A Parametric Study of the Organosolv Fractionation of Norway Spruce Sawdust
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock
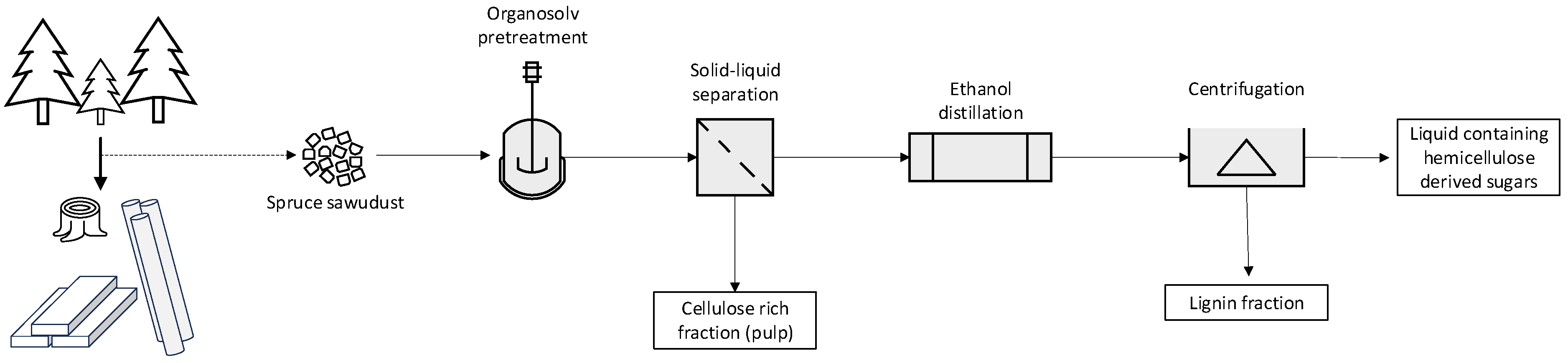
2.2. Pretreatment Procedure
2.3. Analytical Methods
2.4. Calculations
2.5. Enzymatic Saccharification
3. Results and Discussion
3.1. Pulp Composition
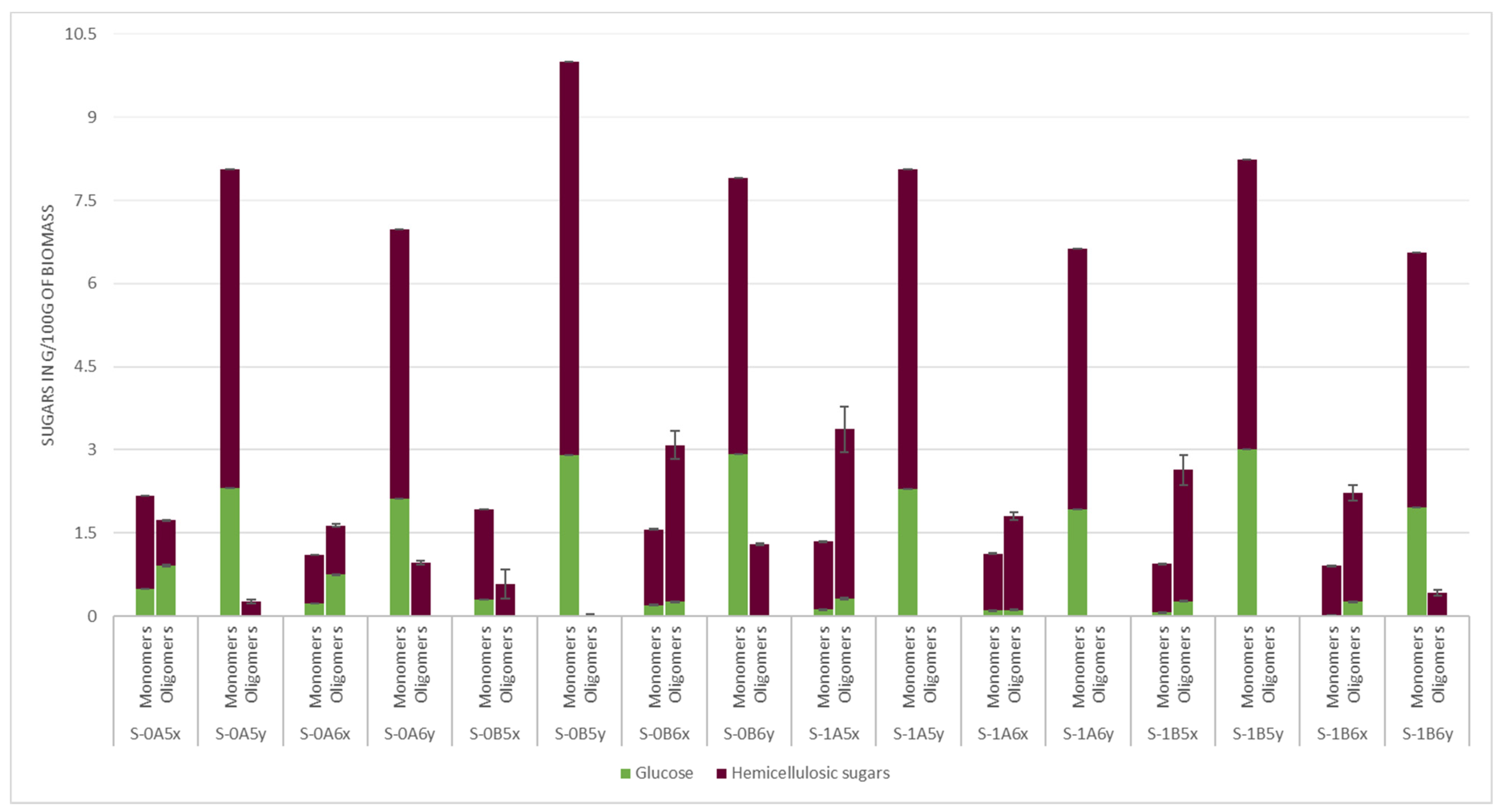
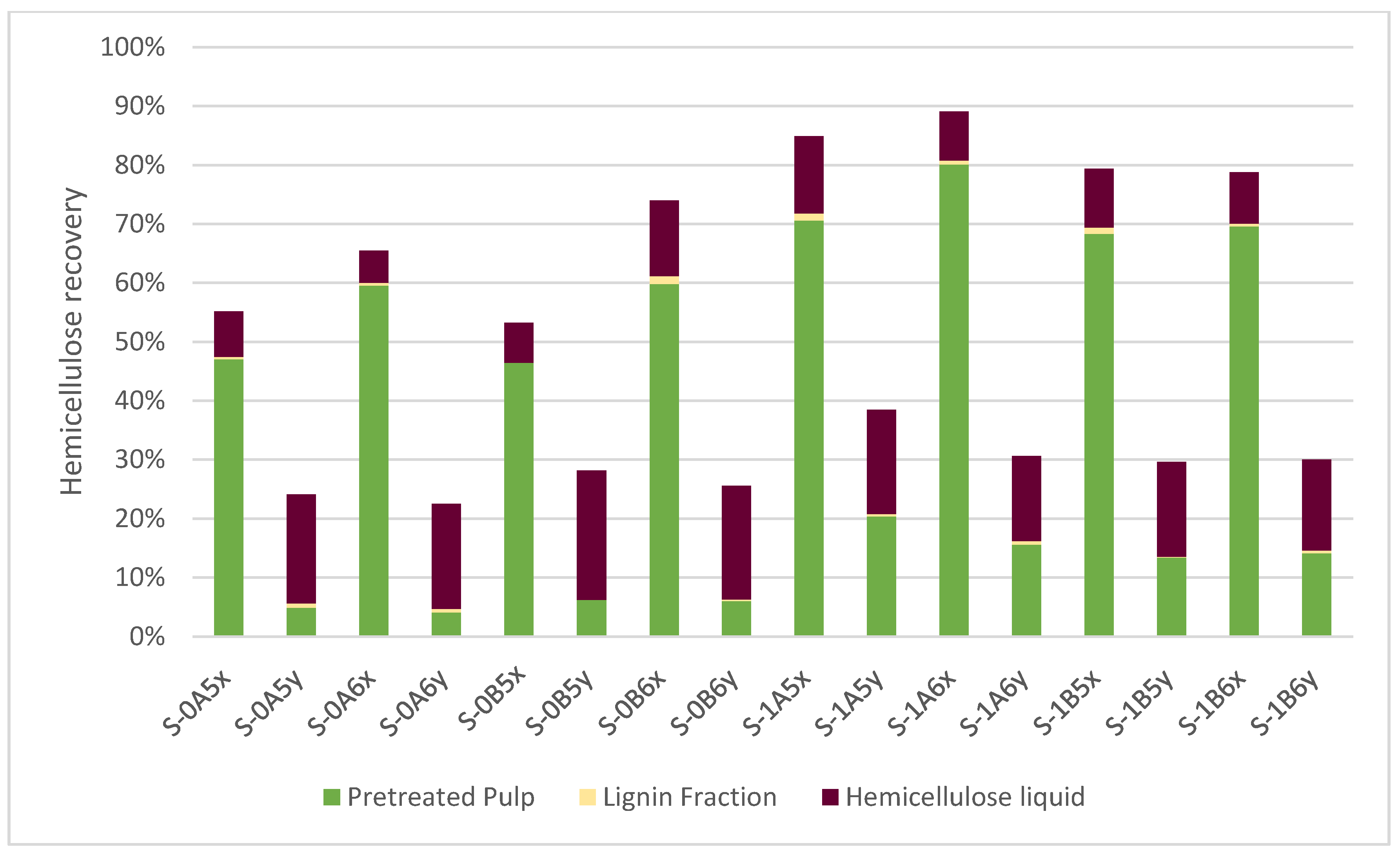
3.2. Hemicellulose Fraction
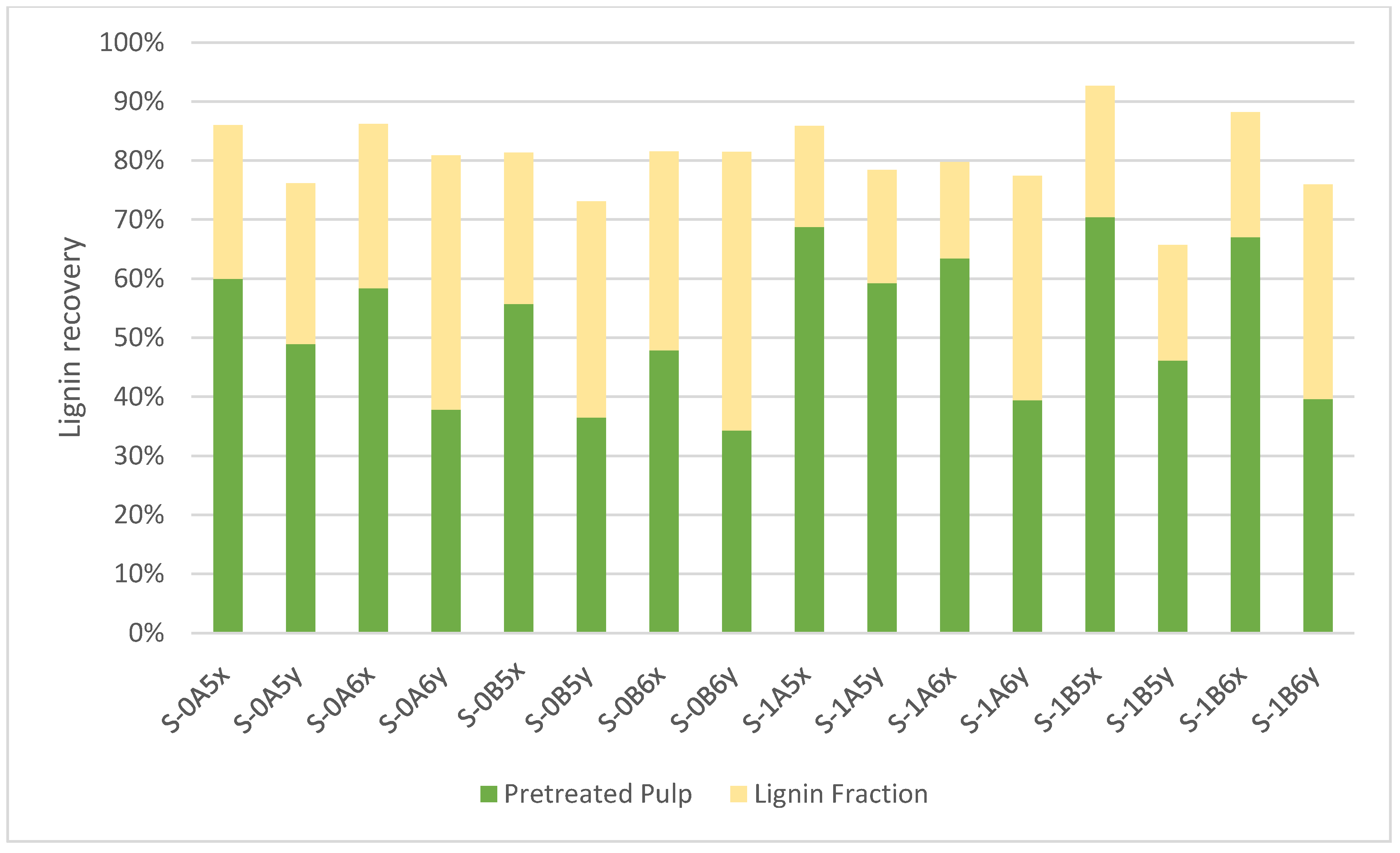
3.3. Lignin Fraction
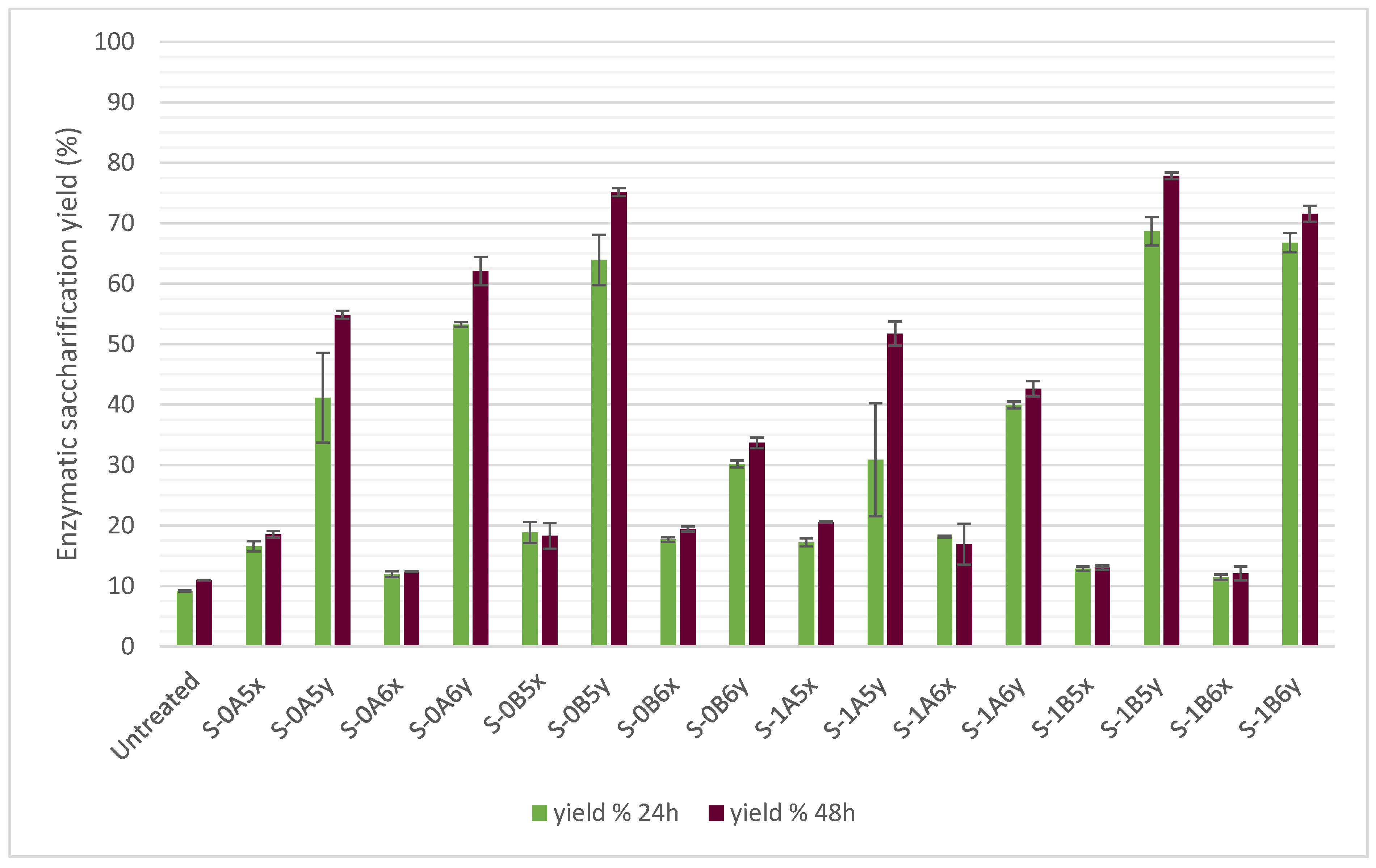
3.4. Enzymatic Saccharification
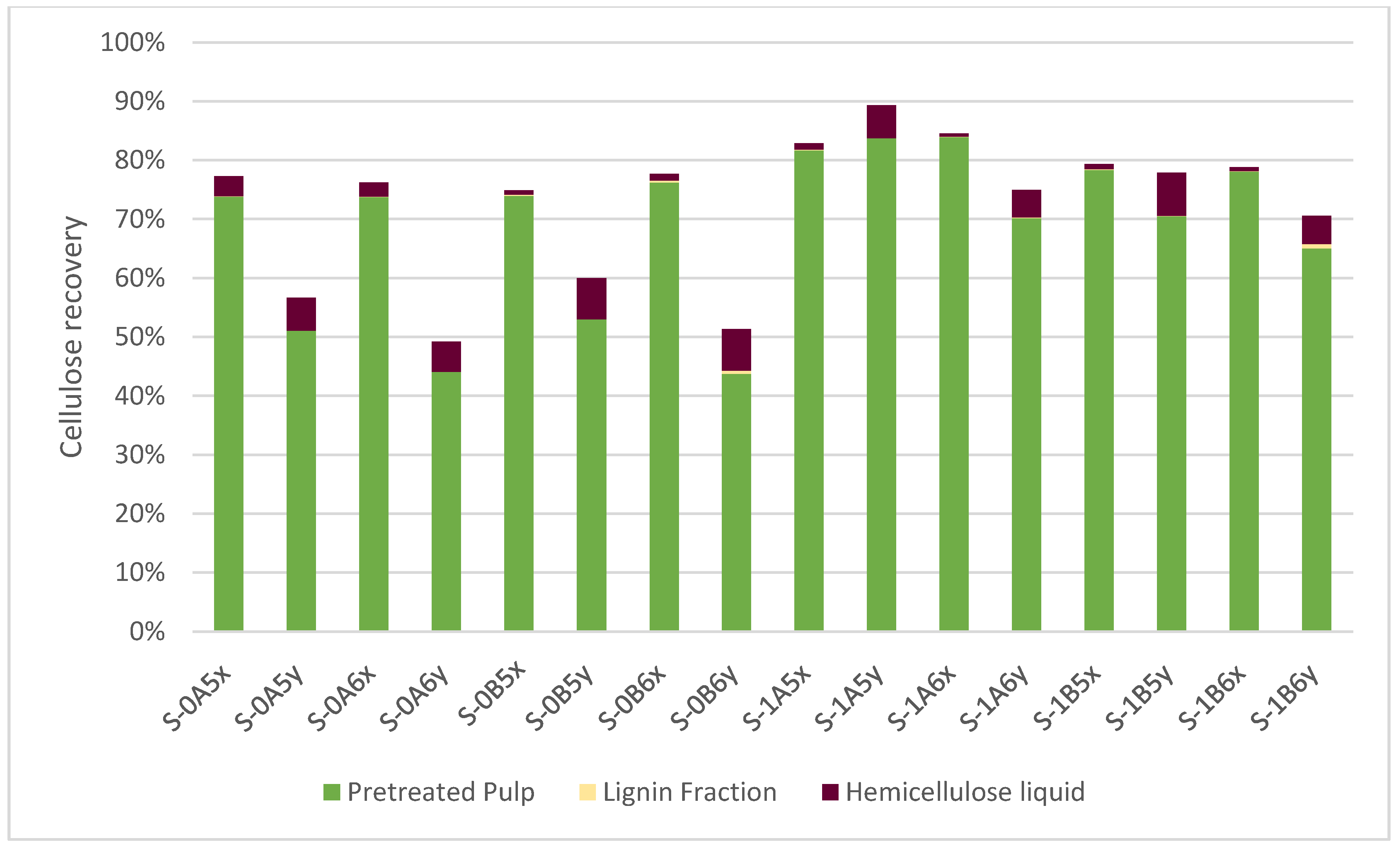
3.5. Mass Balances of the Major Biomass Fractions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumar, A.; Adamopoulos, S.; Jones, D.; Amiandamhen, S.O. Forest Biomass Availability and Utilization Potential in Sweden: A Review. Waste Biomass Valorization 2020, 12, 65–80. [Google Scholar] [CrossRef]
- Lundmark, R.; Athanassiadis, D.; Wetterlund, E. Supply Assessment of Forest Biomass—A Bottom-up Approach for Sweden. Biomass Bioenergy 2015, 75, 213–226. [Google Scholar] [CrossRef]
- Matsakas, L.; Nitsos, C.; Raghavendran, V.; Yakimenko, O.; Persson, G.; Olsson, E.; Rova, U.; Olsson, L.; Christakopoulos, P. A Novel Hybrid Organosolv: Steam Explosion Method for the Efficient Fractionation and Pretreatment of Birch Biomass. Biotechnol. Biofuels 2018, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, F. The Biorefinery Concept: Using Biomass Instead of Oil for Producing Energy and Chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Viell, J.; Harwardt, A.; Seiler, J.; Marquardt, W. Is Biomass Fractionation by Organosolv-like Processes Economically Viable? A Conceptual Design Study. Bioresour. Technol. 2013, 150, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Monção, M.; Wretborn, T.; Rova, U.; Matsakas, L.; Christakopoulos, P. Salicornia Dolichostachya Organosolv Fractionation: Towards Establishing a Halophyte Biorefinery. RSC Adv. 2022, 12, 28599–28607. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.M.; Rigual, V.; Domínguez, J.C.; Alonso, M.V.; Oliet, M.; Rodriguez, F. Fractionation of Pinus Radiata by Ethanol-Based Organosolv Process. Biomass Convers. Biorefinery 2024, 14, 451–464. [Google Scholar] [CrossRef]
- Haus, S.; Björnsson, L.; Börjesson, P. Lignocellulosic Ethanol in a Greenhouse Gas Emission Reduction Obligation System—A Case Study of Swedish Sawdust Based-Ethanol Production. Energies 2020, 13, 1048. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef]
- Vaidya, A.A.; Murton, K.D.; Smith, D.A.; Dedual, G. A Review on Organosolv Pretreatment of Softwood with a Focus on Enzymatic Hydrolysis of Cellulose. Biomass Convers. Biorefinery 2022, 12, 5427–5442. [Google Scholar] [CrossRef]
- Monção, M.; Hrůzová, K.; Rova, U.; Matsakas, L.; Christakopoulos, P. Organosolv Fractionation of Birch Sawdust: Establishing a Lignin-First Biorefinery. Molecules 2021, 26, 6754. [Google Scholar] [CrossRef] [PubMed]
- Weidener, D.; Dama, M.; Dietrich, S.K.; Ohrem, B.; Pauly, M.; Leitner, W.; Domínguez de María, P.; Grande, P.M.; Klose, H. Multiscale Analysis of Lignocellulose Recalcitrance towards OrganoCat Pretreatment and Fractionation. Biotechnol. Biofuels 2020, 13, 155. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Sun, J.; Wang, Z.; Zhao, X.; Hu, Y. Organosolv Fractionation and Simultaneous Conversion of Lignocellulosic Biomass in Aqueous 1,4-Butanediol/Acidic Ionic-Liquids Solution. Ind. Crops Prod. 2019, 138, 111573. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP) (Revised July 2011); National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples; National Renewable Energy Laboratory: Golden, CO, USA, 2006.
- Lange, H.; Rulli, F.; Crestini, C. Gel Permeation Chromatography in Determining Molecular Weights of Lignins: Critical Aspects Revisited for Improved Utility in the Development of Novel Materials. ACS Sustain. Chem. Eng. 2016, 4, 5167–5180. [Google Scholar] [CrossRef]
- Agnihotri, S.; Johnsen, I.A.; Bøe, M.S.; Øyaas, K.; Moe, S. Ethanol Organosolv Pretreatment of Softwood (Picea abies) and Sugarcane Bagasse for Biofuel and Biorefinery Applications. Wood Sci. Technol. 2015, 49, 881–896. [Google Scholar] [CrossRef]
- Goldmann, W.M.; Ahola, J.; Mikola, M.; Tanskanen, J. Solubility and Fractionation of Indulin AT Kraft Lignin in Ethanol-Water Media. Sep. Purif. Technol. 2019, 209, 826–832. [Google Scholar] [CrossRef]
- Hrůzová, K.; Matsakas, L.; Rova, U.; Christakopoulos, P. Organosolv Fractionation of Spruce Bark Using Ethanol–Water Mixtures: Towards a Novel Bio-Refinery Concept. Bioresour. Technol. 2021, 341, 125855. [Google Scholar] [CrossRef]
- Díaz, M.J.; Huijgen, W.J.J.; van der Laan, R.R.; Reith, J.H.; Cara, C.; Castro, E. Organosolv Pretreatment of Olive Tree Biomass for Fermentable Sugars. Holzforschung 2011, 65, 177–183. [Google Scholar] [CrossRef]
- Monção, M.; Thoresen, P.P.; Wretborn, T.; Lange, H.; Rova, U.; Christakopoulos, P.; Matsakas, L. A Novel Biorefinery Concept Based on Marginally Used Halophyte Biomass. Sustain. Energy Fuels 2023, 7, 3902–3918. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.M. Biomass Pretreatment, Biorefineries, and Potential Products for a Bioeconomy Development. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–22. [Google Scholar]
- Nitsos, C.; Rova, U.; Christakopoulos, P. Organosolv Fractionation of Softwood Biomass for Biofuel and Biorefinery Applications. Energies 2017, 11, 50. [Google Scholar] [CrossRef]
- Zhang, Z.; Harrison, M.D.; Rackemann, D.W.; Doherty, W.O.S.; O’Hara, I.M. Organosolv Pretreatment of Plant Biomass for Enhanced Enzymatic Saccharification. Green. Chem. 2016, 18, 360–381. [Google Scholar] [CrossRef]

| Code | Temperature (°C) | Time (min) | Ethanol (%, v/v) | Catalyst (w/wbiomass) |
|---|---|---|---|---|
| S-0A5x | 200 | 15 | 50% | - |
| S-0A5y | 200 | 15 | 50% | 1% |
| S-0A6x | 200 | 15 | 60% | - |
| S-0A6y | 200 | 15 | 60% | 1% |
| S-0B5x | 200 | 30 | 50% | - |
| S-0B5y | 200 | 30 | 50% | 1% |
| S-0B6x | 200 | 30 | 60% | - |
| S-0B6y | 200 | 30 | 60% | 1% |
| S-1A5x | 180 | 15 | 50% | - |
| S-1A5y | 180 | 15 | 50% | 1% |
| S-1A6x | 180 | 15 | 60% | - |
| S-1A6y | 180 | 15 | 60% | 1% |
| S-1B5x | 180 | 30 | 50% | - |
| S-1B5y | 180 | 30 | 50% | 1% |
| S-1B6x | 180 | 30 | 60% | - |
| S-1B6y | 180 | 30 | 60% | 1% |
| Codes | Pulp Recovery (% w/w) | Cellulose (% w/w) (% Retained) | Hemicelluloses (% w/w) (% Retained) | Klason Lignin (% w/w) (% Retained) | Ashes (% w/w) | Total (% w/w) |
|---|---|---|---|---|---|---|
| S-0A5x | 63.65 | 48.06 ± 0.25 (73.82) | 24.07 ± 0.27 (47.01) | 30.69 ± 0.41 (59.97) | 0.15 ± 0.00 | 102.96 |
| S-0A5y | 43.19 | 49.02 ± 0.67 (51.10) | 3.69 ± 0.21 (4.89) | 36.92 ± 0.37 (48.96) | 0.44 ± 0.06 | 90.07 |
| S-0A6x | 67.11 | 45.55 ± 0.05 (73.77) | 28.90 ± 0.07 (59.53) | 28.34 ± 0.77 (58.39) | 0.17 ± 0.06 | 102.96 |
| S-0A6y | 36.20 | 50.46 ± 0.77 (44.09) | 3.67 ± 0.48 (4.08) | 34.06 ± 0.07 (37.86) | 0.47 ± 0.06 | 88.66 |
| S-0B5x | 63.47 | 48.31 ± 0.48 (73.99) | 23.85 ± 0.78 (46.46) | 28.60 ± 0.31 (55.74) | 0.34 ± 0.03 | 101.10 |
| S-0B5y | 40.34 | 54.47 ± 0.15 (53.02) | 5.04 ± 0.35 (6.25) | 29.49 ± 0.45 (36.52) | 0.83 ± 0.12 | 89.84 |
| S-0B6x | 65.56 | 48.18 ± 0.63 (76.23) | 29.72 ± 0.32 (59.80) | 23.79 ± 0.64 (47.88) | 0.90 ± 0.08 | 102.59 |
| S-0B6y | 35.02 | 51.75 ± 1.06 (43.74) | 5.64 ± 0.09 (6.06) | 31.90 ± 1.31 (34.31) | 0.56 ± 0.08 | 89.85 |
| S-1A5x | 74.38 | 45.50 ± 0.31 (81.67) | 30.93 ± 0.24 (70.62) | 30.12 ± 1.33 (68.77) | 0.18 ± 0.00 | 106.73 |
| S-1A5y | 64.89 | 53.46 ± 0.88 (83.72) | 10.26 ± 0.08 (20.43) | 29.73 ± 1.88 (59.22) | 0.26 ± 0.02 | 93.70 |
| S-1A6x | 78.82 | 44.14 ± 0.30 (83.95) | 33.11 ± 0.08 (80.11) | 26.21 ± 0.05 (63.42) | 0.23 ± 0.04 | 103.69 |
| S-1A6y | 53.08 | 54.80 ± 0.84 (70.20) | 9.57 ± 0.13 (15.59) | 24.19 ± 0.16 (39.43) | 0.55 ± 0.02 | 89.11 |
| S-1B5x | 74.55 | 43.55 ± 0.36 (78.36) | 29.86 ± 0.16 (68.32) | 30.76 ± 0.00 (70.40) | 0.17 ± 0.00 | 104.34 |
| S-1B5y | 53.51 | 54.61 ± 0.33 (70.52) | 8.17 ± 0.18 (13.42) | 28.07 ± 0.01 (46.13) | 0.19 ± 0.08 | 91.05 |
| S-1B6x | 74.33 | 43.52 ± 0.17 (78.07) | 30.52 ± 0.11 (69.63) | 29.37 ± 0.31 (67.02) | 0.93 ± 0.12 | 104.35 |
| S-1B6y | 48.96 | 55.05 ± 0.43 (65.04) | 9.42 ± 0.03 (14.16) | 26.35 ± 0.45 (39.61) | 0.02 ± 0.00 | 90.84 |
| Codes | Acetic Acid (g/100 g) | Formic Acid (g/100 g) | Levulinic Acid (g/100 g) | HMF (g/100 g) | Furfural (g/100 g) |
|---|---|---|---|---|---|
| S-0A5x | 0.00 ± 0.00 | 0.25 ± 0.02 | 0.00 ± 0.00 | 1.66 ± 0.05 | 0.98 ± 0.02 |
| S-0A5y | 6.77 ± 0.26 | 0.00 ± 0.00 | 0.00 ± 0.00 | 4.89 ± 0.04 | 2.09 ± 0.21 |
| S-0A6x | 0.00 ± 0.00 | 0.28 ± 0.02 | 0.00 ± 0.00 | 0.83 ± 0.01 | 0.25 ± 0.01 |
| S-0A6y | 4.58 ± 0.20 | 0.00 ± 0.00 | 0.00 ± 0.00 | 4.25 ± 0.44 | 4.27 ± 0.68 |
| S-0B5x | 0.00 ± 0.00 | 0.37 ± 0.03 | 0.00 ± 0.00 | 1.86 ± 0.01 | 0.25 ± 0.00 |
| S-0B5y | 0.24 ± 0.12 | 0.00 ± 0.00 | 0.00 ± 0.00 | 2.90 ± 0.03 | 0.85 ± 0.05 |
| S-0B6x | 0.00 ± 0.00 | 0.24 ± 0.01 | 0.00 ± 0.00 | 1.07 ± 0.00 | 0.25 ± 0.01 |
| S-0B6y | 7.26 ± 0.62 | 0.00 ± 0.00 | 0.00 ± 0.00 | 3.95 ± 0.15 | 5.99 ± 0.72 |
| S-1A5x | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.22 ± 0.00 | 0.35 ± 0.00 |
| S-1A5y | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 4.54 ± 0.30 | 5.23 ± 0.73 |
| S-1A6x | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.20 ± 0.00 | 0.09 ± 0.00 |
| S-1A6y | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 3.25 ± 0.01 | 7.18 ± 0.13 |
| S-1B5x | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.40 ± 0.05 | 0.30 ± 0.05 |
| S-1B5y | 8.33 ± 0.12 | 0.00 ± 0.00 | 0.00 ± 0.00 | 3.64 ± 0.00 | 6.80 ± 0.01 |
| S-1B6x | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.25 ± 0.01 | 0.28 ± 0.01 |
| S-1B6y | 5.22 ± 0.22 | 0.00 ± 0.00 | 0.00 ± 0.00 | 2.68 ± 0.32 | 2.32 ± 0.05 |
| Codes | Cellulose (% w/w) | Hemicelluloses (% w/w) | Klason Lignin (% w/w) | Ashes (% w/w) | Total (% w/w) | Mn (g/mol) | Mw (g/mol) | DI |
|---|---|---|---|---|---|---|---|---|
| S-0A5x | 0.40 ± 0.03 | 1.74 ± 0.10 | 97.02 ± 7.38 | 0.11 ± 0.02 | 99.27 | 2000 | 12,500 | 6.25 |
| S-0A5y | 0.00 ± 0.00 | 2.48 ± 0.00 | 93.64 ± 0.00 | 0.06 ± 0.01 | 96.18 | 900 | 1500 | 1.67 |
| S-0A6x | 0.96 ± 0.02 | 0.82 ± 0.15 | 88.63 ± 7.11 | 0.30 ± 0.18 | 90.71 | 1900 | 13,600 | 7.16 |
| S-0A6y | 0.02 ± 0.00 | 1.28 ± 0.00 | 93.49 ± 0.00 | 0.01 ± 0.01 | 94.81 | 1000 | 1700 | 1.70 |
| S-0B5x | 0.51 ± 0.00 | 0.00 ± 0.00 | 85.23 ± 1.32 | 3.31 ± 0.74 | 89.04 | 2000 | 13,100 | 6.55 |
| S-0B5y | 0.00 ± 0.00 | 0.00 ± 0.00 | 85.23 ± 0.07 | 1.85 ± 0.42 | 87.08 | 1000 | 2700 | 5.76 |
| S-0B6x | 1.00 ± 0.00 | 2.60 ± 0.01 | 87.85 ± 0.40 | 0.07 ± 0.01 | 91.51 | 2000 | 14,900 | 7.45 |
| S-0B6y | 1.34 ± 0.28 | 0.44 ± 0.08 | 95.25 ± 4.38 | 0.16 ± 0.00 | 97.19 | 900 | 1500 | 1.67 |
| S-1A5x | 0.84 ± 0.00 | 6.09 ± 0.06 | 81.59 ± 0.99 | 0.27 ± 0.05 | 88.80 | 2000 | 15,000 | 7.50 |
| S-1A5y | 0.27 ± 0.00 | 1.68 ± 0.00 | 91.99 ± 0.00 | 0.03 ± 0.01 | 93.97 | 800 | 1200 | 1.50 |
| S-1A6x | 1.10 ± 0.03 | 1.27 ± 0.09 | 86.68 ± 9.27 | 0.10 ± 0.03 | 89.14 | 1800 | 11,200 | 6.22 |
| S-1A6y | 0.28 ± 0.00 | 1.58 ± 0.00 | 92.68 ± 0.00 | 0.03 ± 0.01 | 94.58 | 1000 | 1700 | 1.70 |
| S-1B5x | 2.23 ± 0.04 | 2.03 ± 0.18 | 85.16 ± 0.67 | 0.22 ± 0.00 | 89.64 | 1800 | 10,600 | 5.89 |
| S-1B5y | 0.48 ± 0.01 | 0.65 ± 0.01 | 93.62 ± 0.13 | 0.16 ± 0.00 | 94.91 | 800 | 1100 | 1.38 |
| S-1B6x | 0.38 ± 0.09 | 1.67 ± 0.09 | 88.21 ± 0.44 | 0.22 ± 0.00 | 90.48 | 1800 | 11,000 | 6.11 |
| S-1B6y | 2.38 ± 0.11 | 1.19 ± 0.03 | 90.10 ± 0.75 | 0.08 ± 0.00 | 93.76 | 900 | 1300 | 1.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monção, M.; Anukam, A.I.; Hrůzová, K.; Rova, U.; Christakopoulos, P.; Matsakas, L. A Parametric Study of the Organosolv Fractionation of Norway Spruce Sawdust. Energies 2024, 17, 3276. https://doi.org/10.3390/en17133276
Monção M, Anukam AI, Hrůzová K, Rova U, Christakopoulos P, Matsakas L. A Parametric Study of the Organosolv Fractionation of Norway Spruce Sawdust. Energies. 2024; 17(13):3276. https://doi.org/10.3390/en17133276
Chicago/Turabian StyleMonção, Maxwel, Anthony Ike Anukam, Kateřina Hrůzová, Ulrika Rova, Paul Christakopoulos, and Leonidas Matsakas. 2024. "A Parametric Study of the Organosolv Fractionation of Norway Spruce Sawdust" Energies 17, no. 13: 3276. https://doi.org/10.3390/en17133276
APA StyleMonção, M., Anukam, A. I., Hrůzová, K., Rova, U., Christakopoulos, P., & Matsakas, L. (2024). A Parametric Study of the Organosolv Fractionation of Norway Spruce Sawdust. Energies, 17(13), 3276. https://doi.org/10.3390/en17133276









