Review of Organic Waste-to-Energy (OWtE) Technologies as a Part of a Sustainable Circular Economy
Abstract
:1. Introduction
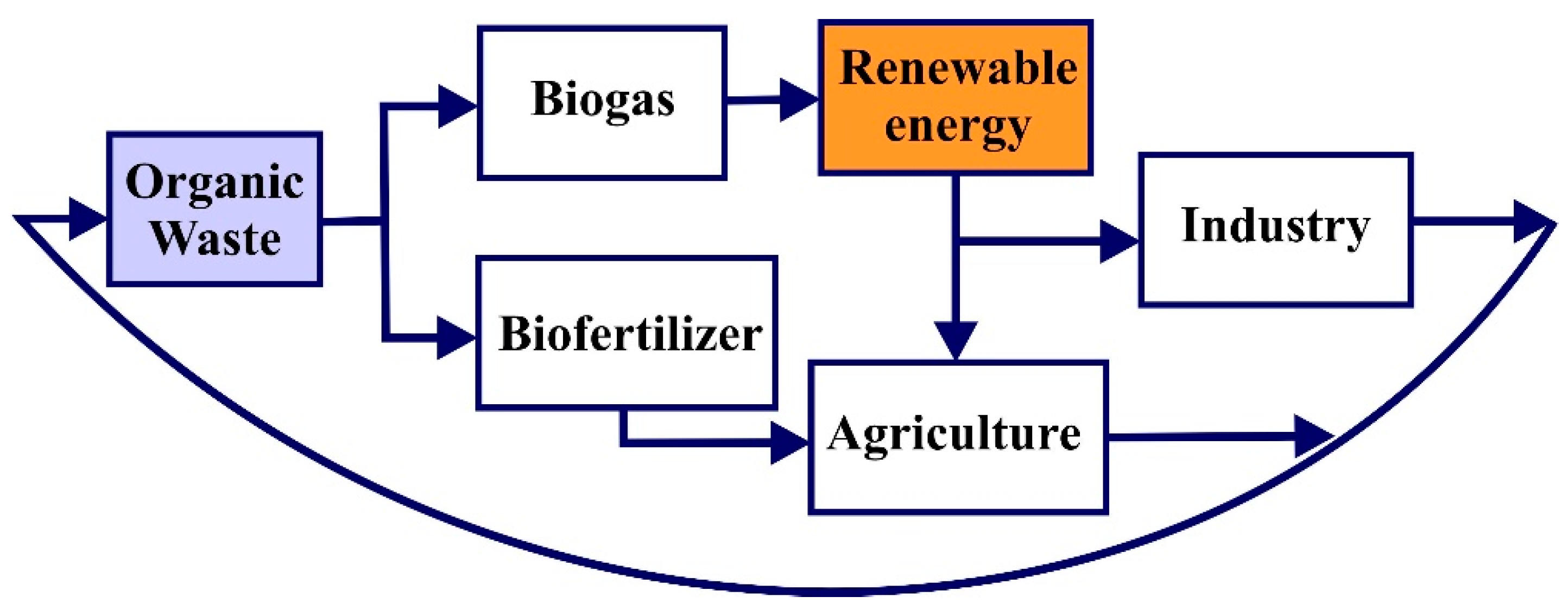
2. Strategies for Effective and Sustainable QWtE Cycle
3. Biochemical OWtE Conversion
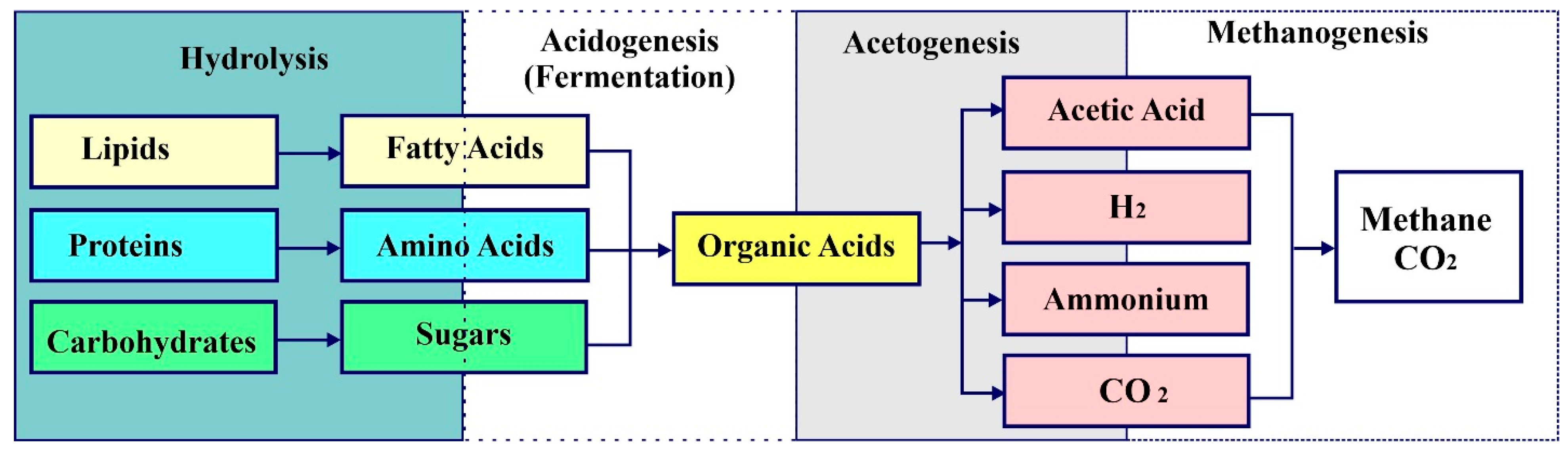
3.1. Anaerobic Fermentation (AF)
3.2. Anaerobic Digestion (AD)
- -
- It can be upgraded to 98% pure biomethane for use as a substitute fuel for natural gas. In this case, a treatment step is required: the removal of carbon dioxide and the scrubbing of toxic and corrosive H2S and other impurities.
- -
- It can be combusted directly to produce heat and power.
- -
- It can be used as row material for hydrogen, methanol, and dimethyl ether production by means of its additional processing through biomethane reforming in the presence of steam.
- Biogas, or biomethane obtained from it, is a renewable energy source.
- The use of biogas can reduce the cost of energy production since it is a cheap and easily accessible source.
- The use of biogas makes it possible to reduce dependence on oil and gas, which cause emissions of greenhouse gases and other harmful substances.
3.3. Joint AD/AF Process
4. Thermochemical OWtE Technologies
- -
- solid (ash), which can be used as a plant nutrient enhancer due to its phosphorus content;
- -
- aqueous (nutrient-rich), which can be used to water plants because it is rich in potassium;
- -
- gas phases (mostly CO2).
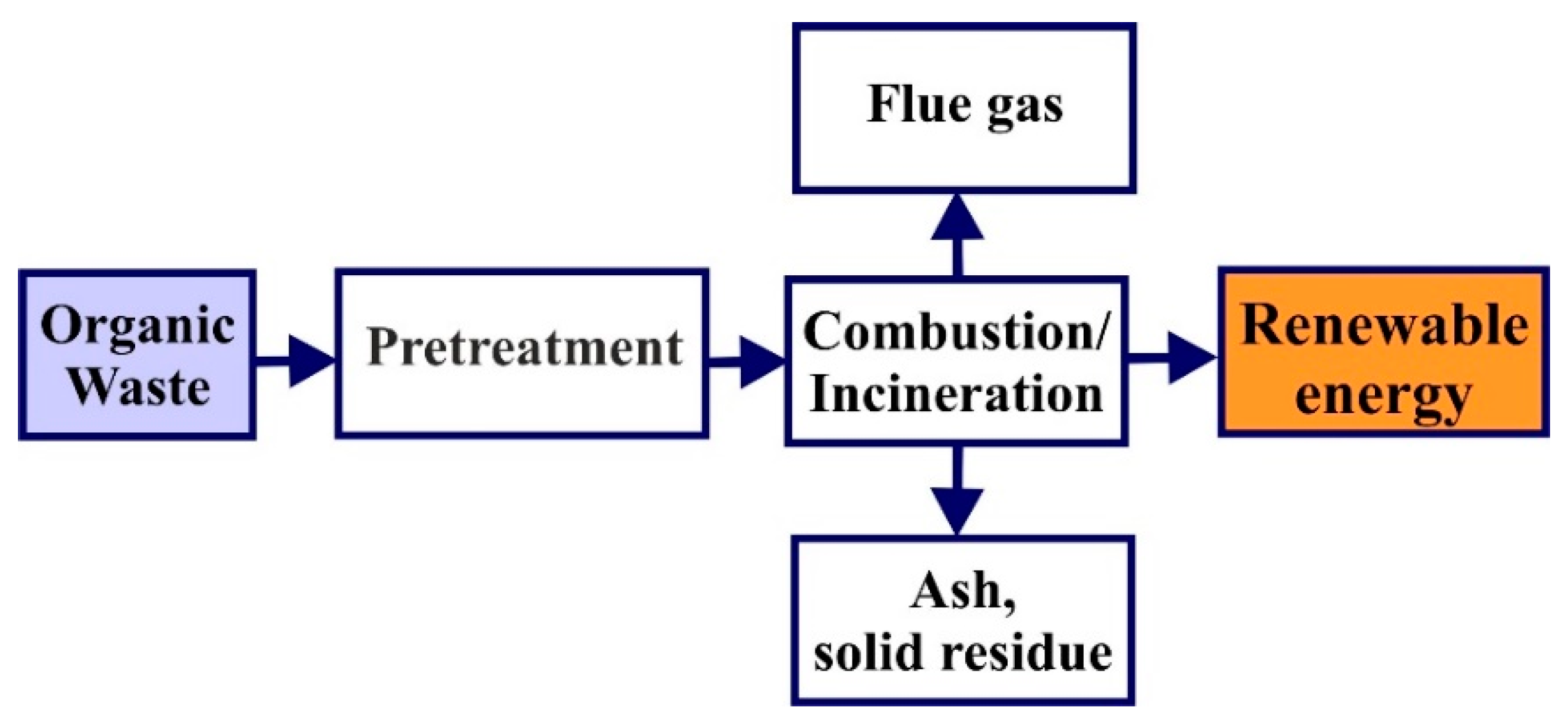
4.1. Incineration
4.2. Gasification
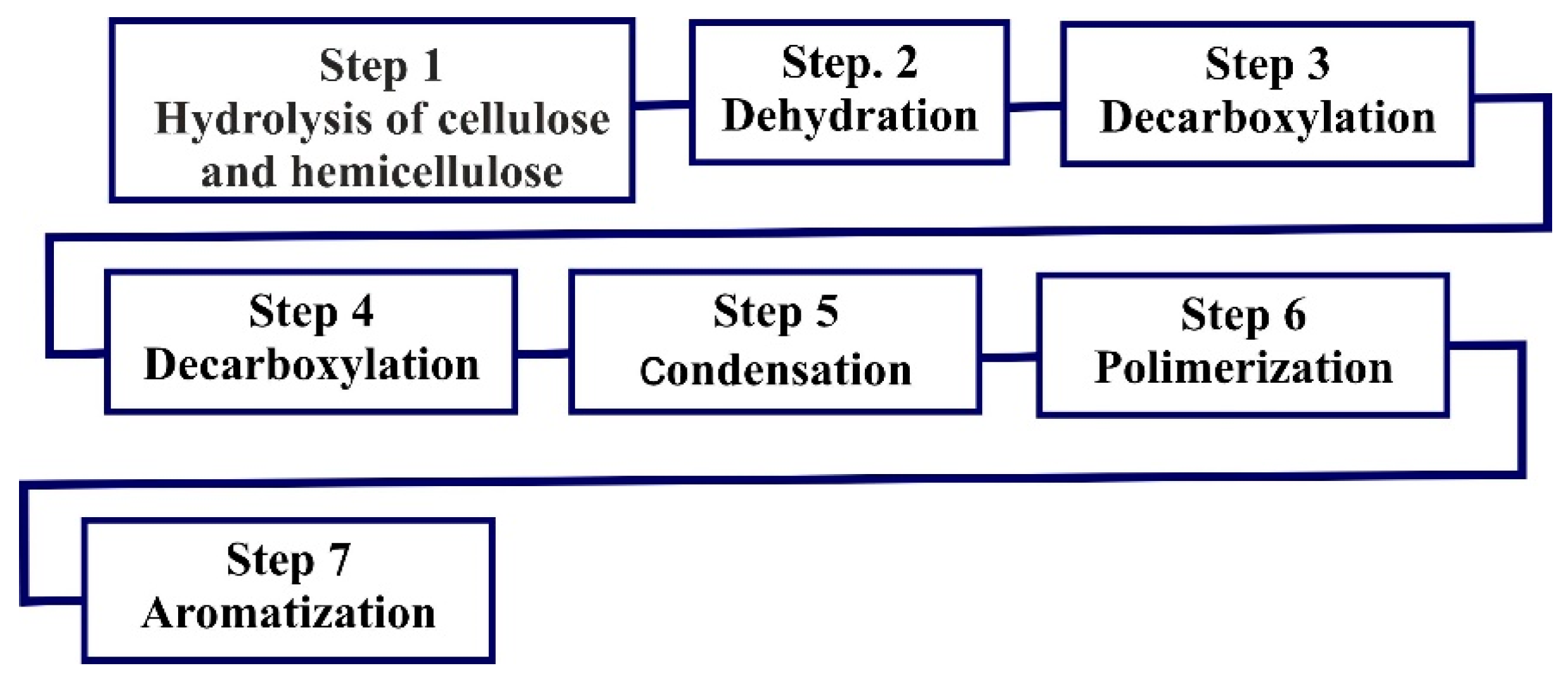
4.3. Pyrolysis
- Placing waste in a special chamber and drying it;
- The dry distillation of waste;
- The combustion of solid residues;
- The formation of gas, coal, and oil (end products of pyrolysis).
4.4. Hydrothermal Carbonization (HTC)
- -
- The waste can be wet, which avoids the previous stage of dehydration;
- -
- Relatively low temperatures compared to other thermochemical processes, thereby reducing energy costs;
- -
- Higher product yield in the case of solid product (coke) compared to other thermochemical methods—20–80% with hydrothermal carbonization versus 12–35% with pyrolysis;
- -
- The resulting liquid and gas phases can be valorized after further processing.
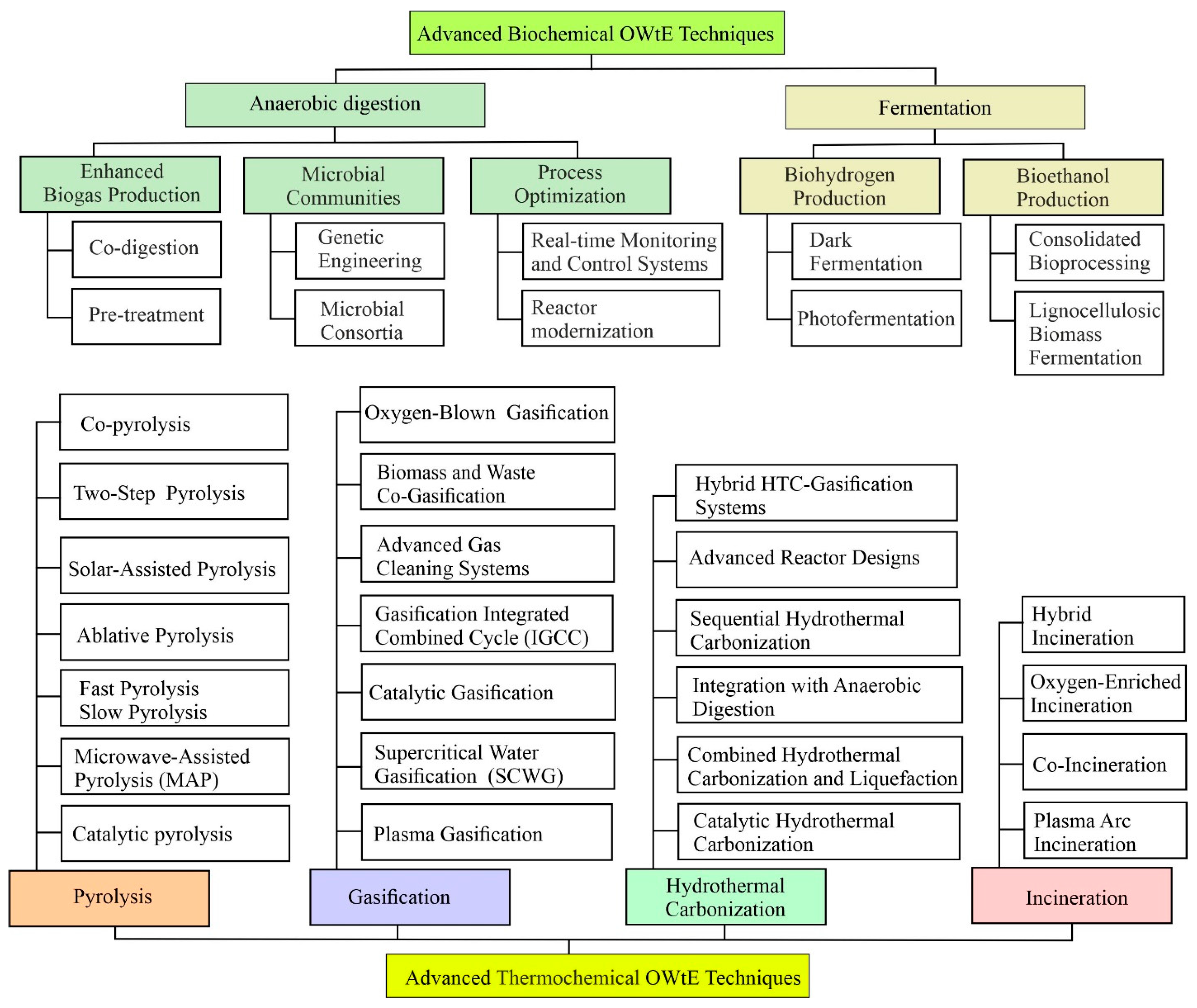
5. Advanced OWtE Techniques
- -
- Applying high temperature and pressure to hydrolyze complex organic compounds into simpler molecules is called thermal hydrolysis [79,80]. Furthermore, thermal hydrolysis destroys pathogens; enhances the solubility of organic molecules, making them more accessible to microbial action; and reduces raw material viscosity, which improves mixing and reduces energy consumption during the breakdown process. As a result, this produces excellent biogas yields.
- -
- -
- Adding chemicals (e.g., acids, alkalis) to solubilize organic matter is called chemical pre-treatment.
6. OWtE Technologies, Practical Application in EU
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CE | Circular economy |
| OWtE | Organic waste-to-energy |
| WtE | Waste-to-energy |
| GHGs | Greenhouse gasses |
| RRfW | Resource recovery from waste |
| OSW | Organic solid waste |
| AF | Anaerobic fermentation |
| ETP | Electron transport phosphorylation |
| AD | Anaerobic digestion |
| VFAs | Volatile fatty acids |
| MSW | Municipal solid waste |
| COD | Chemical oxygen demand |
| HTC | Hydrothermal carbonization |
| HTL | Hydrothermal liquefaction |
| HTV | Hydrothermal vaporization |
| HTG | Hydrothermal gasification |
| DCWG | Super critical water gasification |
References
- Velenturf, A.; Purnell, P. Principles for a Sustainable Circular Economy. Sustain. Prod. Consum. 2021, 27, 1437–1457. [Google Scholar] [CrossRef]
- Lag-Brotons, A.J.; Velenturf, A.P.M.; Crane, R.; Head, I.M.; Purnell, P.; Semple, K.T. Editorial: Resource Recovery From Waste. Front. Environ. Sci. Sec. Microbiol. Chem. Geomicrobiol. 2020, 8, 35. [Google Scholar] [CrossRef]
- Regulation (EU) 2020/852 of the European Parliament and of the Council of 18 June 2020 on the Establishment of a Framework to Facilitate Sustainable Investment, and Amending Regulation (EU) 2019/2088. 2020. Available online: http://data.europa.eu/eli/reg/2020/852/oj (accessed on 10 April 2024).
- Waste Framework Directive. Available online: https://environment.ec.europa.eu/topics/waste-and-recycling/waste-framework-directive_en#ref-2023-amendment-to-the-waste-framework-directive (accessed on 10 March 2024).
- Bakan, B.; Bernet, N.; Bouchez, T. Circular Economy Applied to Organic Residues and Wastewater: Research Challenges. Waste Biomass-Valorization 2022, 13, 1267–1276. [Google Scholar] [CrossRef]
- Bioenergy Report Outlines Progress Being Made across the EU. Available online: https://energy.ec.europa.eu/news/bioenergy-report-outlines-progress-being-made-across-eu-2023-10-27_en (accessed on 10 March 2024).
- Silva-Martínez, R.D.; Sanches-Pereira, A.; Ortiz, W.; Gómez Galindo, M.F.; Coelho, S.T. The state-of-the-art of organic waste to energy in Latin America and the Caribbean: Challenges and opportunities Renew. Energy 2020, 156, 509–525. [Google Scholar] [CrossRef]
- Wainaina, S.; Awasthi, M.K.; Sarsaiya, S.; Chen, H.; Singh, E.; Kumar, A.; Ravindran, B.; Awasthi, S.K.; Liu, T.; Duan, Y.; et al. Resource recovery and circular economy from organic solid waste using aerobic and anaerobic digestion technologies. Bioresour. Technol. 2020, 301, 122778. [Google Scholar] [CrossRef] [PubMed]
- Primary Energy—Global Consumption 2023. Statista. Available online: https://www.statista.com/statistics/265598/consumption-of-primary-energy-worldwide/ (accessed on 15 July 2024).
- Kalair, A.R.; Seyedmahmoudian, M.; Stojcevski, A.; Abas, N.; Khan, N. Waste to energy conversion for a sustainable future. Heliyon 2021, 7, e08155. [Google Scholar] [CrossRef]
- Kataya, G.; Cornu, D.; Bechelany, M.; Hijazi, A.; Issa, M. Biomass Waste Conversion Technologies and Its Application for Sustainable Environmental Development—A Review. Agronomy 2023, 13, 2833. [Google Scholar] [CrossRef]
- Elalami, D.; Barakat, A. State of the art of energy production from agricultural residues using thermochemical and biological processes. In Clean Energy and Resources Recovery; Volume 1; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–24. [Google Scholar]
- Zhang, J.; Du, Z.; Fu, L.; Han, Y.; Zheng, W.; Yu, F.; Chen, H.; Feng, L.; Li, Y.; Ping, W. Novel Anaerobic Digestion and Carbon Dioxide Emissions Efficiency Analysis of Food Waste Treatment Based on SBM-DEA Model. J. Clean. Prod. 2021, 328, 129591. [Google Scholar] [CrossRef]
- Unegg, M.C.; Steininger, K.W.; Ramsauer, C.; Rivera-Aguilar, M. Assessing the Environmental Impact of Waste Management: A Comparative Study of CO2 Emissions with a Focus on Recycling and Incineration. J. Clean. Prod. 2023, 415, 137745. [Google Scholar] [CrossRef]
- Integrated Gasification Combined-Cycle|Climate Technology Centre & Network|1181699. Available online: https://www.ctc-n.org/technology-library/production-efficiency/integrated-gasification-combined-cycle (accessed on 27 June 2024).
- Schwartz, N.R.; Paulsen, A.D.; Blaise, M.J.; Wagner, A.L.; Yelvington, P.E. Analysis of Emissions from Combusting Pyrolysis Products. Fuel 2020, 274, 117863. [Google Scholar] [CrossRef]
- Yu, S.; He, J.; Zhang, Z.; Sun, Z.; Xie, M.; Xu, Y.; Bie, X.; Li, Q.; Zhang, Y.; Sevilla, M.; et al. Towards Negative Emissions: Hydrothermal Carbonization of Biomass for Sustainable Carbon Materials (Adv. Mater. 18/2024). Adv. Mater. 2024, 36, 2470139. [Google Scholar] [CrossRef]
- Carbon Dioxide Emissions Factor, kg CO2 per MWh. Our World in Data. Available online: https://ourworldindata.org/grapher/carbon-dioxide-emissions-factor (accessed on 27 June 2024).
- Vikjær-Andresen, E. How Can Biogenic CO2 Be Used to Address the Climate Crisis? World Economic Forum. Available online: https://www.weforum.org/agenda/2023/10/biogenic-co2-climate-green-hydrogen/ (accessed on 11 July 2024).
- Jarunglumlert, T.; Bampenrat, A.; Sukkathanyawat, H.; Prommuak, C. Enhanced Energy Recovery from Food Waste by Co-Production of Bioethanol and Biomethane Process. Fermentation 2021, 7, 265. [Google Scholar] [CrossRef]
- Kumar, H.; Vijay, V.K.; Subbarao, P.M.; Chandra, R. Studies on the Application of Bio-Carbon Dioxide as Controlled Atmosphere on Pest Management in Wheat Grain Storage. J. Stored Prod. Res. 2022, 95, 101911. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC) Emission Factor Database (EFDB). Available online: https://www.ipcc-nggip.iges.or.jp/EFDB/ (accessed on 27 October 2023).
- Lanza, L.J. Tech’s Bacteria Carbon Capture Tech. Carbon Credits. Available online: https://carboncredits.com/lanzatech-capture-carbon/ (accessed on 24 June 2024).
- Velvizhi, G.; Sarkar, O.; Rovira-Alsina, L.; Puig, S.; Mohan, S.V. Conversion of Carbon Dioxide to Value Added Products through Anaerobic Fermentation and Electro Fermentation: A Comparative Approach. Int. J. Hydrogen Energy 2022, 47, 15442–15455. [Google Scholar] [CrossRef]
- Kwon, E.E.; Kim, S.; Lee, J. Pyrolysis of Waste Feedstocks in CO2 for Effective Energy Recovery and Waste Treatment. J. CO2 Util. 2019, 31, 173–180. [Google Scholar] [CrossRef]
- Williams, C.L.; Dahiya, A.; Porter, P. Introduction to bioenergy and waste to energy. In Bioenergy, 2nd ed.; Cambridge University Press: Cambridge, UK, 2020; pp. 5–44. [Google Scholar]
- Manikandan, S.; Vickram, S.; Sirohi, R.; Subbaiya, R.; Krishnan, R.Y.; Karmegam, N.; Sumathijones, C.; Rajagopal, R.; Chang, S.W.; Ravindran, B.; et al. Critical review of biochemical pathways to transformation of waste and biomass into bioenergy. Bioresour. Technol. 2023, 372, 128679. [Google Scholar] [CrossRef] [PubMed]
- Buckel, W. Energy Conservation in Fermentations of Anaerobic. Bacteria. Front. Microbiol. 2021, 12, 703525. [Google Scholar]
- Zhang, Y.; Wang, X.; Zhu, W.; Zhao, Y.; Wang, N.; Gao, M.; Wang, Q. Anaerobic fermentation of organic solid waste: Recent updates in substrates, products, and the process with multiple products co-production. Environ. Res. 2023, 233, 116444. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, Y.; Song, T.; Su, H. Waste Fermentation for Energy Recovery. In Waste-to-Energy: Recent Developments and Future Perspectives towards Circular Economy; Springer: Cham, Switzerland, 2022; pp. 207–225. [Google Scholar]
- Hegde, S.; Trabold, T.A. Sustainable Waste-to-Energy Technologies: Fermentation. In Sustainable Food Waste-To-Energy Systems; Chapter 5; Academic Press: Cambridge, MA, USA, 2018; pp. 69–88. [Google Scholar]
- Fan, Y.X.; Zhang, J.Z.; Zhang, Q.; Ma, X.Q.; Liu, Z.Y.; Lu, M.; Qiao, K.; Li, F.L. Biofuel and chemical production from carbon one industry flux gas by acetogenic bacteria. In Advances in Applied Microbiology; Chapter 1; Academic Press: Cambridge, MA, USA, 2021; Volume 117, pp. 1–34. [Google Scholar]
- Zueva, S.; Kovalev, A.A.; Litti, Y.V.; Ippolito, N.M.; Innocenzi, V.; De Michelis, I. Environmental and Economic Aspects of Biomethane Production from Organic Waste in Russia. Energies 2021, 14, 5244. [Google Scholar] [CrossRef]
- Yan, J.; Salman, C.A. Waste-to-energy (WtE): Current technologies and their future potential. In Waste Biorefineries; Elsevier: Amsterdam, The Netherlands, 2023; pp. 83–97. [Google Scholar]
- Dong, H.; Mangino, J.; Mcallister, T.A.; Hatfield, J.L.; Johnson, D.E.; Bartram, D.; Gibb, D. Emissions from Livestock and Manure Management. 2006. Available online: http://www.ipcc-nggip.iges.or.jp/public/2006gl/pdf/4_Volume4/V4_10_Ch10_Livestock.pdf (accessed on 16 July 2024).
- Gopal, P.M.; Sivaram, N.M.; Barik, D. Paper Industry Wastes and Energy Generation From Wastes. In Energy from Toxic Organic Waste for Heat and Power Generation; Woodhead Publishing Series in Energy; Chapter 7; Woodhead Publishing: Sawston, UK, 2019; pp. 83–97. [Google Scholar]
- Singh, V.; Das, D. Chapter 3. Potential of Hydrogen Production From Biomass. In Science and Engineering of Hydrogen-Based Energy Technologies; Academic Press: Cambridge, MA, USA, 2019; pp. 123–164. [Google Scholar]
- Sharma, H.B.; Venna, S.; Dubey, B.K. Resource recovery and circular economy approach in organic waste management using hydrothermal carbonization. In Clean Energy and Resources Recovery; Chapter 13; Elsevier: Amsterdam, The Netherlands, 2021; pp. 313–326. [Google Scholar]
- Thabit, Q.; Nassour, A.; Nelles, M. Flue Gas Composition and Treatment Potential of a Waste Incineration Plant. Appl. Sci. 2022, 12, 5236. [Google Scholar] [CrossRef]
- Alao, M.A.; Popoola, O.M.; Ayodele, T.R. Waste-To-Energy Nexus: An Overview of Technologies and Implementation for Sustainable Development. Clean. Energy Syst. 2022, 3, 100034. [Google Scholar] [CrossRef]
- Wei, R.; Zhang, R.; Song, L. Incineration disposal of organic waste bio-residue via a deep dewatering process using refuse incineration bottom ash: Moisture transfer and low calorific value improvement. Environ. Sci. Pollut. Res. 2022, 29, 78107–78119. [Google Scholar] [CrossRef] [PubMed]
- Dal Pozzo, A.; Antonioni, G.; Guglielmi, D.; Stramigioli, C.; Cozzani, V. Comparison of alternative flue gas dry treatment technologies in waste-to-energy processes. Waste Manag. 2016, 51, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Moharir, R.V.; Gautam, P.; Kumar, S. Waste Treatment Processes/Technologies for Energy Recovery. In Current Developments in Biotechnology and Bioengineering; Chapter 4; Elsevier: Amsterdam, The Netherlands, 2019; pp. 53–77. [Google Scholar]
- Lam, C.H.K.; Ip, A.W.M.; Barford, J.P.; McKay, G. Use of Incineration MSW Ash: A Review. Sustainability 2010, 2, 1943–1968. [Google Scholar] [CrossRef]
- Murugesan, P.; Raja, V.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Food waste valorisation via gasification. A review on emerging concepts, prospects and challenges. Sci. Total Environ. 2022, 851, 157955. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Upadhyay, R.K. Review on biomass gasification: Gasifiers, gasifying mediums, and operational parameters. Mater. Sci. Energy Technol. 2021, 4, 329–340. [Google Scholar] [CrossRef]
- Yang, H.; Chen, H. Biomass gasification for synthetic liquid fuel production. In Gasification for Synthetic Fuel Production; Woodhead Publishing: Sawston, UK, 2015; pp. 241–275. [Google Scholar]
- McCaffrey, Z.; Thy, P.; Long, M.; Oliveira, M.; Wang, L.; Torres, L.; Aktas, T.; Chiou, B.S.; Orts, W.; Jenkins, B.M. Air and steam gasification of almond biomass. Front. Energy Res. 2019, 7, 84. [Google Scholar] [CrossRef]
- Tezer, Ö.; Karabağ, N.; Öngen, N.; Çolpan, C.Ö.; Ayol, A. Biomass gasification for sustainable energy production: A review. Int. J. Hydrogen Energy 2022, 47, 15419–15433. [Google Scholar] [CrossRef]
- Sansaniwal, S.K.; Pal, K.; Rosen, M.A.; Tyagi, S.K. Recent advances in the development of biomass gasification technology: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 72, 363–384. [Google Scholar] [CrossRef]
- Kane, S.; Ulrich, R.; Harrington, A.; Stadie, N.P.; Ryan, C. Physical and chemical mechanisms that influence the electrical conductivity of lignin-derived biochar. Carbon Trends. 2021, 5, 100088. [Google Scholar] [CrossRef]
- Su, H.; Hantoko, D.; Yan, M.; Cai, Y.; Kanchanatip, E.; Liu, J.; Zhou, X.; Zhang, S. Evaluation of catalytic subcritical water gasification of food waste for hydrogen production: Effect of process conditions and different types of catalyst loading. Int. J. Hydrogen Energy 2019, 44, 21451–21463. [Google Scholar] [CrossRef]
- Su, H.; Liao, W.; Wang, J.; Hantoko, D.; Zhou, Z.; Feng, H.; Jiang, J.; Yan, M. Assessment of supercritical water gasification of food waste under the background of waste sorting: Influences of plastic waste contents. Int. J. Hydrogen Energy 2020, 45, 21138–21147. [Google Scholar] [CrossRef]
- Czajczyńska, D.; Anguilano, L.; Ghazal, H.; Krzyżyńska, R.; Reynolds, A.J.; Spencer, N.; Jouhara, H. Potential of pyrolysis processes in the waste management sector. Therm. Sci. Eng. Prog. 2017, 3, 171–197. [Google Scholar] [CrossRef]
- Shahbaz, M.; AlNouss, A.; Parthasarathy, P.; Abdelaal, A.H.; Mackey, H.; McKay, G. Investigation of biomass components on the slow pyrolysis products yield using Aspenlus for techno-economic analysis. Biomass Conv. Bioref. 2020, 12, 669–681. [Google Scholar] [CrossRef]
- Chen, W.H.; Farooq, W.; Shahbaz, M.; Naqvi, S.R.; Ali, I.; Al-Ansari, T. Current status of biohydrogen production from lignocellulosic biomass, technical challenges and commercial potential through pyrolysis process. Energy 2021, 226, 120433. [Google Scholar] [CrossRef]
- Venderbosch, R.H.; Prins, W. Fast pyrolysis technology development. Biofuels Bioprod. Biorefining 2010, 4, 178–208. [Google Scholar] [CrossRef]
- Uddin, M.N.; Techato, K.; Taweekun, J.; Rahman, M.M.; Rasul, M.G.; Mahlia, T.M.I. An overview of recent developments in biomass pyrolysis technologies. Energies 2018, 11, 3115. [Google Scholar] [CrossRef]
- Amenaghawon, N.; Anyalewechi, C.L.; Okieimen, C.O.; Kusuma, H.S. Biomass pyrolysis technologies for value-added products: A state-of-the-art review. Environ. Dev. Sustain. 2021, 23, 14324–14378. [Google Scholar] [CrossRef]
- Al-Rumaihi, A.; Shahbaz, M.; Mckay, G.; Mackey, H.; Al-Ansari, T. A review of pyrolysis technologies and feedstock: A blending approach for plastic and biomass towards optimum biochar yield. Renew. Sustain. Energy 2022, 167, 112715. [Google Scholar] [CrossRef]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Development of biochar-based functional materials: Toward a sustainable platform carbon material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef]
- Onay, O.; Kockar, O.M. Slow, fast and flash pyrolysis of rapeseed Renew. Energy 2003, 28, 2417–2433. [Google Scholar]
- Andooz, A.; Eqbalpour, M.; Kowsari, E.; Ramakrishna, S.; Cheshmeh, Z.A. A comprehensive review on pyrolysis from the circular economy point of view and its environmental and social effects. J. Clean. Prod. 2023, 388, 136021. [Google Scholar] [CrossRef]
- Fang, J.; Zhan, L.; Ok, Y.S.; Gao, B. Minireview of potential applications of hydrochar derived from hydrothermal carbonization of biomass. J. Ind. Eng. Chem. 2018, 57, 15–21. [Google Scholar] [CrossRef]
- Czerwińska, K.; Śliz, M.; Wilk, M. Hydrothermal carbonization process: Fundamentals, main parameter characteristics and possible applications including an effective method of SARS-CoV-2 mitigation in sewage sludge. A review. Renew. Sustain. Energy Rev. 2022, 154, 111873. [Google Scholar] [CrossRef]
- González-Arias, J.; Sánchez, M.E.; Cara-Jiménez, J. Hydrothermal carbonization of biomass and waste: A review. Environ. Chem. Lett. 2022, 20, 211–221. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Liu, Z.; Jain, A.; Srinivasan, M.P.; Balasubramanian, R. Hydrothermal carbonization of sewage sludge for energy production with coal. Fuel 2013, 111, 201–210. [Google Scholar] [CrossRef]
- Antero, R.V.P.; Alves, A.C.F.; De Oliveira, S.B.; Ojala, S.A.; Brum, S.S. Challenges and alternatives for the adequacy of hydrothermal carbonization of lignocellulosic biomass in cleaner production systems: A review. J. Clean. Prod. 2020, 252, 119899. [Google Scholar] [CrossRef]
- Wang, L.; Chang, Y.; Li, A. Hydrothermal carbonization for energy-efficient processing of sewage sludge: A review. Renew. Sustain. Energy Rev. 2019, 108, 423–440. [Google Scholar] [CrossRef]
- Tekin, K.; Karagöz, S.; Bektaş, S. A review of hydrothermal biomass processing. Renew. Sustain. Energy Rev. 2014, 40, 673–687. [Google Scholar] [CrossRef]
- Yan, M.; He, L.; Prabowo, B.; Fang, Z.; Lin, J.; Xu, Z.; Hu, Y. Effect of pressure and atmosphere during hydrothermal treatment on the properties of sewage sludge derived solid fuel. J. Mater. Cycles Waste Manag. 2018, 20, 1594–1604. [Google Scholar] [CrossRef]
- He, C.; Wang, K.; Giannis, A.; Yang, Y.; Wang, J.Y. Products evolution during hydrothermal conversion of dewatered sewage sludge in sub- and near-critical water: Effects of reaction conditions and calcium oxide additive. Int. J. Hydrogen Energy 2015, 40, 5776–5787. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.; Park, K.Y. Hydrothermal carbonization of anaerobically digested sludge for solid fuel production and energy recovery. Fuel 2014, 130, 120–125. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Z.; Wang, J.; Zhao, X.; Zhao, Y.; Qian, J.; Wang, T. Pyrolysis and hydrothermal carbonization of biowaste: A comparative review on the conversion pathways and potential applications of char product. Sustain. Chem. Pharm. 2023, 33, 101106. [Google Scholar] [CrossRef]
- Vinardell, S.; Astals, S.; Koch, K.; Mata-Alvarez, J.; Dosta, J. Co-Digestion of Sewage Sludge and Food Waste in a Wastewater Treatment Plant Based on Mainstream Anaerobic Membrane Bioreactor Technology: A Techno-Economic Evaluation. Bioresour. Technol. 2021, 330, 124978. [Google Scholar] [CrossRef] [PubMed]
- Morelli, B.; Cashman, S.; Ma, X.C.; Turgeon, J.; Arden, S.; Garland, J. Environmental and Cost Benefits of Co-Digesting Food Waste at Wastewater Treatment Facilities. Water Sci. Technol. 2020, 82, 227–241. [Google Scholar] [CrossRef]
- Pramanik, S.K. Anaerobic Co-Digestion of Municipal Organic Solid Waste: Achievements and Perspective. Bioresour. Technol. Rep. 2022, 20, 101284. [Google Scholar] [CrossRef]
- Zhang, W.; Kong, T.; Xing, W.; Li, R.; Yang, T.; Yao, N.; Lv, D. Links between Carbon/Nitrogen Ratio, Synergy and Microbial Characteristics of Long-Term Semi-Continuous Anaerobic Co-Digestion of Food Waste, Cattle Manure and Corn Straw. Bioresour. Technol. 2022, 343, 126094. [Google Scholar] [CrossRef]
- Chuenchart, W.; Logan, M.; Leelayouthayotin, C.; Visvanathan, C. Enhancement of Food Waste Thermophilic Anaerobic Digestion through Synergistic Effect with Chicken Manure. Biomass Bioenergy 2020, 136, 105541. [Google Scholar] [CrossRef]
- Liu, X.; Lee, C.; Kim, J.Y. Thermal Hydrolysis Pre-Treatment Combined with Anaerobic Digestion for Energy Recovery from Organic Wastes. J. Mater. Cycles Waste Manag. 2020, 22, 1370–1381. [Google Scholar] [CrossRef]
- Kim, S.; Lee, C.; Kim, J.; Kim, J.Y. Feasibility of Thermal Hydrolysis Pretreatment to Reduce Hydraulic Retention Time of Anaerobic Digestion of Cattle Manure. Bioresour. Technol. 2023, 384, 129308. [Google Scholar] [CrossRef]
- Li, X.; Guo, S.; Peng, Y.; He, Y.; Wang, S.; Li, L.; Zhao, M. Anaerobic Digestion Using Ultrasound as Pretreatment Approach: Changes in Waste Activated Sludge, Anaerobic Digestion Performances and Digestive Microbial Populations. Biochem. Eng. J. 2018, 139, 139–145. [Google Scholar] [CrossRef]
- Pasalari, H.; Gholami, M.; Rezaee, A.; Esrafili, A.; Farzadkia, M. Perspectives on Microbial Community in Anaerobic Digestion with Emphasis on Environmental Parameters: A Systematic Review. Chemosphere 2021, 270, 128618. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, P.; Rajendran, K. Dynamic Simulation and Optimization of Anaerobic Digestion Processes Using MATLAB. Bioresour. Technol. 2022, 351, 126970. [Google Scholar] [CrossRef]
- Ahmad, A.; Rambabu, K.; Hasan, S.W.; Show, P.L.; Banat, F. Biohydrogen Production through Dark Fermentation: Recent Trends and Advances in Transition to a Circular Bioeconomy. Int. J. Hydrogen Energy 2023, 52, 335–357. [Google Scholar] [CrossRef]
- Nadeem, F.; Zhang, H.; Tahir, N.; Zhang, Z.; Singhania, R.R.; Shahzaib, M.; Ramzan, H.; Usman, M.; Rahman, M.U.; Zhang, Q. Advances in the Catalyzed Photo-Fermentative Biohydrogen Production through Photo Nanocatalysts with the Potential of Selectivity, and Customization. Bioresour. Technol. 2023, 382, 129221. [Google Scholar] [CrossRef] [PubMed]
- Phwan, C.K.; Ong, H.C.; Chen, W.-H.; Ling, T.C.; Ng, E.P.; Show, P.L. Overview: Comparison of Pretreatment Technologies and Fermentation Processes of Bioethanol from Microalgae. Energy Convers. Manag. 2018, 173, 81–94. [Google Scholar] [CrossRef]
- Maleki, F.; Changizian, M.; Zolfaghari, N.; Rajaei, S.; Noghabi, K.A.; Zahiri, H.S. Consolidated Bioprocessing for Bioethanol Production by Metabolically Engineered Bacillus Subtilis Strains. Sci. Rep. 2021, 11, 13731. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Yamakawa, C.K.; van der Maas, L.; Dragone, G. New Trends in Bioprocesses for Lignocellulosic Biomass and CO2 Utilization. Renew. Sustain. Energy Rev. 2021, 152, 111620. [Google Scholar] [CrossRef]
- Malik, K.; Sharma, P.; Yang, Y.; Zhang, P.; Zhang, L.; Xing, X.; Yue, J.; Song, Z.; Nan, L.; Yujun, S.; et al. Lignocellulosic Biomass for Bioethanol: Insight into the Advanced Pretreatment and Fermentation Approaches. Ind. Crops Prod. 2022, 188, 115569. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Zuhara, S.; Al-Ansari, T.; McKay, G. A Review on Catalytic CO2 Pyrolysis of Organic Wastes to High-Value Products. Fuel 2023, 335, 127073. [Google Scholar] [CrossRef]
- Ren, X.; Shanb Ghazani, M.; Zhu, H.; Ao, W.; Zhang, H.; Moreside, E.; Zhu, J.; Yang, P.; Zhong, N.; Bi, X. Challenges and Opportunities in Microwave-Assisted Catalytic Pyrolysis of Biomass: A Review. Appl. Energy 2022, 315, 118970. [Google Scholar] [CrossRef]
- Zaman, C.Z.; Pal, K.; Yehye, W.A.; Sagadevan, S.; Shah, S.T.; Adebisi, G.A.; Marliana, E.; Rafique, R.F.; Johan, R.B. Pyrolysis: A Sustainable Way to Generate Energy from Waste. In Pyrolysis; IntechOpen: London, UK, 2017; pp. 3–36. [Google Scholar] [CrossRef]
- Mong, G.R.; Chong, C.T.; Chong, W.W.F.; Ng, J.-H.; Ong, H.C.; Ashokkumar, V.; Tran, M.-V.; Karmakar, S.; Goh, B.H.H.; Mohd Yasin, M.F. Progress and Challenges in Sustainable Pyrolysis Technology: Reactors, Feedstocks and Products. Fuel 2022, 324, 124777. [Google Scholar] [CrossRef]
- Ndukwu, M.C.; Horsfall, I.T.; Ubouh, E.A.; Ekop, I.E.; Ezejiofor, N.R. Review of Solar-Biomass Pyrolysis Systems: Focus on the Configuration of Thermal-Solar Systems and Reactor Orientation. J. King Saud Univ.—Eng. Sci. 2020, 33, 413–423. [Google Scholar] [CrossRef]
- Xing, B.; Hu, Y.; Xu, D.; Li, B.; Li, Y.; Guan, W.; Li, R.; Wang, S. Recent Advances and Perspectives of Copyrolysis of Biomass and Organic Solid Waste. Energy Fuels 2023, 37, 4769–4790. [Google Scholar] [CrossRef]
- Sanjaya, E.; Abbas, A. Plasma Gasification as an Alternative Energy-From-Waste (EFW) Technology for the Circular Economy: An Environmental Review. Resour. Conserv. Recycl. 2023, 189, 106730. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, J.; Feng, Y.; Wang, R.; Zhao, Z.; Chen, H. Supercritical Water Gasification of Organic Solid Waste: H2 Yield and Cold Gas Efficiency Optimization Considering Modeling Uncertainties. Int. J. Hydrogen Energy 2023, 48, 30702–30717. [Google Scholar] [CrossRef]
- Faizan, M.; Song, H. Critical Review on Catalytic Biomass Gasification: State-of-Art Progress, Technical Challenges, and Perspectives in Future Development. J. Clean. Prod. 2023, 408, 137224. [Google Scholar] [CrossRef]
- Choudhary, N.K.; Deep, A.P.; Karmakar, S. Thermodynamic Analysis of Integrated Gasification Combined Cycle Integrated with Organic Rankine Cycle for Waste Heat Utilization. Waste Biomass Valorization 2024, 15, 3691–3709. [Google Scholar] [CrossRef]
- Ramos, A.; Monteiro, E.; Silva, V.; Rouboa, A. Co-Gasification and Recent Developments on Waste-To-Energy Conversion: A Review. Renew. Sustain. Energy Rev. 2018, 81, 380–398. [Google Scholar] [CrossRef]
- Djandja, O.S.; Liew, R.K.; Liu, C.; Liang, J.; Yuan, H.; He, W.; Feng, Y.; Lougou, B.; Duan, P.-G.; Lu, X.; et al. Catalytic Hydrothermal Carbonization of Wet Organic Solid Waste: A Review. Sci. Total Environ. 2023, 873, 162119. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; César Torres-Mayanga, P.; Abaide, E.R.; Zabot, G.L.; De Castilhos, F. Hydrothermal Carbonization and Liquefaction: Differences, Progress, Challenges, and Opportunities. Bioresour. Technol. 2022, 343, 126084. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Andreottola, G.; Elagroudy, S.; Negm, M.S.; Fiori, L. Coupling Hydrothermal Carbonization and Anaerobic Digestion for Sewage Digestate Management: Influence of Hydrothermal Treatment Time on Dewaterability and Bio-Methane Production. J. Environ. Manag. 2021, 281, 111910. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, R.; Giannis, A.; Li, C.; He, C. Sequential Hydrothermal Carbonization and CO2 Gasification of Sewage Sludge for Improved Syngas Production with Mitigated Emissions of NOx Precursors. Chem. Eng. J. 2023, 454, 140239. [Google Scholar] [CrossRef]
- Zeng, J.; Mustafa, A.B.; Liu, M.; Huang, G.; Shang, N.; Liu, X.; Wei, K.; Wang, P.; Dong, H. Environmental, Energy, and Techno-Economic Assessment of Waste-To-Energy Incineration. Sustainability 2024, 16, 4140. [Google Scholar] [CrossRef]
- Comoglio, C.; Castelluccio, S.; Scarrone, A.; Fiore, S. Analysis of Environmental Sustainability Reporting in the Waste-To-Energy Sector: Performance Indicators and Improvement Targets of the EMAS-Registered Waste Incineration Plants in Italy. J. Clean. Prod. 2022, 378, 134546. [Google Scholar] [CrossRef]
- Odunlami, O.A.; Vershima, D.A.; Oladimeji, T.E.; Nkongho, S.; Ogunlade, S.K.; Fakinle, B.S. Advanced Techniques for the Capturing and Separation of CO2—A Review. Results Eng. 2022, 15, 100512. [Google Scholar] [CrossRef]
- CEWEP—The Confederation of European Waste-to-Energy Plants. Available online: https://www.cewep.eu/what-cewep-does/ (accessed on 13 July 2024).
- Turning Waste into Energy at Högdalenverket|Best Practice. Smart City Sweden. Available online: https://smartcitysweden.com/best-practice/76/waste-incineration-at-hogdalenverket/ (accessed on 25 June 2024).
- Svenselius, M.W. Linköping Home to Sweden’s Largest Biogas Facility. Available online: https://liu.se/en/news-item/storsta-biogasanlaggningen-finns-i-linkoping (accessed on 13 July 2024).
- Pio, D.T.; Tarelho, L.A.C. Industrial Gasification Systems (>3 MWth) for Bioenergy in Europe: Current Status and Future Perspectives. Renew. Sustain. Energy Rev. 2021, 145, 111108. [Google Scholar] [CrossRef]
- Green Gasmill Submitted into Planning in Gloucestershire. Available online: https://www.ecotricity.co.uk/our-news/2016/green-gasmill-submitted-into-planning-in-gloucestershire#:~:text=Ecotricity%2C%20Britain%E2%80%99s%20leading%20green%20energy%20company%2C%20has%20submitted (accessed on 13 July 2024).
- Kymijärvi II, the World’s First SRF Gasification Power Plant—Nordregio. Available online: https://archive.nordregio.se/en/Publications/Publications-2016/GREEN-GROWTH-IN-NORDIC-REGIONS-50-ways-to-make-/Clean-tech-and-renewable-energy--/Kymij/index.html (accessed on 27 June 2024).
- Lahti Energia’s Unique Gasification Plant Reference. Makron. Available online: https://makron.com/en/references/lahti-energia-s-unique-gasification-plant/ (accessed on 26 June 2024).
- Eni Sustainable Mobility: Il Biocarburante 100% da Materie Prime Rinnovabili Arriva Nelle Stazioni di Servizio. Available online: https://www.eni.com/it-IT/media/news/2023/02/eni-sustainable-mobility-biocarburante-100-materie-prime-rinnovabili-arriva-stazioni-servizio.html (accessed on 29 June 2024).
- About Us. Available online: https://ingelia.com/index.php/quienes-somos/?lang=en (accessed on 29 June 2024).
- Sustainable and Optimal Use of Biomass for Energy in the EU beyond 2020 Final Report PricewaterhouseCoopers EU Services EESV’s Consortium to EC Directorate General for Energy Directorate C1—Renewables and CCS Policy; 2017. Available online: https://energy.ec.europa.eu/document/download/e23523df-5718-439d-94da-58c1b647e0d4_en?filename=biosustain_report_final.pdf (accessed on 29 June 2024).
- Biogas Outlook 2024 Production and Use of Biogas in Denmark. Available online: https://www.biogas.dk/wp-content/uploads/2024/05/Biogas-Outlook-2024-05-30-WEB-engelsk.pdf (accessed on 12 July 2024).
- ISWA White Book on Energy-From-Waste (EfW) Technologies Working Together for a Cleaner, Healthier Planet 3 2 ISWA White Book on Energy-From-Waste (EfW) Technologies Contents. Available online: https://www.iswa.org/wp-content/uploads/2023/07/ISWA-Whitebook-on-Energy-from-Waste-Technologies.pdf (accessed on 28 June 2024).







| Processes | Description | Products | Disadvantages |
|---|---|---|---|
| Biochemical Conversion | |||
| Anaerobic digestion | Biological process (hydrolysis, acidogenesis, acetogenesis, and methanogenesis) in which an organic matter is broken down into smaller compounds using anaerobic microorganisms | Biogas (50–75%), CO2, digestate (biofertilizer). H2 and Lactic acid are other by-products from the AD process which isare used for the production of several useful products such as acrylic acid, pyruvic acid, and biodegradable polymers | Low CH4 content in biogas quality, process inefficiencies, and inhibition due to intermediates Emission of 11 kg of CO2/kWh |
| Anaerobic fermentation | Fermentation is the process by which microorganisms (yeast or bacteria) convert biomolecules (glucose) into alcohol or acid under anaerobic conditions | Biofuels (Bioethanol) Hydrogen gas (H2) By-products can be utilized as fuel for boilers or processed further through gasification | The need for energy-intensive pretreatment processes |
| Thermochemical process | |||
| Incineration | Thermal oxidation in presence of oxygen (from air) 950–1100 °C | Produce heat: 544 kWh/ton of waste to the grid By-products: flue gases, ash | Dewatering step is required Recommended moisture content should be less than 45% Emission of 14–35 kg of CO2/kWh |
| Gasification | Gasification is conversion of biomass into combustible synthetic gas under an oxygen-deficient or low oxygen environment 700–1200 °C | Produce heat: 685 kWh/ton of waste Products: biochar, bio-oil, and syngas, which could be adapted for energy and heat By-products: tar (complex mixture of condensable hydrocarbons), condensates, ash and slag | Recommended moisture content 10–20% Emission of 8 kg of CO2/kWh |
| Pyrolisis | Thermal decomposition of organic matter in an oxygen-free environment or inert gas. 300–1200 °C | Produces heat: 571 kWh/ton Products: biochar, a solid rich in carbon; bio-oil, obtained as a liquid after condensation of the volatile organic matter By-products: tar (complex mixture of condensable hydrocarbons) | A drying step is required before pyrolysis Moisture content of less than 7% is recommended Emission of 9.5 kg of CO2/kWh |
| Hydrothermal carbonization | Thermochemical conversion process, under hydrothermal conditions in hot pressurized water 180–260 °C and 10–40 bars | Product: biofuel (hydrochar), a carbonaceous solid with carbon content usually greater than 80% by weight By-products: process water, biogas | Wastewater formation |
| Technique | CO2 Emission | Residual Waste Generation | Efficiency | Ref. |
|---|---|---|---|---|
| Anaerobic digestion | Low to moderate (biogenic) (50–200 kg of CO2/kWh) | Low (digestate as fertilizer) | Biogas can be converted to electricity using combined heat and power (CHP) systems, with conversion efficiencies of 30–40% for electricity and 50–60% for heat. Energy content around 20–25 MJ/m3. | [13] |
| Incineration | High (600–1200 kg of CO2/MWh) | Moderate to high (Ash) | Overall efficiency can range from 20 to 30% for electricity generation and up to 80–90% if combined heat and power (CHP) systems are used. | [14] |
| Gasification | Moderate (200–600 kg of CO2/MWh) | Low to moderate (char, tar) | The overall efficiency of the gasification process (from biomass to syngas) can range from 60% to 80%. | [15] |
| Pyrolisis | Low to moderate 150–500 kg of CO2/MWh | Low (biochar) | Typical efficiencies of electricity generation range from 20% to 40%, reaching 80% in case of combined heat and power (CHP). | [16] |
| Hydrothermal carbonization | Low (biogenic) (100 kg of CO2/MWh) | Moderate (hydrochar, process water) | The efficiency of converting hydrochar into heat or electricity depends on combustion technologies and can range from 20% to 40%. | [17] |
| Advantages | Shortcomings |
|---|---|
| Biogas plants are independent of external conditions (solar activity, the presence of constant winds or rivers) and can continuously generate electricity and heat, in the case of constant access to a stable supply of organic waste. | There is a need for a guaranteed supply of waste generation facilities. |
| Biogas can be stored, transported, and used as a fuel, unlike the energy of the sun, water, or wind, the operation of which requires batteries or power lines. | The need for guaranteed sales of produced electricity. |
| Biogas plants are compact and quiet, and the spread of unpleasant odors is minimized, which makes choosing a site easier. The modularity of biogas plants makes them cost-effective both on sites with low waste flows and in large complexes with high needs for recycling raw materials and energy consumption. | Significant capital costs per unit of power. |
| Pyrolysis | Definition | Advantages | Conditions |
|---|---|---|---|
| Slow pyrolysis | Slowly heat the organic matter in an anaerobic environment. | Results in a greater yield of high-quality char, while minimizing the production of liquid and gaseous products. | Temperature: 300–800 °C. Residence time: >1 h. Heating rate: 5–7 °C/min. Main products: solid residue or char [61]. |
| Rapid (fast) pyrolysis | Organic waste is treated in the absence of oxygen with a high rate that causes the organic matter to decompose rapidly, producing mostly vapors and aerosols, with small amounts of gas and coal. | Maximizes the production of high-quality liquid oil. The process is highly scalable and economically feasible. Gas production is higher than slow pyrolysis. Can be conducted in various reactors. | Temperature: 400–600 °C. Residence time: 0.5–2 s. Heating rate: 300–1000 °C/min. Main products: liquid or oil [61]. |
| Flash pyrolysis | Produces dark brown pyrolysis oil post the feedstock decomposition, cooling, and condensation. The pyrolysis generates mainly vapours and aerosols with a small quantity of char. | Yields 75% of pyrolysis oil by the weight of the product. | Temperature: <650 °C. Residence time: <0.5 s. Heating rate: 104 K/s. Main products: liquid or oil [62]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zueva, S.; Ferella, F.; Corradini, V.; Vegliò, F. Review of Organic Waste-to-Energy (OWtE) Technologies as a Part of a Sustainable Circular Economy. Energies 2024, 17, 3797. https://doi.org/10.3390/en17153797
Zueva S, Ferella F, Corradini V, Vegliò F. Review of Organic Waste-to-Energy (OWtE) Technologies as a Part of a Sustainable Circular Economy. Energies. 2024; 17(15):3797. https://doi.org/10.3390/en17153797
Chicago/Turabian StyleZueva, Svetlana, Francesco Ferella, Valentina Corradini, and Francesco Vegliò. 2024. "Review of Organic Waste-to-Energy (OWtE) Technologies as a Part of a Sustainable Circular Economy" Energies 17, no. 15: 3797. https://doi.org/10.3390/en17153797
APA StyleZueva, S., Ferella, F., Corradini, V., & Vegliò, F. (2024). Review of Organic Waste-to-Energy (OWtE) Technologies as a Part of a Sustainable Circular Economy. Energies, 17(15), 3797. https://doi.org/10.3390/en17153797










