Defect Engineering of Nickel-Based Compounds for Energy-Saving H2 Production
Abstract
1. Introduction
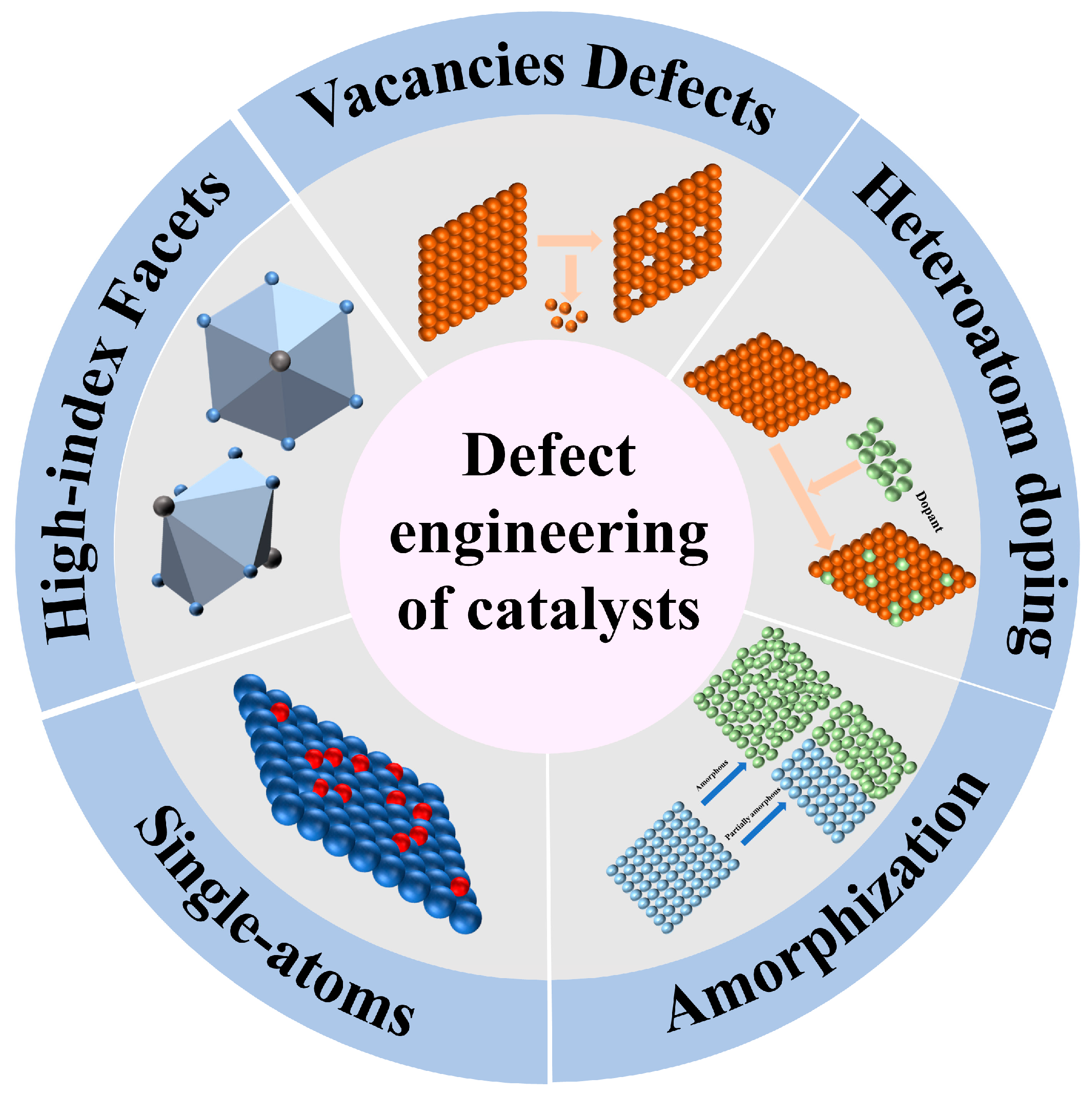
2. Defect Engineering of Catalysts
2.1. Vacancy Defects
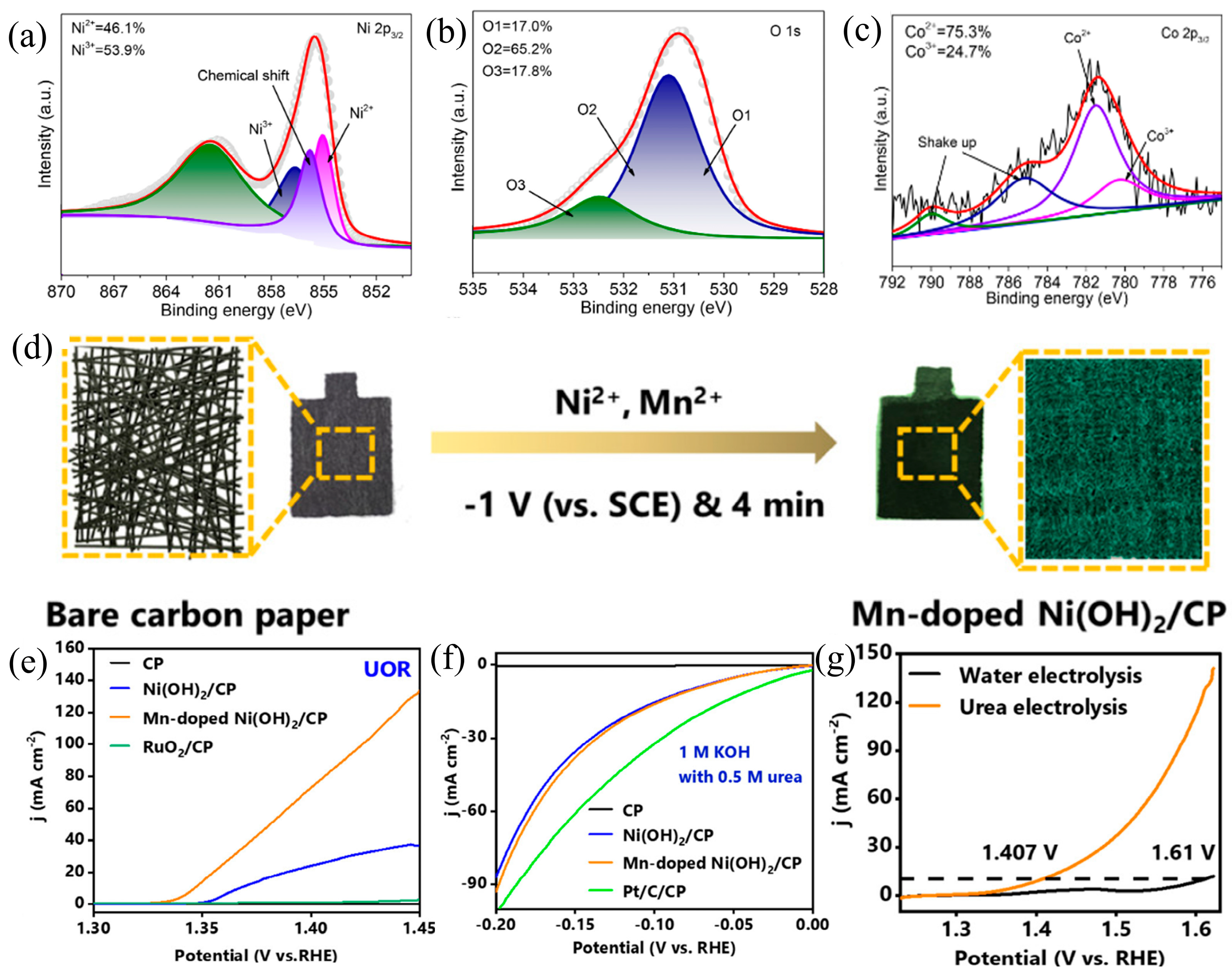
2.2. Heteroatom Doping
2.3. Amorphization
2.4. Single-Atom Design
2.5. Other Defects
3. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Huber, G.W.; Shabaker, J.W.; Dumesic, J.A. Raney Ni-Sn Catalyst for H2 Production from Biomass-Derived Hydrocarbons. Science 2003, 300, 2075–2077. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ling, Y.; Lu, X.; Wang, H.; Qian, F.; Tong, Y.; Li, Y. Solar Driven Hydrogen Releasing from Urea and Human Urine. Energy Environ. Sci. 2012, 5, 8215–8219. [Google Scholar] [CrossRef]
- Yang, X.; Lu, A.-Y.; Zhu, Y.; Hedhili, M.N.; Min, S.; Huang, K.-W.; Han, Y.; Li, L.-J. CoP Nanosheet Assembly Grown on Carbon Cloth: A Highly Efficient Electrocatalyst for Hydrogen Generation. Nano Energy 2015, 15, 634–641. [Google Scholar] [CrossRef]
- Yu, H.; Qi, L.; Hu, Y.; Qu, Y.; Yan, P.; Isimjan, T.T.; Yang, X. Nanowire-Structured FeP-CoP Arrays as Highly Active and Stable Bifunctional Electrocatalyst Synergistically Promoting High-Current Overall Water Splitting. J. Colloid Interface Sci. 2021, 600, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Cheng, X.; Chen, J.; Wang, D.; Wang, B.; Tian, J.; Isimjan, T.T.; Yang, X. Temperature Effect on Crystallinity and Chemical States of Nickel Hydroxide as Alternative Superior Catalyst for Urea Electrooxidation. Electrochim. Acta 2019, 301, 47–54. [Google Scholar] [CrossRef]
- Li, Y.; Wei, X.; Chen, L.; Shi, J. Electrocatalytic Hydrogen Production Trilogy. Angew. Chem. Int. Ed. 2021, 60, 19550–19571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhou, W.; Yan, X.; Huang, X.; Wu, S.; Pan, J.; Shahnavaz, Z.; Li, T.; Yu, X. Sodium dodecyl sulfate intercalated two-dimensional nickel-cobalt layered double hydroxides to synthesize multifunctional nanomaterials for supercapacitors and electrocatalytic hydrogen evolution. Fuel 2023, 333, 126323. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Chang, J.; Feng, L. A Review of Ni Based Powder Catalyst for Urea Oxidation in Assisting Water Splitting Reaction. Adv. Powder Mater. 2022, 1, 100030. [Google Scholar] [CrossRef]
- Sun, X.; Ding, R. Recent Progress with Electrocatalysts for Urea Electrolysis in Alkaline Media for Energy-Saving Hydrogen Production. Catal. Sci. Technol. 2020, 10, 1567–1581. [Google Scholar] [CrossRef]
- Liu, Y.; Xing, Y.; Xu, S.; Lu, Y.; Sun, S.; Jiang, D. Interfacing Co3Mo with CoMoOx for synergistically boosting electrocatalytic hydrogen and oxygen evolution reactions. Chem. Eng. J. 2022, 431, 144660. [Google Scholar] [CrossRef]
- Xu, X.; Shao, Z.; Jiang, S.P. High-entropy Materials for Water Electrolysis. Energy Technol. 2022, 10, 2200573. [Google Scholar] [CrossRef]
- She, S.; Zhu, Y.; Wu, X.; Hu, Z.; Shelke, A.; Pong, W.-F.; Chen, Y.; Song, Y.; Liang, M.; Chen, C.-T. Realizing High and Stable Electrocatalytic Oxygen Evolution for Iron-based Perovskites by Co-doping-induced Structural and Electronic Modulation. Adv. Funct. Mater. 2022, 32, 2111091. [Google Scholar] [CrossRef]
- Lu, S.; Wang, Q.; Fang, L.; Zheng, X.; Yin, F.; Liu, H. Bimetallic Platinum-Nickel Nanoparticles Modified on the Screen-Printed Electrode for Highly Sensitive Urea Determination. IEEE Sens. J. 2024, 24, 4221–4227. [Google Scholar] [CrossRef]
- Yao, Z.-C.; Tang, T.; Jiang, Z.; Wang, L.; Hu, J.-S.; Wan, L.-J. Electrocatalytic Hydrogen Oxidation in Alkaline Media: From Mechanistic Insights to Catalyst Design. ACS Nano 2022, 16, 5153–5183. [Google Scholar] [PubMed]
- Ji, Q.; Yu, X.; Chen, L.; Okonkwo, C.E.; Zhou, C. Effect of cobalt doping and sugarcane bagasse carbon on the electrocatalytic performance of MoS2 nanocomposites. Fuel 2022, 324, 124814. [Google Scholar] [CrossRef]
- Lu, S.; Hummel, M.; Gu, Z.; Wang, Y.; Wang, K.; Pathak, R.; Zhou, Y.; Jia, H.; Qi, X.; Zhao, X.; et al. Highly efficient urea oxidation via nesting nano-nickel oxide in eggshell membrane-derived carbon. ACS Sustain. Chem. Eng. 2021, 9, 1703–1713. [Google Scholar]
- Zhang, Z.; Li, L.; Li, Y.; Zheng, Y.; Wu, Q.; Xie, L.; Luo, B.; Hao, J.; Shi, W. Activating MoO4 tetrahedrons to MoOx species in MoNi alloy for boosting performance in alkaline hydrogen evolution reaction. Chem. Eng. J. 2023, 469, 143846. [Google Scholar]
- Zheng, X.; Lu, S. Theoretical Exploration of Anchoring Type Effects of C2N Nanosheet-Supported Nickel Atoms for Urea Electrooxidation. ACS Appl. Nano Mater. 2024, 7, 3361–3372. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, J.; Li, T.; Zhang, T.; Arbiol, J.; Yan, S.; Wang, Y.; Li, H.; Cabot, A. Pd2Ga nanorods as highly active bifunctional catalysts for electrosynthesis of acetic acid coupled with hydrogen production. Chem. Eng. J. 2022, 446, 136878. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, X.; Fang, L.; Lu, S. Urea electrooxidation in alkaline environment: Fundamentals and applications. ChemElectroChem 2023, 10, e202300138. [Google Scholar] [CrossRef]
- Peng, X.; Wang, T.; Liu, B.; Li, Y.; Zhao, T. A Solvent Molecule Reconstruction Strategy Enabling a High-Voltage Ether-Based Electrolyte. Energy Environ. Sci. 2022, 15, 5350–5361. [Google Scholar] [CrossRef]
- Lu, S.; Zheng, X.; Fang, L.; Yin, F.; Liu, H. Rational engineering design of nickel hydroxides for urea oxidation reaction: A mini-review. Electrochem. Commun. 2023, 157, 107599. [Google Scholar] [CrossRef]
- Wang, H.; Guo, Y.; Mao, Q.; Yu, H.; Deng, K.; Wang, Z.; Li, X.; Xu, Y.; Wang, L. Sulfur and Phosphorus Co-Doping Optimized Electronic Structure and Modulated Intermediate Affinity on PdSP Metallene for Ethanol-Assisted Energy-Saving H2 Production. Nanoscale 2023, 15, 7765–7771. [Google Scholar] [CrossRef]
- Meng, N.; Ma, X.; Wang, C.; Wang, Y.; Yang, R.; Shao, J.; Huang, Y.; Xu, Y.; Zhang, B.; Yu, Y. Oxide-Derived Core–Shell Cu@ Zn Nanowires for Urea Electrosynthesis from Carbon Dioxide and Nitrate in Water. ACS Nano 2022, 16, 9095–9104. [Google Scholar] [CrossRef]
- Lu, S.; Zheng, X.; Wang, H.; Wang, C.; Akinlabi, E.; Xu, B.; Yang, X.; Hua, Q.; Liu, H. Energy-saving hydrogen production by heteroatom modulations coupling urea electrooxidation. EcoMat 2024, e12477. [Google Scholar] [CrossRef]
- Boggs, B.K.; King, R.L.; Botte, G.G. Urea Electrolysis: Direct Hydrogen Production from Urine. Chem. Commun. 2009, 4859–4861. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.; Tao, S.; Irvine, J.T. A Direct Urea Fuel Cell–Power from Fertiliser and Waste. Energy Environ. Sci. 2010, 3, 438–441. [Google Scholar] [CrossRef]
- Xu, W.; Wu, Z.; Tao, S. Urea-based Fuel Cells and Electrocatalysts for Urea Oxidation. Energy Technol. 2016, 4, 1329–1337. [Google Scholar] [CrossRef]
- Liao, W.; Zhao, Q.; Wang, S.; Ran, Y.; Su, H.; Gan, R.; Lu, S.; Zhang, Y. Insights into mechanisms on electrochemical oxygen evolution substitution reactions. J. Catal. 2023, 428, 115161. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Offen-Polak, I.; Burshtein, T.Y.; Farber, E.M.; Kornblum, L.; Eisenberg, D. Urea Oxidation Electrocatalysis on Nickel Hydroxide: The Role of Disorder. J. Solid State Electrochem. 2021, 25, 159–171. [Google Scholar] [CrossRef]
- Urbańczyk, E.; Sowa, M.; Simka, W. Urea Removal from Aqueous Solutions—A Review. J. Appl. Electrochem. 2016, 46, 1011–1029. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Ji, Y.; Liu, X.; Su, D.; Zhuang, Z.; Chang, Y.-C.; Pao, C.-W.; Shao, Q.; Hu, Z. Atomic-Thick Metastable Phase RhMo Nanosheets for Hydrogen Oxidation Catalysis. Nat. Commun. 2023, 14, 1761. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, L.; He, W.; Li, L.; Lu, S. Heteroatom-doped nickel sulfide for efficient electrochemical oxygen evolution reaction. Energies 2023, 16, 881. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Ma, W.; Wan, H.; Zhang, X.; Zhang, X.; Jiang, S.; Zheng, J.; Zhou, Z. In Situ Anchoring Massive Isolated Pt Atoms at Cationic Vacancies of α-NixFe1-x(OH)2 to Regulate the Electronic Structure for Overall Water Splitting. Adv. Funct. Mater. 2022, 32, 2203342. [Google Scholar] [CrossRef]
- Li, L.; Lu, S.; Dai, Y.; Li, H.; Wang, X.; Zhang, Y. Controlled synthesis of hierarchical nanostructured metal ferrite microspheres for enhanced electrocatalytic oxygen evolution reaction. ACS Appl. Nano Mater. 2023, 6, 2184–2192. [Google Scholar] [CrossRef]
- Wu, Z.; Guo, X.; Zhang, Z.; Song, M.; Jiao, T.; Zhu, Y.; Wang, J.; Liu, X. Interface Engineering of MoS2 for Electrocatalytic Performance Optimization for Hydrogen Generation via Urea Electrolysis. ACS Sustain. Chem. Eng. 2019, 7, 16577–16584. [Google Scholar] [CrossRef]
- Zeng, Y.; Xiang, S.; Lu, S.; Qi, X. Structural Design of Nickel Hydroxide for Efficient Urea Electrooxidation. Materials 2024, 17, 2617. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Gu, Z.; Hummel, M.; Zhou, Y.; Wang, K.; Xu, B.B.; Wang, Y.; Li, Y.; Qi, X.; Liu, X. Nickel Oxide Immobilized on the Carbonized Eggshell Membrane for Electrochemical Detection of Urea. J. Electrochem. Soc. 2020, 167, 106509. [Google Scholar] [CrossRef]
- Li, F.-M.; Xia, C.; Xia, B.Y. Exploration and Insight of Dynamic Structure Evolution for Electrocatalysts. Acc. Mater. Res. 2023, 4, 427–437. [Google Scholar] [CrossRef]
- He, F.; Zheng, Q.; Yang, X.; Wang, L.; Zhao, Z.; Xu, Y.; Hu, L.; Kuang, Y.; Yang, B.; Li, Z. Spin-State Modulation on Metal–Organic Frameworks for Electrocatalytic Oxygen Evolution. Adv. Mater. 2023, 35, 2304022. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, X.; Song, Z.; Zhu, K.; Cao, D.; Yao, J.; Wang, G.; Yan, Q. Peony-like CuxSy Hybrid Iron-Nickel Sulfide Heterogeneous Catalyst for Boosting Alkaline Oxygen Evolution Reaction. Surf. Interfaces 2023, 38, 102788. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, Y.; Yin, X.; Shen, L.; Zhu, K.; Huang, X.; Cao, D.; Yao, J.; Wang, G.; Yan, Q. Defect-Rich MnxOy Complex Fe–Ni Sulfide Heterogeneous Electrocatalyst for a Highly Efficient Hydrogen Evolution Reaction. J. Power Sources 2022, 540, 231664. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Z.; Yan, Q.; Cong, W.; Yang, L.; Zhu, K.; Ye, K.; Yan, J.; Cao, D.; Wang, G. Tremella-like Manganese Dioxide Complex (Fe, Ni) 3S4 Hybrid Catalyst for Highly Efficient Oxygen Evolution Reaction. J. Power Sources 2021, 515, 230627. [Google Scholar] [CrossRef]
- Qiu, Y.; Dai, X.; Wang, Y.; Ji, X.; Ma, Z.; Liu, S. The Polyoxometalates Mediated Preparation of Phosphate-Modified NiMoO4− x with Abundant O-Vacancies for H2 Production via Urea Electrolysis. J. Colloid Interface Sci. 2023, 629, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Dong, Y.; Liu, F.; Wang, H.; Wang, X.; Li, X.; Ren, J.; Wang, R. The Ni–P Dual Vacancies Crystalline/Amorphous Bifunctional Electrode with ZnO “Electron Pump” for Urea-Assisted Electrolysis of Water to Produce Hydrogen. Int. J. Hydrogen Energy 2024, 49, 984–994. [Google Scholar] [CrossRef]
- Liu, X.; Qin, H.; Ye, Z.; Yao, D.; Miao, W.; Mao, S. Interconnected Mn-Doped Ni (OH) 2 Nanosheet Layer for Bifunctional Urea Oxidation and Hydrogen Evolution: The Relation between Current Drop and Urea Concentration during the Long-Term Operation. ACS EST Eng. 2022, 2, 853–862. [Google Scholar] [CrossRef]
- Yuan, W.; Jiang, T.; Fang, X.; Fan, Y.; Qian, S.; Gao, Y.; Cheng, N.; Xue, H.; Tian, J. Interface Engineering of S-Doped Co2P@ Ni2P Core–Shell Heterostructures for Efficient and Energy-Saving Water Splitting. Chem. Eng. J. 2022, 439, 135743. [Google Scholar] [CrossRef]
- Guo, L.; Chi, J.; Zhu, J.; Cui, T.; Lai, J.; Wang, L. Dual-Doping NiMoO4 with Multi-Channel Structure Enable Urea-Assisted Energy-Saving H2 Production at Large Current Density in Alkaline Seawater. Appl. Catal. B Environ. 2023, 320, 121977. [Google Scholar] [CrossRef]
- Chen, N.; Du, Y.-X.; Zhang, G.; Lu, W.-T.; Cao, F.-F. Amorphous Nickel Sulfoselenide for Efficient Electrochemical Urea-Assisted Hydrogen Production in Alkaline Media. Nano Energy 2021, 81, 105605. [Google Scholar] [CrossRef]
- Qiao, L.; Zhu, A.; Liu, D.; Feng, J.; Chen, Y.; Chen, M.; Zhou, P.; Yin, L.; Wu, R.; Ng, K.W. Crystalline Phosphides/Amorphous Oxides Composite for Energy-Saving Hydrogen Production Assisted by Efficient Urea Oxidation Reaction. Chem. Eng. J. 2023, 454, 140380. [Google Scholar] [CrossRef]
- Khalafallah, D.; Farghaly, A.A.; Ouyang, C.; Huang, W.; Hong, Z. Atomically Dispersed Pt Single Sites and Nanoengineered Structural Defects Enable a High Electrocatalytic Activity and Durability for Hydrogen Evolution Reaction and Overall Urea Electrolysis. J. Power Sources 2023, 558, 232563. [Google Scholar] [CrossRef]
- Kumar, A.; Liu, X.; Lee, J.; Debnath, B.; Jadhav, A.R.; Shao, X.; Bui, V.Q.; Hwang, Y.; Liu, Y.; Kim, M.G. Discovering Ultrahigh Loading of Single-Metal-Atoms via Surface Tensile-Strain for Unprecedented Urea Electrolysis. Energy Environ. Sci. 2021, 14, 6494–6505. [Google Scholar] [CrossRef]
- Huo, J.-M.; Wang, Y.; Xue, J.-N.; Yuan, W.-Y.; Zhai, Q.-G.; Hu, M.-C.; Li, S.-N.; Chen, Y. High-Valence Metal Doping Induced Lattice Expansion for M-FeNi LDH toward Enhanced Urea Oxidation Electrocatalytic Activities. Small 2024, 20, 2305877. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Yu, L.; Xiong, K.; Chen, J.; Zhang, H.; Deng, M.; Shi, X. An Energy-Efficient H2 Production Based on Urea-Aided Water Splitting Enhanced by Ru Induced in-Situ Speciation of NiO Nanosheets on Porous Ni. J. Alloys Compd. 2024, 983, 173938. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Deng, S.; Zhang, K.; Xie, D.; Zhang, Y.; Zhang, Y.; Wang, Y.; Fan, H.J.; Xia, X. High-Index-Faceted Ni3S2 Branch Arrays as Bifunctional Electrocatalysts for Efficient Water Splitting. Nano-Micro Lett. 2019, 11, 12. [Google Scholar]
- Li, Y.; Min, K.-A.; Han, B.; Lee, L.Y.S. Ni Nanoparticles on Active (001) Facet-Exposed Rutile TiO2 Nanopyramid Arrays for Efficient Hydrogen Evolution. Appl. Catal. B Environ. 2021, 282, 119548. [Google Scholar] [CrossRef]
- Li, Y.; Luo, F.; Xie, Y.; Chang, C.; Xie, M.; Yang, Z. Oxygen Vacancies in α-Ni(OH)2 Porous Nanoflowers Promote Urea Oxidation. Int. J. Hydrogen Energy 2023, 48, 9155–9162. [Google Scholar] [CrossRef]
- Qin, H.; Ye, Y.; Li, J.; Jia, W.; Zheng, S.; Cao, X.; Lin, G.; Jiao, L. Synergistic Engineering of Doping and Vacancy in Ni(OH) 2 to Boost Urea Electrooxidation. Adv. Funct. Mater. 2023, 33, 2209698. [Google Scholar] [CrossRef]
- Zhang, K.; Duan, Y.; Graham, N.; Yu, W. Unveiling the Synergy of Polymorph Heterointerface and Sulfur Vacancy in NiS/Ni3S2 Electrocatalyst to Promote Alkaline Hydrogen Evolution Reaction. Appl. Catal. B Environ. 2023, 323, 122144. [Google Scholar]
- He, Q.; Wan, Y.; Jiang, H.; Pan, Z.; Wu, C.; Wang, M.; Wu, X.; Ye, B.; Ajayan, P.M.; Song, L. Nickel Vacancies Boost Reconstruction in Nickel Hydroxide Electrocatalyst. ACS Energy Lett. 2018, 3, 1373–1380. [Google Scholar] [CrossRef]
- Ye, Y.; Gan, Y.; Cai, R.; Dai, X.; Yin, X.; Nie, F.; Ren, Z.; Wu, B.; Cao, Y.; Zhang, X. Oxygen Vacancies and Surface Reconstruction on NiFe LDH@Ni(OH)2 Heterojunction Synergistically Triggering Oxygen Evolution and Urea Oxidation Reaction. J. Alloys Compd. 2022, 921, 166145. [Google Scholar] [CrossRef]
- Zhou, X.; Du, X.; Zhang, X. Controlled Synthesis of M Doped NiMoO4 (M = Co, Cu and Fe) for Urea, Freshwater and Seawater Oxidation Reaction. Fuel 2024, 371, 132050. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, Y.; Wang, Y.; Li, B.; Zhang, Y.; Ma, Z.; Liu, S. Coaxial Ni–S@N-Doped Carbon Nanofibers Derived Hierarchical Electrodes for Efficient H 2 Production via Urea Electrolysis. ACS Appl. Mater. Interfaces 2021, 13, 3937–3948. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wei, G.; Wang, C.; Zhou, X.; Zhao, X.; Zhao, Q.; Wang, S.; Kong, F. In Situ Topologically Induced Metastable Phase Ni/r-Ni(OH)2@C Heterostructures with Abundant Oxygen Vacancies as Efficient Bifunctional Electrocatalysts for Energy-Saving Hydrogen Production. J. Alloys Compd. 2023, 959, 170545. [Google Scholar] [CrossRef]
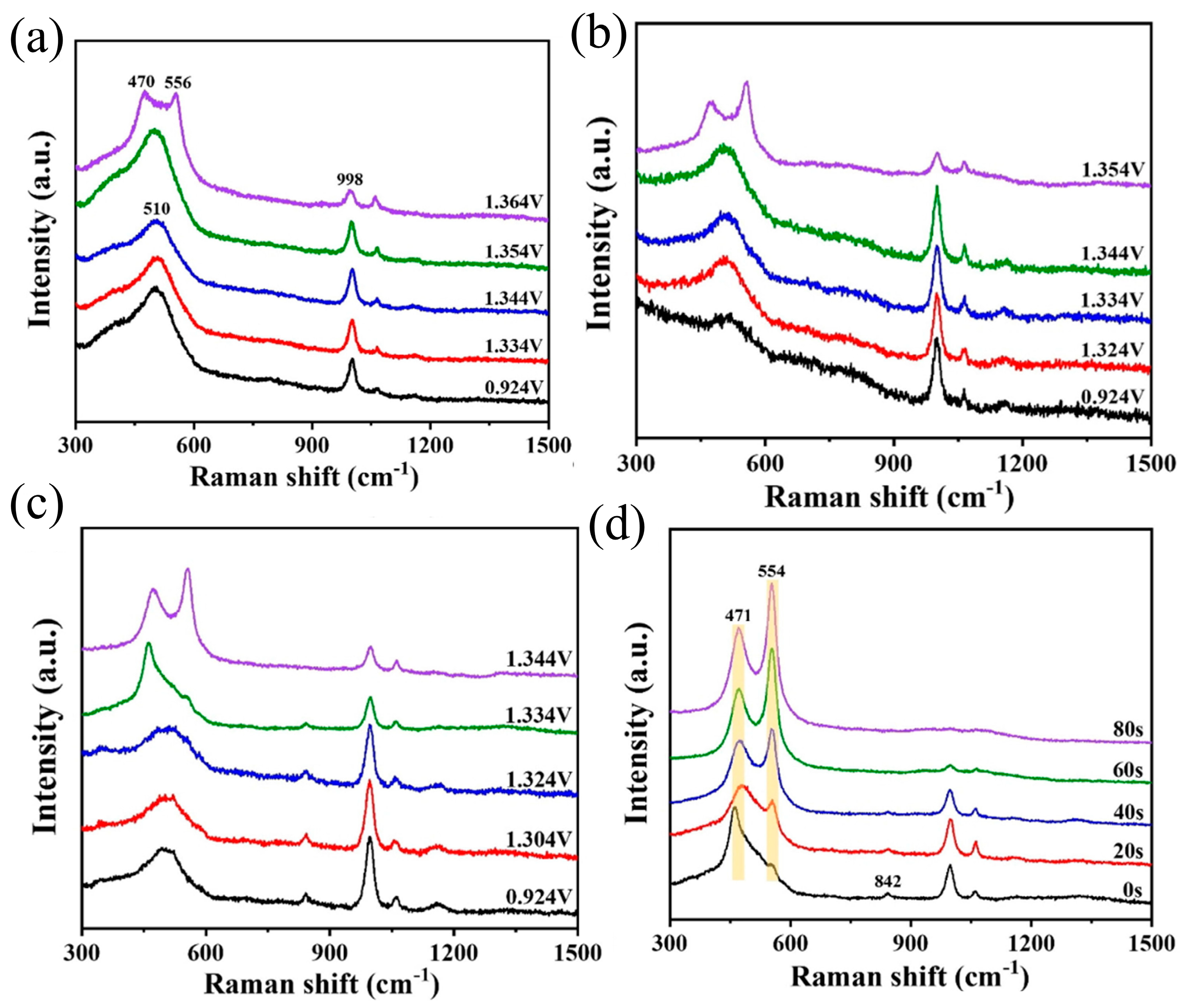
- Yang, X.; Zhang, H.; Xu, W.; Yu, B.; Liu, Y.; Wu, Z. A Doping Element Improving the Properties of Catalysis: In Situ Raman Spectroscopy Insights into Mn-Doped NiMn Layered Double Hydroxide for the Urea Oxidation Reaction. Catal. Sci. Technol. 2022, 12, 4471–4485. [Google Scholar] [CrossRef]
- Jin, L.; Ji, R.; Wan, H.; He, J.; Gu, P.; Lin, H.; Xu, Q.; Lu, J. Boosting the Electrocatalytic Urea Oxidation Performance by Amorphous–Crystalline Ni-TPA@ NiSe Heterostructures and Mechanism Discovery. ACS Catal. 2022, 13, 837–847. [Google Scholar] [CrossRef]
- Dong, Z.; Lin, F.; Yao, Y.; Jiao, L. Crystalline Ni (OH) 2/Amorphous NiMoOx Mixed-catalyst with Pt-like Performance for Hydrogen Production. Adv. Energy Mater. 2019, 9, 1902703. [Google Scholar] [CrossRef]
- Jia, X.; Kang, H.; Yang, X.; Li, Y.; Cui, K.; Wu, X.; Qin, W.; Wu, G. Amorphous Ni(III)-Based Sulfides as Bifunctional Water and Urea Oxidation Anode Electrocatalysts for Hydrogen Generation from Urea-Containing Water. Appl. Catal. B Environ. 2022, 312, 121389. [Google Scholar] [CrossRef]
- Liu, S.-S.; Ma, L.-J.; Li, J.-S. Facile Preparation of Amorphous NiFe Hydroxide by Corrosion Engineering for Electrocatalytic Water and Urea Oxidation. J. Alloys Compd. 2023, 936, 168271. [Google Scholar]
- Liu, H.; Wang, P.; Qi, X.; Liu, J.; Yin, A.; Wang, Y.; Ye, Y.; Luo, J.; Ren, Z.; Yu, S. An Amorphous Nickel Carbonate Catalyst for Superior Urea Oxidation Reaction. J. Electroanal. Chem. 2023, 949, 117856. [Google Scholar]
- Li, Y.; Wang, H.; Wang, R.; He, B.; Gong, Y. 3D Self-Supported FeOP Film on Nickel Foam as a Highly Active Bifunctional Electrocatalyst for Urea-Assisted Overall Water Splitting. Mater. Res. Bull. 2018, 100, 72–75. [Google Scholar] [CrossRef]
- Shekhawat, A.; Samanta, R.; Panigrahy, S.; Barman, S. Electrocatalytic Oxidation of Urea and Ethanol on Two-Dimensional Amorphous Nickel Oxide Encapsulated on N-Doped Carbon Nanosheets. ACS Appl. Energy Mater. 2023, 6, 3135–3146. [Google Scholar] [CrossRef]
- Chen, X.; Wan, J.; Chai, J.; Zhang, L.; Zhang, F.; Zhang, Q.; Gu, L.; Zheng, L.; Yu, R. Nickel-Iron in the Second Coordination Shell Boost Single-Atomic-Site Iridium Catalysts for High-Performance Urea Electrooxidation. Nano Res. 2024, 17, 3919–3926. [Google Scholar] [CrossRef]
- Feng, L.-L.; Yu, G.; Wu, Y.; Li, G.-D.; Li, H.; Sun, Y.; Asefa, T.; Chen, W.; Zou, X. High-Index Faceted Ni 3 S 2 Nanosheet Arrays as Highly Active and Ultrastable Electrocatalysts for Water Splitting. J. Am. Chem. Soc. 2015, 137, 14023–14026. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Zhang, W.-D.; Xu, H.; Hu, M.; Yang, J.; Liu, J.; Gu, Z.-G.; Yan, X. Hierarchical Microspheres Constructed by Hexagonal NiCo (OH) 2 Nanosheets with Rich Ni3+ Species and Carboxylic Groups for Efficient Urea Oxidation Reaction. J. Alloys Compd. 2023, 930, 167453. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Zou, Y.; Wang, S.; He, J. Trace Cobalt Doping and Defect Engineering of High Surface Area α-Ni (OH) 2 for Electrocatalytic Urea Oxidation. Energy Environ. Mater. 2024, 7, e12576. [Google Scholar] [CrossRef]
- Cao, X.; Wang, T.; Qin, H.; Lin, G.; Zhao, L.; Jiao, L. Crystalline—Amorphous Interfaces of NiO-CrO x Electrocatalysts for Boosting the Urea Oxidation Reaction. Nano Res. 2023, 16, 3665–3671. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, W.-D.; Yao, Y.; Yang, J.; Liu, J.; Gu, Z.-G.; Yan, X. Amorphous Chromium Oxide Confined Ni/NiO Nanoparticles-Assembled Nanosheets for Highly Efficient and Stable Overall Urea Splitting. J. Colloid Interface Sci. 2023, 629, 501–510. [Google Scholar] [CrossRef] [PubMed]
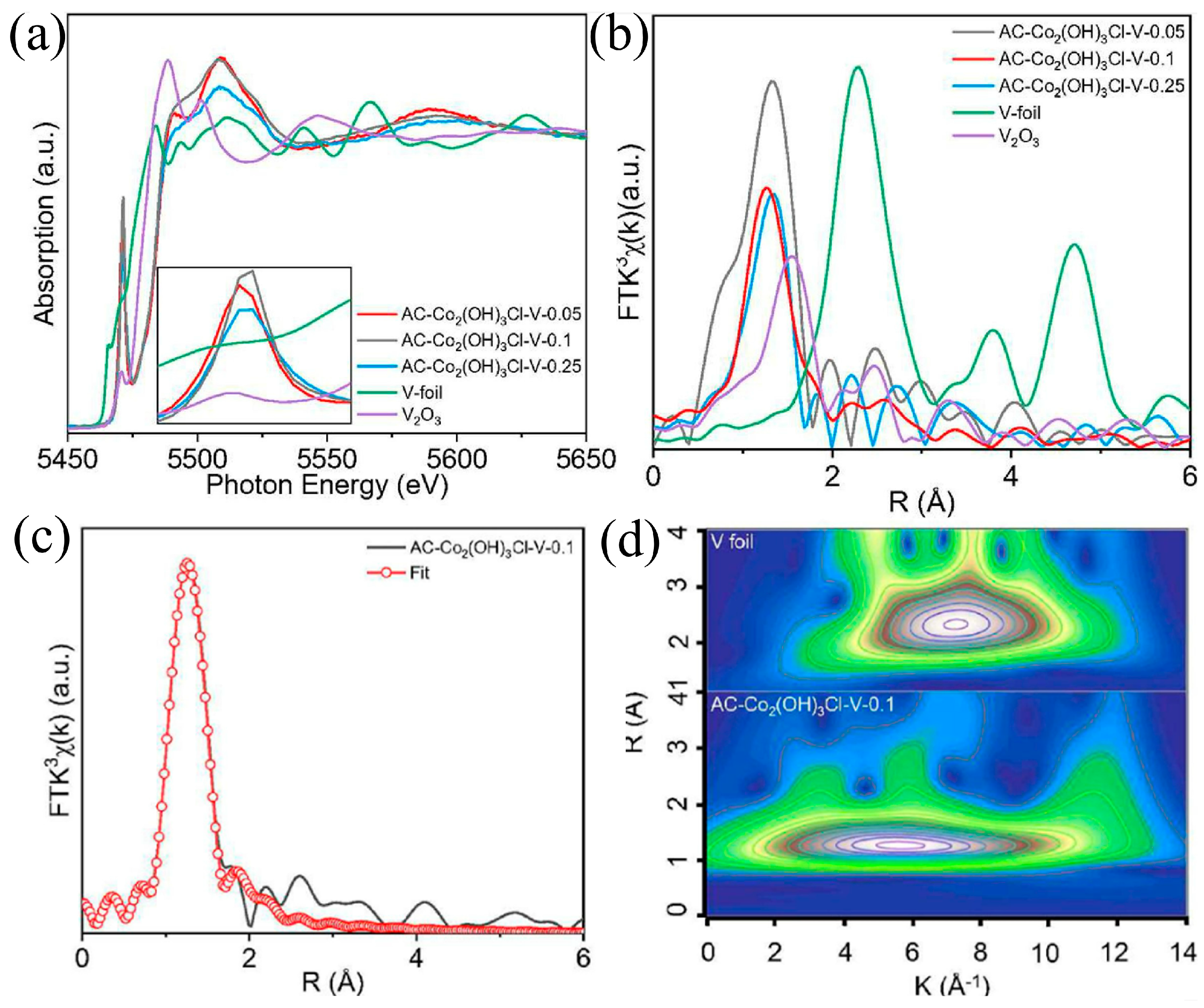
- Zhang, B.; Pan, C.; Liu, H.; Wu, X.; Jiang, H.; Yang, L.; Qi, Z.; Li, G.; Shan, L.; Lin, Y. Achieving High-Efficient Urea Oxidation via Regulating the Rate-Determining Step over a V Single Atom Incorporated Co Hydroxide Electrocatalyst. Chem. Eng. J. 2022, 439, 135768. [Google Scholar] [CrossRef]
- Behrens, M.; Studt, F.; Kasatkin, I.; Kühl, S.; Hävecker, M.; Abild-Pedersen, F.; Zander, S.; Girgsdies, F.; Kurr, P.; Kniep, B.-L. The Active Site of Methanol Synthesis over Cu/ZnO/Al2O3 Industrial Catalysts. Science 2012, 336, 893–897. [Google Scholar] [CrossRef]
- Liu, Y.; Hua, X.; Xiao, C.; Zhou, T.; Huang, P.; Guo, Z.; Pan, B.; Xie, Y. Heterogeneous Spin States in Ultrathin Nanosheets Induce Subtle Lattice Distortion to Trigger Efficient Hydrogen Evolution. J. Am. Chem. Soc. 2016, 138, 5087–5092. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Li, Y.; Huo, J.; Chen, R.; Dai, L.; Wang, S. Defect Chemistry of Nonprecious-metal Electrocatalysts for Oxygen Reactions. Adv. Mater. 2017, 29, 1606459. [Google Scholar] [CrossRef]
- Lu, S.; Hummel, M.; Kang, S.; Pathak, R.; He, W.; Qi, X.; Gu, Z. Density Functional Theory Investigation of the NiO@ Graphene Composite as a Urea Oxidation Catalyst in the Alkaline Electrolyte. ACS Omega 2021, 6, 14648–14654. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Liu, X.; Xiong, D.; Wang, J.; Gong, S.; Xu, C.; Wu, D.; Chen, Z. Ultrafast Room-Temperature Activation of Nickel Foams as Highly Efficient Electrocatalysts. Chem. Eng. J. 2023, 475, 146253. [Google Scholar] [CrossRef]
- He, B.; Kuang, Y.; Hou, Z.; Zhou, M.; Chen, X. Enhanced Electrocatalytic Hydrogen Evolution Activity of Nickel Foam by Low-Temperature-Oxidation. J. Mater. Res. 2018, 33, 213–224. [Google Scholar] [CrossRef]



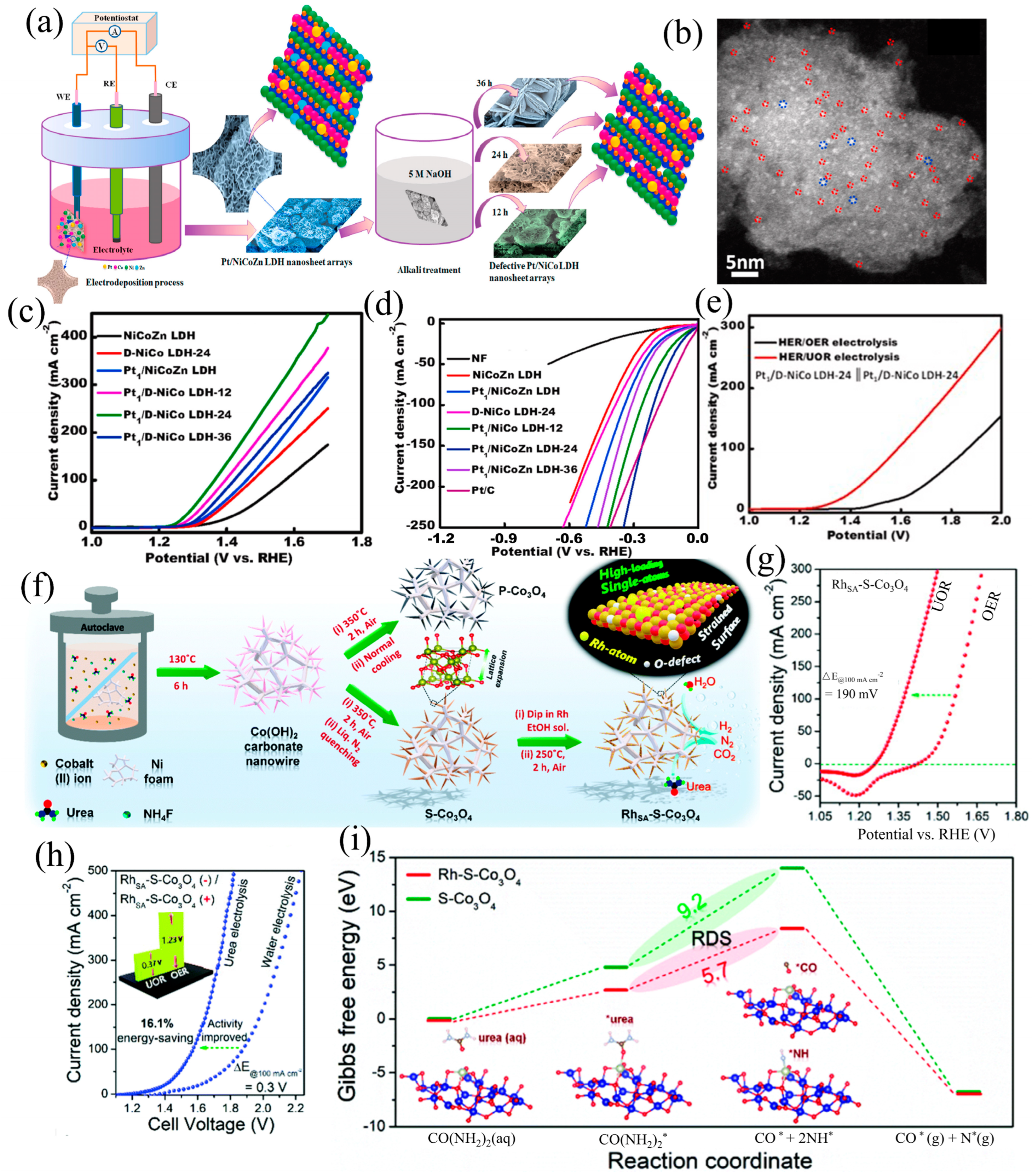
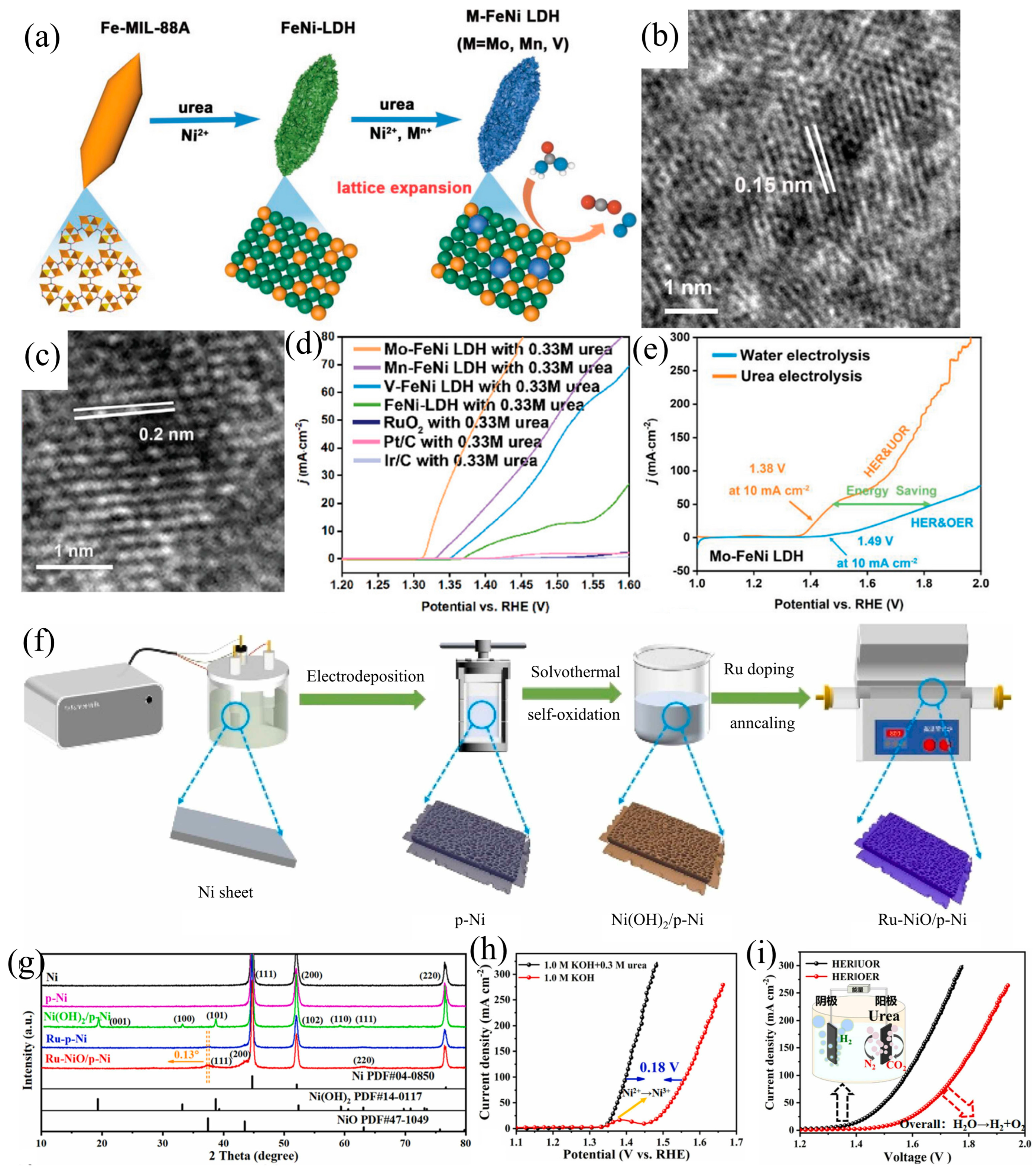
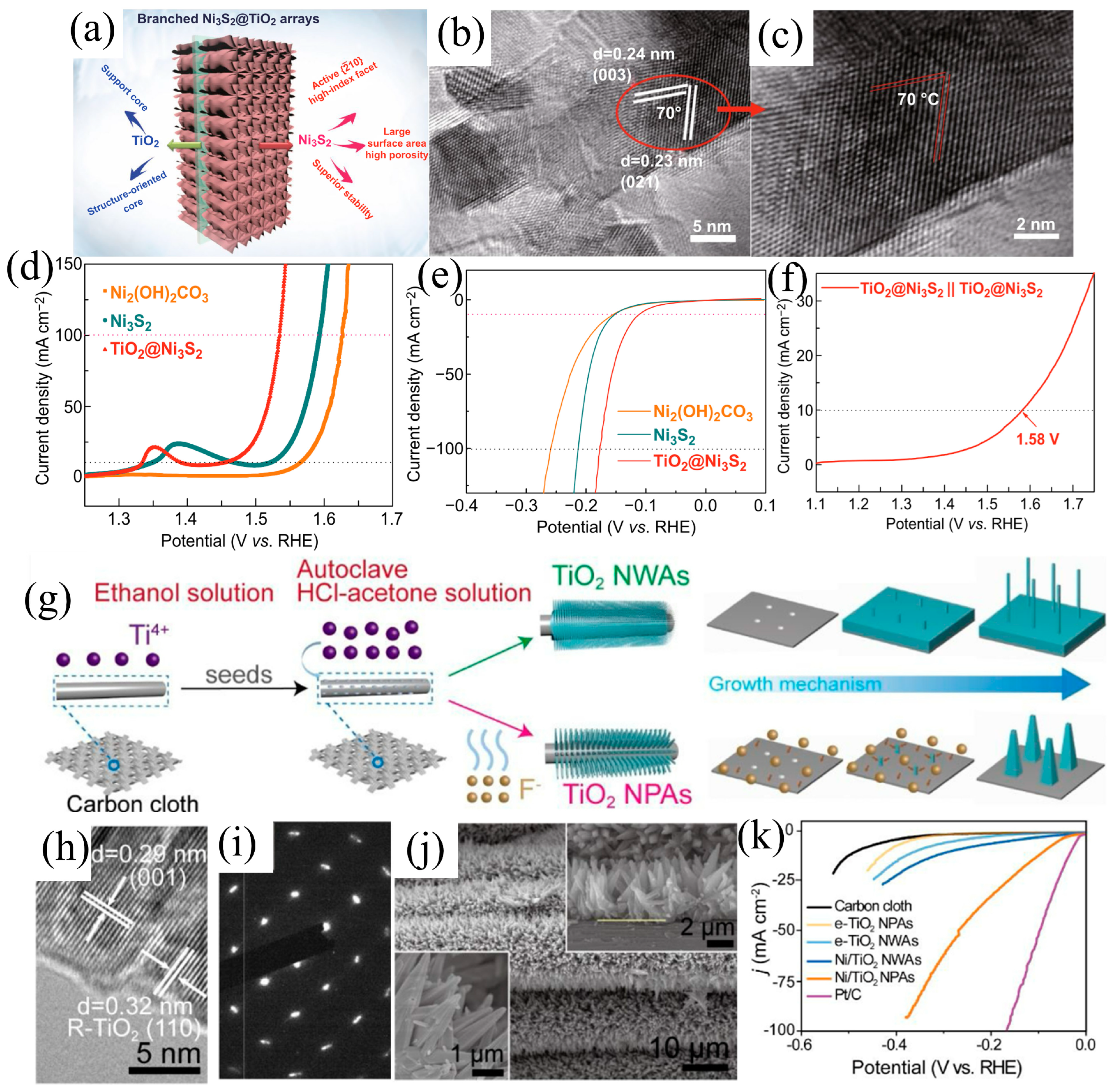
| Catalysts | HER Activity | UOR Activity | Water Electrolysis | Urea Electrolysis | Refs. |
|---|---|---|---|---|---|
| NF/P-NiMoO4− x | 116 mV (vs. RHE) 10 mA cm−2 | 1.59 V (vs. RHE) 100 mA cm−2 | 1.66 V 10 mA cm−2 | 1.48 V 10 mA cm−2 | [44] |
| ZnO-Ni2P/NF (a) | - | 1.347 V (vs. RHE) 50 mA cm−2 | 1.667 V 50 mA cm−2 | 1.529 V 50 mA cm−2 | [45] |
| Mn-Ni(OH)2/CP | −76 mV (vs. RHE) 10 mA cm−2 | 1.347 V (vs. RHE) 10 mA cm−2 | 1.61 V 10 mA cm−2 | 1.407 V 10 mA cm−2 | [46] |
| S-Co2P@Ni2P | 103 mV (vs. RHE) 100 mA cm−2 | 1.36 V (vs. RHE) 100 mA cm−2 | 1.52 V 10 mA cm−2 | 1.43 V 10 mA cm−2 | [47] |
| Ru/P-NiMoO4@NF | 0.23 mV (vs. RHE) 3000 mA cm−2 | 1.46 V (vs. RHE) 1000 mA cm−2 | - | 1.73 V 500 mA cm−2 | [48] |
| Ni-S-Se/NF | 98 mV (vs. RHE) 10 mA cm−2 | 1.38 V (vs. RHE) 10 mA cm−2 | 1.57 V 10 mA cm−2 | 1.47 V 10 mA cm−2 | [49] |
| c-CoNiPx/a-P-MnOy | 100 mV (vs. RHE) 10 mA cm−2 | 1.35 V (vs. RHE) 100 mA cm−2 | 2.0 V 100 mA cm−2 | 1.67 V 100 mA cm−2 | [50] |
| Pt1/D-NiCo LDH (b)-24 | 37 mV (vs. RHE) 10 mA cm−2 | 1.25 V (vs. RHE) 10 mA cm−2 | 1.52 V 10 mA cm−2 | 1.32 V 10 mA cm−2 | [51] |
| RhSA(c)-S-Co3O4/NF | 45 mV (vs. RHE) 10 mA cm−2 | 1.28 V (vs. RHE) 10 mA cm−2 | - | 1.33 V 10 mA cm−2 | [52] |
| Mo-FeNi-LDH (b) | - | 1.32 V (vs. RHE) 10 mA cm−2 | 1.49 V 10 mA cm−2 | 1.38 V 10 mA cm−2 | [53] |
| Ru-NiO/p-Ni | 127 mV (vs. RHE) 10 mA cm−2 | 1.39 V (vs. RHE) 100 mA cm−2 | 1.75 V 100 mA cm−2 | 1.58 V 100 mA cm−2 | [54] |
| TiO2@Ni3S2 | 112 mV (vs. RHE) 10 mA cm−2 | - | 1.58 V 10 mA cm−2 | - | [55] |
| Ni/TiO2 NPAs (d) | 88 mV (vs. RHE) 10 mA cm−2 | - | - | - | [56] |
| Catalysts | Preparation Method | Type of Defects | Refs. |
|---|---|---|---|
| a-Ni(OH)2 | Template Method | Vacancy defects | [57] |
| V-Ni(OH)2 | One-step hydrothermal method | Vacancy defects | [58] |
| Mo-NiS/Ni3S2 | Hydrothermal sulfurization–acid-assisted etching | Vacancy defects | [59] |
| (a) VNi-α-Ni(OH)2 | Alkaline precipitation method | Vacancy defects | [60] |
| NiFe LDH (b)@Ni(OH)2 | Electrodeposition and in situ etching | Vacancy defects | [61] |
| Co-NiMoO4/NF | Hydrothermal and impregnation | Heteroatom doping | [62] |
| P-NiMoO4 | Acid etching and oxidative synthesis | Heteroatom doping | [44] |
| Ru/P-NiMoO4@NF | Hydrothermal synthesis and thermal treatment | Heteroatom doping | [48] |
| S-Co2P@Ni2P | Chemical deposition and low-temperature phosphidation | Heteroatom doping | [47] |
| Ni/Ni3S2@N | Clectrodeposition and thermal treatment | Heteroatom doping | [63] |
| Ni/r-Ni(OH)2@C | Plasma-enhanced chemical vapor deposition | Heteroatom doping | [64] |
| Mn-Ni(OH)2 | Electrodeposition | Heteroatom doping | [65] |
| Ni-TPA (c)@NiSe | Hydrothermal synthesis | Amorphization | [66] |
| Ni(OH)2–NiMoOx/NF | Template method and electrodeposition | Amorphization | [67] |
| Ni(OH)S/NF | Solvothermal synthesis and annealing | Amorphization | [68] |
| NiFe hydroxide | Chemical corrosion | Amorphization | [69] |
| Nickel carbonate | Chemical precipitation | Amorphization | [70] |
| Fe-O-P | Atomic layer deposition–phosphorization | Amorphization | [71] |
| NiOx/N-doped | Chemical reduction | Amorphization | [72] |
| RhSA-S-Co3O4 | Hydrothermal synthesis and rapid quenching | Single-atom design | [52] |
| Pt/D-NiCo LDH SACs (d) | Electrodeposition and etching treatment | Single-atom design | [51] |
| Ir-NiFe-OH | Hydrothermal synthesis | Single-atom design | [73] |
| TiO2@Ni2(OH)2CO3 | Atomic layer deposition | High-index facets | [55] |
| Ni3S2 Nanosheet | Hydrothermal synthesis | High-index facets | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Qi, X.; Lu, S.; Khalil, M.N.; Dong, X.; Wang, H. Defect Engineering of Nickel-Based Compounds for Energy-Saving H2 Production. Energies 2024, 17, 3801. https://doi.org/10.3390/en17153801
Zeng Y, Qi X, Lu S, Khalil MN, Dong X, Wang H. Defect Engineering of Nickel-Based Compounds for Energy-Saving H2 Production. Energies. 2024; 17(15):3801. https://doi.org/10.3390/en17153801
Chicago/Turabian StyleZeng, Yi, Xueqiang Qi, Shun Lu, Mohamed N. Khalil, Xiuxiu Dong, and Haoqi Wang. 2024. "Defect Engineering of Nickel-Based Compounds for Energy-Saving H2 Production" Energies 17, no. 15: 3801. https://doi.org/10.3390/en17153801
APA StyleZeng, Y., Qi, X., Lu, S., Khalil, M. N., Dong, X., & Wang, H. (2024). Defect Engineering of Nickel-Based Compounds for Energy-Saving H2 Production. Energies, 17(15), 3801. https://doi.org/10.3390/en17153801







