 | 0.08 | 0.02 | 0.12 |
| C6+ | C6+ | v/v % | 0.00 | 0.32 | - | 0.23 | 0.14 | 0.06 | 0.31 |
| Nitrogen | N2 | v/v % | 0.06 | 0.07 | - | 0.48 | 0.05 | 0.05 | 0.07 |
| Units | Catalyst Conditioning | Cond. 1 | Cond. 2 | Cond. 3 | Cond. 4 | Cond. 5 | ||
|---|---|---|---|---|---|---|---|---|
| DOS | - | 1–3 | 4–5 | 6 | 7–8 | 9–10 | 11–13 | 14–15 |
| Pres. | MPa | 14.8 | 14.8 | 8.3 | 8.3 | 8.3 | 8.3 | 8.3 |
| Temp. | K | 613 | 653 | 603 | 653 | 603 | 573 | 588 |
| H2/oil | scfb | 3640 | 3640 | 2500 | 3000 | 2500 | 3000 | 3000 |
| LHSV | hr−1 | 2.18 | 2.18 | 1 | 1 | 1 | 1 | 1 |
| Feed | - | HAGO | HAGO | wax | wax | wax | wax | wax |
| Units | Cond. 1 | Cond. 2 | Cond. 3 | Cond. 4 | Cond. 5 | ||
|---|---|---|---|---|---|---|---|
| Hydrogen | H2 | v/v % | - | - | 93.49 | 99.33 | 98.84 |
| Methane | CH4 | v/v % | - | - | 0.07 | 0.01 | 0.01 |
| Propane | C3H8 | v/v % | - | - | 1.73 | 0.08 | 0.19 |
| Isobutane | i-C4H10 | v/v % | - | - | 2.36 | 0.15 | 0.32 |
| N-Butane | n-C4H10 | v/v % | - | - | 1.05 | 0.07 | 0.15 |
| Isopentane | i-C5H12 | v/v % | - | - | 0.00 | 0.13 | 0.21 |
| N-Pentane | n-C5H12 | v/v % | - | - | 0.47 | 0.04 | 0.07 |
| C6+ | C6+ | v/v % | - | - | 0.72 | 0.15 | 0.14 |
| Nitrogen | N2 | v/v % | - | - | 0.10 | 0.05 | 0.05 |
| Units | Cat. Conditioning | Cat. Conditioning | Cond. 1 | Cond. 2 | Cond. 3 | |
|---|---|---|---|---|---|---|
| DOS | - | 1–3 | 4–5 | 6 | 7–8 | 9–10 |
| Pres. | MPa | 14.8 | 13.8 | 8.3 | 13.8 | 10.3 |
| Temp. | K | 613 | 653 | 603 | 623 | 623 |
| H2/oil | scfb | 3640 | 3000 | 2500 | 2500 | 3000 |
| LHSV | hr−1 | 2.18 | 1 | 1 | 1 | 1 |
| Feed | - | HAGO | wax | wax | wax | wax |
| Units | Cond. 1 | Cond. 2 | Cond. 3 | |
|---|---|---|---|---|
| Hydrogen | v/v % | 99.84 | 99.72 | 99.72 |
| Methane | v/v % | 0.01 | 0.04 | 0.04 |
| Ethane | v/v % | 0.00 | 0.02 | 0.02 |
| N-Butane | v/v % | 0.01 | 0.03 | 0.03 |
| N-Pentane | v/v % | 0.02 | 0.03 | 0.03 |
| C6+ | v/v % | 0.03 | 0.06 | 0.06 |
| Carbon dioxide | v/v % | 0.01 | 0.04 | 0.03 |
| Nitrogen | v/v % | 0.06 | 0.06 | 0.05 |
| Properties | Units | Gasoline EN 228 | e-Gasoline Fraction | Diesel EN 590 | e-Diesel Fraction | Analysis/Method |
|---|---|---|---|---|---|---|
| Kinematic viscosity at 313 K | mm2s−1 | - | - | 2.0–4.5 | 2.427 | ASTM D445 |
| Density at 288 K | Kg m−3 | 720–775 | 716 | 820–835 | 774 | ASTM D4052 |
| RVP at 311 K (summer grade) | kPa | 45–60 | 26.29 | - | - | ASTM D323 |
| Cetane number, min | - | - | - | Min. 51 | 77.38 * | ASTM D613 |
| RON | - | Min. 95 | NA ** | - | - | ASTM D2699 |
| Distillation temperature T10 | K | Max. 343 | 340 | - | 490 | ASTM D86 |
| T50 | K | 339–383 | 424 | - | 538 | ASTM D86 |
| T90 | K | Max. 463 | 470 | 555–611 | 604 | ASTM D86 |
| Oxidation stability at 383 K | For gasoline: minutes For diesel: g m−3 | Min. 144 | NA ** | Max. 25 | NA ** | Gasoline: ASTM D525 Diesel: EN ISO 12205 |
| Oxygen content | wt% | Max. 2.7 | 0.0 | - | 0.0 | ASTM D4815 |
| Benzene | v/v % | Max. 1.0 | NA ** | - | - | ASTM D4420 |
| Sulfur content | wt% | Max. 0.001 | 0.002 | Max. 0.001 | 0.001 | ASTM D381 |
| Flash point | K | - | - | Min. 329 | 368 | ASTM D93 |
| Lubricity at 333 K | μm | - | - | Max. 460 | - | ASTM D6079 |
| Ash | wt% | - | - | Max. 0.01 | 0 | ASTM D482 |
| Water and sediment | v/v % | - | - | Max. 0.05 | 0.0029 | ASTM D2709 |
| Calorific value | MJ/kg | 34.84 | NA ** | 43.8 | 47.35 | Calculated |
| Stoichiometric air fuel ratio | - | 1/14.7 | NA ** | 1/14.67 | NA ** | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitriadis, A.; Chrysikou, L.P.; Bezergianni, S. Automotive e-Fuels via Hydrocracking of FT-Wax: e-Gasoline and e-Diesel Production. Energies 2024, 17, 2756. https://doi.org/10.3390/en17112756
Dimitriadis A, Chrysikou LP, Bezergianni S. Automotive e-Fuels via Hydrocracking of FT-Wax: e-Gasoline and e-Diesel Production. Energies. 2024; 17(11):2756. https://doi.org/10.3390/en17112756
Chicago/Turabian StyleDimitriadis, Athanasios, Loukia P. Chrysikou, and Stella Bezergianni. 2024. "Automotive e-Fuels via Hydrocracking of FT-Wax: e-Gasoline and e-Diesel Production" Energies 17, no. 11: 2756. https://doi.org/10.3390/en17112756
APA StyleDimitriadis, A., Chrysikou, L. P., & Bezergianni, S. (2024). Automotive e-Fuels via Hydrocracking of FT-Wax: e-Gasoline and e-Diesel Production. Energies, 17(11), 2756. https://doi.org/10.3390/en17112756







