Graphitic Carbon Nitride (g-C3N4) in Photocatalytic Hydrogen Production: Critical Overview and Recent Advances
Abstract
:1. Introduction
2. Synthesis Methods
2.1. Bulk g-C3N4 Synthesis
| Precursor | Temperature (°C) | Heating Time (h) | Ramp Rate (°C/min) | Color | Structure | Ref. |
|---|---|---|---|---|---|---|
| Melamine | 550 | 3 | 2.5 | Lemon chiffon | [27] | |
| Melamine | 400, 550 | 1, 2 | 3 | Pale yellow | - | [61] |
| Melamine | 500 | 4 | - | Yellowish | Bulk | [41] |
| Melamine | 550 | 2 | 5 | Yellow | Bulk | [32,52] |
| Melamine | 650 | 2 | 2 | Yellow | Bulk | [60] |
| Urea | 520 | 4 | 10 | Pale yellow | Bulk | [36] |
| Melamine | 600 | 2 | 5 | Pale yellow | - | [48] |
| Urea and dicyandiamide | 550 | 4 | - | - | Bulk | [66] |
| Melamine | 550 | 5 | 1 | Bright yellow | [67] | |
| Melamine | 550 | 4 | 2 | - | Flakes | [40] |
| Urea | 550 | 4 | - | Flakes | [40] | |
| Melamine | 550 | 1 | 5 | Light yellow | Bulk | [67] |
| Melamine | 600 | 2 | 5 | Yellow | Bulk | [42] |
| Urea | 550 | 3 | 1 | - | Semi-stacked sheets | [26] |
| Melamine | 520 | 4 | 10 | - | Bulk | [65] |
| Dicyandiamide | 600 | 2 | 2 | Pale yellow | Bulk | [68] |
2.2. g-C3N4 Nanosheet Synthesis
Exfoliation Methods
2.3. g-C3N4 Nanotube Synthesis
2.4. g-C3N4 Nanodot Synthesis
2.5. Doping and Composite Synthesis
3. Characterization
3.1. Structural Characteristics
3.1.1. XRD
3.1.2. XPS
3.1.3. FTIR
3.2. Surface and Morphological Characteristics
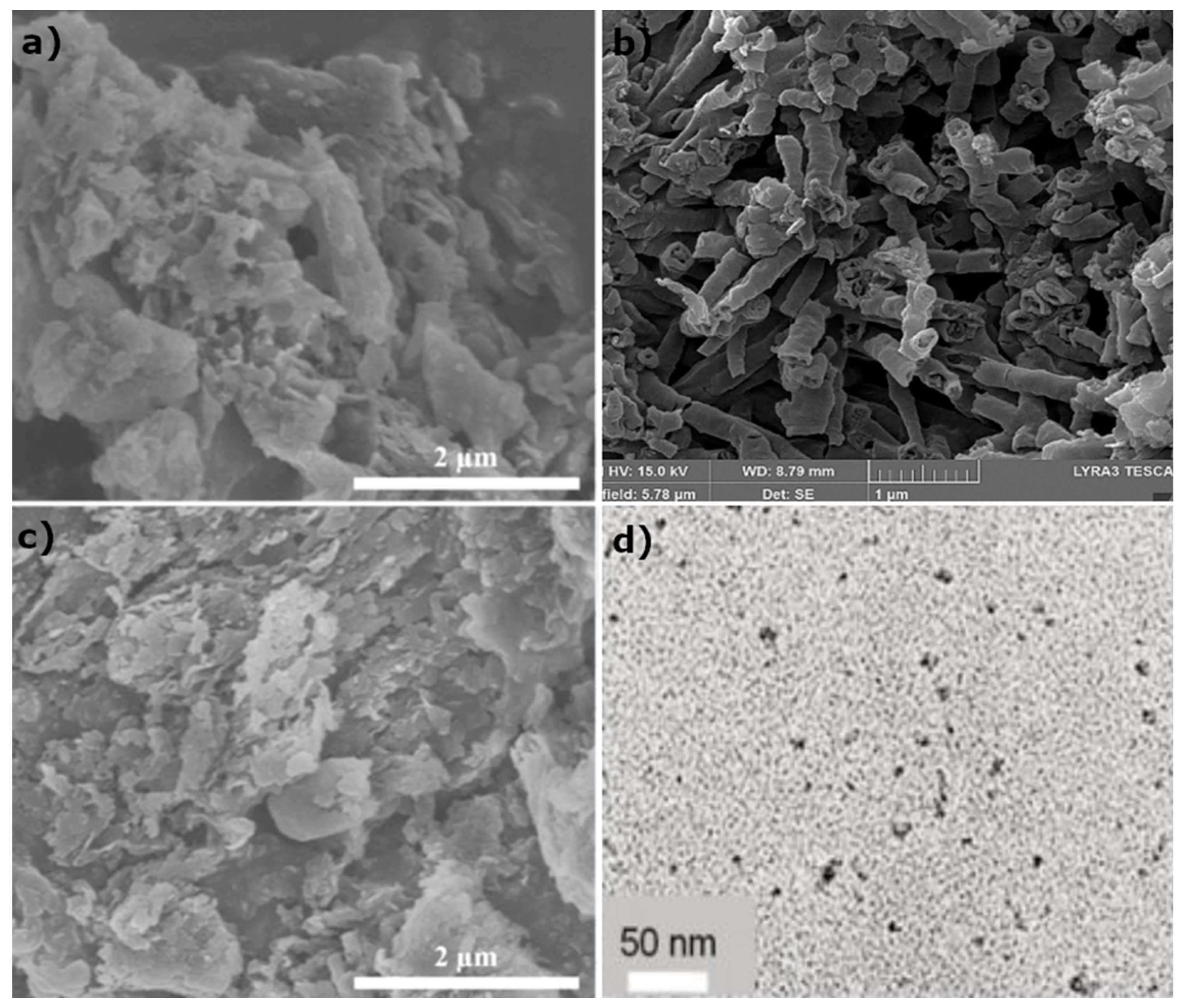
3.2.1. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
3.2.2. Brunauer–Emmett–Teller (BET) Analysis
3.3. Optical–Electrical Properties: Improvement Methods
3.3.1. Ultraviolet-Visual Spectrometry (UV-Vis): Light Response
3.3.2. Photo Luminescence Steady-State and Transient Spectroscopy: Recombination Prevention
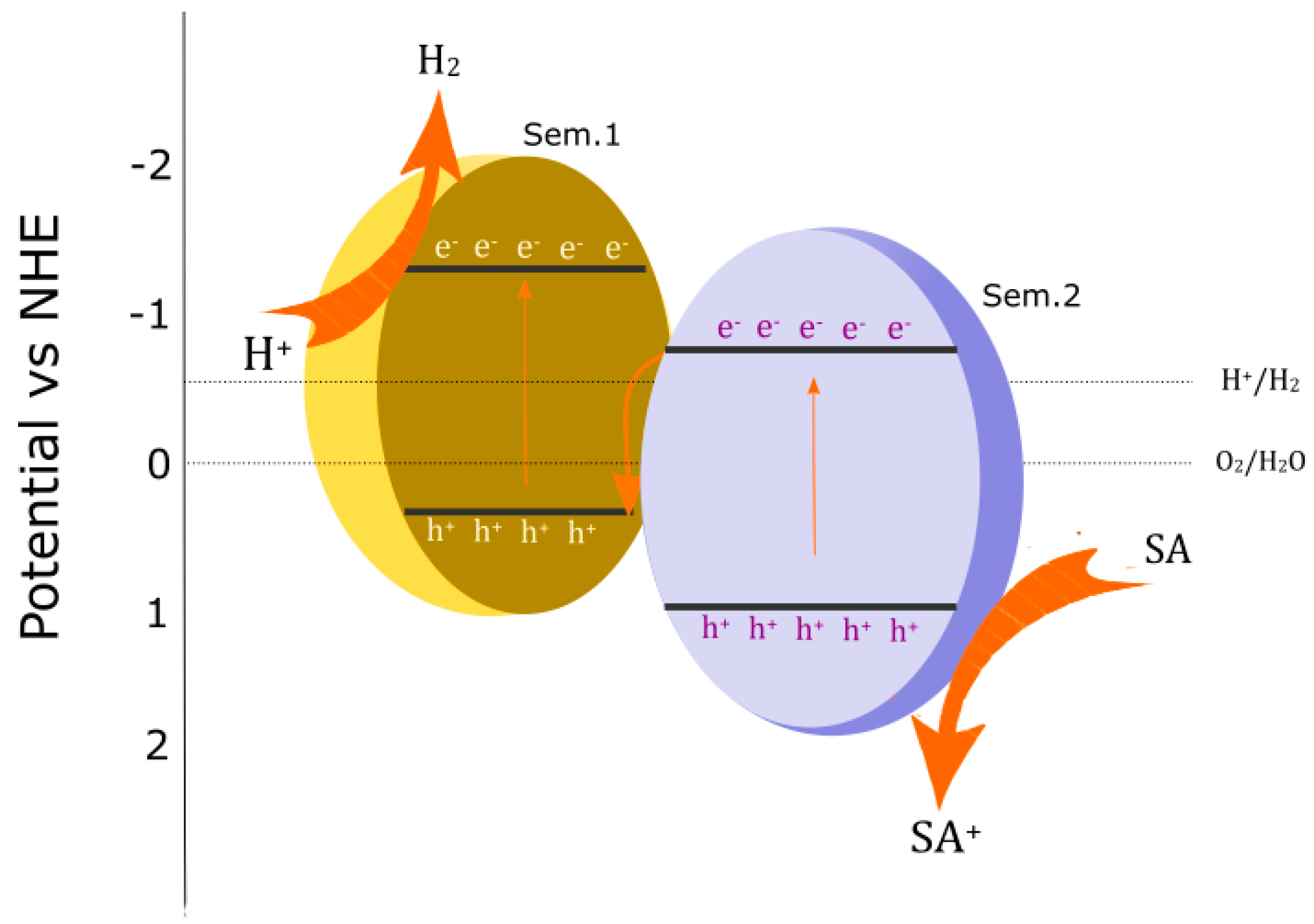
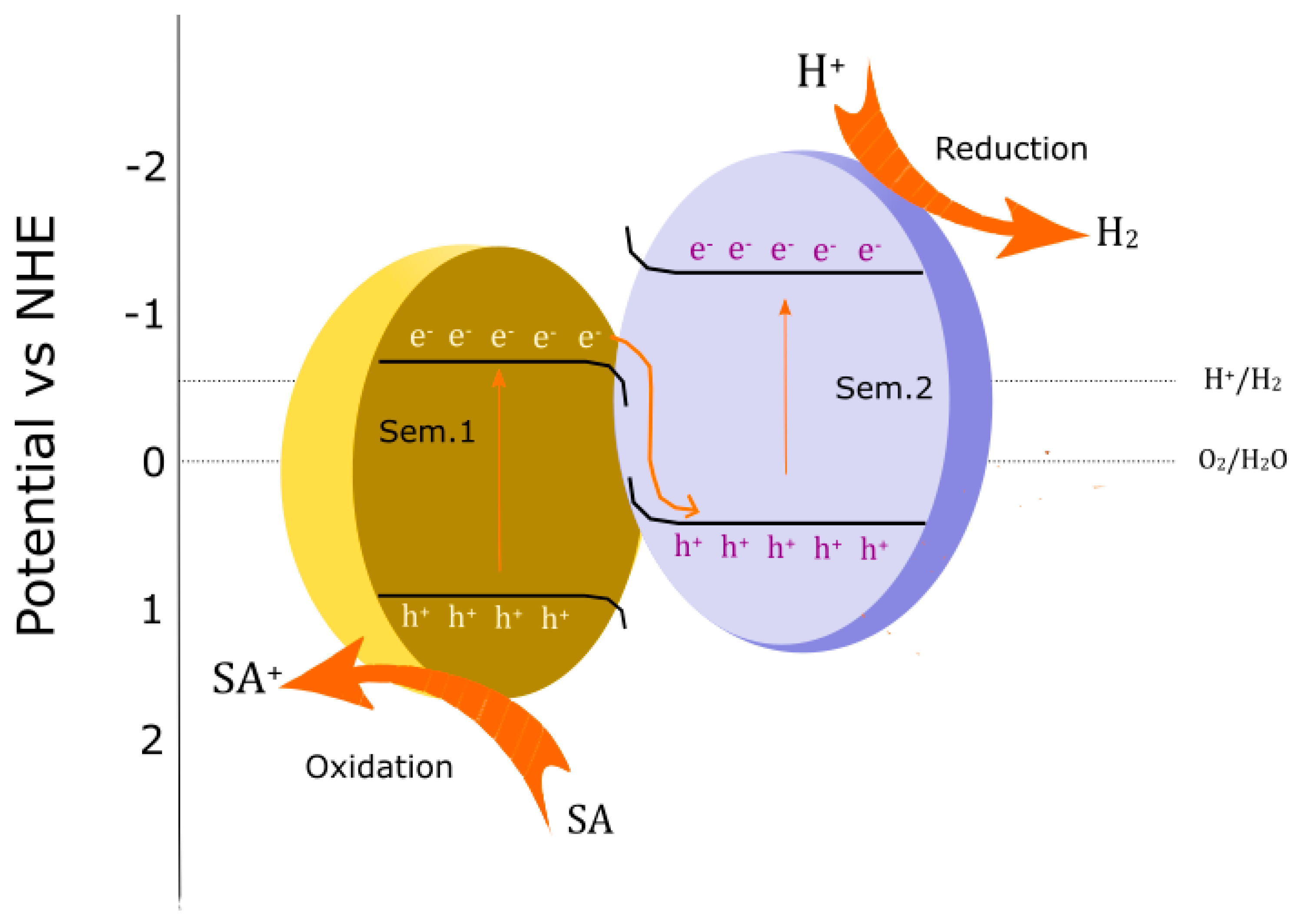
4. Mechanism of g-C3N4 in Photocatalytic Composites and Applications
4.1. Basic Mechanism of Water Splitting
4.2. Function of Structurally Modified g-C3N4 in Photocatalytic Mechanisms
4.3. Function of g-C3N4 in Composites
4.4. Comparative Overview of Hydrogen Production by g-C3N4-Based Materials
5. Challenges, Perspectives, and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sun, R.; Wang, X.; Gao, Y.; Yao, Y.; Xin, L.; Wang, D.; Wang, Y. Rapid charge migration of hierarchical S-Scheme g-C3/N4/CdS@C for efficient photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2024, 55, 635–644. [Google Scholar] [CrossRef]
- Emran, M.Y.; Miran, W.; Gomaa, H.; Ibrahim, I.; Belessiotis, G.V.; Abdelwahab, A.A.; Othman, M. Ben Biowaste Materials for Advanced Biodegradable Packaging Technology. In Handbook of Biodegradable Materials; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–37. [Google Scholar] [CrossRef]
- Acheampong, A.O.; Opoku, E.E.O. Environmental degradation and economic growth: Investigating linkages and potential pathways. Energy Econ. 2023, 123, 106734. [Google Scholar] [CrossRef]
- López, L.R.; Dessì, P.; Cabrera-Codony, A.; Rocha-Melogno, L.; Kraakman, B.; Naddeo, V.; Balaguer, M.D.; Puig, S. CO2 in indoor environments: From environmental and health risk to potential renewable carbon source. Sci. Total Environ. 2023, 856, 159088. [Google Scholar] [CrossRef]
- Wang, J.; Azam, W. Natural resource scarcity, fossil fuel energy consumption, and total greenhouse gas emissions in top emitting countries. Geosci. Front. 2024, 15, 101757. [Google Scholar] [CrossRef]
- Stathopoulos, N.; Belessiotis, G.; Oikonomou, P.; Papanicolaou, E. Experimental investigation of thermal degradation of phase change materials for medium-temperature thermal energy storage and tightness during cycling inside metal spheres. J. Energy Storage 2020, 31, 101618. [Google Scholar] [CrossRef]
- Belessiotis, G.V.; Ibrahim, I.; Falaras, P. Sensitizer effects on DSSC performance under pan-illumination conditions. J. Photochem. Photobiol. A Chem. 2022, 433, 114201. [Google Scholar] [CrossRef]
- Belessiotis, G.V.; Antoniadou, M.; Ibrahim, I.; Karagianni, C.S.; Falaras, P. Universal electrolyte for DSSC οperation under both simulated solar and indoor fluorescent lighting. Mater. Chem. Phys. 2021, 277, 125543. [Google Scholar] [CrossRef]
- Dincer, I.; Aydin, M.I. New paradigms in sustainable energy systems with hydrogen. Energy Convers. Manag. 2023, 283, 116950. [Google Scholar] [CrossRef]
- Sun, D.W.; Long, C.C.; Huang, J.H. Highly dispersed platinum-anchored g-C3N4 nanotubes for photocatalytic hydrogen generation. Int. J. Hydrogen Energy 2023, 48, 943–952. [Google Scholar] [CrossRef]
- Dong, X.; Pang, D.; Luo, G.; Zhu, X. Microbial Water Electrolysis Cells for Efficient Wastewater Treatment and H2 Production. ACS Sustain. Chem. Eng. 2024, 12, 4203–4212. [Google Scholar] [CrossRef]
- Patil, S.A.; Khot, A.C.; Chavan, V.D.; Rabani, I.; Kim, D.K.; Jung, J.; Im, H.; Shrestha, N.K. Electrostatically robust CoFeOF nanosheet against chloride for green-H2 production in alkaline seawater electrolysis. Chem. Eng. J. 2024, 480, 146545. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, P.; Chen, Q.; Yin, X.; Chen, K.; Lu, Z.; Wen, Z. Development of high-efficiency alkaline OER electrodes for hybrid acid-alkali electrolytic H2 generation. J. Colloid Interface Sci. 2023, 636, 610–617. [Google Scholar] [CrossRef]
- Belessiotis, G.V.; Falara, P.; Ibrahim, I.; Kontos, A.G. Magnetic Metal Oxide-Based Photocatalysts with Integrated Silver for Water Treatment. Materials 2022, 15, 4629. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.; Rauf, S.; Irfan, M.; Hayat, A.; Alghamdi, M.M.; El-Zahhar, A.A.; Ghernaout, D.; Al-Hadeethi, Y.; Lv, W. Recent developments, advances and strategies in heterogeneous photocatalysts for water splitting. Nanoscale Adv. 2024, 6, 1286–1330. [Google Scholar] [CrossRef]
- Shah, S.S.A.; Nazir, M.A.; Khan, K.; Hussain, I.; Tayyab, M.; Alarfaji, S.S.; Hassan, A.M.; Sohail, M.; Javed, M.S.; Najam, T. Solar energy storage to chemical: Photocatalytic CO2 reduction over pristine metal-organic frameworks with mechanistic studies. J. Energy Storage 2024, 75, 109725. [Google Scholar] [CrossRef]
- Feng, S.; Su, R. Synthetic Chemistry in Flow: From Photolysis & Homogeneous Photocatalysis to Heterogeneous Photocatalysis. ChemSusChem 2024, e202400064. [Google Scholar] [CrossRef]
- Ibrahim, I.; Belessiotis, G.V.; Antoniadou, M.; Kaltzoglou, A.; Sakellis, E.; Katsaros, F.; Sygellou, L.; Arfanis, M.K.; Salama, T.M.; Falaras, P. Silver decorated TiO2/g-C3N4 bifunctional nanocomposites for photocatalytic elimination of water pollutants under UV and artificial solar light. Results Eng. 2022, 14, 100470. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based Heterostructured Photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Liu, X.; Ma, R.; Zhuang, L.; Hu, B.; Chen, J.; Liu, X.; Wang, X. Recent developments of doped g-C3N4 photocatalysts for the degradation of organic pollutants. Crit. Rev. Environ. Sci. Technol. 2021, 51, 751–790. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Zhang, H.; Fan, J.; Xiang, Q. Design and application of active sites in g-C3N4-based photocatalysts. J. Mater. Sci. Technol. 2020, 56, 69–88. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, H.; Wu, Z.; Wang, L. g-C3N4 based composite photocatalysts for photocatalytic CO2 reduction. Catal. Today 2018, 300, 160–172. [Google Scholar] [CrossRef]
- Patnaik, S.; Sahoo, D.P.; Parida, K. Recent advances in anion doped g-C3N4 photocatalysts: A review. Carbon 2021, 172, 682–711. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Ghandi, K. A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)–Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing. Nanomaterials 2022, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ye, Y.; Sun, J.; Li, Z.; Ping, J.; Sun, X. Recent Advances in g-C3N4-Based Photocatalysts for Pollutant Degradation and Bacterial Disinfection: Design Strategies, Mechanisms, and Applications. Small 2022, 18, 2105089. [Google Scholar] [CrossRef]
- Saman, F.; Bahruji, H.; Mahadi, A.H.; Ling, C.H.S. Pd/g-C3N4 photocatalyst for hydrogen production: Role of experimental condition for Schottky barrier. Fuel 2023, 349, 128725. [Google Scholar] [CrossRef]
- Bharagav, U.; Ramesh Reddy, N.; Nava Koteswara Rao, V.; Ravi, P.; Sathish, M.; Rangappa, D.; Prathap, K.; Shilpa Chakra, C.; Shankar, M.V.; Appels, L.; et al. Bifunctional g-C3N4/carbon nanotubes/WO3 ternary nanohybrids for photocatalytic energy and environmental applications. Chemosphere 2023, 311, 137030. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Zhang, Y.; Chang, H.; Qiu, R.; Xie, H.; Yang, W.; Zhang, M.; Wang, W.; Liu, Q.; Wang, X.; et al. When 2D g-C3N4 meets 0D CNQDs and 1D CNTs: A strategy for photocatalytic hydrogen production over a ternary non-metallic catalyst. Sep. Purif. Technol. 2024, 343, 127161. [Google Scholar] [CrossRef]
- Xu, C.; Li, D.; Liu, X.; Ma, R.; Sakai, N.; Yang, Y.; Lin, S.; Yang, J.; Pan, H.; Huang, J.; et al. Direct Z-scheme construction of g-C3N4 quantum dots / TiO2 nanoflakes for efficient photocatalysis. Chem. Eng. J. 2022, 430, 132861. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Zhong, J.; Li, J. Facile construction of CuO/g-C3N4 heterojunctions with promoted photocatalytic hydrogen generation behaviors. Fuel 2023, 353, 129224. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, Z.; Zou, Y.; Chen, J.; Shi, J.W. The progress of g-C3N4 in photocatalytic H2 evolution: From fabrication to modification. Coord. Chem. Rev. 2024, 500, 215489. [Google Scholar] [CrossRef]
- Humayun, M.; Khan, A.; Ali Marwat, M.; Bououdina, M.; Ali, S.; Ur Rahman, A.; Ali, F.; Wang, C. Au decorated chemically exfoliated g-C3N4 as highly efficient visible light catalyst for hydrogen production. J. Photochem. Photobiol. A Chem. 2024, 450, 115472. [Google Scholar] [CrossRef]
- Djurišić, A.B.; He, Y.; Ng, A.M.C. Visible-light photocatalysts: Prospects and challenges. APL Mater. 2020, 8, 030903. [Google Scholar] [CrossRef]
- Niu, P.; Qiao, M.; Li, Y.; Huang, L.; Zhai, T. Distinctive defects engineering in graphitic carbon nitride for greatly extended visible light photocatalytic hydrogen evolution. Nano Energy 2018, 44, 73–81. [Google Scholar] [CrossRef]
- Liao, G.; Gong, Y.; Zhang, L.; Gao, H.; Yang, G.J.; Fang, B. Semiconductor polymeric graphitic carbon nitride photocatalysts: The “holy grail” for the photocatalytic hydrogen evolution reaction under visible light. Energy Environ. Sci. 2019, 12, 2080–2147. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Qi, K.; Liu, S. yuan CuInS2 quantum-dot-modified g-C3N4 S-scheme heterojunction photocatalyst for hydrogen production and tetracycline degradation. J. Mater. Sci. Technol. 2024, 172, 145–155. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Morales-Mendoza, J.E.; Paraguay-Delgado, F. Widening UV–Vis absorption band by Cu doped ZnO and ZnO/CuO composite. Mater. Lett. 2021, 291, 129494. [Google Scholar] [CrossRef]
- Ibrahim, I.; Belessiotis, G.V.; Elseman, A.M.; Mohamed, M.M.; Ren, Y.; Salama, T.M.; Mohamed, M.B.I. Magnetic TiO2/CoFe2O4 Photocatalysts for Degradation of Organic Dyes and Pharmaceuticals without Oxidants. Nanomaterials 2022, 12, 3290. [Google Scholar] [CrossRef]
- Sharma, P.; Sarngan, P.P.; Lakshmanan, A.; Sarkar, D. One-step synthesis of highly reactive g-C3N4. J. Mater. Sci. Mater. Electron. 2022, 33, 9116–9125. [Google Scholar] [CrossRef]
- Iqbal, N.; Afzal, A.; Khan, I.; Khan, M.S.; Qurashi, A. Molybdenum impregnated g-C3N4 nanotubes as potentially active photocatalyst for renewable energy applications. Sci. Rep. 2021, 11, 16886. [Google Scholar] [CrossRef]
- Vega, M.F.; Olivas, C.; Díaz-Faes, E.; Barriocanal, C. Evaluation of water splitting efficiency of g-C3N4 thermally etched/exfoliated under air and CO2 atmospheres. Catal. Today 2024, 427, 114412. [Google Scholar] [CrossRef]
- Qin, J.; Jiao, Y.; Liu, M.; Li, Y.; Wang, J. Heat treatment to prepare boron doped g-C3N4 nanodots/carbon-rich g-C3N4 nanosheets heterojunction with enhanced photocatalytic performance for water splitting hydrogen evolution. J. Alloys Compd. 2022, 898, 162846. [Google Scholar] [CrossRef]
- Ding, Q.; Zou, X.; Ke, J.; Dong, Y.; Cui, Y.; Lu, G.; Ma, H. S-scheme 3D/2D NiCo2O4@g-C3N4 hybridized system for boosting hydrogen production from water splitting. Renew. Energy 2023, 203, 677–685. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiao, H.; Yan, Z.H.; Duan, L.; Ni, L.; Fan, J. Bin PtS2/g-C3N4 van der Waals heterostructure: A direct Z-scheme photocatalyst with high optical absorption, solar-to-hydrogen efficiency and catalytic activity. Int. J. Hydrogen Energy 2023, 48, 14659–14669. [Google Scholar] [CrossRef]
- Yang, W.; Jia, L.; Wu, P.; Zhai, H.; He, J.; Liu, C.; Jiang, W. Effect of thermal program on structure–activity relationship of g-C3N4 prepared by urea pyrolysis and its application for controllable production of g-C3N4. J. Solid State Chem. 2021, 304, 122545. [Google Scholar] [CrossRef]
- Gao, J.; Li, M.; Chen, H.; Guo, L.; Li, Z.; Wang, X. Microstructure regulation of graphitic carbon nitride nanotubes via quick thermal polymerization process for photocatalytic hydrogen evolution. J. Photochem. Photobiol. A Chem. 2023, 441, 114747. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, J.; Qian, S.; Xue, H.; Tian, J.; Jiang, T. Assembling carbon nitride quantum dots into hollow fusiformis and loading CoP for photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2024, 667, 128–135. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, Q.; Wang, Y.; Zhou, M.; Li, X.; Xu, S.; Li, Z. Construction of Z-scheme heterojunction CoS/CdS@g-C3N4 hollow sphere with spatical charge separation for enhanced photocatalytic hydrogen production. Appl. Surf. Sci. 2023, 626, 157214. [Google Scholar] [CrossRef]
- Su, Z.; Zhang, J.; Tan, Z.; Hu, J.; Zhang, F.; Duan, R.; Yao, L.; Han, B.; Zhao, Y.; Yang, Y. Facilitated photocatalytic H2 production on Cu-coordinated mesoporous g-C3N4 nanotubes. Green Chem. 2023, 25, 2577–2582. [Google Scholar] [CrossRef]
- Zhen, X.; Fan, C.; Tang, L.; Luo, J.; Zhong, L.; Gao, Y.; Zhang, M.; Zheng, J. Advancing charge carriers separation and transformation by nitrogen self-doped hollow nanotubes g-C3N4 for enhancing photocatalytic degradation of organic pollutants. Chemosphere 2023, 312, 137145. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, A.; Ding, J.; Hu, R.; Gong, Y.; Li, X.; Chen, L.; Chen, P.; Tian, X. Amino modulation on the surface of graphitic carbon nitride for enhanced photocatalytic hydrogen production. Carbon 2024, 219, 118841. [Google Scholar] [CrossRef]
- Ge, Y.; Shen, Q.; Zhang, Q.; Li, N.; Lu, D.; Zhang, Z.; Fu, Z.; Zhou, J. Sulfur and carbon co-doped g-C3N4 microtubes with enhanced photocatalytic H2 production activity. Front. Energy 2024, 18, 110–121. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Olatunde, O.C.; Nkwe, V.M.; Oyewo, O.A.; Muliwa, A.M.; Ferjani, H.; Yousef, T.A. Synthesis of p-n GCN/CuBi2O4 heterostructures for enhanced visible light photocatalysis. Inorg. Chem. Commun. 2024, 162, 112193. [Google Scholar] [CrossRef]
- Li, J.; Wei, W.; Mu, C.; Huang, B.; Dai, Y. Electronic properties of g-C3N4/CdS heterojunction from the first-principles. Phys. E Low-Dimens. Syst. Nanostructures 2018, 103, 459–463. [Google Scholar] [CrossRef]
- Belessiotis, G.V.; Kontos, A.G. Plasmonic silver (Ag)-based photocatalysts for H2 production and CO2 conversion: Review, analysis and perspectives. Renew. Energy 2022, 195, 497–515. [Google Scholar] [CrossRef]
- Zhang, X.; Ran Zhang, X.; Yang, P.; Chen, H.S.; Ping Jiang, S. Black magnetic Cu-g-C3N4 nanosheets towards efficient photocatalytic H2 generation and CO2/benzene conversion. Chem. Eng. J. 2022, 450, 138030. [Google Scholar] [CrossRef]
- Yang, S.; Liu, C.; Wang, J.; Lin, X.; Hong, Y.; Guo, F.; Shi, J. Enhanced photocatalytic activity of g-C3N4 quantum dots/Bi3.64Mo0.36O6.55 nanospheres composites. J. Solid State Chem. 2020, 287, 121347. [Google Scholar] [CrossRef]
- Ma, P.; Zhang, X.; Wang, C.; Wang, Z.; Wang, K.; Feng, Y.; Wang, J.; Zhai, Y.; Deng, J.; Wang, L.; et al. Band alignment of homojunction by anchoring CN quantum dots on g-C3N4 (0D/2D) enhance photocatalytic hydrogen peroxide evolution. Appl. Catal. B Environ. 2022, 300, 120736. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Jiang, Z.; Chen, H.S.; Yang, P. Phosphorus and sulphur co-doping of g-C3N4 nanotubes with tunable architectures for superior photocatalytic H2 evolution. Int. J. Hydrogen Energy 2019, 44, 20042–20055. [Google Scholar] [CrossRef]
- Alam, U.; Pandey, A.; Verma, N. An anthraquinone-integrated S-scheme-based NiTiO3-g-C3N4 composite with enhanced hydrogen production activity. Int. J. Hydrogen Energy 2023, 48, 2532–2541. [Google Scholar] [CrossRef]
- Liu, X.; Xu, X.; Gan, H.; Yu, M.; Huang, Y. The Effect of Different g-C3N4 Precursor Nature on Its Structural Control and Photocatalytic Degradation Activity. Catalysts 2023, 13, 848. [Google Scholar] [CrossRef]
- Xia, Z.; Chen, C.; Qi, X.; Xu, Q.; Tang, H.; Liu, G. Delocalized Electrons via In Situ CNT Growth on Au/g-C3N4 for Boosting Photocatalytic H2 Evolution. Adv. Sustain. Syst. 2023, 7, 2200134. [Google Scholar] [CrossRef]
- Li, S.; Wang, K.; Fan, Z.; Xie, H.; Lin, S.; Jin, Z. Efficient photocatalytic hydrogen evolution via interfacial engineering and charge carrier utilization with WSP/g-C3N4 S-scheme heterojunction. Int. J. Hydrogen Energy 2024, 51, 1070–1079. [Google Scholar] [CrossRef]
- Ismael, M. Construction of novel Ru-embedded bulk g-C3N4 photocatalysts toward efficient and sustainable photocatalytic hydrogen production. Diam. Relat. Mater. 2024, 144, 111024. [Google Scholar] [CrossRef]
- Liu, X.; Guo, Y.; Wang, P.; Zhang, Q.; Wang, Z.; Liu, Y.; Zheng, Z.; Cheng, H.; Dai, Y.; Huang, B. The synergy of thermal exfoliation and phosphorus doping in g-C3N4 for improved photocatalytic H2 generation. Int. J. Hydrogen Energy 2021, 46, 3595–3604. [Google Scholar] [CrossRef]
- Dharman, R.K.; Oh, T.H. Fabrication of g-C3N4@N-doped Bi2MoO6 heterostructure for enhanced visible-light-driven photocatalytic degradation of tetracycline pollutant. Chemosphere 2023, 338, 139513. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Zhang, X.; Yang, P. G-C3N4 sheet nanoarchitectonics with island-like crystalline/amorphous homojunctions towards efficient H2 and H2O2 evolution. Environ. Res. 2023, 236, 116805. [Google Scholar] [CrossRef] [PubMed]
- Che, W.; Cheng, W.; Yao, T.; Tang, F.; Liu, W.; Su, H.; Huang, Y.; Liu, Q.; Liu, J.; Hu, F.; et al. Fast Photoelectron Transfer in (Cring)-C3N4 Plane Heterostructural Nanosheets for Overall Water Splitting. J. Am. Chem. Soc. 2017, 139, 3021–3026. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Chen, S.; Xie, Y.; Ma, Y.; Luo, Y.; Huang, J.; Ling, Y.; Ye, J.; Liang, Y.; et al. In situ loading of ZnIn2S4 nanosheets onto S doped g-C3N4 nanosheets to construct type II heterojunctions for improving photocatalytic hydrogen production. J. Alloys Compd. 2022, 924, 166569. [Google Scholar] [CrossRef]
- Zhang, X.; Matras-Postolek, K.; Yang, P.; Ping Jiang, S. Z-scheme WOx/Cu-g-C3N4 heterojunction nanoarchitectonics with promoted charge separation and transfer towards efficient full solar-spectrum photocatalysis. J. Colloid Interface Sci. 2023, 636, 646–656. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, X.; Chen, H.S.; Hu, X.; Yang, P. Formation of g-C3N4 Nanotubes towards Superior Photocatalysis Performance. ChemCatChem 2019, 11, 4558–4567. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M.; Kumar, N. Synergistic effect of gold NPs modified graphitic carbon nitride nanotubes (g-CNT) with the role of hot electrons and hole scavengers for boosting solar hydrogen production. Int. J. Hydrogen Energy 2023, 48, 15504–15521. [Google Scholar] [CrossRef]
- Yang, B.; Han, J.; Zhang, Q.; Liao, G.; Cheng, W.; Ge, G.; Liu, J.; Yang, X.; Wang, R.; Jia, X. Carbon defective g-C3N4 thin-wall tubes for drastic improvement of photocatalytic H2 production. Carbon 2023, 202, 348–357. [Google Scholar] [CrossRef]
- Qi, K.; Lv, W.; Khan, I.; Liu, S. yuan Photocatalytic H2 generation via CoP quantum-dot-modified g-C3N4 synthesized by electroless plating. Chin. J. Catal. 2020, 41, 114–121. [Google Scholar] [CrossRef]
- Bharagav, U.; Reddy, N.R.; Koteswararao, V.N.; Ravi, P.K.P.; Sathish, M.; Cheralathan, K.K.; Shankar, M.V.; Kumari, M.M. Heterojunction of CdS Nanocapsules-WO3Nanosheets Composite as a Stable and Efficient Photocatalyst for Hydrogen Evolution. Energy Fuels 2020, 34, 14598–14610. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, S.; Liang, Y.; Li, Z.; Wu, S.; Chang, C.; Luo, S.; Cui, Z. One-step synthesis of Mo and S co-doped porous g-C3N4 nanosheets for efficient visible-light photocatalytic hydrogen evolution. Appl. Surf. Sci. 2021, 536, 147743. [Google Scholar] [CrossRef]
- Dong, H.; Hong, S.; Zhang, P.; Yu, S.; Wang, Y.; Yuan, S.; Li, H.; Sun, J.; Chen, G.; Li, C. Metal-free Z-scheme 2D/2D VdW heterojunction for high-efficiency and durable photocatalytic H2 production. Chem. Eng. J. 2020, 395, 125150. [Google Scholar] [CrossRef]
- Liu, M.; Jiao, Y.; Qin, J.; Li, Z.; Wang, J. Boron doped C3N4 nanodots/nonmetal element (S, P, F, Br) doped C3N4 nanosheets heterojunction with synergistic effect to boost the photocatalytic hydrogen production performance. Appl. Surf. Sci. 2021, 541, 148558. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, J.; Yu, L.; Peng, J.; Song, Z.; Xiong, Z.; Li, N.; Xiang, K.; Zou, J.; Hsu, J.P.; et al. Tailoring Advanced N-Defective and S-Doped g-C3N4 for Photocatalytic H2 Evolution. Small 2023, 19, 2301116. [Google Scholar] [CrossRef]
- Yuan, B.; Zou, X.; Yan, T.; Fei, J.; Chu, Z. Green synthesis of graphitic carbon nitride nanodots using sodium chloride template. J. Nanoparticle Res. 2016, 18, 110. [Google Scholar] [CrossRef]
- Kamalakannan, S.; Balasubramaniyan, N.; Bernaurdshaw, N.; Vattikondala, G. Impact of nitrogen doping on triazole-based graphitic carbon Nitride-TiO2 (P25) S-scheme heterojunction for improved photocatalytic hydrogen production. Nanoscale Adv. 2023, 5, 5907–5922. [Google Scholar] [CrossRef]
- Xing, W.; Tu, W.; Ou, M.; Wu, S.; Yin, S.; Wang, H.; Chen, G.; Xu, R. Anchoring Active Pt2+/Pt0 Hybrid Nanodots on g-C3N4 Nitrogen Vacancies for Photocatalytic H2 Evolution. ChemSusChem 2019, 12, 2029–2034. [Google Scholar] [CrossRef]
- Yang, S.; Guo, X.; Liu, K.; Li, Y.; Li, T.; Gu, X.; Arenal, R.; Zheng, X.; Li, W.; Sun, C.; et al. Size effect of CoS2 cocatalyst on photocatalytic hydrogen evolution performance of g-C3N4. J. Colloid Interface Sci. 2023, 635, 305–315. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W. Photochemical splitting of water for hydrogen production by photocatalysis: A review. Sol. Energy Mater. Sol. Cells 2014, 128, 85–101. [Google Scholar] [CrossRef]
- Razzaq, A.; In, S., II. TiO2 based nanostructures for photocatalytic CO2 conversion to valuable chemicals. Micromachines 2019, 10, 326. [Google Scholar] [CrossRef]
- Fern, J.; Greco, R.; Navlani-garc, M.; Cao, W.; Berenguer-murcia, Á.; Cazorla-amor, D. g-C3N4-Based Direct Z-Scheme Photocatalysts for Environmental Applications. Catalysts 2022, 12, 1137. [Google Scholar] [CrossRef]
- Wageh, S.; Al-Ghamdi, A.A.; Jafer, R.; Li, X.; Zhangc, P. A new heterojunction in photocatalysis: S-scheme heterojunction. Chin. J. Catal. 2021, 42, 667–669. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, X.; Zhao, Y.; Zhang, K.; Yan, Y.; Qi, K. Photocatalytic Hydrogen Production and Tetracycline Degradation Using ZnIn2S4 Quantum Dots Modified g-C3N4 Composites. Nanomaterials 2023, 13, 305. [Google Scholar] [CrossRef]
- Ren, Z.; Xie, J.; Li, X.; Guo, L.; Zhang, Q.; Wu, J.; Li, Y.; Liu, W.; Li, P.; Fu, Y.; et al. Rational design of graphite carbon nitride-decorated zinc oxide nanoarrays on three-dimensional nickel foam for the efficient production of reactive oxygen species through stirring-promoted piezo–photocatalysis. J. Colloid Interface Sci. 2023, 632, 271–284. [Google Scholar] [CrossRef]
- Yang, S.; Wang, K.; Chen, Q.; Wu, Y. Enhanced photocatalytic hydrogen production of S-scheme TiO2/g-C3N4 heterojunction loaded with single-atom Ni. J. Mater. Sci. Technol. 2024, 175, 104–114. [Google Scholar] [CrossRef]


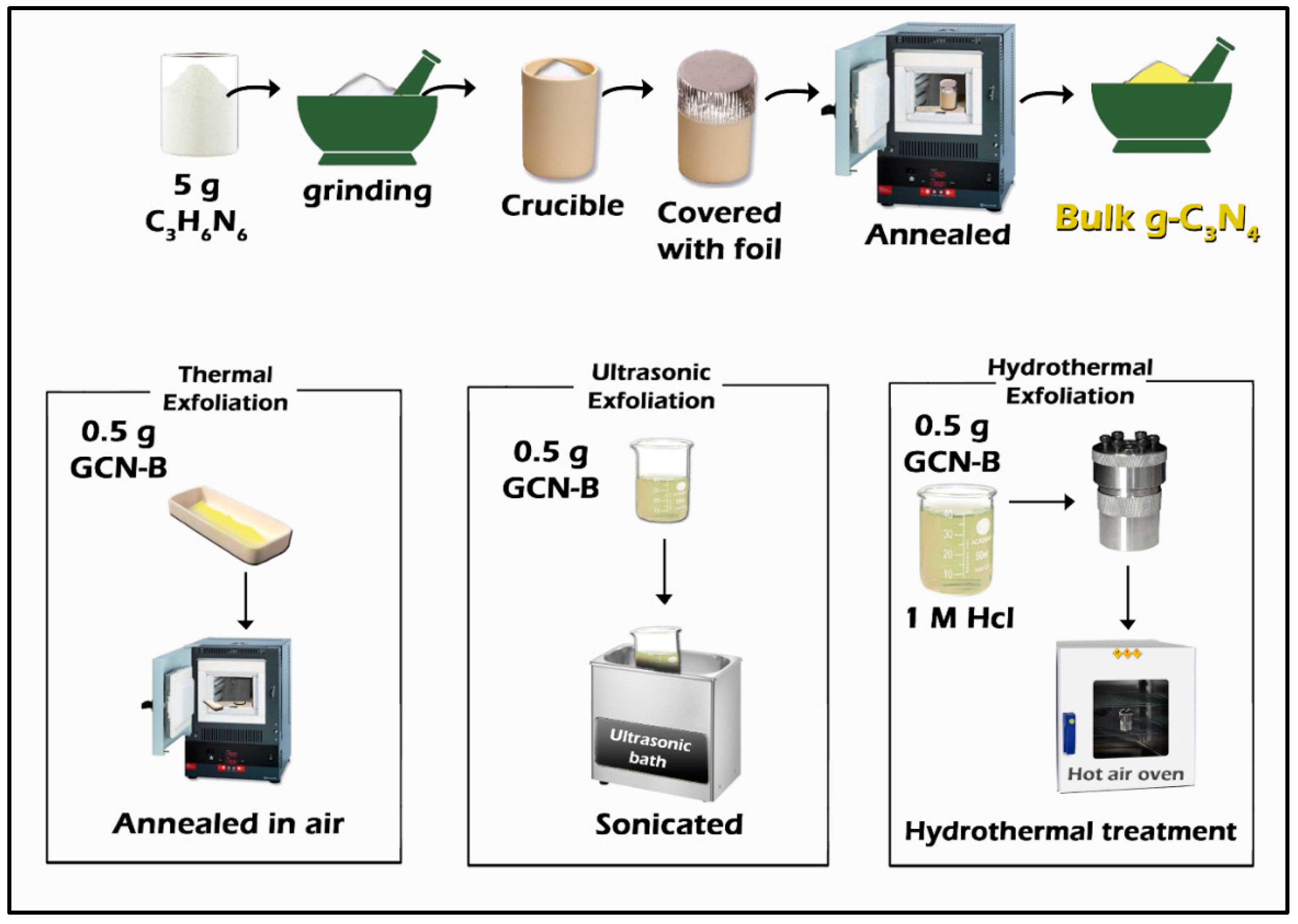


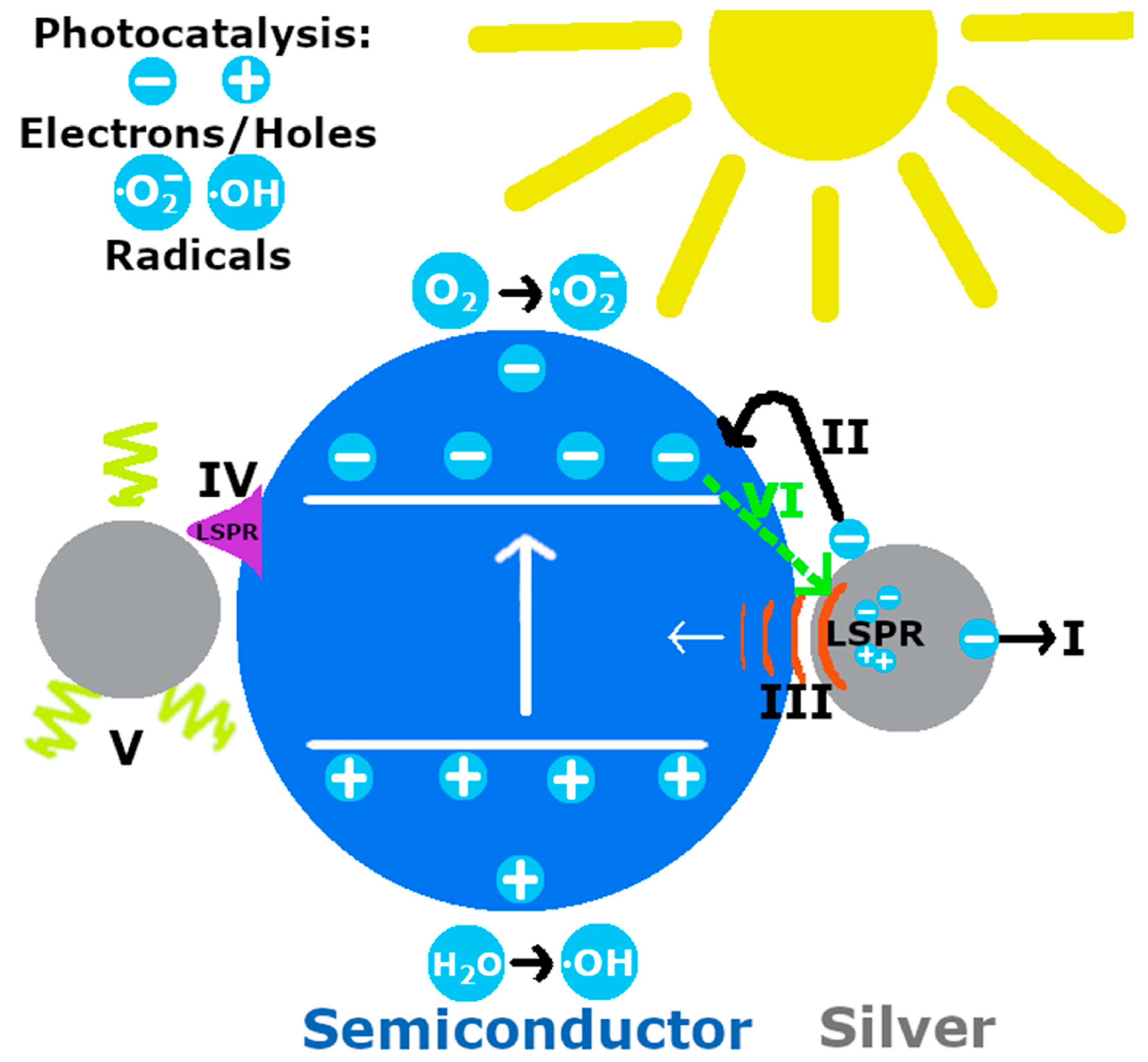
| Heat Treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Precursor | Type of Method | Method | Temperature (°C) | Time (h) | Temperature Ramp Rate (°C/min) | Additional Treatment | Color | Structure | Ref. |
| Melamine | Direct | Thermal | 520 | 4 | 20 | (Post) Milling Washing Drying | Yellow | Single plane | [59] |
| Melamine | Exfoliation | Ultrasonic | - | - | Ultrasonication for 1 h 27 °C and centrifugation for 16 cycles | Pale yellow | Semi-folded sheet aggregates | [27] | |
| Melamine | Exfoliation | Thermal | 520 | 2 | 5 | - | White with a yellow tint | Re-stacked layers | [27] |
| Melamine | Exfoliation | Hydrothermal | 120 (Autoclave) | 10 h | - | Dissolved in acidic solution (HCl) for 1 h and stirred | - | Semi-clusters of folded nanosheets | [27] |
| Melamine | Exfoliation | Chemical | Calcination of sediment 550 | 2 h | - | Dissolved in acidic solution (H2SO4) for 1 h and stirred | White | Pristine nanosheets | [32] |
| Urea and dicyandiamide | Exfoliation | Thermal | 520 | 2 | - | - | Pristine nanosheets | [66] | |
| Thiourea-urea | Direct | Thermal | 550 | 4 | - | - | S-doped nanosheets | [70] | |
| dicyandiamide | Direct | Thermal | 720 | - | 7 | - | Nanosheets | [57] | |
| Melamine | Exfoliation | Thermal | 500 | 6 | - | - | - | Clustered sheets | [42] |
| Melamine | Exfoliation | Thermal -CO2 Atmosphere | 560 | 2 | - | - | - | Clustered sheets | [42] |
| Melamine | Exfoliation | Thermal | 560 | 2 | - | - | - | Porous nanosheet | [42] |
| Melamine | Exfoliation | Thermal | 500 | 2 | 2 | - | Yellowish | Nanosheets | [43] |
| Melamine | Direct | Mechanochemical | 700 | 2 | 5 | (Pre)grinding | Cu-dopped CN sheets | [71] | |
| Precursor | 1 | 2 | 3 | 4 | 5 | Ref. |
|---|---|---|---|---|---|---|
| Melamine | Acid dissolving at 85 °C/24 h HNO3 | Centrifuge washing pH neutralization | Solvothermal Treatment 200 °C for 12 h | 0.22 μm filtration | 4 h sonication Freeze-dry | [48] |
| Urea | Acid dissolving at 80 °C/24 h HCl | Centrifuge washing pH neutralization | Water mix ultrasonicated 20 h | 0.22 μm filtration | Freeze-dry | [29] |
| Urea | Acid dissolving at 80 °C/24 h HNO3/H2SO4 | Centrifuge washing pH neutralization | Solvothermal treatment 180 °C for 10 h | Freeze-dry | [28] |
| C atoms in Groups/Excitations | Names | Peaks (eV) | Ref. |
|---|---|---|---|
| N−C=N | sp2 hybridized carbon in aromatic rings | ~288 | [27,28,36,40,41,46] |
| C-C | Graphitic carbon [53] Adventitious carbon Contaminant carbon | ~284.5 | [28,36,41,46,51,53,72] |
| C-NH, C-NH2 | Amorphous carbon | ~287 | [28,36,51,53] |
| N–C–O/C–O | - | ~288.5 | [46] |
| N Atoms in Groups/Excitations | Names | Peaks (eV) | Ref. |
|---|---|---|---|
| C-N=C | Pyridinic-N, sp2 hybridized N in triazine rings | ~398.7 | [36,46,60] |
| N-(C)3 | Graphitic-N and tertiary N | ~400 | [28,36,40,46,60] |
| C-NH2 and N-H | Surface amino groups Pyrrol-N | ~401 | [36,40,46,60]. |
| π-excitations | π–π bonds | ~404.5 | [28,51,72] |
| π-excitations | π–π bonds | ~404.5 |
| Material | Specific Surface Area (m2/g) | Pore Size (nm) | Ref. |
|---|---|---|---|
| Bulk CN | 17.02 | 24.01 | [27,28] |
| CNQDs/CN | 56.30 | 23.48 | [28] |
| CNTs/CN | 53.07 | 27.28 | [28] |
| CNQDs/CN/CNTs | 74.64 | 24.15 | [28] |
| CN sheets-Therm. | 100.77 | 6.63 | [27] |
| CN sheets-Ultrasonic | 54.97 | 31.27 | [27] |
| CN sheets-Hydrotherm. | 23.95 | 20.56 | [27] |
| Cu/g-C3N4 porous nanotubes | 45.44 | - | [30] |
| BCN | 12.7 | - | [32] |
| ECN | 26.4 | - | [32] |
| Au-ECN | 26.9 | - | [32] |
| Bulk CN | 23 | - | [65] |
| CoS2/CN | 35 | - | [65] |
| Material | Band Gap (V) | Ref. |
|---|---|---|
| H+/H2 | 0 | [72] |
| O2/H2O | 1.23 | [72] |
| g-C3N4 bulk | 2.78 | [28] |
| Pristine CN (650 °C) | 2.88 | [72] |
| Fe3+-doped CNTs | 2.44 | [72] |
| g-C3N4 bulk | 2.83 | [73] |
| g-C3N4 nanotubes | 2.94 | [73] |
| Au/g-C3N4 NT | 2.95 | [73] |
| CuO | 1.7 | [30] |
| g-C3N4 bulk | 2.7 | [30] |
| CuO/g-C3N4 bulk | 2.33 | [30] |
| 5%Mo-CN | 2.63 | [57] |
| Bulk | 2.59 | [47] |
| CN tubes | 2.72 | [47] |
| CN | 2.54 | [60] |
| 0.25 wt% S/0.25% P-dopped nanotubes | 2.71 | [60] |
| 0.5 wt% S/0.5% P-dopped nanotubes | 2.83 | [60] |
| CN | 2.7 | [32] |
| CN thermally exfoliated sheets | 2.7 | [32] |
| 1 wt% @ CN thermally exfoliated sheets | 2.65 | [32] |
| CN sheets | 2.78 | [28] |
| CNQDs/CN | 2.76 | [28] |
| CNT/CN | 2.73 | [28] |
| CNQDs/CN /CNT | 2.65 | [28] |
| CN 600 °C 2 h/5 °C/min | 2.76 | [42] |
| CN 560 °C 2 h/5 °C/min | 2.98 | [42] |
| S-doped nanosheets | 2.64 | [70] |
| Sample | Treatment | Co-Catalyst | Sacrificial Agent | Irradiation Cut Filter (nm) | H2 Production (μmol h−1g−1) | Role of CN | Ref. |
|---|---|---|---|---|---|---|---|
| CN bulk | Powder calcination | - | RhB | 20.5 | Catalyst | [60] | |
| CN bulk | Powder calcination | 3.0 wt% Pt | TEOA | 420 | 76.55 | Catalyst | [66] |
| CN P-doped bulk | Powder calcination | 3.0 wt% Pt | TEOA | 420 | 423.82 | Catalyst | [66] |
| CN 550 °C | Calcination 550 °C | - | Ethylene glycol | 306.4 | Catalyst | [28] | |
| CN 650 °C | Calcination 650 °C | Pt wt% in situ | Ethylene glycol | 420 | 557.5 | Catalyst | [28] |
| CN bulk | Calcination 600 °C | 3 wt% Pt in situ | TEOA | 320 | 2243 | Catalyst | [42] |
| CN nanosheets | Thermal exfoliation | 3 wt% Pt | TEOA | 420 | 389.86 | Catalyst | [66] |
| CN P-doped nanosheets | Thermal exfoliation | 3 wt% Pt | TEOA | 420 | 1146.8 | Catalyst | [66] |
| CN bulk Fe3+-doped | Re-crystallization with TM salts | Pt wt% in situ | TEOA | 420 | 2524.5 | Catalyst | [53,72] |
| CN nanosheets | Mechanochemical | - | TEOA | 280 | Catalyst | [57] | |
| CN nanosheets Deposited 5 wt% Cu | Mechanochemical Deposition by high temp. (720 °C) calcination | 5 wt% Cu | TEOA | 526 | Base catalyst for doping | [57] | |
| CN nanotubes: 0.5% S/P co-doped | Powder calcination | RhB | 8163.5 | Base catalyst for doping | [60] | ||
| CN nanosheets | Thermal exfoliation | - | TEOA-seawater | - | 1629 | Catalyst | [27] |
| WO3 nanosheets @ CN nanosheets | Thermal exfoliation and wet impregnation | - | TEOA-seawater | - | 4328 | Binary comp. base Reduction agent | [27] |
| WO3 nanosheets and CNTs @ CN nanosheets | Thermal exfoliation and wet impregnation | - | TEOA- seawater | - | 11,520 | Ternary comp. base Reduction agent | [27] |
| Fe3+-doped CNTs | Re-crystallization of melamine Calcination 650 °C | Pt | TEOA | 420 | 7538.3 | Base-doped catalyst | [72] |
| CNTs/CN sheets | Microwave 100 °C | - | Ethylene glycol | - | 411.3 | Base-doped catalyst | [28] |
| CNQDs/CN sheets | Microwave 100 °C | - | Ethylene glycol | 683.2 | Base-doped catalyst | [28] | |
| CNQDs/CN/CNTs | Microwave 100 °C | Ethylene glycol | 1109.4 | Base-doped catalyst | [28] | ||
| g-C3N4 bulk | - | TEOA | 420 | 4.8 | Catalyst | [52] | |
| 0.5 wt% Pt @urea-treated g-C3N4 | 0.5 wt% Pt in situ | TEOA | 420 | Base-doped catalyst | [52] | ||
| urea-treated g-C3N4 | - | TEOA | 420 | 6.28 | Catalyst | ||
| CuInS nanotubes | CuInS | TEOA | 365 | Binary comp | [36] | ||
| TiO2 nanoflakes | 3 wt% Pt in situ | Methanol | 380 | 10 | Base-doped catalyst | [29] | |
| 15 wt% g-C3N4 sheets @ TiO2 nanoflakes | 3 wt% Pt in situ | Methanol | 380 | 180 | Surface-mounted | [29] | |
| Bulk CN | Thermal | - | Methanol | 420 | 15 | Catalyst | [32] |
| CN nanosheets | acid exfoliation | - | Methanol | 420 | 44 | Catalyst | [32] |
| CN nanosheets Au 1 wt% loaded | Photo-deposition 200–400 nm | Au 1 wt% | Methanol | 420 | 410 | Catalyst | [32] |
| S-doped CN nanosheets | Calcination | 0.6 wt% Pt | TEOA | 420 | 210 | Catalyst | [70] |
| 20% S-doped CN nanosheets/ZnIn2S4 | Calcination | 0.6 wt% Pt | TEOA | 420 | 1630 | Catalyst | [70] |
| Nanosheets | Thermal exfoliation 500 °C | 3 wt% Pt in situ | TEOA | 320 | 6137 | Catalyst | [42] |
| Nanosheets | Thermal exfoliation 520 °C | 3 wt% Pt in situ | TEOA | 320 | 6515 | Catalyst | [42] |
| Nanosheets | Thermal exfoliation 540 °C | 3wt% Pt in situ | TEOA | 320 | 4749 | Catalyst | [42] |
| Nanosheets | Thermal exfoliation 540 °C/6 h—CO2 atmosphere | 3 wt% Pt in situ | TEOA | 320 | 4241 | Catalyst | [42] |
| Pd/g-C3N4 (bulk-sheet-tube mix) | Thermal exfoliation 550 °C | 0.3 wt% Pd wet-impreg. | TEOA | - | 5000 | Base catalyst | [26] |
| Bulk | Calcination 550 °C, 5 °C/min | 35.7 | Catalyst | [47] | |||
| Nanosheet melamine and urea 1/10 | Calcination 550 °C/5 min | - | TEOA | 420 | 266.4 | Catalyst | [47] |
| 1.5 wt% CuO/ CN bulk | Calcination 550 °C Wet impregnation | TEOA | - | 130.1 | Base catalyst | [30] | |
| Amorphous/crystalline g-C3N4 | Thermal | 1.23 wt% Pt in situ | TEOA | 420 | 799.2 | SC1 and SC2 | [68] |
| Ni@TiO2/g-C3 N4 | Calcination hydrothermal | 7.5 wt% Ni | TEOA | 420, 500, 550 | 134 | Reduction SC | [91] |
| Bulk CN | Thermal (550 °C, 4 h, 5 °C/min) | 1 wt% Pt in situ | TEOA | 420 | 60 | Catalyst | [74] |
| Hexagonal rod CN | Hydrothermal and re-calcination (550 °C, 4 h, and 5 °C/min) | 1 wt% Pt in situ | TEOA | 420 | 343.5 | Catalyst | [74] |
| Hollow nanotube | Re-recalcination (500 °C, 2 h, and 5 °C/min, Ar atm) | 1 wt% Pt in situ | TEOA | 420 | 1534 | Catalyst | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyriakos, P.; Hristoforou, E.; Belessiotis, G.V. Graphitic Carbon Nitride (g-C3N4) in Photocatalytic Hydrogen Production: Critical Overview and Recent Advances. Energies 2024, 17, 3159. https://doi.org/10.3390/en17133159
Kyriakos P, Hristoforou E, Belessiotis GV. Graphitic Carbon Nitride (g-C3N4) in Photocatalytic Hydrogen Production: Critical Overview and Recent Advances. Energies. 2024; 17(13):3159. https://doi.org/10.3390/en17133159
Chicago/Turabian StyleKyriakos, Periklis, Evangelos Hristoforou, and George V. Belessiotis. 2024. "Graphitic Carbon Nitride (g-C3N4) in Photocatalytic Hydrogen Production: Critical Overview and Recent Advances" Energies 17, no. 13: 3159. https://doi.org/10.3390/en17133159
APA StyleKyriakos, P., Hristoforou, E., & Belessiotis, G. V. (2024). Graphitic Carbon Nitride (g-C3N4) in Photocatalytic Hydrogen Production: Critical Overview and Recent Advances. Energies, 17(13), 3159. https://doi.org/10.3390/en17133159










