A Review of Life Cycle Assessment (LCA) Studies for Hydrogen Production Technologies through Water Electrolysis: Recent Advances
Abstract
1. Introduction
2. Literature Review
2.1. Hydrogen Production Methods
2.2. Water Electrolysis Technology as Green Hydrogen Production Solution
2.2.1. Alkaline Water Electrolyzers (AWE)
2.2.2. Anion Exchange Membrane Water Electrolysis (AEMWE)
2.2.3. Solid Oxide Electrolysis Cells (SOEC)
2.2.4. Proton Exchange Membrane Water Electrolysis (PEMWE)
2.2.5. Comparison of Key Water Electrolysis Technologies
2.3. Concept of Fluctuation of Power Supply in Electrolysis
2.4. LCA of Hydrogen Production
2.4.1. LCA in Water Electrolysis
2.4.2. Supply Chain Analysis of Water Electrolysis
2.5. LCA of WE in Recent Studies
2.5.1. LCA in Hydrogen Production Based on the Hydrogen Council’s Report
2.5.2. LCA of the Solid Oxide Electrolysis Cell (SOEC)
2.5.3. LCA of the Proton Exchange Membrane Water Electrolysis (PEMWE)
2.5.4. LCA of the Alkaline Water Electrolysis
2.5.5. Broad Reviews of Electrolysis Technologies’s LCA
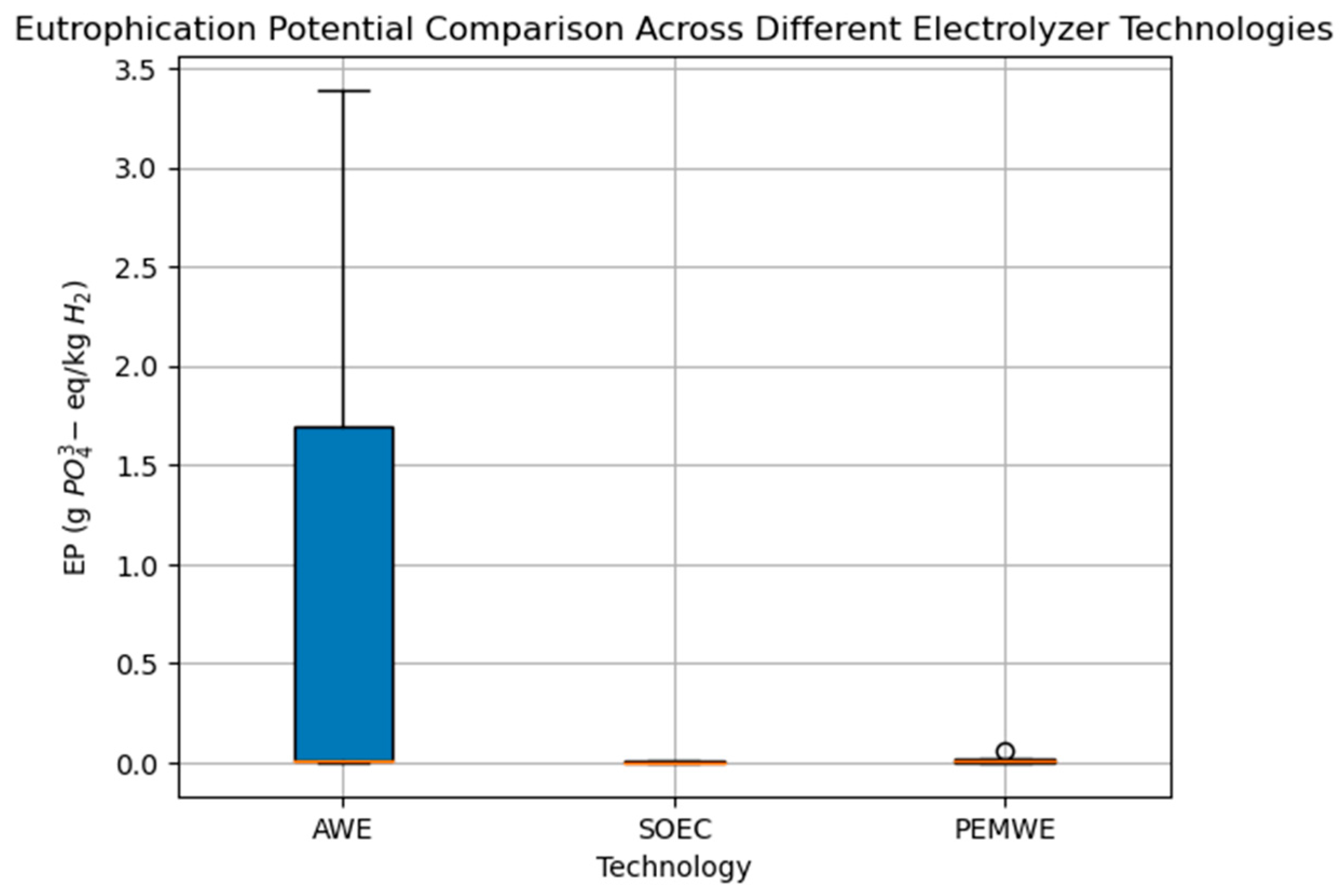
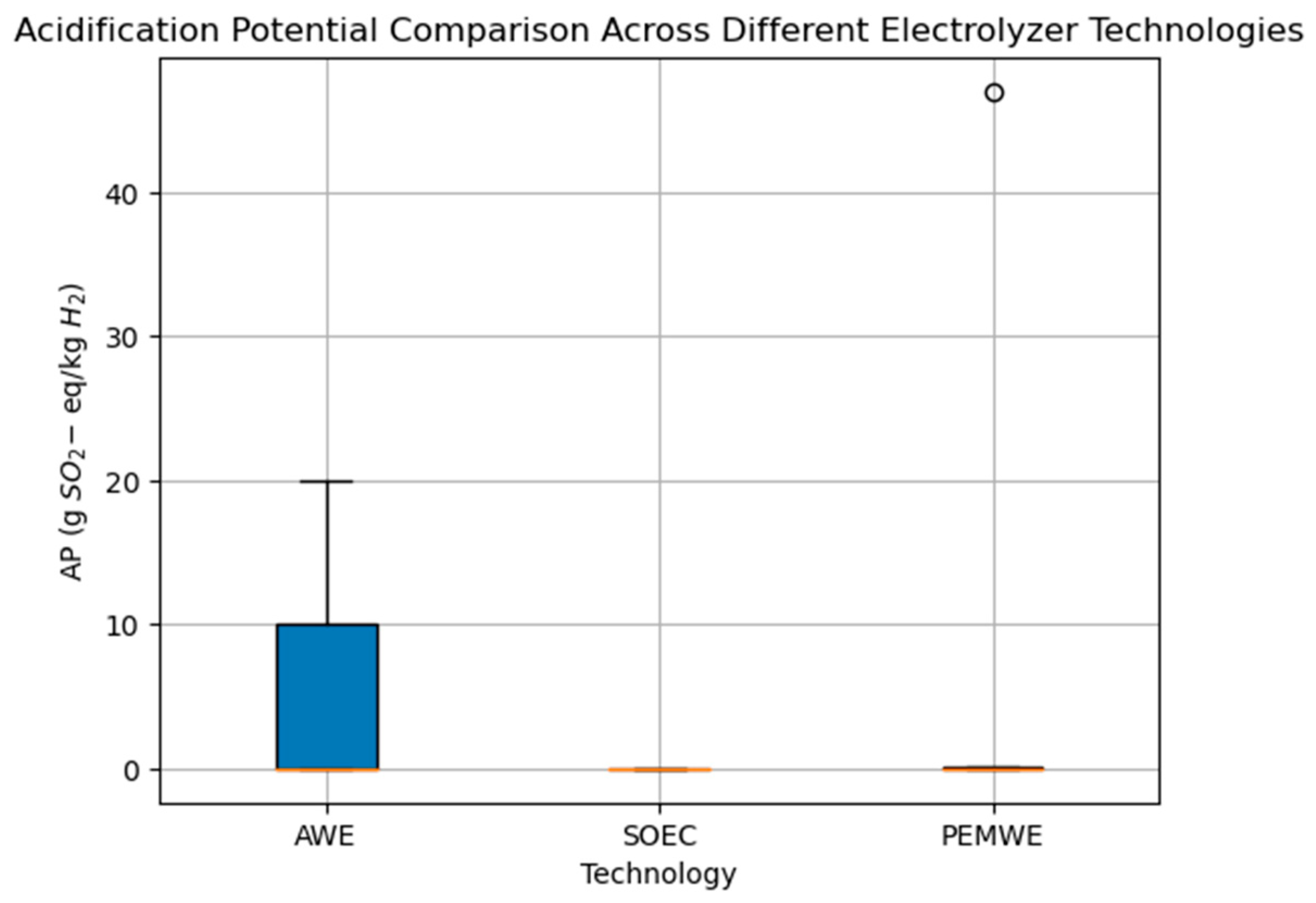
3. Summary of Environmental Impacts Result
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bareiß, K.; Rua, C.; Möckl, M.; Hamacher, T. Life cycle assessment of hydrogen from proton exchange membrane water electrolysis in future energy systems. Appl. Energy 2019, 237, 862–872. [Google Scholar] [CrossRef]
- Meyer, Q.; Zeng, Y.; Zhao, C. In Situ and Operando Characterization of Proton Exchange Membrane Fuel Cells. Adv. Mater. 2019, 31, 1901900. [Google Scholar] [CrossRef] [PubMed]
- Aouali, F.Z.; Becherif, M.; Tabanjat, A.; Emziane, M.; Mohammedi, K.; Krehi, S.; Khellaf, A. Modelling and Experimental Analysis of a PEM Electrolyser Powered by a Solar Photovoltaic Panel. Energy Procedia 2014, 62, 714–722. [Google Scholar] [CrossRef]
- BP P.L.C. Full Report—BP Statistical Review of World Energy. 2019. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2019-full-report.pdf (accessed on 1 May 2024).
- BP P.L.C. Full Report—BP Statistical Review of World Energy. 2022. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2022-full-report.pdf (accessed on 1 May 2024).
- Kumar, S.S.; Lim, H. An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Rat: EUCO. Tagung des Europäischen Rates—Schlussfolgerungen. Available online: https://www.parlament.gv.at/gegenstand/XXVII/EU/190883 (accessed on 1 May 2024).
- Gül, M.; Akyüz, E. Hydrogen Generation from a Small-Scale Solar Photovoltaic Thermal (PV/T) Electrolyzer System: Numerical Model and Experimental Verification. Energies 2020, 13, 2997. [Google Scholar] [CrossRef]
- Miller, H.A.; Bouzek, K.; Hnat, J.; Loos, S.; Bernäcker, C.I.; Weißgärber, T.; Röntzsch, L.; Meier-Haack, J. Green hydrogen from anion exchange membrane water electrolysis: A review of recent developments in critical materials and operating conditions. Sustain. Energy Fuels 2020, 4, 2114–2133. [Google Scholar] [CrossRef]
- Zhao, G.; Kraglund, M.R.; Frandsen, H.L.; Wulff, A.C.; Jensen, S.H.; Chen, M.; Graves, C.R. Life cycle assessment of H2O electrolysis technologies. Int. J. Hydrogen Energy 2020, 45, 23765–23781. [Google Scholar] [CrossRef]
- Fraunhofer. Economical and Resource-Saving Green Hydrogen. 2023. Available online: https://www.fraunhofer.de/content/dam/zv/en/press-media/2023/ifam-economical-and-resource-saving-green-hydrogen-2.pdf (accessed on 29 August 2023).
- Strogies, M.; Gniffke, P. Berichterstattung unter der Klimarahmenkonvention der Vereinten Nationen und dem Kyoto-Protokoll; Tech. Rep.; Umweltbundesamt: Vienna, Austria, 2017. [Google Scholar]
- Hydrogen Council. Hydrogen Decarbonization Pathways—A Life-Cycle Assessment. 2021. Available online: https://hydrogencouncil.com/wp-content/uploads/2021/01/Hydrogen-Council-Report_Decarbonization-Pathways_Part-1-Lifecycle-Assessment.pdf (accessed on 12 July 2023).
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Kumar, S.S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- European Commission; Joint Research Centre; Carrara, S.; Bobba, S.; Blagoeva, D.; Dias, P.A.; Cavalli, A.; Georgitzikis, K.; Grohol, M.; Itul, A.; et al. Supply Chain Analysis and Material Demand Forecast in Strategic Technologies and Sectors in the EU—A Foresight Study; Publications Office of the European Union: Luxembourg, 2023. [Google Scholar]
- Ajanovic, A.; Sayer, M.; Haas, R. The economics and the environmental benignity of different colors of hydrogen. Int. J. Hydrogen Energy 2022, 47, 24136–24154. [Google Scholar] [CrossRef]
- Woody, A.; Carlson, H. State of Play: Hydrogen in 2020|White & Case LLP. 2023. Available online: https://www.whitecase.com/insight-alert/state-play-hydrogen-2020 (accessed on 6 July 2023).
- Ingale, G.U.; Kwon, H.-M.; Jeong, S.; Park, D.; Kim, W.; Bang, B.; Lim, Y.-I.; Kim, S.W.; Kang, Y.-B.; Mun, J.; et al. Assessment of Greenhouse Gas Emissions from Hydrogen Production Processes: Turquoise Hydrogen vs. Steam Methane Reforming. Energies 2022, 15, 8679. [Google Scholar] [CrossRef]
- Okoye, C.O.; Jones, I.; Zhu, M.; Zhang, Z.; Zhang, D. Manufacturing of carbon black from spent tyre pyrolysis oil—A literature review. J. Clean. Prod. 2021, 279, 123336. [Google Scholar] [CrossRef]
- Sundin, C. Environmental Assessment of Electrolyzers for Hydrogen Gas Production. 2019. Available online: https://www.semanticscholar.org/paper/Environmental-Assessment-of-Electrolyzers-for-Gas-Sundin/1a6dcdd528ef4a9f303f5311e564f1a2efb586a0 (accessed on 1 May 2024).
- Lundberg, S. Comparative LCA of Electrolyzers for Hydrogen Gas Production. KTH, Sustainable Development, Environmental science and Engineering, Document Number 19543. Available online: https://www.semanticscholar.org/paper/Comparative-LCA-of-Electrolyzers-for-Hydrogen-Gas-Lundberg/b28594280c1940ba426b1a6f7b05e47205933164 (accessed on 1 May 2024).
- Papakonstantinou, G.; Algara-Siller, G.; Teschner, D.; Vidaković-Koch, T.; Schlögl, R.; Sundmacher, K. Degradation study of a proton exchange membrane water electrolyzer under dynamic operation conditions. Appl. Energy 2020, 280, 115911. [Google Scholar] [CrossRef]
- Kanz, O.; Bittkau, K.; Ding, K.; Rau, U.; Reinders, A. Review and harmonization of the life-cycle global warming impact of PV-powered hydrogen production by electrolysis. Front. Electron. 2021, 2, 711103. [Google Scholar] [CrossRef]
- Schmidt, P.; Weindorf, W.; Roth, A.; Batteiger, V.; Riegel, F. Power-to-Liquids Potentials and Perspectives for the Future Supply of Renewable Aviation Fuel; Umweltbundesamt: Dessau-Roßlau, Germany, 2016; Available online: https://www.umweltbundesamt.de/sites/default/files/medien/377/publikationen/161005_uba_hintergrund_ptl_barrierrefrei.pdf (accessed on 1 May 2024).
- Peng, L.; Wei, Z. Catalyst Engineering for Electrochemical Energy Conversion from Water to Water: Water Electrolysis and the Hydrogen Fuel Cell. Engineering 2020, 6, 653–679. [Google Scholar] [CrossRef]
- Liu, T.; Wang, X.; Jiang, X.; Deng, C.; Niu, S.; Mao, J.; Zeng, W.; Liu, M.; Liao, H. Mechanism of corrosion and sedimentation of nickel electrodes for alkaline water electrolysis. Mater. Chem. Phys. 2023, 303, 127806. [Google Scholar] [CrossRef]
- Brauns, J.; Turek, T. Alkaline Water Electrolysis Powered by Renewable Energy: A Review. Processes 2020, 8, 248. [Google Scholar] [CrossRef]
- Vincent, I.; Bessarabov, D. Low cost hydrogen production by anion exchange membrane electrolysis: A review. Renew. Sustain. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future cost and performance of water electrolysis: An expert elicitation study. Int. J. Hydrogen Energy 2017, 42, 30470–30492. [Google Scholar] [CrossRef]
- Faid, A.Y.; Xie, L.; Barnett, A.O.; Seland, F.; Kirk, D.; Sunde, S. Effect of anion exchange ionomer content on electrode performance in AEM water electrolysis. Int. J. Hydrogen Energy 2020, 45, 28272–28284. [Google Scholar] [CrossRef]
- Ferreira, A.P.R.A.; Oliveira, R.C.P.; Mateus, M.M.; Santos, D.M.F. A Review of the Use of Electrolytic Cells for Energy and Environmental Applications. Energies 2023, 16, 1593. [Google Scholar] [CrossRef]
- Sugawara, Y.; Sankar, S.; Miyanishi, S.; Illathvalappil, R.; Gangadharan, P.K.; Kuroki, H.; Anilkumar, G.M.; Yamaguchi, T. Anion Exchange Membrane Water Electrolyzers: An Overview. J. Chem. Eng. Jpn. 2023, 56, 2210195. [Google Scholar] [CrossRef]
- Riedel, M.; Heddrich, M.P.; Ansar, A.; Fang, Q.; Blum, L.; Friedrich, K.A. Pressurized operation of solid oxide electrolysis stacks: An experimental comparison of the performance of 10-layer stacks with fuel electrode and electrolyte supported cell concepts. J. Power Sources 2020, 475, 228682. [Google Scholar] [CrossRef]
- Bender, G.; Carmo, M.; Smolinka, T.; Gago, A.; Danilovic, N.; Mueller, M.; Ganci, F.; Fallisch, A.; Lettenmeier, P.; Friedrich, K.A.; et al. Initial approaches in benchmarking and round robin testing for proton exchange membrane water electrolyzers. Int. J. Hydrogen Energy 2019, 44, 9174–9187. [Google Scholar] [CrossRef]
- Bock, R.; Karoliussen, H.; Seland, F.; Pollet, B.G.; Thomassen, M.S.; Holdcroft, S.; Burheim, O.S. Measuring the thermal conductivity of membrane and porous transport layer in proton and anion exchange membrane water electrolyzers for temperature distribution modeling. Int. J. Hydrogen Energy 2020, 45, 1236–1254. [Google Scholar] [CrossRef]
- Shirvanian, P.; van Berkel, F. Novel components in Proton Exchange Membrane (PEM) Water Electrolyzers (PEMWE): Status, challenges and future needs. A mini review. Electrochem. Commun. 2020, 114, 106704. [Google Scholar] [CrossRef]
- Siracusano, S.; Trocino, S.; Briguglio, N.; Baglio, V.; Aricò, A.S. Electrochemical Impedance Spectroscopy as a Diagnostic Tool in Polymer Electrolyte Membrane Electrolysis. Materials 2018, 11, 1368. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.; Mays, T.; McManus, M. Review and meta-analysis of recent life cycle assessments of hydrogen production. Clean. Environ. Syst. 2023, 9, 100116. [Google Scholar] [CrossRef]
- Laveissiere, G.; Demoulin, M.; Calvet, R. Overall Assessment of Electrolyser Sustainability. Available online: https://www.sia-partners.com/system/files/document_download/file/2022-11/Electrolyzer%20materials.pdf (accessed on 1 May 2024).
- Eikeng, E.; Makhsoos, A.; Pollet, B.G. Critical and strategic raw materials for electrolysers, fuel cells, metal hydrides and hydrogen separation technologies. Int. J. Hydrogen Energy 2024, 71, 433–464. [Google Scholar] [CrossRef]
- Zignani, S.C.; Faro, M.L.; Carbone, A.; Italiano, C.; Trocino, S.; Monforte, G.; Aricò, A.S. Performance and stability of a critical raw materials-free anion exchange membrane electrolysis cell. Electrochim. Acta 2022, 413, 140078. [Google Scholar] [CrossRef]
- Enapter. Enapter Datasheet EL 2.1 EN. Available online: https://handbook.enapter.com/electrolyser/el21/downloads/Enapter_Datasheet_EL21_EN.pdf (accessed on 1 May 2024).
- Anantharaj, S.; Aravindan, V. Developments and Perspectives in 3d Transition-Metal-Based Electrocatalysts for Neutral and Near-Neutral Water Electrolysis. Adv. Energy Mater. 2020, 10, 1902666. [Google Scholar] [CrossRef]
- Lei, Z.; Wang, T.; Zhao, B.; Cai, W.; Liu, Y.; Jiao, S.; Li, Q.; Cao, R.; Liu, M. Recent Progress in Electrocatalysts for Acidic Water Oxidation. Adv. Energy Mater. 2020, 10, 2000478. [Google Scholar] [CrossRef]
- Zhang, H.; Su, S.; Chen, X.; Lin, G.; Chen, J. Performance evaluation and optimum design strategies of an acid water electrolyzer system for hydrogen production. Int. J. Hydrogen Energy 2012, 37, 18615–18621. [Google Scholar] [CrossRef]
- Koponen, J.; Kosonen, A.; Ruuskanen, V.; Huoman, K.; Niemelä, M.; Ahola, J. Control and energy efficiency of PEM water electrolyzers in renewable energy systems. Int. J. Hydrogen Energy 2017, 42, 29648–29660. [Google Scholar] [CrossRef]
- Dobó, Z.; Palotás, Á. Impact of the voltage fluctuation of the power supply on the efficiency of alkaline water electrolysis. Int. J. Hydrogen Energy 2016, 41, 11849–11856. [Google Scholar] [CrossRef]
- Bundesministerium für Wirtschaft und Energie (BMWi). Nationales Reformprogramm. Available online: https://www.bmwk.de/Redaktion/DE/Publikationen/Energie/die-nationale-wasserstoffstrategie.pdf?__blob=publicationFile&v=11 (accessed on 1 May 2024).
- International Energy Agency—IEA. Renewable Energy Statistics 2022; The International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2022. [Google Scholar]
- Ozbilen, A.; Dincer, I.; Rosen, M.A. Environmental evaluation of hydrogen production via thermochemical water splitting using the Cu–Cl Cycle: A parametric study. Int. J. Hydrogen Energy 2011, 36, 9514–9528. [Google Scholar] [CrossRef]
- DIN 14040; Environmental Management-Life Cycle Assessment-Principles and Framework (ISO 14040:2006 + Amd 1:2020); German Version EN ISO14040:2006 + A1:2020. DIN: Berlin, Germany, 2006. [CrossRef]
- DIN 14044; Environmental Management-Life Cycle Assessment-Requirements and Guidelines (ISO 14044:2006 + Amd 1:2017 + Amd 2:2020); German Version EN ISO 14044:2006 + A1:2018 + A2:2020. DIN: Berlin, Germany, 2006. [CrossRef]
- Lozanovski, A.; Schuller, O.; Faltenbacher, M. FC-Hy Guide—Guidance Document for Performing LCAs on Fuel Cells and H2 Technologies. Available online: https://hytechcycling.eu/wp-content/uploads/HY-Guidance-Document.pdf (accessed on 1 May 2024).
- Ecoinvent. System Models. 2018. Available online: https://support.ecoinvent.org/system-models#Allocation_classification (accessed on 1 May 2024).
- Zhao, G.; Pedersen, A.S. Life Cycle Assessment of Hydrogen Production and Consumption in an Isolated Territory. Procedia CIRP 2018, 69, 529–533. [Google Scholar] [CrossRef]
- Häfele, S.; Hauck, M.; Dailly, J. Life cycle assessment of the manufacture and operation of solid oxide electrolyser components and stacks. Int. J. Hydrogen Energy 2016, 41, 13786–13796. [Google Scholar] [CrossRef]
- Giraldi, M.R.; François, J.-L.; Cecilia, M. Life cycle assessment of hydrogen production from a high temperature electrolysis process coupled to a high temperature gas nuclear reactor. Int. J. Hydrogen Energy 2015, 40, 4019–4033. [Google Scholar] [CrossRef]
- Bhandari, R.; Trudewind, C.A.; Zapp, P. Life cycle assessment of hydrogen production via electrolysis—A review. J. Clean. Prod. 2014, 85, 151–163. [Google Scholar] [CrossRef]
- LBST. E3database. 2009. Available online: https://www.e3database.com/index.html (accessed on 17 May 2024).
- Gerhardt-Mörsdorf, J.; Peterssen, F.; Burfeind, P.; Benecke, M.; Bensmann, B.; Hanke-Rauschenbach, R.; Minke, C. Life Cycle Assessment of a 5 MW Polymer Exchange Membrane Water Electrolysis Plant. Adv. Energy Sustain. Res. 2024, 5, 2300135. [Google Scholar] [CrossRef]
- Yang, S.; Mao, D.; Yu, Z.; Ma, W.; Ma, L.; Li, X.; Xi, F. Comparison of life cycle assessment between hydrogen production from silicon waste and alkaline water electrolysis. Sci. Total Environ. 2024, 920, 171065. [Google Scholar] [CrossRef] [PubMed]
- IRENA. Renewable Energy Statistics 2022; The International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2022. [Google Scholar]




| TRL | Description |
| 1 | basic principles observed |
| 2 | technology concept formulated |
| 3 | experimental proof of concept |
| 4 | technology validated in the lab |
| 5 | technology validated in relevant environment (industrially relevant environment in the case of key enabling technologies) |
| 6 | technology demonstrated in relevant environment (industrially relevant environment in the case of key enabling technologies) |
| 7 | system prototype demonstration in an operational environment |
| 8 | the system completed and qualified |
| 9 | the actual system proved in an operational environment (competitive manufacturing in the case of key enabling technologies or in space) |
| Technology | Advantages | Disadvantages | Critical Raw Material | Technology Maturity | Operating Temperature | System Lifetime (h) | Source |
|---|---|---|---|---|---|---|---|
| AWE | Non-noble catalyst layer (CL) Low-cost and non-PGM CL Energy efficiency 70–80%. Stable over long periods. Low system costs (around 800–1000 EUR/kW installed capacity). | Formation of carbonate on the electrode. Low purity and crossover of gases. Low operational pressure. Low dynamic operation. Corrosive liquid electrolyte. | Nickel Chromium Zinc | Commercially mature TRL 9 | 70–90 °C | 60,000–90,000 | [9,15,39,40,41] |
| PEMWE | High current densities. Compact system design. Fast responses. High purity of gases. Energy efficiency 80%. High dynamic operation. | Noble and expensive metal CL. Acidic corrosive. Possible low durability. Currently, high system costs of around 1000–1500 EUR/kW installed capacity. | Titanium Platinum Iridium Chromium | Commercialization at small scale TRL 6–8 | 50–80 °C | 20,000–60,000 | [15,30,34,39,40,41,42] |
| AEMWE | Low-cost transition metal catalysts. Non-corrosive electrolyte. High operating pressure. Compact cell design. Absence of leaking. | Low current densities. Membrane degradation. Excessive catalyst loading. | Critical raw material-free | Laboratory stage TRL 2–3 | 40–60 °C | - | [9,16,29,31,39,41,42,43] |
| SOEC | High working Pressure. Non-noble CL. Energy efficiency 90–100%. | Large system design. Low durability. High system costs around 1800–2300 €/kW installed capacity. | Yttrium Zirconium Gallium | Commercialization in the near term TRL 5 | 700–850 °C | 10,000 | [9,15,16,31,39,40,41] |
| Supply Risk | Strategic Raw Material | Supply Risk | Critical Raw Material |
|---|---|---|---|
| 4.1 | Magnesium | 5.3 | HREE (rest) |
| 4.0 | REE(Magnet) | 4.4 | Niobium |
| 3.8 | Boron | 3.5 | LREE (rest) |
| 2.7 | PGM | 2.6 | Strontium |
| 1.8 | Natural graphite | 2.4 | Scandium |
| 1.7 | Cobalt | 2.3 | Vanadium |
| 1.4 | Silicon metal | 1.3 | Baryte |
| 1.2 | Tungsten | 1.3 | Tantalum |
| 1.2 | Manganese | 1.2 | Aluminium |
| 0.5 | Nickel | ||
| 0.1 | Copper | ||
| Study | Technology | Power Supply | Highest Factor on Environmental Impact Category or Status of It | The LCIA Method |
|---|---|---|---|---|
| Wilkinson, et al. (2023) [39] | literature data | literature data | Global warming potential (GWP) | Literature data |
| Sundin (2019) [21] | PEMWE, AWE | Grid mix | Electrolyzer lifetime and current density | CML 2001 |
| Zhao and Schrøder Pedersen, (2018) [56] | PEMWE | Wind turbines | Global warming potential | ILCD 2011 Midpoint |
| Häfele, et al. (2016) [57] | SOEC | 94% nuclear primary energy | Highest from the electrolysis itself | Not mentioned |
| Giraldi, et al. (2015) [58] | SOEC | Nuclear power supply | The electrolysis cell and hydrogen production processes. | ReCiPe midpoint |
| Bhandari, et al. (2014) [59] | PEMWE, AWE, SOEC | × | Global warming potential (GWP) and Acidification potential (AP) | literature data |
| Our Study | literature data | literature data | GWP, AP, and Eutrophication potential | Literature data |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaya, N.; Glöser-Chahoud, S. A Review of Life Cycle Assessment (LCA) Studies for Hydrogen Production Technologies through Water Electrolysis: Recent Advances. Energies 2024, 17, 3968. https://doi.org/10.3390/en17163968
Shaya N, Glöser-Chahoud S. A Review of Life Cycle Assessment (LCA) Studies for Hydrogen Production Technologies through Water Electrolysis: Recent Advances. Energies. 2024; 17(16):3968. https://doi.org/10.3390/en17163968
Chicago/Turabian StyleShaya, Negar, and Simon Glöser-Chahoud. 2024. "A Review of Life Cycle Assessment (LCA) Studies for Hydrogen Production Technologies through Water Electrolysis: Recent Advances" Energies 17, no. 16: 3968. https://doi.org/10.3390/en17163968
APA StyleShaya, N., & Glöser-Chahoud, S. (2024). A Review of Life Cycle Assessment (LCA) Studies for Hydrogen Production Technologies through Water Electrolysis: Recent Advances. Energies, 17(16), 3968. https://doi.org/10.3390/en17163968








