Investigation of the Pore Characteristics and Capillary Forces in Shale before and after Reaction with Supercritical CO2 and Slickwater
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Description and Preparation
2.2. CO2 Treatment Procedure
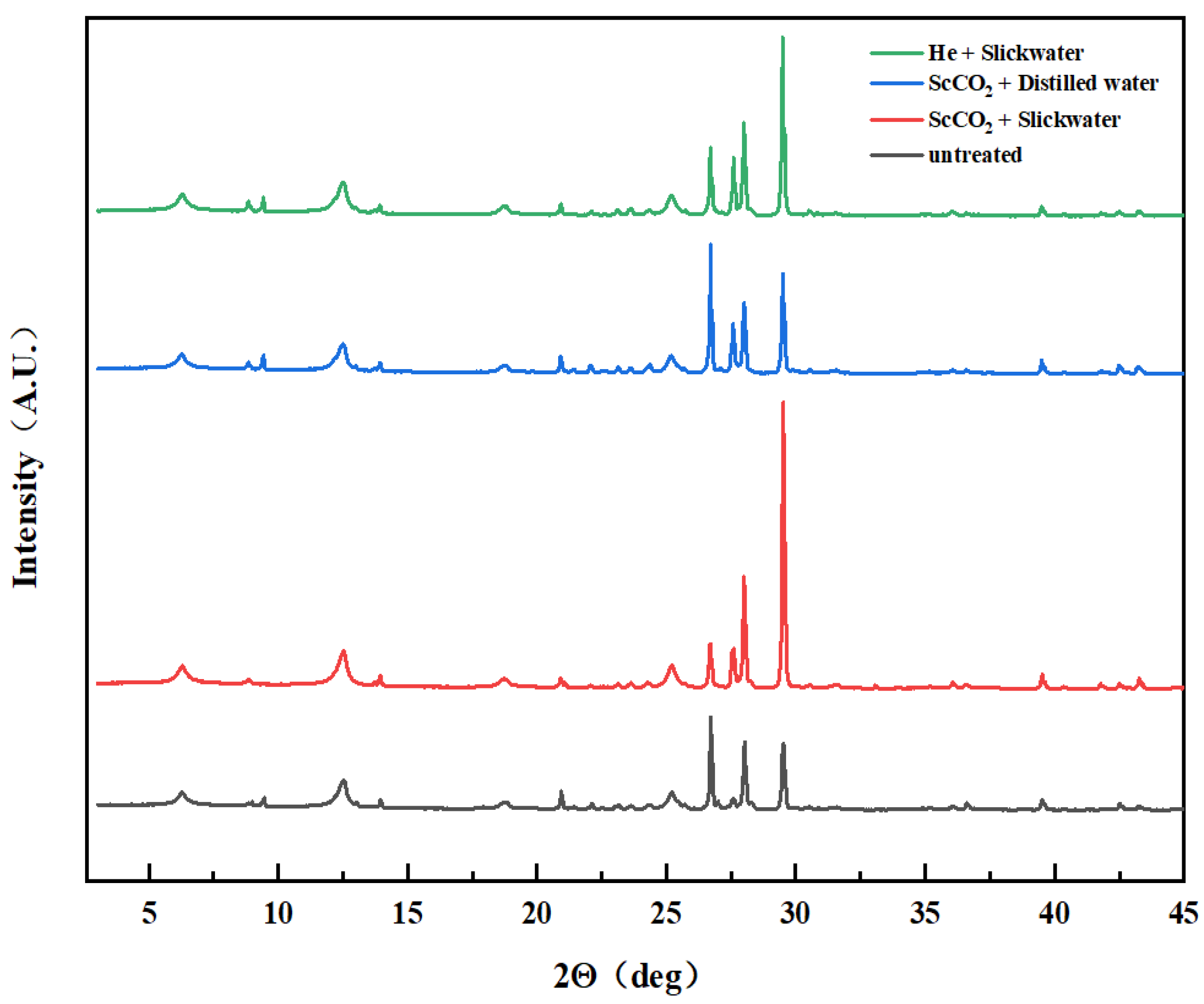
2.3. XRD Analysis
2.4. N2 Adsorption Analysis
2.5. Mercury Intrusion Porosimetry (MIP) Test
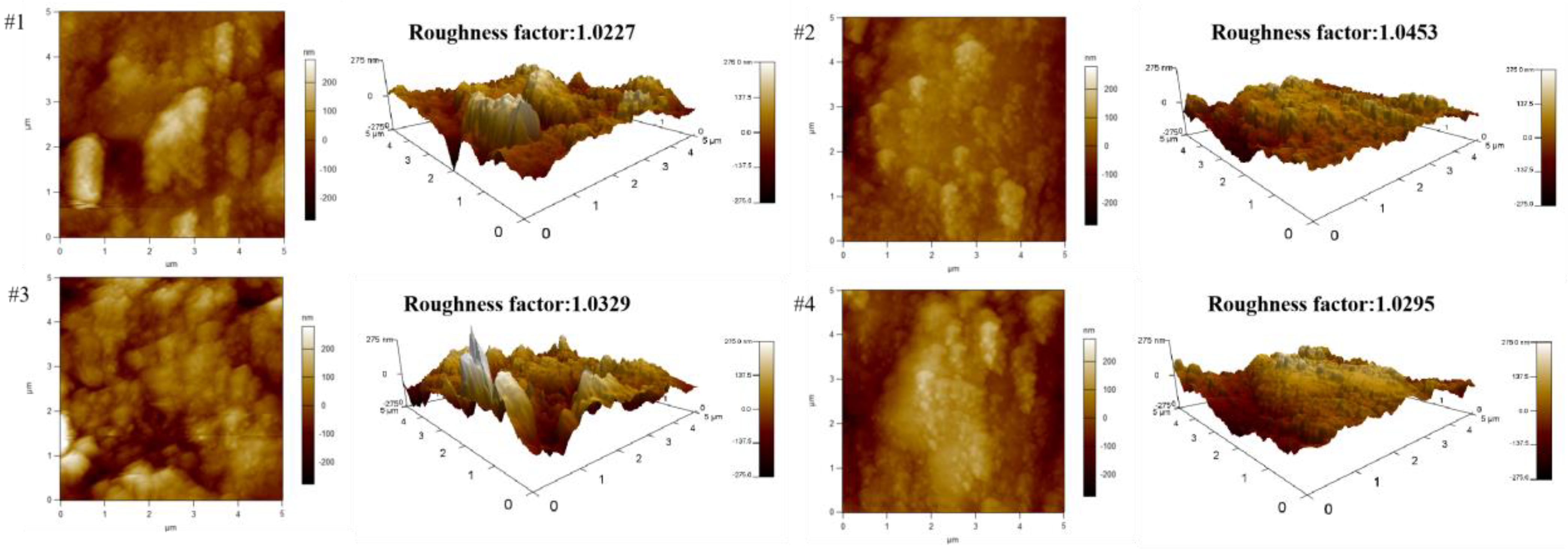
2.6. Contact Angle Measurement
3. Results
3.1. Mineral Compositions before and after Treatment
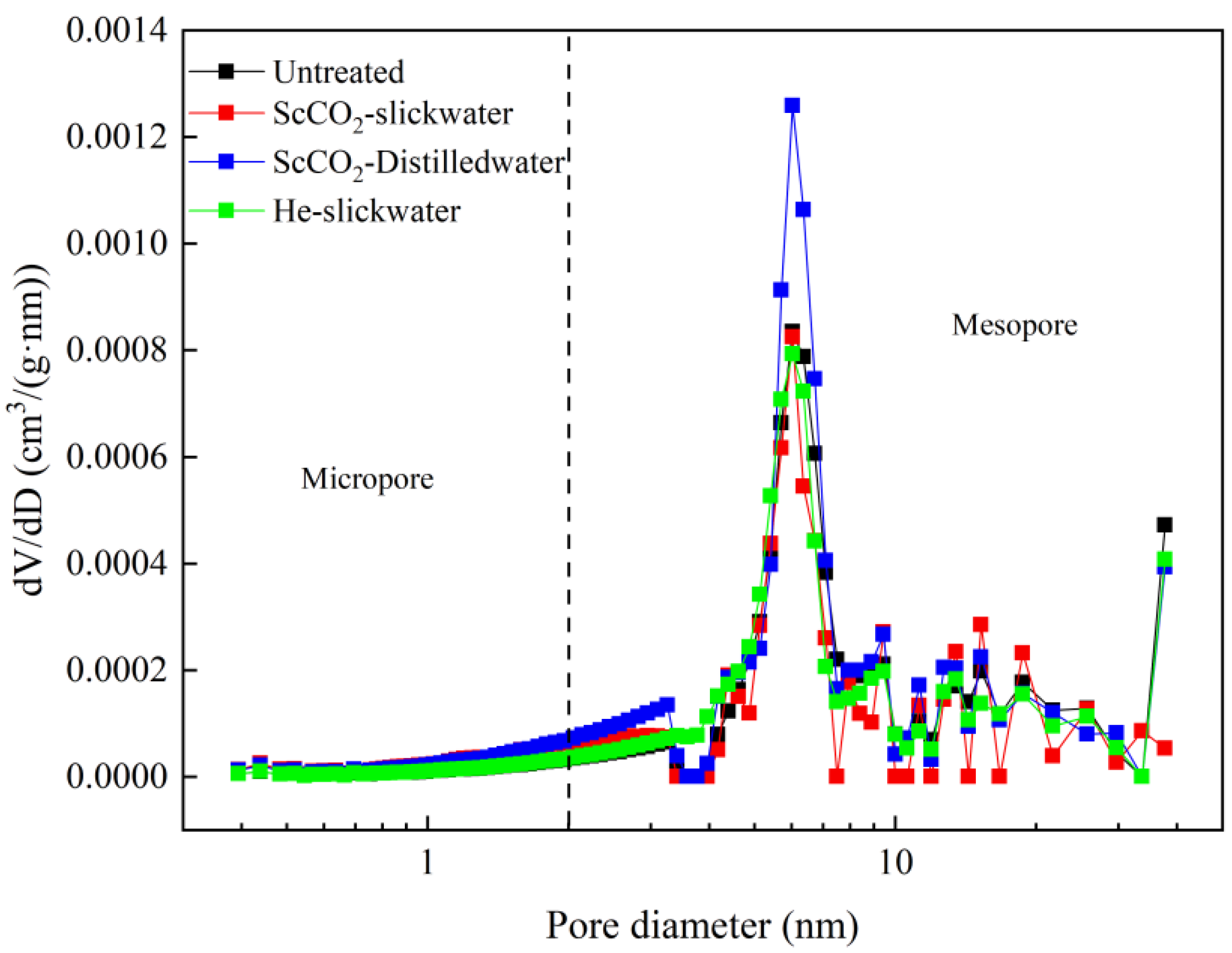
3.2. Analysis of the Pore Characteristics Based on N2 Adsorption Isotherms
3.2.1. Analysis of the Pore Characteristics Based on N2
3.2.2. Changes of the Pore-Structure Parameters Based on N2
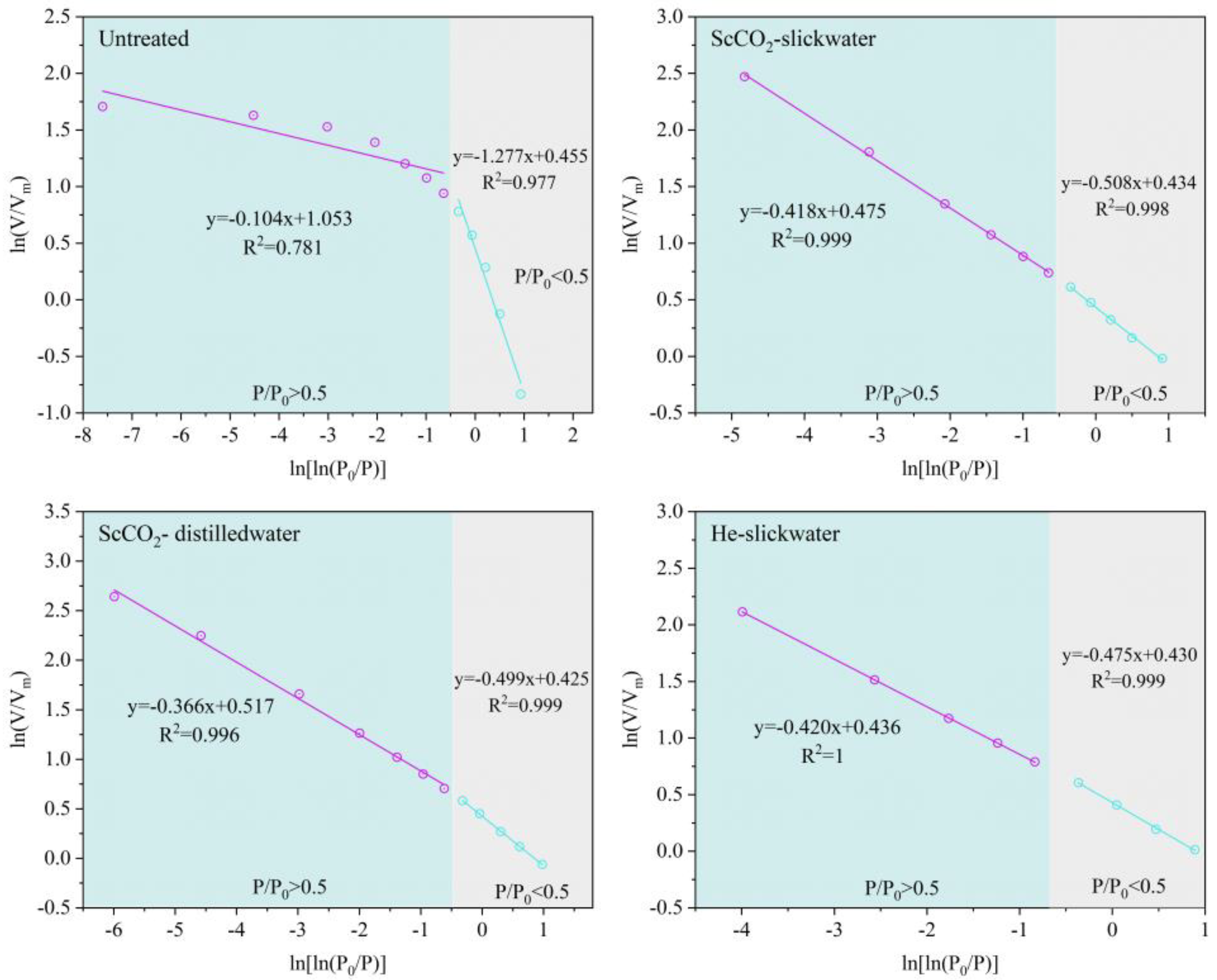
3.2.3. Fractal Dimensions of Sample Based on N2 Isotherms
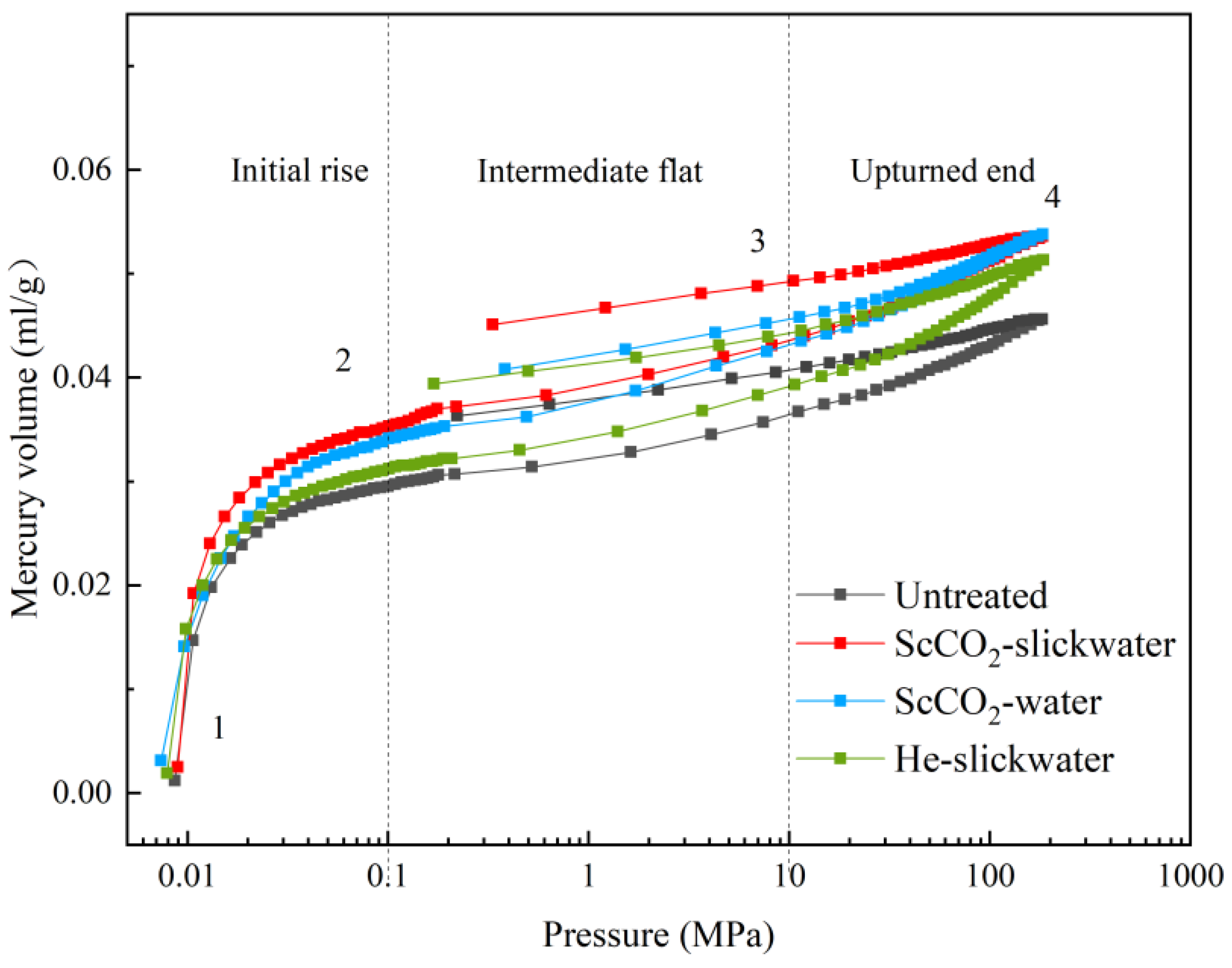
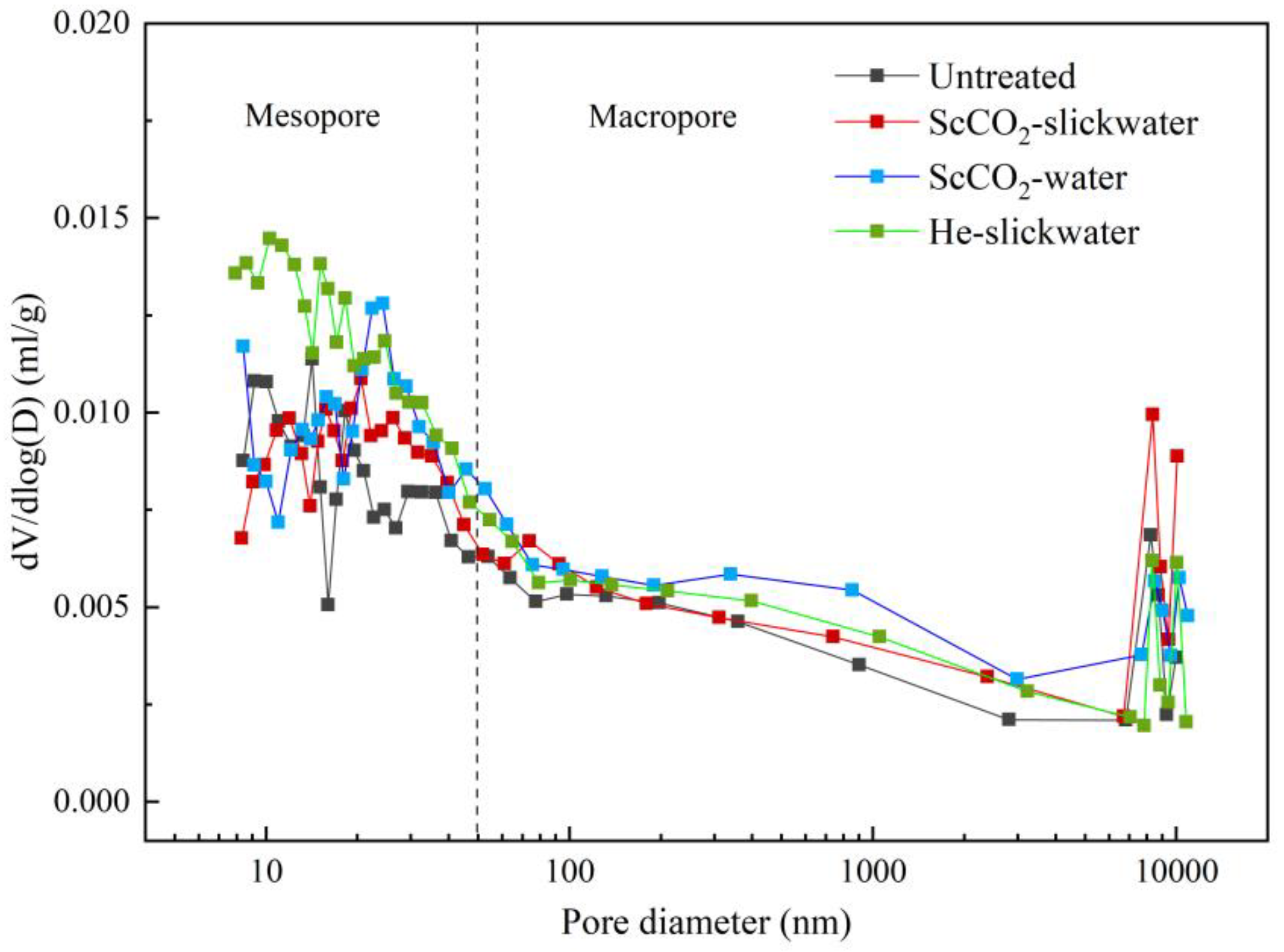
3.3. Analysis of the Pore Characteristics Based on MIP
3.4. Effect of ScCO2 and Slickwater on the Wettability of Shale Surface
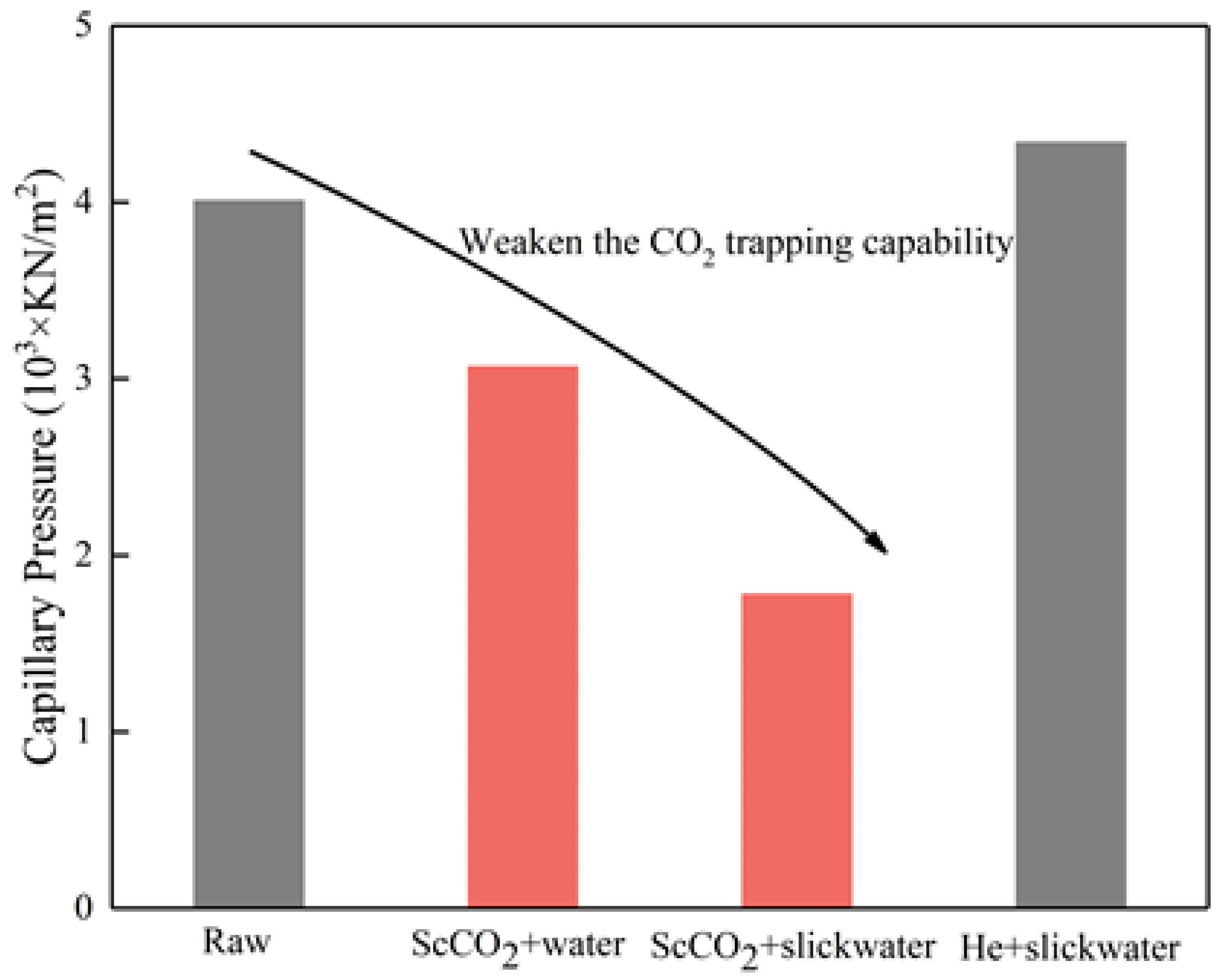
3.5. Implications for the CO2 Geological Sequestration and Shale Gas Recovery
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meakin, P.; Huang, H.; Malthe-Sørenssen, A.; Thøgersen, K. Shale gas: Opportunities and challenges. Environ. Geosci. 2013, 20, 151–164. [Google Scholar] [CrossRef]
- Shakya, S.; Li, B.; Etienne, X. Shale revolution, oil and gas prices, and drilling activities in the United States. Energy Econ. 2022, 108, 105877. [Google Scholar] [CrossRef]
- Zhong, C.; Hou, D.; Liu, B.; Zhu, S.; Wei, T.; Gehman, J.; Alessi, D.S.; Qian, P.-Y. Water footprint of shale gas development in China in the carbon neutral era. J. Environ. Manag. 2023, 331, 117238. [Google Scholar] [CrossRef]
- Sakhaee-Pour, A.; Bryant, S.L. Gas permeability of shale. SPE Reserv. Eval. Eng. 2012, 15, 401–409. [Google Scholar] [CrossRef]
- Clarkson, C.; Haghshenas, B.; Ghanizadeh, A.; Qanbari, F.; Williams-Kovacs, J.D.; Riazi, N.; Debuhr, C.; Deglint, H.J. Nanopores to megafractures: Current challenges and methods for shale gas reservoir and hydraulic fracture characterization. J. Nat. Gas Sci. Eng. 2016, 31, 612–657. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, W.; Xu, Z.; Liu, S.; Wei, C. A review of experimental apparatus for supercritical CO2 fracturing of shale. J. Pet. Sci. Eng. 2022, 208, 109515. [Google Scholar] [CrossRef]
- Memon, S.; Feng, R.; Ali, M.; Bhatti, M.A.; Giwelli, A.; Keshavarz, A.; Xie, Q.; Sarmadivaleh, M. Supercritical CO2-Shale interaction induced natural fracture closure: Implications for scCO2 hydraulic fracturing in shales. Fuel 2022, 313, 122682. [Google Scholar] [CrossRef]
- Xie, W.; Chen, S.; Wang, M.; Yu, Z.; Wang, H. Progress and prospects of supercritical CO2 application in the exploitation of shale gas reservoirs. Energy Fuels 2021, 35, 18370–18384. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, J.; Tang, J.; Ao, X.; Li, H.; Zhou, J.; Sun, X. Pore changes of slickwater-containing shale under supercritical CO2 treatment. Fuel 2022, 312, 122775. [Google Scholar] [CrossRef]
- Ao, X.; Wang, B.; Qu, H.; Xiang, Z.; Luo, Z. Swelling of shales with slickwater in carbon dioxide. Energy Fuels 2021, 35, 5122–5129. [Google Scholar] [CrossRef]
- Dayan, A.; Stracener, S.M.; Clark, P.E. Proppant transport in slick-water fracturing of shale-gas formations. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 4–7 October 2009. [Google Scholar]
- Ribeiro, L.H.; Li, H.; Bryant, J.E. Use of a CO2-hybrid fracturing design to enhance production from unpropped-fracture networks. SPE Prod. Oper. 2017, 32, 28–40. [Google Scholar] [CrossRef]
- Qin, C.; Jiang, Y.; Zhou, J.; Song, X.; Liu, Z.; Li, D.; Zhou, F.; Xie, Y.; Xie, C. Effect of supercritical CO2 extraction on CO2/CH4 competitive adsorption in Yanchang shale. Chem. Eng. J. 2021, 412, 128701. [Google Scholar] [CrossRef]
- Jiang, K.; Ashworth, P.; Zhang, S.; Hu, G. Print media representations of carbon capture utilization and storage (CCUS) technology in China. Renew. Sustain. Energy Rev. 2022, 155, 111938. [Google Scholar] [CrossRef]
- Isah, A.; Arif, M.; Hassan, A.; Mahmoud, M.; Iglauer, S. A systematic review of Anhydrite-Bearing Reservoirs: EOR Perspective, CO2-Geo-storage and future research. Fuel 2022, 320, 123942. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Tang, J.; Jia, Y.; Tian, R.; Zhou, J.; Chen, X.; Xu, Z.; Cheng, Q. Water wettability alternation of CO2-water-shale system due to nanoparticles: Implications for CO2 geo-storage. Int. J. Greenh. Gas Control 2023, 124, 103836. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, J.; Jia, Y.; Lu, Y.; Shang, C.; Tian, R.; Sun, X.; Cheng, Q.; Zhao, G. Investigation on Capillary Force Recovery of Nanoparticle-Coated Shale after Supercritical CO2 Exposure: Implications for CO2 Sequestration. Energy Fuels 2023, 37, 13137–13150. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Hu, Q.; Cheng, H.; Ilavsky, J.; Kuzmenko, I.; Shang, F.; Liu, L. Pore Structure and Wettability of Bossier Shale, East Texas, United States: Insights from Integrated Porosimetry, Scattering, and Imbibition Approaches. Energy Fuels 2023, 37, 11042–11054. [Google Scholar] [CrossRef]
- Khandoozi, S.; Hazlett, R.; Fustic, M. A critical review of CO2 mineral trapping in sedimentary reservoirs–from theory to application: Pertinent parameters, acceleration methods and evaluation workflow. Earth-Sci. Rev. 2023, 244, 104515. [Google Scholar] [CrossRef]
- Iglauer, S. CO2–water–rock wettability: Variability, influencing factors, and implications for CO2 geostorage. Acc. Chem. Res. 2017, 50, 1134–1142. [Google Scholar] [CrossRef]
- Ao, X.; Lu, Y.; Tang, J.; Chen, Y.; Li, H. Investigation on the physics structure and chemical properties of the shale treated by supercritical CO2. J. CO2 Util. 2017, 20, 274–281. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, K.; Zhou, L.; Jiang, Y.; Xian, X.; Zhang, C.; Tian, S.; Fan, M.; Lu, Z. Microstructure and mechanical properties alterations in shale treated via CO2/CO2-water exposure. J. Pet. Sci. Eng. 2021, 196, 108088. [Google Scholar] [CrossRef]
- Qin, C.; Jiang, Y.; Zuo, S.; Chen, S.; Xiao, S.; Liu, Z. Investigation of adsorption kinetics of CH4 and CO2 on shale exposure to supercritical CO2. Energy 2021, 236, 121410. [Google Scholar] [CrossRef]
- Liu, B.; Teng, J.; Mastalerz, M.; Schieber, J.; Schimmelmann, A.; Bish, D. Compositional control on shale pore structure characteristics across a maturation gradient: Insights from the Devonian New Albany Shale and Marcellus Shale in the eastern United States. Energy Fuels 2021, 35, 7913–7929. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Zhang, R.; Anovitz, L.M.; Bleuel, M.; Liu, S.; Chen, S. SANS coupled with fluid invasion approaches for characterization of overall nanopore structure and mesopore connectivity of organic-rich marine shales in China. Int. J. Coal Geol. 2020, 217, 103343. [Google Scholar] [CrossRef]
- Lu, Y.; Tian, R.; Tang, J.; Jia, Y.; Lu, Z.; Sun, X. Investigating the mineralogical and chemical effects of CO2 injection on shale wettability at different reservoir temperatures and pressures. Energy Fuels 2021, 35, 14838–14851. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, H.; Wei, Z.; Wang, Y.; Wu, B.; Zhuo, S.; Zhao, Z.; Li, J.; Hao, L.; Yang, H. Effects of slick water fracturing fluid on pore structure and adsorption characteristics of shale reservoir rocks. J. Nat. Gas Sci. Eng. 2018, 51, 27–36. [Google Scholar] [CrossRef]
- Balashov, V.N.; Guthrie, G.D.; Lopano, C.L.; Hakala, J.A.; Brantley, S.L. Reaction and diffusion at the reservoir/shale interface during CO2 storage: Impact of geochemical kinetics. Appl. Geochem. 2015, 61, 119–131. [Google Scholar] [CrossRef]
- Ilgen, A.G.; Aman, M.; Espinoza, D.N.; Rodriguez, M.A.; Griego, J.M.; Dewers, T.A.; Feldman, J.D.; Stewart, T.A.; Choens, R.C.; Wilson, J. Shale-brine-CO2 interactions and the long-term stability of carbonate-rich shale caprock. Int. J. Greenh. Gas Control 2018, 78, 244–253. [Google Scholar] [CrossRef]
- Jung, H.B.; Um, W.; Cantrell, K.J. Effect of oxygen co-injected with carbon dioxide on Gothic shale caprock–CO2–brine interaction during geologic carbon sequestration. Chem. Geol. 2013, 354, 1–14. [Google Scholar] [CrossRef]
- Gupta, N.; Verma, A. Supercritical Carbon Dioxide Utilization for Hydraulic Fracturing of Shale Reservoir, and Geo-Storage: A Review. Energy Fuels 2023, 37, 14604–14621. [Google Scholar] [CrossRef]
- Mastalerz, M.; Hampton, L.; Drobniak, A.; Loope, H. Significance of analytical particle size in low-pressure N2 and CO2 adsorption of coal and shale. Int. J. Coal Geol. 2017, 178, 122–131. [Google Scholar] [CrossRef]
- Yang, K.; Li, B.; Li, J.; Ren, C. Adsorption characteristics and thermodynamics of CH4, CO2, and N2 on shale at different temperatures. Energy Fuels 2022, 36, 14079–14093. [Google Scholar] [CrossRef]
- Hazra, B.; Wood, D.A.; Vishal, V.; Singh, A.K. Pore characteristics of distinct thermally mature shales: Influence of particle size on low-pressure CO2 and N2 adsorption. Energy Fuels 2018, 32, 8175–8186. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, L.; Mastalerz, M.; Schimmelmann, A. The effect of analytical particle size on gas adsorption porosimetry of shale. Int. J. Coal Geol. 2015, 138, 103–112. [Google Scholar] [CrossRef]
- Liang, L.; Xiong, J.; Liu, X. An investigation of the fractal characteristics of the Upper Ordovician Wufeng Formation shale using nitrogen adsorption analysis. J. Nat. Gas Sci. Eng. 2015, 27, 402–409. [Google Scholar] [CrossRef]
- Li, Z.; Shen, X.; Qi, Z.; Hu, R. Study on the pore structure and fractal characteristics of marine and continental shale based on mercury porosimetry, N2 adsorption and NMR methods. J. Nat. Gas Sci. Eng. 2018, 53, 12–21. [Google Scholar] [CrossRef]
- Yang, R.; He, S.; Yi, J.; Hu, Q. Nano-scale pore structure and fractal dimension of organic-rich Wufeng-Longmaxi shale from Jiaoshiba area, Sichuan Basin: Investigations using FE-SEM, gas adsorption and helium pycnometry. Mar. Pet. Geol. 2016, 70, 27–45. [Google Scholar] [CrossRef]
- Yang, X.; Guo, S. Comparative analysis of shale pore size characterization methods. Pet. Sci. Technol. 2020, 38, 793–799. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Guo, J.; Gou, X.; Jiang, Z.; Pan, B. Reduced adsorption of polyacrylamide-based fracturing fluid on shale rock using urea. Energy Sci. Eng. 2018, 6, 749–759. [Google Scholar] [CrossRef]
- Xu, J.; Georges, M.M.; Chen, J.; Wang, X. Comparison of adsorption ability of PAM, APAM and CPAM on Sodium-Montmorllonite surface: A molecular dynamics study. Chem. Phys. 2023, 574, 112051. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, K.; Tian, S.; Zhou, L.; Xian, X.; Jiang, Y.; Liu, M.; Cai, J. (CO2-water-shale interaction induced shale microstructural alteration. Fuel 2020, 263, 116642. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, J.; Li, H.; Tang, J.; Zhou, L.; Ao, X. Gas flow characteristics in shale fractures after supercritical CO2 soaking. J. Nat. Gas Sci. Eng. 2021, 88, 103826. [Google Scholar] [CrossRef]
- Qin, C.; Jiang, Y.; Luo, Y.; Xian, X.; Liu, H.; Li, Y. Effect of supercritical carbon dioxide treatment time, pressure, and temperature on shale water wettability. Energy Fuels 2017, 31, 493–503. [Google Scholar] [CrossRef]
- Qin, C.; Jiang, Y.; Zhou, J.; Zuo, S.; Chen, S.; Liu, Z.; Yin, H.; Li, Y. Influence of supercritical CO2 exposure on water wettability of shale: Implications for CO2 sequestration and shale gas recovery. Energy 2022, 242, 122551. [Google Scholar] [CrossRef]
- Ali, M.; Pan, B.; Yekeen, N.; Al-Anssari, S.; Al-Anazi, A.; Keshavarz, A.; Iglauer, S.; Hoteit, H. Assessment of wettability and rock-fluid interfacial tension of caprock: Implications for hydrogen and carbon dioxide geo-storage. Int. J. Hydrogen Energy 2022, 47, 14104–14120. [Google Scholar] [CrossRef]
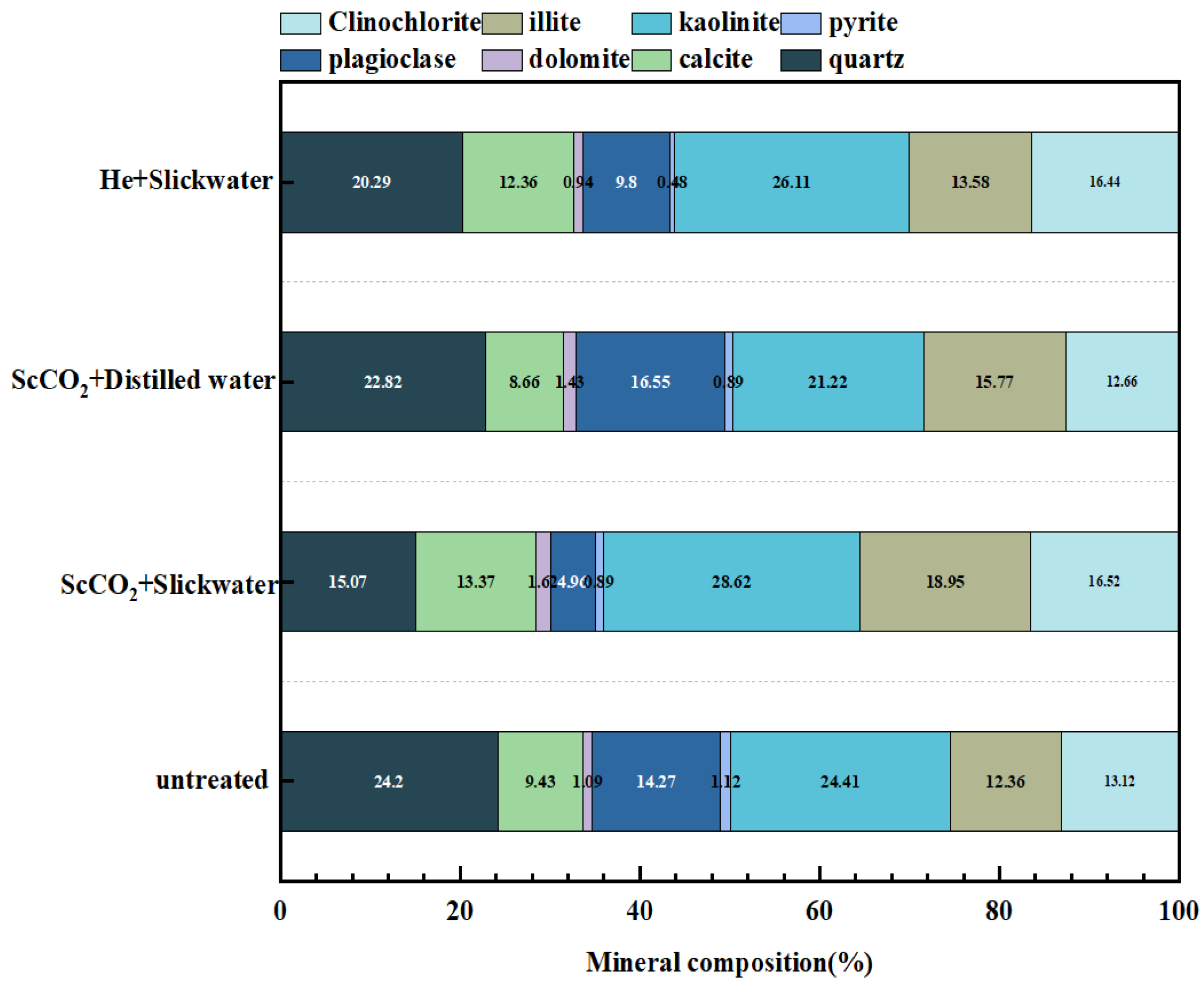




| Sample | Quartz | Calcite | Dolomite | Plagioclase | Pyrite | Total Clay | Kaolinite | Illite | Clinochlore |
|---|---|---|---|---|---|---|---|---|---|
| Untreated | 24.2 | 9.43 | 1.09 | 14.27 | 1.12 | 49.89 | 24.41 | 12.36 | 13.12 |
| ScCO2–slickwater | 15.07 | 13.37 | 1.62 | 4.96 | 0.89 | 64.09 | 28.62 | 18.95 | 16.52 |
| ScCO2–distilled water | 22.82 | 8.66 | 1.43 | 16.55 | 0.89 | 49.65 | 21.22 | 15.77 | 12.66 |
| He–slickwater | 20.29 | 12.36 | 0.94 | 9.8 | 0.48 | 56.13 | 26.11 | 13.58 | 16.44 |
| Sample | Surface Area (m2/g) | Pore Volume (cm3/kg) | SBET (m2/g) | TPV (cm3/kg) | Da (nm) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Micro (HK) | Meso (BJH-ad) | Macro (BJH-ad) | Micro (HK) | Meso (BJH-ad) | Macro (BJH-ad) | ||||
| Untreated | 0.153 | 2.018 | 0.139 | 0.1299 | 5.384 | 6.374 | 2.128 | 16.199 | 30.443 |
| ScCO2–slickwater | 0.114 | 1.712 | 0.090 | 0.0976 | 4.560 | 2.943 | 1.822 | 7.656 | 16.812 |
| ScCO2–distilled water | 0.207 | 1.982 | 0.140 | 0.174 | 5.521 | 6.702 | 2.477 | 12.341 | 19.932 |
| He–slickwater | 0.116 | 1.813 | 0.144 | 0.100 | 4.549 | 6.222 | 2.081 | 14.175 | 27.247 |
| Sample | P/P0 < 0.5 | P/P0 > 0.5 | ||||
|---|---|---|---|---|---|---|
| Fitting Equation | D | R2 | Fitting Equation | D | R2 | |
| Untreated | y = −1.277x + 0.455 | 1.723 | 0.977 | y = −0.104x + 1.053 | 2.896 | 0.781 |
| ScCO2–slickwater | y = −0.508x + 0.434 | 2.492 | 0.998 | y = −0.418x + 0.475 | 2.582 | 0.999 |
| ScCO2–distilled water | y = −0.499x + 0/425 | 2.501 | 0.999 | y = −0.366x + 0.517 | 2.634 | 0.996 |
| He–slickwater | y = −0.475x + 0.430 | 2.525 | 0.999 | y = −0.420x + 0.436 | 2.580 | 1 |
| Sample | SSA (m2/g) | PV (cm3/g) | Da (nm) | ||||
|---|---|---|---|---|---|---|---|
| Meso- | Macro- | TSSA | Meso- | Macro- | TPV | ||
| Untreated | 1.543 | 0.165 | 1.709 | 0.007 | 0.009 | 0.015 | 36.054 |
| ScCO2–slickwater | 1.576 | 0.187 | 1.763 | 0.007 | 0.010 | 0.017 | 38.568 |
| ScCO2–distilled water | 1.635 | 0.200 | 1.835 | 0.008 | 0.011 | 0.019 | 40.976 |
| He–slickwater | 2.328 | 0.181 | 2.509 | 0.010 | 0.010 | 0.020 | 31.253 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Li, Q.; Liu, Y.; Tang, J.; Jia, Y.; Gong, T. Investigation of the Pore Characteristics and Capillary Forces in Shale before and after Reaction with Supercritical CO2 and Slickwater. Energies 2024, 17, 3870. https://doi.org/10.3390/en17163870
Zhang C, Li Q, Liu Y, Tang J, Jia Y, Gong T. Investigation of the Pore Characteristics and Capillary Forces in Shale before and after Reaction with Supercritical CO2 and Slickwater. Energies. 2024; 17(16):3870. https://doi.org/10.3390/en17163870
Chicago/Turabian StyleZhang, Chi, Qian Li, Yanlin Liu, Jiren Tang, Yunzhong Jia, and Tianyi Gong. 2024. "Investigation of the Pore Characteristics and Capillary Forces in Shale before and after Reaction with Supercritical CO2 and Slickwater" Energies 17, no. 16: 3870. https://doi.org/10.3390/en17163870
APA StyleZhang, C., Li, Q., Liu, Y., Tang, J., Jia, Y., & Gong, T. (2024). Investigation of the Pore Characteristics and Capillary Forces in Shale before and after Reaction with Supercritical CO2 and Slickwater. Energies, 17(16), 3870. https://doi.org/10.3390/en17163870






