A Review of the Synthesis of Biopolymer Hydrogel Electrolytes for Improved Electrode–Electrolyte Interfaces in Zinc-Ion Batteries
Abstract
:1. Introduction
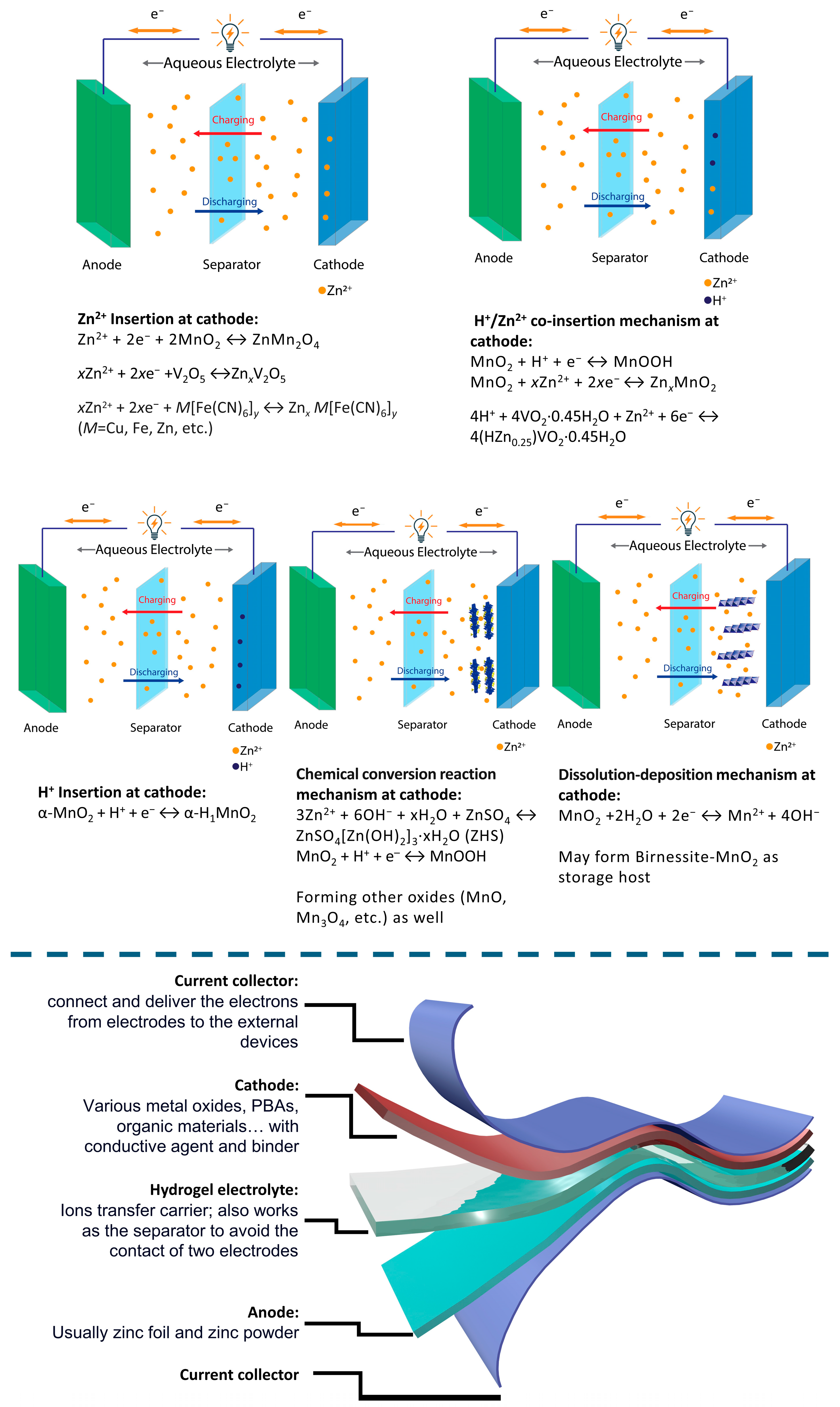
2. Energy Storage Mechanisms of Zinc-Ion Batteries
3. Electrode–Electrolyte Interfaces in Zn-Ion Batteries
4. Biopolymer Hydrogel Electrolytes for Zn-Ion Batteries
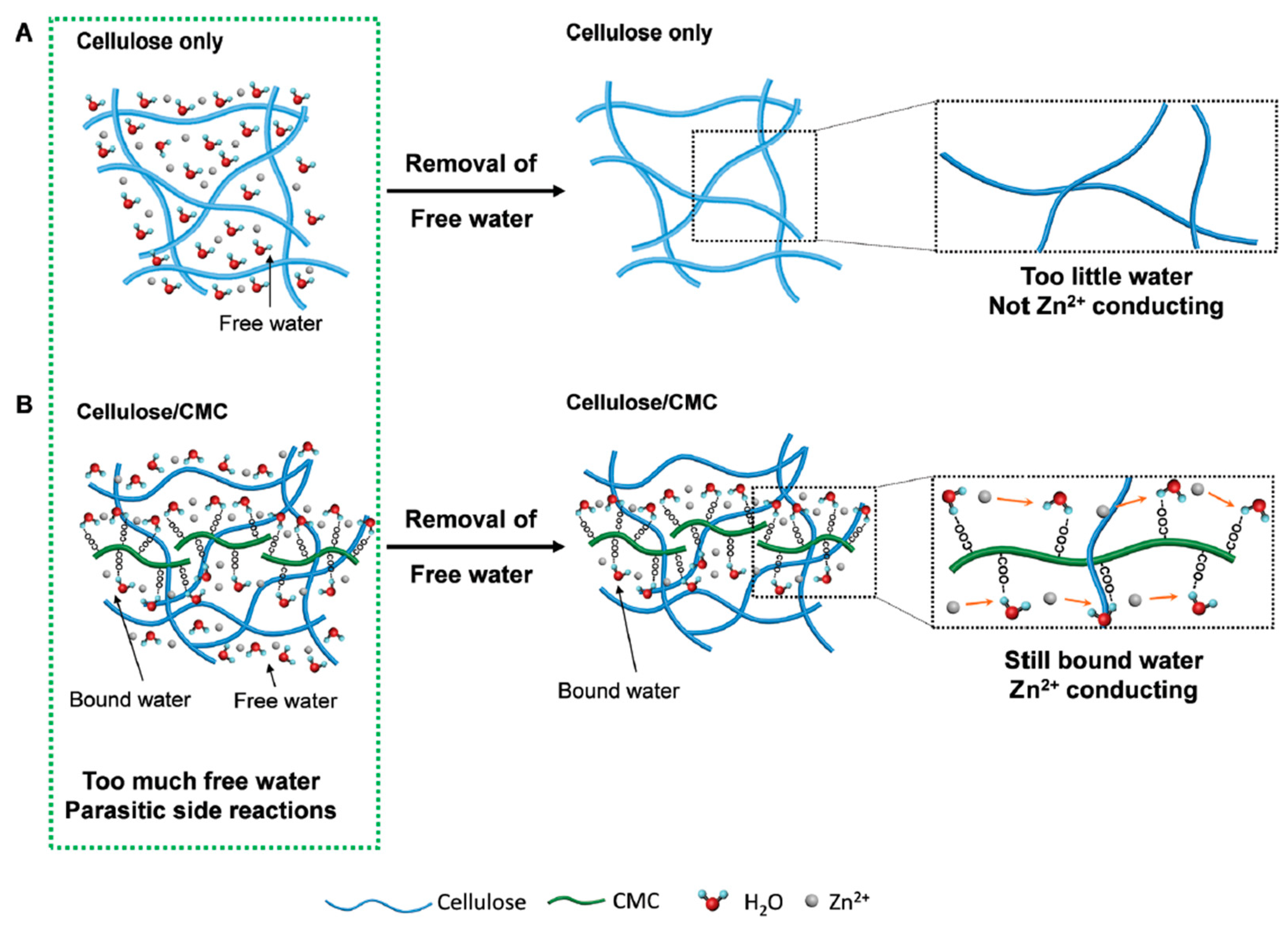
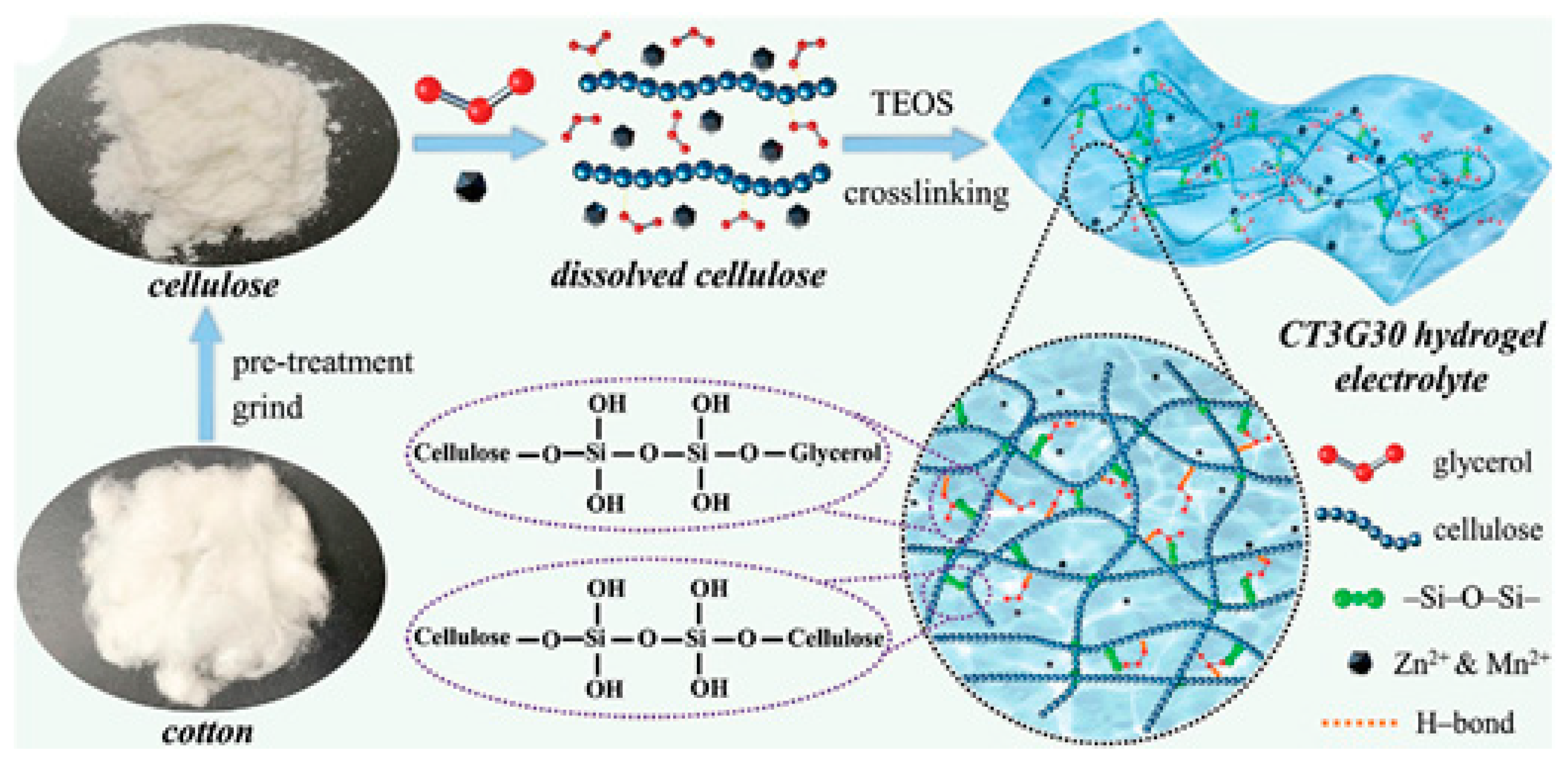
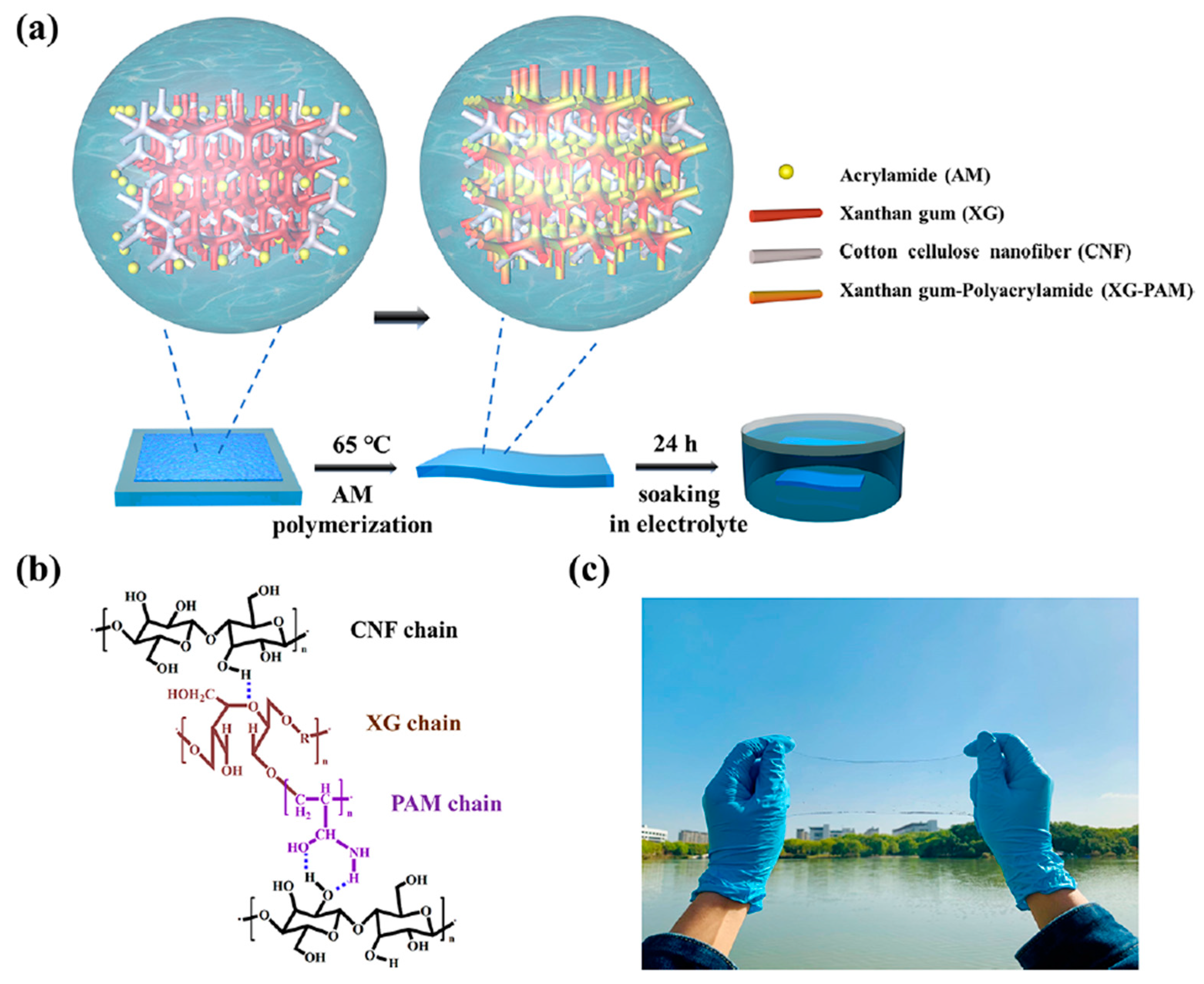
4.1. Cellulose and Its Derivatives
4.1.1. Enhanced Mechanical and Electrochemical Properties
4.1.2. Improved Cycling Stability
4.1.3. Anti-Freezing Properties
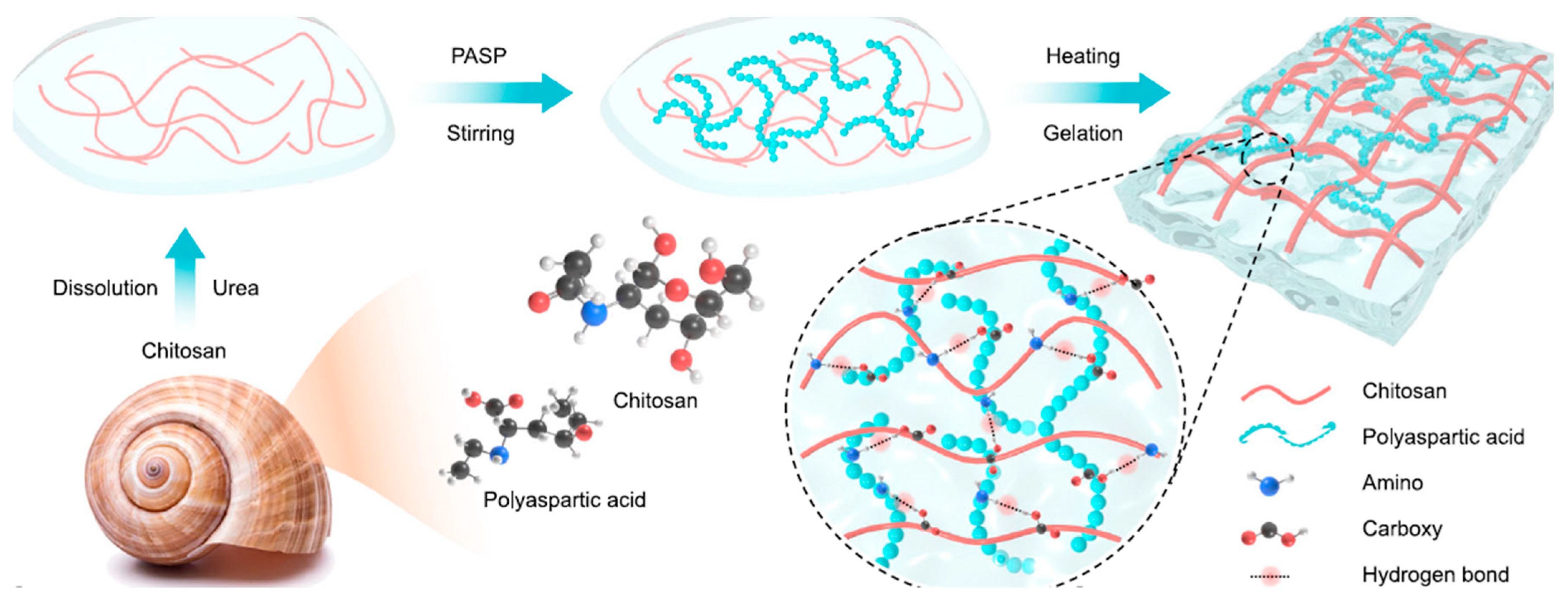
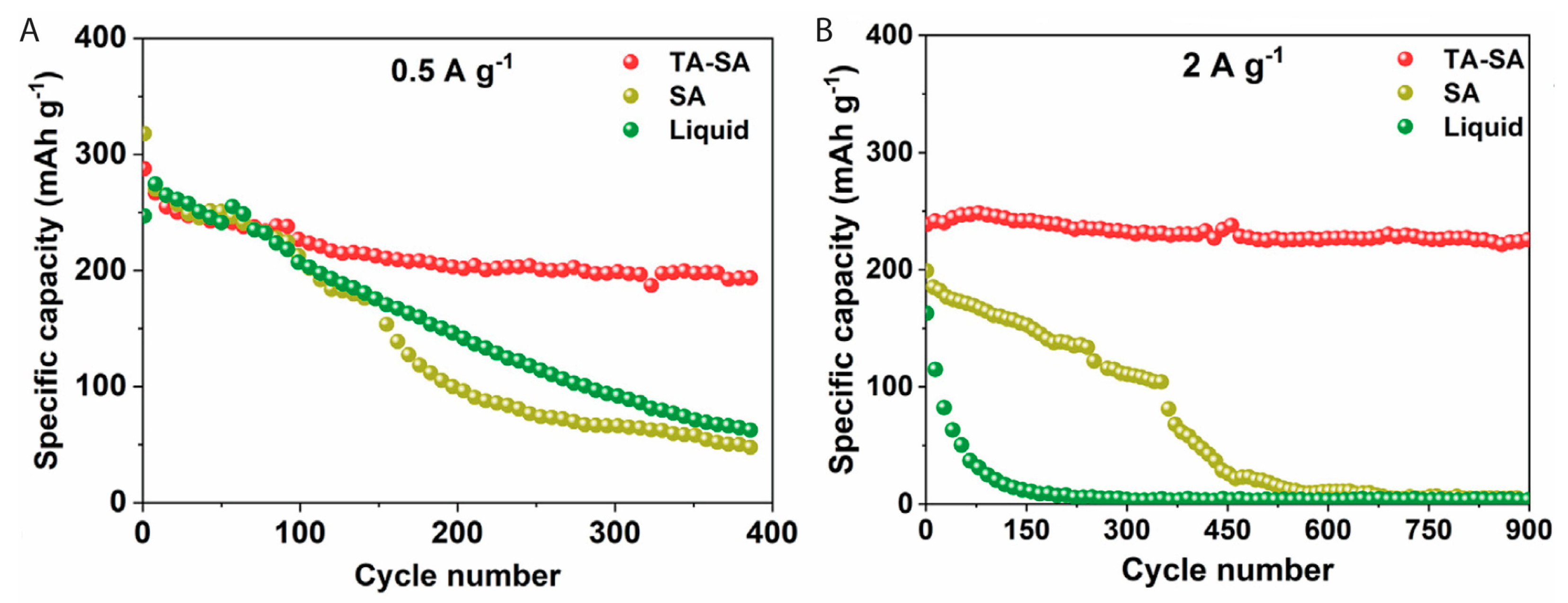
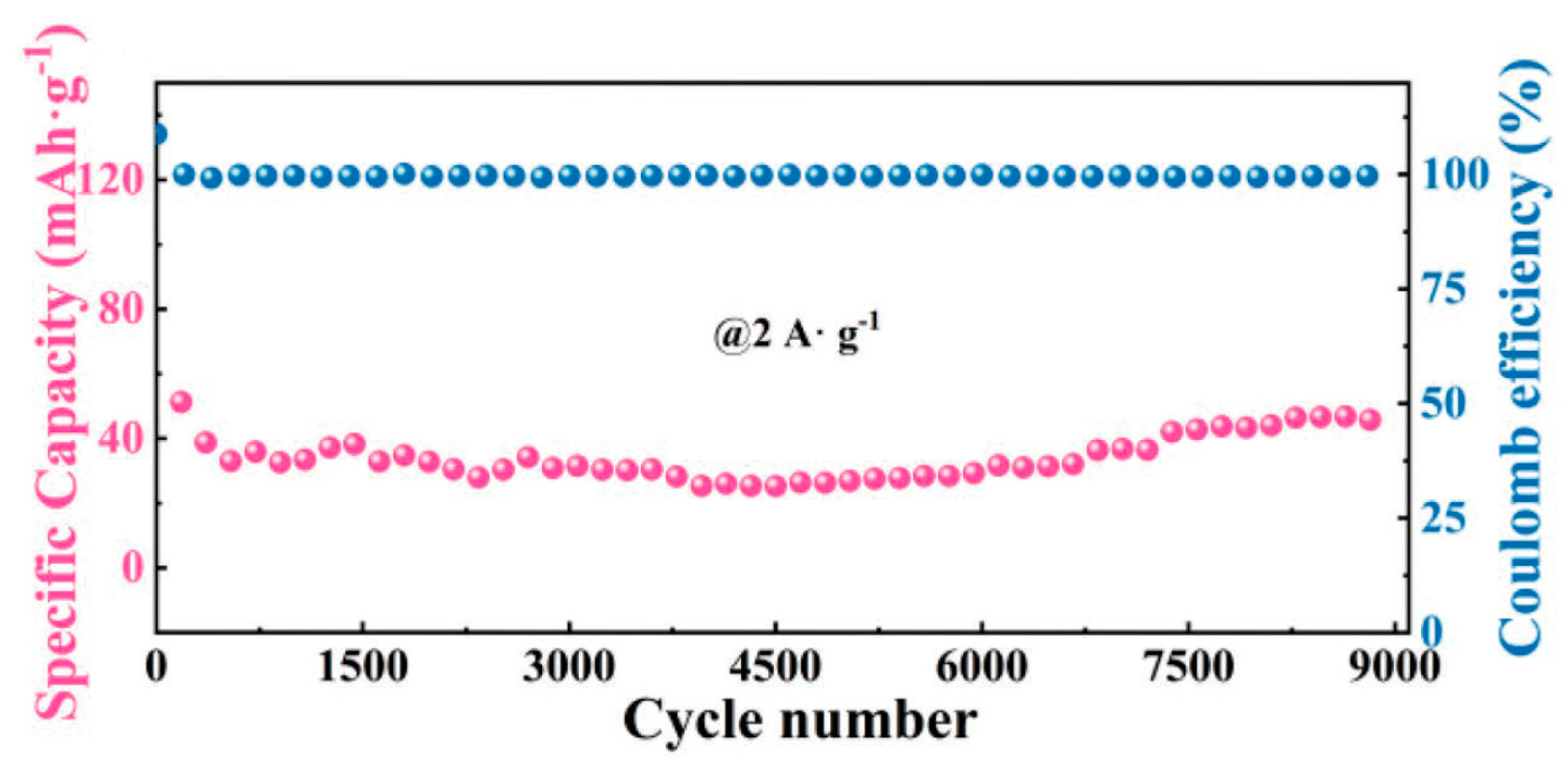
4.2. Chitin and Chitosan
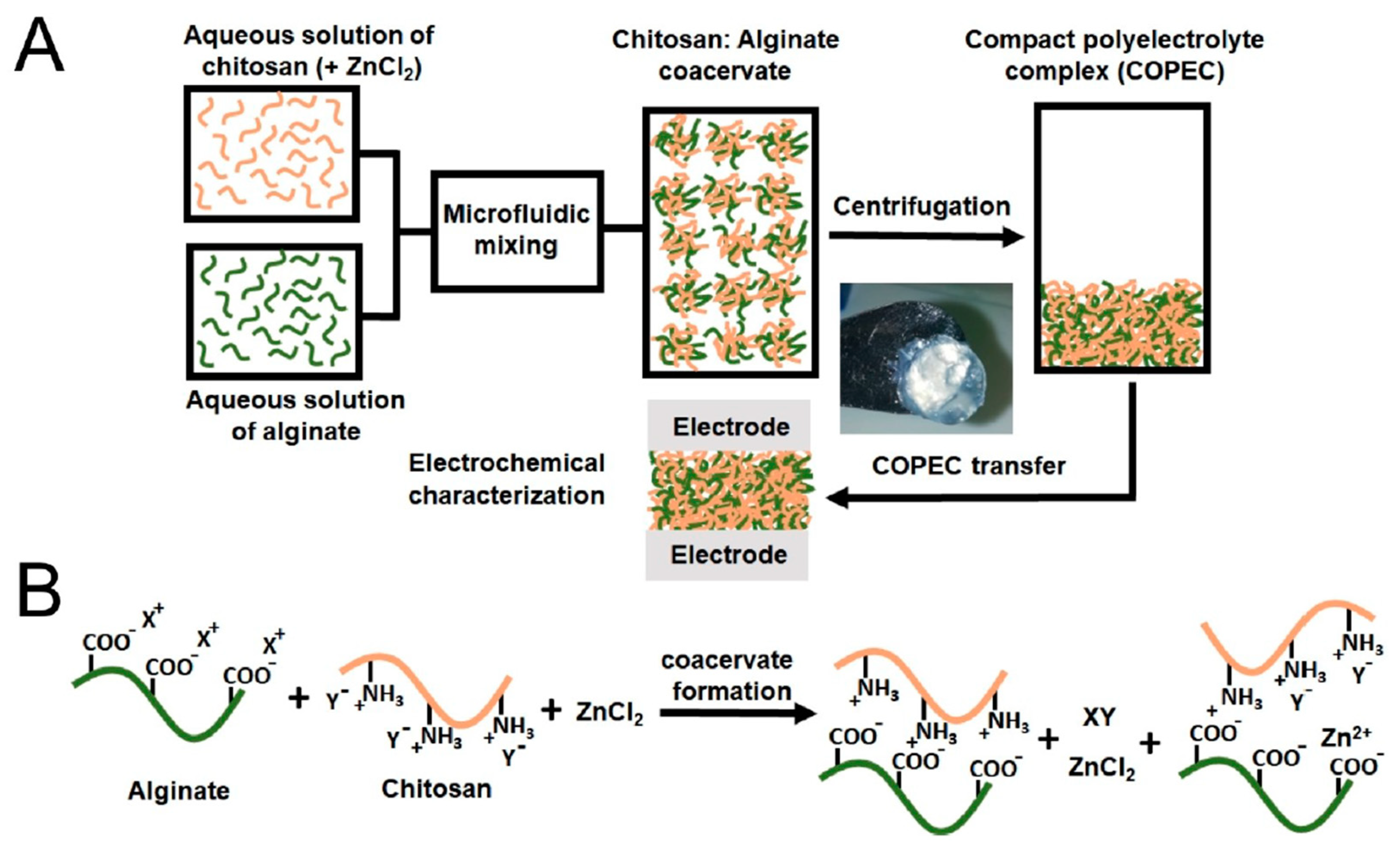
4.3. Alginate
4.4. Gelatin
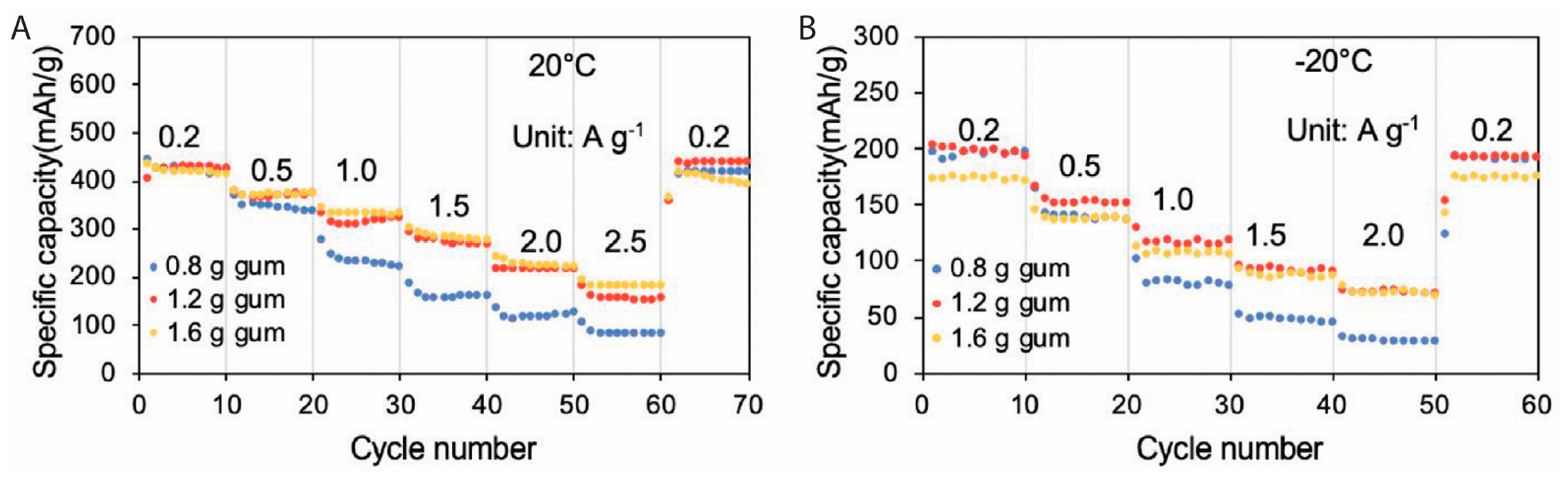
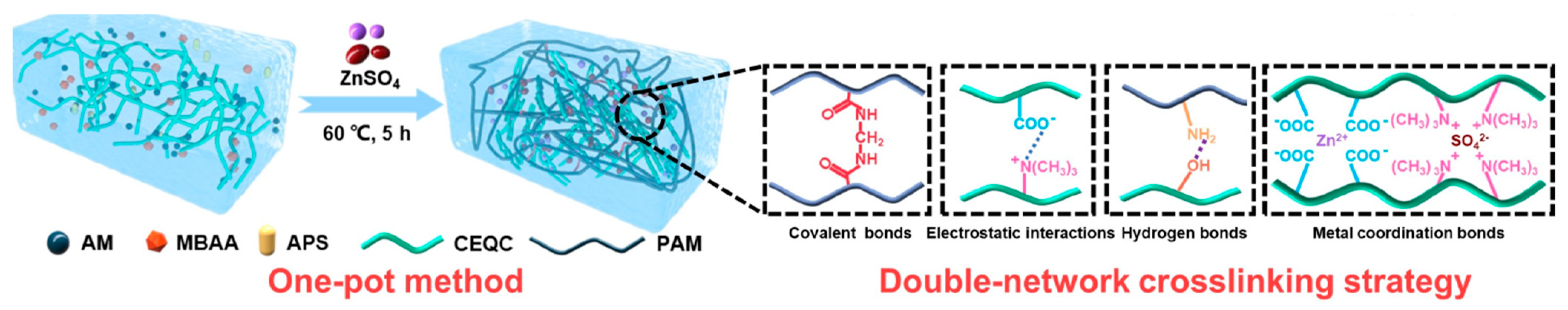
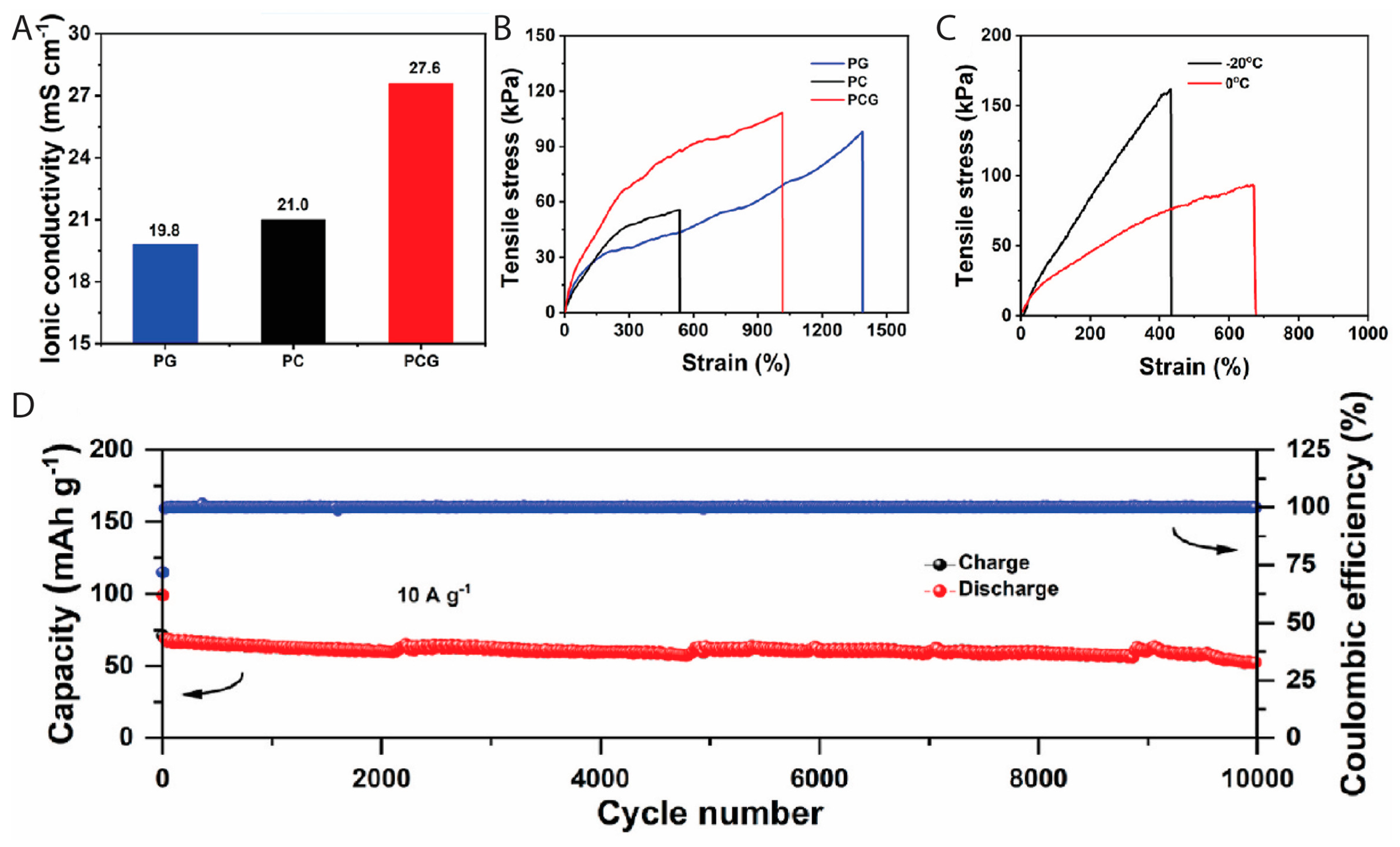
4.5. Xanthan Gum, Guar Gum, and Gum Arabic
| Hydrogels [Ref.] | Electrolyte Salts | Mechanical Strength | Ionic Conductivity | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Cellulose-based hydrogel [97] | 3 M ZnSO4 | 39.5 MPa | 0.643 mS cm−1 | Low cost; Low thickness | Relatively low cycling stability; Low conductivity |
| Bacterial cellulose hydrogel [98] | 2.0 M ZnSO4 and 0.2 M MnSO4 | ~1.75 MPa | 27.8 mS cm−1 | Low cost; High stability; Easy fabrication | Relatively low mechanical properties |
| CMC hydrogel [53] | 7 M KAc and 1 M ZnAc2 | 1.33 MPa | 34.5 mS cm−1 | Low cost; High cycling stability | Complex fabrication; Relatively low mechanical properties |
| Nanocellulose–CMC hydrogel [100] | 2 M ZnSO4 | 70 MPa | 26 mS cm−1 | High cycling stability; Good rate performance; High tensile strength | Relatively complex fabrication |
| CMC with tetraethyl orthosilicate and glycerol [103] | 4.5 M ZnSO4 | 2.11 MPa | 19.4 mS cm−1 @ −40 °C | Wide temperature stable window; Good stability | Relatively complex fabrication |
| Sorbitol-modified cellulose hydrogel electrolyte [52] | 16 M ZnCl2 | 0.62 MPa | 35.4 mS cm−1 | Wide temperature stable window; High conductivity | Low mechanical strength; Relatively high cost |
| Chitosan-Zn membrane electrolyte [105] | 2 M ZnSO4 | 7.4 MPa | 71.8 mS cm−1 | High conductivity; Non-flammability | Relatively complex fabrication |
| Natural chitosan-glass fiber hydrogel [106] | 2 M ZnSO4 | 2.40 MPa | 83.4 mS cm−1 | Low cost; High conductivity | Glass fiber may limit the wearable application |
| Chitosan on cotton pad [107] | 2 M ZnSO4 | ~5 MPa | - | Low cost; Dendrite control | Relatively low stability |
| Kappa (k)-carrageenan–chitosan hydrogel [109] | 2 M ZnSO4 | 14.2 MPa | 5.3 mS cm−1 | High cycling stability; High mechanical strength | Low conductivity |
| Zinc alginate gel [112] | 2 M ZnSO4 and 0.2 M MnSO4 | - | 18.3 mS cm−1 | Relatively high cycling stability | Relatively low temperature compatibility |
| Zinc alginate hydrogel [114] | 2 M ZnSO4 | 4.63 MPa | 0.54 mS cm−1 | Self-healing; Easy fabrication | Low conductivity |
| Guar gum–alginate [116] | 2 M ZnSO4 and 0.1 M MnSO4 | - | 6.19 mS cm−1@ −20 °C | Wide temperature window | Unclear mechanical strength |
| Gelatin-based hydrogel electrolyte [60] | 0.5 M Li2SO4 and 0.5 M ZnSO4 | ~100 kPa | 37.2 mS cm−1 | High conductivity; Easy fabrication | Low mechanical strength |
| Gelatin hydrogel [99] | 2 M ZnSO4 | 1.5 MPa | 23.5 mS cm−1 | Low material cost; High conductivity | Low mechanical strength; Relatively complex fabrication |
| Xanthan gum hydrogel [124] | 2 M ZnSO4 and 0.1 M MnSO4 | - | 14.6 mS cm−1 | High conductivity; Easy fabrication | Low mechanical strength; Relatively low cycling stability |
| Xanthan gum hydrogel [125] | 4 M ZnCl2 | ~0.1 MPa | 2.54 mS cm−1 @ −20 °C | Wide temperature stable window | Relatively low conductivity at room temperature; Low mechanical strength |
| Guar gum biopolymer electrolyte [126] | 2 M ZnSO4 and 0.1 M MnSO4 | 0.65 MPa | 10.7 mS cm−1 | Easy fabrication; Relatively high conductivity | Low mechanical strength |
5. Hybrid Biopolymer–Synthetic Polymer Hydrogel Electrolytes for Zn-Ion Batteries
5.1. Cellulose and Its Derivatives with Synthetic Polymer
5.2. Chitosan, Chitin, or Derivatives with Synthetic Polymer
5.3. Alginate with Synthetic Polymer
5.4. Gelatin with Synthetic Polymer
5.5. Xanthan Gum with Synthetic Polymer
5.6. Agar with Synthetic Polymer
5.7. Soybean Protein with Synthetic Polymer
| Hydrogels [Ref.] | Electrolyte Salts | Mechanical Strength | Ionic Conductivity | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Cellulose nanofiber–PAM hydrogel electrolyte [128] | 1 M Zn(CF3SO3)2 | 192 kPa | 6.8 mS cm−1 | High cycling stability; Wide temperature stable window | Low mechanical strength; Complex fabrication |
| Lignin-containing cellulose nanofiber-PAM hydrogel [129] | 1 M Zn(OTF)2 | 350 kPa | 21.57 mS cm−1 | High cycling stability; Dendrite growth control | Low mechanical strength; Relatively expensive salts |
| CMC-PAM hydrogel electrolyte [130] | 2 M ZnSO4 and 0.1 M MnSO4 | ~35 kPa | 13 mS cm−1 | High stretchability; Relatively low cost | Low mechanical strength |
| PAM/CMC/gelatin hydrogel electrolyte [133] | 2 M ZnSO4 and 0.1 M MnSO4 | 108.3 kPa | 27.0 mS cm−1 | High conductivity; Wide temperature stable window | Low mechanical strength; Complex fabrication |
| PAM-poly(ethylene glycol) diacrylate-CMC hydrogel [134] | 2 M ZnSO4 | 2.25 MPa | 30.24 mS cm−1 | High conductivity; High mechanical strength | Relatively low cycling stability |
| Cellulose nanofiber-PAM hydrogel [135] | 40 wt% Zn(CF3SO3)2 | ~ 250 kPa | - | Wide temperature stable window; High cycling stability | Relatively low mechanical strength |
| PAM-cotton cellulose nanofiber-CMC hydrogel [137] | 1 M ZnSO4 | 60 kPa | 2.492 S m−1 | Very high conductivity; High stretchability | Low mechanical strength |
| PAM-chitin nanofiber hydrogel [139] | 0.01 M Zn(CF3SO3)2 | 114.62 kPa | 15.2 mS cm−1 | High cycling stability | Low mechanical strength; Relatively expensive electrolyte salt |
| Carboxymethyl chitosan-PAM hydrogel [140] | 2 M ZnSO4 and 0.1 M MnSO4 | 67 kPa | 5.6 mS cm−1 | Low conductivity and mechanical strength | Reliable anti-freezing (–48 °C) feature |
| Alginate-PAM hydrogel [141] | 1 M ZnSO4 and 0.2 M MnSO4 | ~500 kPa | 29.2 mS cm−1 | High conductivity | Relatively complex fabrication |
| PAM-gelatin-DMAPS hydrogel [142] | 2 M ZnSO4 | ~35 kPa | 35.1 mS cm−1 | High conductivity | Low mechanical strength |
| Xanthan gum-PAM-cotton cellulose nanofibers [143] | 2 M ZnSO4 and 0.1 M MnSO4 | 84 kPa | 28.8 mS cm−1 | Water proofing; Stability under deformation | Low mechanical strength |
| Agar-PAM hydrogel [146] | 3 M ZnSO4 | 92.34 MPa | - | High mechanical strength; Dendrite growth control; Anti-freezing feature | Relatively low conductivity |
| Soybean protein-PAM hydrogel [147] | 1 M ZnSO4 and 0.1 M MnSO4 | 31.3 KPa | 50.8 mS cm−1 | Relatively high conductivity; High stability under deformation | Low mechanical strength |
6. Future Perspectives
- First of all, the design of ionic conductivity and mechanical properties of the hydrogel electrolyte is still in need of further optimization. The relatively larger thickness of a hydrogel electrolyte, compared to a liquid electrolyte, as well as the lower water content, inevitably reduce conductivity. In this context, additional research into double-network and triple-network hydrogels could be conducted. Such interpenetrating network structures can enhance mechanical strength, durability, and robustness. Moreover, ionic conductivity can be increased by developing polymer networks with a higher number of hydrophilic groups on their chains, improving water retention. Interpenetrating network structures can be further explored for hydrogel electrolytes in ZIBs using multiple biopolymers rather than hybrid biopolymers–synthetic polymers to make the hydrogels greener and more cost-effective, such as further study around relatively rigid CMC and other highly conductive polysaccharides.
- Moreover, further design efforts could focus more on the mechanical properties and electrochemical performance on the entire ZIB cell. Ensuring the firm integration of electrodes with the electrolyte and maintaining stability during large, long-term deformation are essential for the lifespan of ZIBs. Interfaces should also be enhanced to achieve a more stable environment for various interfacial reactions. Simple modifications on the electrode side (such as the addition of non-covalent interactions), and even the integration of electrodes with hydrogel electrolytes, might expand the electrochemical stable window to a higher level or offer better interfacial contact during deformation. Fundamental research focused on understanding interfacial mechanisms, combining DFT calculations, computer simulations, and experimental research involving in operando characterization can provide better insights and help improve the future design and development of hydrogel electrolytes in ZIBs.
- Apart from stability during deformation, durability under harsh environments undeniably impacts the performance of ZIBs. Certain conditions, such as temperature variations, bending, and other deformations, may lead to the aging of hydrogels, including shrinkage, reduction in adhesion, and decreased wettability, all resulting in increased interface resistance and deteriorating efficiency at the electrode–electrolyte interface [91]. Thus, water retention capability still needs further investigation to improve conductivity stability throughout the lifespan, potentially expanding application scenes to low-temperature and large-scale energy storage, offshore energy storage, and smart wearable electronics.
- In addition, better strategies need to be developed for widening the electrochemical stable window for hydrogel ZIBs, as the current additives used may influence the ionic conductivity of the hydrogel or affect aqueous interactions at the interface. The subtle utilization of different polar groups, controlling free water molecules, and using charged or zwitterionic groups to regulate ionic movement may be effective choices for controlling side reactions. However, the use of functional groups or specific polymers may also result in a change in electrochemical performance, and a detailed investigation of compatibility between different components should be performed.
- The development of biopolymer electrolytes also needs more attention regarding the possibility of commercialization. Although the high natural abundance of most biopolymers theoretically improves accessibility, some hydrogels may not be competitive enough compared to mature aqueous systems, limited by their relatively small markets and high prices. Moreover, the synthesis of hybrid electrolytes could introduce complicated processes or high-cost materials, leading to an increase in expenses. More efforts can be dedicated to making modifications based on low-price raw materials, such as CMC, alginate, starch, etc., among biobased hydrogels, as well as synthetic materials with stable and economical supplies, such as PAM and PVA. It is gratifying that increasing applications and patents focusing on ZIBs with hydrogel electrolytes have been launched in recent years, with many exploring the future of biopolymer electrolytes (e.g., CN113644227A; EP4078716A1; US20180166662A1; WO2022197984A1).
- The next generation of battery assembly will pave the way for extensive applications of ZIBs with biohydrogels. Compared to conventional cells, 3D-printed ZIBs have already demonstrated multiple advantages, such as miniaturization, flexibility, customization, and high safety. Very recently, polyacrylamide–hemicellulose/EGaIn microdroplet hydrogel has been successfully applied as hydrogel ink for 3D-printed ZIBs [150]. Similarly, PAM-based cross-linked hydrogel electrolytes have also demonstrated their potential in 3D-printing ZIBs [151]. Utilizing the features of biopolymer hydrogels for further improvement in areal capacity and stability of electrodes, as well as the related development for wearable or even implantable practical applications, will propel the potential usage of ZIBs.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, M.J.; Hwang, Y.C. Exploring the Factors Affecting the Continued Usage Intention of IoT-Based Healthcare Wearable Devices Using the TAM Model. Sustainability 2022, 14, 12492. [Google Scholar] [CrossRef]
- Ding, Y.L.; Cano, Z.P.; Yu, A.P.; Lu, J.; Chen, Z.W. Automotive Li-Ion Batteries: Current Status and Future Perspectives. Electrochem. Energy Rev. 2019, 2, 1–28. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Ge, M.Y.; Rong, J.P.; Zhou, C.W. Free-Standing LiNi0.5Mn1.5O4/Carbon Nanofiber Network Film as Lightweight and High-Power Cathode for Lithium Ion Batteries. ACS Nano 2014, 8, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.F.; Zheng, J.M.; Liu, J.; Wang, B.Q.; Cheng, X.P.; Zhang, Y.F.; Sun, X.L.; Wang, C.M.; Zhang, J.G. Tailoring grain boundary structures and chemistry of Ni-rich layered cathodes for enhanced cycle stability of lithium-ion batteries. Nat. Energy 2018, 3, 600–605. [Google Scholar] [CrossRef]
- Bresser, D.; Hosoi, K.; Howell, D.; Li, H.; Zeisel, H.; Amine, K.; Passerini, S. Perspectives of automotive battery R&D in China, Germany, Japan, and the USA. J. Power Sources 2018, 382, 176–178. [Google Scholar] [CrossRef]
- Fang, G.Z.; Zhou, J.; Pan, A.Q.; Liang, S.Q. Recent Advances in Aqueous Zinc-Ion Batteries. ACS Energy Lett. 2018, 3, 2480–2501. [Google Scholar] [CrossRef]
- Golmohammadzadeh, R.; Faraji, F.; Jong, B.; Pozo-Gonzalo, C.; Banerjee, P.C. Current challenges and future opportunities toward recycling of spent lithium-ion batteries. Renew. Sustain. Energy Rev. 2022, 159, 112202. [Google Scholar] [CrossRef]
- Baird, A.R.; Archibald, E.J.; Marr, K.C.; Ezekoye, O.A. Explosion hazards from lithium-ion battery vent gas. J. Power Sources 2020, 446, 227257. [Google Scholar] [CrossRef]
- Xie, X.S.; Li, J.J.; Xing, Z.Y.; Lu, B.A.; Liang, S.Q.; Zhou, J. Biocompatible zinc battery with programmable electro-cross-linked electrolyte. Natl. Sci. Rev. 2023, 10, nwac281. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.D. Materials Perspectives for Self-Powered Cardiac Implantable Electronic Devices toward Clinical Translation. Acc. Mater. Res. 2021, 2, 739–750. [Google Scholar] [CrossRef]
- Li, J.J.; Ruan, P.C.; Chen, X.H.; Lei, S.R.; Lu, B.A.; Chen, Z.Z.; Zhou, J. Aqueous Batteries for Human Body Electronic Devices Focus Review. ACS Energy Lett. 2023, 8, 2904–2918. [Google Scholar] [CrossRef]
- Duan, J.J.; Xie, W.K.; Yang, P.H.; Li, J.; Xue, G.B.; Chen, Q.; Yu, B.Y.; Liu, R.; Zhou, J. Tough hydrogel diodes with tunable interfacial adhesion for safe and durable wearable batteries. Nano Energy 2018, 48, 569–574. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [PubMed]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-Ion Batteries. Adv. Funct. Mater. 2013, 23, 947–958. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef]
- Pramudita, J.C.; Sehrawat, D.; Goonetilleke, D.; Sharma, N. An Initial Review of the Status of Electrode Materials for Potassium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1602911. [Google Scholar] [CrossRef]
- Zhang, W.C.; Liu, Y.J.; Guo, Z.P. Approaching high-performance potassium-ion batteries via advanced design strategies and engineering. Sci. Adv. 2019, 5, eaav7412. [Google Scholar] [CrossRef]
- Rajagopalan, R.; Tang, Y.G.; Ji, X.B.; Jia, C.K.; Wang, H.Y. Advancements and Challenges in Potassium Ion Batteries: A Comprehensive Review. Adv. Funct. Mater. 2020, 30, 1909486. [Google Scholar] [CrossRef]
- Huie, M.M.; Bock, D.C.; Takeuchi, E.S.; Marschilok, A.C.; Takeuchi, K.J. Cathode materials for magnesium and magnesium-ion based batteries. Coord. Chem. Rev. 2015, 287, 15–27. [Google Scholar] [CrossRef]
- Wang, F.; Fan, X.L.; Gao, T.; Sun, W.; Ma, Z.H.; Yang, C.Y.; Han, F.; Xu, K.; Wang, C.S. High-Voltage Aqueous Magnesium Ion Batteries. ACS Cent. Sci. 2017, 3, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Gummow, R.J.; Vamvounis, G.; Kannan, M.B.; He, Y.H. Calcium-Ion Batteries: Current State-of-the-Art and Future Perspectives. Adv. Mater. 2018, 30, 1801702. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Xu, Z.L.; Yoon, G.; Park, S.K.; Wang, J.; Hyun, H.; Park, H.; Lim, J.; Ko, Y.J.; Yun, Y.S.; et al. Stable and High-Power Calcium-Ion Batteries Enabled by Calcium Intercalation into Graphite. Adv. Mater. 2020, 32, 1904411. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, C.L.; Zhang, S.Q.; Song, X.H.; Tang, Y.B.; Cheng, H.M. Reversible calcium alloying enables a practical room-temperature rechargeable calcium-ion battery with a high discharge voltage. Nat. Chem. 2018, 10, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chen, X.Y.; Yu, M.; Niu, Z.Q.; Cheng, F.Y.; Chen, J. Materials chemistry for rechargeable zinc-ion batteries. Chem. Soc. Rev. 2020, 49, 4203–4219. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.Y.; Shan, L.T.; Liang, S.Q.; Zhou, J. Issues and opportunities facing aqueous zinc-ion batteries. Energy Environ. Sci. 2019, 12, 3288–3304. [Google Scholar] [CrossRef]
- Konarov, A.; Voronina, N.; Jo, J.H.; Bakenov, Z.; Sun, Y.K.; Myung, S.T. Present and Future Perspective on Electrode Materials for Rechargeable Zinc-Ion Batteries. ACS Energy Lett. 2018, 3, 2620–2640. [Google Scholar] [CrossRef]
- Das, S.K.; Mahapatra, S.; Lahan, H. Aluminium-ion batteries: Developments and challenges. J. Mater. Chem. A 2017, 5, 6347–6367. [Google Scholar] [CrossRef]
- Lin, M.C.; Gong, M.; Lu, B.G.; Wu, Y.P.; Wang, D.Y.; Guan, M.Y.; Angell, M.; Chen, C.X.; Yang, J.; Hwang, B.J.; et al. An ultrafast rechargeable aluminium-ion battery. Nature 2015, 520, 324–328. [Google Scholar] [CrossRef]
- Wang, D.Y.; Wei, C.Y.; Lin, M.C.; Pan, C.J.; Chou, H.L.; Chen, H.A.; Gong, M.; Wu, Y.P.; Yuan, C.Z.; Angell, M.; et al. Advanced rechargeable aluminium ion battery with a high-quality natural graphite cathode. Nat. Commun. 2017, 8, 14283. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.Q.; Ji, Y.J.; Ma, J.M.; Yu, H.J. Emerging Nonaqueous Aluminum-Ion Batteries: Challenges, Status, and Perspectives. Adv. Mater. 2018, 30, 1706310. [Google Scholar] [CrossRef] [PubMed]
- Kundu, D.; Vajargah, S.H.; Wan, L.W.; Adams, B.; Prendergast, D.; Nazar, L.F. Aqueous vs. nonaqueous Zn-ion batteries: Consequences of the desolvation penalty at the interface. Energy Environ. Sci. 2018, 11, 881–892. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Kim, J.M.; Chun, Y.T.; Zhang, J.; Jun, S.C. Recent Advances in Vanadium-Based Aqueous Rechargeable Zinc-Ion Batteries. Adv. Energy Mater. 2020, 10, 2000477. [Google Scholar] [CrossRef]
- Jia, X.X.; Liu, C.F.; Neale, Z.G.; Yang, J.H.; Cao, G.Z. Active Materials for Aqueous Zinc Ion Batteries: Synthesis, Crystal Structure, Morphology, and Electrochemistry. Chem. Rev. 2020, 120, 7795–7866. [Google Scholar] [CrossRef]
- Wan, F.; Niu, Z.Q. Design Strategies for Vanadium-based Aqueous Zinc-Ion Batteries. Angew. Chem.-Int. Ed. 2019, 58, 16358–16367. [Google Scholar] [CrossRef]
- Zhu, X.D.; Cao, Z.Y.; Wang, W.J.; Li, H.J.; Dong, J.C.; Gao, S.P.; Xu, D.X.; Li, L.; Shen, J.F.; Ye, M.X. Superior-Performance Aqueous Zinc-Ion Batteries Based on the In Situ Growth of MnO2 Nanosheets on V2CTX MXene. ACS Nano 2021, 15, 2971–2983. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, Q.; Liu, Z.X.; Wang, D.H.; Guo, Y.; Li, X.L.; Tang, Y.C.; Li, H.F.; Dong, B.B.; Zhi, C.Y. Dendrites in Zn-Based Batteries. Adv. Mater. 2020, 32, 2001854. [Google Scholar] [CrossRef]
- Zhang, Q.; Luan, J.Y.; Tang, Y.G.; Ji, X.B.; Wang, H.Y. Interfacial Design of Dendrite-Free Zinc Anodes for Aqueous Zinc-Ion Batteries. Angew. Chem.-Int. Ed. 2020, 59, 13180–13191. [Google Scholar] [CrossRef]
- Cao, Z.Y.; Zhuang, P.Y.; Zhang, X.; Ye, M.X.; Shen, J.F.; Ajayan, P.M. Strategies for Dendrite-Free Anode in Aqueous Rechargeable Zinc Ion Batteries. Adv. Energy Mater. 2020, 10, 2001599. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, D.D.; Zhang, X.Y.; Zeng, Z.Y.; Qin, J.Q.; Huang, Y.H. Strategies of regulating Zn2+ solvation structures for dendrite-free and side reaction-suppressed zinc-ion batteries. Energy Environ. Sci. 2022, 15, 499–528. [Google Scholar] [CrossRef]
- Wu, B.K.; Zhang, G.B.; Yan, M.Y.; Xiong, T.F.; He, P.; He, L.; Xu, X.; Mai, L.Q. Graphene Scroll-Coated α-MnO2 Nanowires as High-Performance Cathode Materials for Aqueous Zn-Ion Battery. Small 2018, 14, 1703850. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.L.; Mao, J.F.; Pang, W.K.; Vongsvivut, J.; Zeng, X.H.; Thomsen, L.; Wang, Y.Y.; Liu, J.W.; Li, D.; Guo, Z.P. Tuning the Electrolyte Solvation Structure to Suppress Cathode Dissolution, Water Reactivity, and Zn Dendrite Growth in Zinc-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2104281. [Google Scholar] [CrossRef]
- Zhang, D.D.; Cao, J.; Zhang, X.Y.; Insin, N.; Wang, S.M.; Han, J.T.; Zhao, Y.S.; Qin, J.Q.; Huang, Y.H. Inhibition of Manganese Dissolution in Mn2O3 Cathode with Controllable Ni2+ Incorporation for High-Performance Zinc Ion Battery. Adv. Funct. Mater. 2021, 31, 2009412. [Google Scholar] [CrossRef]
- Bayaguud, A.; Fu, Y.P.; Zhu, C.B. Interfacial parasitic reactions of zinc anodes in zinc ion batteries: Underestimated corrosion and hydrogen evolution reactions and their suppression strategies. J. Energy Chem. 2022, 64, 246–262. [Google Scholar] [CrossRef]
- Cai, Y.; Chua, R.; Srinivasan, M. Anode Materials for Rechargeable Aqueous Al-Ion Batteries: Progress and Prospects. Chemnanomat 2022, 8, e202100507. [Google Scholar] [CrossRef]
- Lv, Y.Q.; Xiao, Y.; Ma, L.T.; Zhi, C.Y.; Chen, S.M. Recent Advances in Electrolytes for “Beyond Aqueous” Zinc-Ion Batteries. Adv. Mater. 2022, 34, 2106409. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Tang, Z.H.; Luo, L.F.; Yang, W.L.; Liu, Y.; Shen, Z.H.; Fan, X.H. Self-Healing Solid Polymer Electrolyte with High Ion Conductivity and Super Stretchability for All-Solid Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 36320–36329. [Google Scholar] [CrossRef]
- Zeng, Y.X.; Zhang, X.Y.; Meng, Y.; Yu, M.H.; Yi, J.N.; Wu, Y.Q.; Lu, X.H.; Tong, Y.X. Achieving Ultrahigh Energy Density and Long Durability in a Flexible Rechargeable Quasi-Solid-State Zn-MnO2 Battery. Adv. Mater. 2017, 29, 1700274. [Google Scholar] [CrossRef]
- Zhao, J.W.; Sonigara, K.K.; Li, J.J.; Zhang, J.; Chen, B.B.; Zhang, J.J.; Soni, S.S.; Zhou, X.H.; Cui, G.L.; Chen, L.Q. A Smart Flexible Zinc Battery with Cooling Recovery Ability. Angew. Chem.-Int. Ed. 2017, 56, 7871–7875. [Google Scholar] [CrossRef]
- Deng, Y.Q.; Wu, Y.H.; Wang, L.L.; Zhang, K.F.; Wang, Y.; Yan, L.F. Polysaccharide hydrogel electrolytes with robust interfacial contact to electrodes for quasi-solid state flexible aqueous zinc ion batteries with efficient suppressing of dendrite growth. J. Colloid Interface Sci. 2023, 633, 142–154. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Lim, G.J.H.; Verma, V.; Cai, Y.; Chua, R.; Lim, J.J.N.; Srinivasan, M. Solid State Zinc and Aluminum ion batteries: Challenges and Opportunities. Chemsuschem 2023, 16, e202202297. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.H.; Zhou, W.J.; Wu, T.; Chen, M.F.; Han, X.; Tian, Q.H.; Xu, J.L.; Chen, J.Z. Sorbitol-modified cellulose hydrogel electrolyte derived from wheat straws towards high-performance environmentally adaptive flexible zinc-ion batteries. Chem. Eng. J. 2022, 446, 137056. [Google Scholar] [CrossRef]
- Quan, Y.H.; Ma, H.; Chen, M.F.; Zhou, W.J.; Tian, Q.H.; Han, X.; Chen, J.Z. Salting-Out Effect Realizing High-Strength and Dendrite-Inhibiting Cellulose Hydrogel Electrolyte for Durable Aqueous Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2023, 15, 44974–44983. [Google Scholar] [CrossRef]
- Wang, X.L.; Wang, B.; Cheng, J.L. Multi-Healable, Mechanically Durable Double Cross-Linked Polyacrylamide Electrolyte Incorporating Hydrophobic Interactions for Dendrite-Free Flexible Zinc-Ion Batteries. Adv. Funct. Mater. 2023, 33, 2304470. [Google Scholar] [CrossRef]
- Xia, H.; Xu, G.; Cao, X.; Miao, C.Y.; Zhang, H.N.; Chen, P.Y.; Zhou, Y.; Zhang, W.; Sun, Z.M. Single-Ion-Conducting Hydrogel Electrolytes Based on Slide-Ring Pseudo-Polyrotaxane for Ultralong-Cycling Flexible Zinc-Ion Batteries. Adv. Mater. 2023, 35, e2301996. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.Y.; Wang, Z.Q.; Jia, H.; Ng, P.F.; Chow, L.; Fei, B. Recent advances of hydrogel electrolytes in flexible energy storage devices. J. Mater. Chem. A 2021, 9, 2043–2069. [Google Scholar] [CrossRef]
- Leng, K.T.; Li, G.J.; Guo, J.J.; Zhang, X.Y.; Wang, A.X.; Liu, X.J.; Luo, J.Y. A Safe Polyzwitterionic Hydrogel Electrolyte for Long-Life Quasi-Solid State Zinc Metal Batteries. Adv. Funct. Mater. 2020, 30, 2001317. [Google Scholar] [CrossRef]
- Chen, G.Q.; Huang, J.R.; Gu, J.F.; Peng, S.J.; Xiang, X.T.; Chen, K.; Yang, X.X.; Guan, L.H.; Jiang, X.C.; Hou, L.X. Highly tough supramolecular double network hydrogel electrolytes for an artificial flexible and low-temperature tolerant sensor. J. Mater. Chem. A 2020, 8, 6776–6784. [Google Scholar] [CrossRef]
- Ma, R.J.; Xu, Z.X.; Wang, X.L. Polymer Hydrogel Electrolytes for Flexible and Multifunctional Zinc-Ion Batteries and Capacitors. Energy Environ. Mater. 2023, 6, e12464. [Google Scholar] [CrossRef]
- Han, Q.; Chi, X.W.; Zhang, S.M.; Liu, Y.Z.; Zhou, B.A.; Yang, J.H.; Liu, Y. Durable, flexible self- standing hydrogel electrolytes enabling high- safety rechargeable solid- state zinc metal batteries. J. Mater. Chem. A 2018, 6, 23046–23054. [Google Scholar] [CrossRef]
- Xu, T.; Liu, K.; Sheng, N.; Zhang, M.H.; Liu, W.; Liu, H.Y.; Dai, L.; Zhang, X.Y.; Si, C.L.; Du, H.S.; et al. Biopolymer-based hydrogel electrolytes for advanced energy storage/conversion devices: Properties, applications, and perspectives. Energy Storage Mater. 2022, 48, 244–262. [Google Scholar] [CrossRef]
- Wang, Z.F.; Li, H.F.; Tang, Z.J.; Liu, Z.X.; Ruan, Z.H.; Ma, L.T.; Yang, Q.; Wang, D.H.; Zhi, C.Y. Hydrogel Electrolytes for Flexible Aqueous Energy Storage Devices. Adv. Funct. Mater. 2018, 28, 1804560. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.; Zhou, W.X.; Meng, S.; Qi, C.; Liu, Z.; Kong, T.T. Biocompatible, High-Performance, Wet-Adhesive, Stretchable All-Hydrogel Supercapacitor Implant Based on PANI@rGO/Mxenes Electrode and Hydrogel Electrolyte. Adv. Energy Mater. 2021, 11, 2101329. [Google Scholar] [CrossRef]
- Mo, F.N.; Chen, Z.; Liang, G.J.; Wang, D.H.; Zhao, Y.W.; Li, H.F.; Dong, B.B.; Zhi, C.Y. Zwitterionic Sulfobetaine Hydrogel Electrolyte Building Separated Positive/Negative Ion Migration Channels for Aqueous Zn-MnO2 Batteries with Superior Rate Capabilities. Adv. Energy Mater. 2020, 10, 2000035. [Google Scholar] [CrossRef]
- Li, J.; Yu, P.F.; Zhang, S.T.; Wen, Z.L.; Wen, Y.H.; Zhou, W.Y.; Dong, X.M.; Liu, Y.L.; Liang, Y.R. Mild synthesis of superadhesive hydrogel electrolyte with low interfacial resistance and enhanced ionic conductivity for flexible zinc ion battery. J. Colloid Interfaces Sci. 2021, 600, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Owusu, K.A.; Pan, X.; Yu, R.; Qu, L.; Liu, Z.; Wang, Z.; Tahir, M.; Haider, W.A.; Zhou, L.; Mai, L. Introducing Na2SO4 in aqueous ZnSO4 electrolyte realizes superior electrochemical performance in zinc-ion hybrid capacitor. Mater. Today Energy 2020, 18, 100529. [Google Scholar] [CrossRef]
- Liu, C.X.; Xie, X.S.; Lu, B.G.; Zhou, J.; Liang, S.Q. Electrolyte Strategies toward Better Zinc-Ion Batteries. ACS Energy Lett. 2021, 6, 1015–1033. [Google Scholar] [CrossRef]
- Wang, Y.B.; Li, Q.; Hong, H.; Yang, S.; Zhang, R.; Wang, X.Q.; Jin, X.; Xiong, B.; Bai, S.C.; Zhi, C.Y. Lean-water hydrogel electrolyte for zinc ion batteries. Nat. Commun. 2023, 14, 3890. [Google Scholar] [CrossRef]
- Xu, P.J.; Wang, C.Y.; Zhao, B.X.; Zhou, Y.; Cheng, H.F. A high-strength and ultra-stable halloysite nanotubes-crosslinked polyacrylamide hydrogel electrolyte for flexible zinc-ion batteries. J. Power Sources 2021, 506, 230196. [Google Scholar] [CrossRef]
- Yan, Y.C.; Duan, S.D.; Liu, B.; Wu, S.W.; Alsaid, Y.; Yao, B.W.; Nandi, S.; Du, Y.J.; Wang, T.W.; Li, Y.Z.; et al. Tough Hydrogel Electrolytes for Anti-Freezing Zinc-Ion Batteries. Adv. Mater. 2023, 35, 230196. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Gao, A.M.; Hao, J.N.; Li, X.L.; Ling, J.Z.; Yi, F.Y.; Li, Q.Z.; Shu, D. Soaking-free and self-healing hydrogel for wearable zinc-ion batteries. Chem. Eng. J. 2023, 452, 139605. [Google Scholar] [CrossRef]
- Ni, Q.; Kim, B.; Wu, C.A.; Kang, K. Non-Electrode Components for Rechargeable Aqueous Zinc Batteries: Electrolytes, Solid-Electrolyte-Interphase, Current Collectors, Binders, and Separators. Adv. Mater. 2022, 34, 2108206. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; Zhang, Z.; Mao, F.F.; Liu, P.G.; Zhou, Z. Advances and strategies of electrolyte regulation in Zn-ion batteries. Mater. Chem. Front. 2023, 7, 3232–3258. [Google Scholar] [CrossRef]
- Xu, C.J.; Li, B.H.; Du, H.D.; Kang, F.Y. Energetic Zinc Ion Chemistry: The Rechargeable Zinc Ion Battery. Angew. Chem.-Int. Ed. 2012, 51, 933–935. [Google Scholar] [CrossRef]
- Zhu, Q.C.; Xiao, Q.; Zhang, B.W.; Yan, Z.C.; Liu, X.; Chen, S.; Ren, Z.F.; Yu, Y. VS4 with a chain crystal structure used as an intercalation cathode for aqueous Zn-ion batteries. J. Mater. Chem. A 2020, 8, 10761–10766. [Google Scholar] [CrossRef]
- Zhang, N.; Dong, Y.; Jia, M.; Bian, X.; Wang, Y.Y.; Qiu, M.D.; Xu, J.Z.; Liu, Y.C.; Jiao, L.F.; Cheng, F.Y. Rechargeable Aqueous Zn-V2O5 Battery with High Energy Density and Long Cycle Life. ACS Energy Lett. 2018, 3, 1366–1372. [Google Scholar] [CrossRef]
- Jia, Z.J.; Wang, B.G.; Wang, Y. Copper hexacyanoferrate with a well-defined open framework as a positive electrode for aqueous zinc ion batteries. Mater. Chem. Phys. 2015, 149, 601–606. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Gim, J.; Kim, S.; Song, J.; Pham, D.T.; Jo, J.; Xiu, Z.; Mathew, V.; Kim, J. A layered δ-MnO2 nanoflake cathode with high zinc-storage capacities for eco-friendly battery applications. Electrochem. Commun. 2015, 60, 121–125. [Google Scholar] [CrossRef]
- Zhang, N.; Ji, Y.R.; Wang, J.C.; Wang, P.F.; Zhu, Y.R.; Yi, T.F. Understanding of the charge storage mechanism of MnO2-based aqueous zinc-ion batteries: Reaction processes and regulation strategies. J. Energy Chem. 2023, 82, 423–463. [Google Scholar] [CrossRef]
- Tie, Z.W.; Liu, L.J.; Deng, S.Z.; Zhao, D.B.; Niu, Z.Q. Proton Insertion Chemistry of a Zinc-Organic Battery. Angew. Chem.-Int. Ed. 2020, 59, 4920–4924. [Google Scholar] [CrossRef]
- Liao, Y.X.; Chen, H.C.; Yang, C.; Liu, R.; Peng, Z.W.; Cao, H.J.; Wang, K.K. Unveiling performance evolution mechanisms of MnO2 polymorphs for durable aqueous zinc-ion batteries. Energy Storage Mater. 2022, 44, 508–516. [Google Scholar] [CrossRef]
- Sun, W.; Wang, F.; Hou, S.Y.; Yang, C.Y.; Fan, X.L.; Ma, Z.H.; Gao, T.; Han, F.D.; Hu, R.Z.; Zhu, M.; et al. Zn/MnO2 Battery Chemistry with H+ and Zn2+ Coinsertion. J. Am. Chem. Soc. 2017, 139, 9775–9778. [Google Scholar] [CrossRef]
- Li, L.Y.; Hoang, T.K.A.; Zhi, J.; Han, M.; Li, S.K.; Chen, P. Functioning Mechanism of the Secondary Aqueous Zn-β-MnO2 Battery. ACS Appl. Mater. Interfaces 2020, 12, 12834–12846. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.Y.; Wu, T.; Sun, S.C.; van den Bergh, W.; Stefik, M.; Huang, K. Synergistic H+/Zn2+ dual ion insertion mechanism in high-capacity and ultra-stable hydrated VO2 cathode for aqueous Zn-ion batteries. Energy Storage Mater. 2020, 29, 60–70. [Google Scholar] [CrossRef]
- Tian, Y.P.; Ju, M.M.; Bin, X.Q.; Luo, Y.J.; Que, W.X. Long cycle life aqueous rechargeable battery Zn/Vanadium hexacyanoferrate with H+/Zn2+ coinsertion for high capacity. Chem. Eng. J. 2022, 430, 132864. [Google Scholar] [CrossRef]
- Liu, W.B.; Zhang, X.Y.; Huang, Y.F.; Jiang, B.Z.; Chang, Z.W.; Xu, C.J.; Kang, F.Y. β-MnO2 with proton conversion mechanism in rechargeable zinc ion battery. J. Energy Chem. 2021, 56, 365–373. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, J.; Bai, C.L.; Li, X.K.; Fang, G.Z.; Liang, S.Q. Zn/MnO2 battery chemistry with dissolution-deposition mechanism. Mater. Today Energy 2020, 16, 100396. [Google Scholar] [CrossRef]
- Yan, H.H.; Zhang, X.K.; Yang, Z.W.; Xia, M.T.; Xu, C.W.; Liu, Y.W.; Yu, H.X.; Zhang, L.Y.; Shu, J. Insight into the electrolyte strategies for aqueous zinc ion batteries. Coord. Chem. Rev. 2022, 452, 214297. [Google Scholar] [CrossRef]
- Li, Y.F.; Yuan, J.J.; Qiao, Y.F.; Xu, H.; Zhang, Z.H.; Zhang, W.Y.; He, G.Y.; Chen, H.Q. Recent progress in structural modification of polymer gel electrolytes for use in solid-state zinc-ion batteries. Dalton Trans. 2023, 52, 11780–11796. [Google Scholar] [CrossRef]
- Hu, F.L.; Li, M.Y.; Gao, G.W.; Fan, H.Q.; Ma, L.T. The Gel-State Electrolytes in Zinc-Ion Batteries. Batteries 2022, 8, 214. [Google Scholar] [CrossRef]
- Dong, H.B.; Li, J.W.; Guo, J.; Lai, F.L.; Zhao, F.J.; Jiao, Y.D.; Brett, D.J.L.; Liu, T.X.; He, G.J.; Parkin, I.P. Insights on Flexible Zinc-Ion Batteries from Lab Research to Commercialization. Adv. Mater. 2021, 33, 2007548. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Ali, Y.; Lee, S. Debonding Mechanisms at the Particle-Binder Interface in the Li-Ion Battery Electrode. J. Electrochem. Soc. 2020, 167, 060515. [Google Scholar] [CrossRef]
- Iqbal, N.; Choi, J.; Lee, C.; Ayub, H.M.U.; Kim, J.; Kim, M.; Kim, Y.; Moon, D.; Lee, S. Effects of Diffusion-Induced Nonlinear Local Volume Change on the Structural Stability of NMC Cathode Materials of Lithium-Ion Batteries. Mathematics 2022, 10, 4697. [Google Scholar] [CrossRef]
- Iqbal, N.; Ali, Y.; Lee, S. Mechanical degradation analysis of a single electrode particle with multiple binder connections: A comparative study. Int. J. Mech. Sci. 2020, 188, 105943. [Google Scholar] [CrossRef]
- Ali, Y.; Iqbal, N.; Lee, S. Role of SEI layer growth in fracture probability in lithium-ion battery electrodes. Int. J. Energy Res. 2021, 45, 5293–5308. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem.-Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.Y.; Yan, S.Y.; Zhang, Y.F.; Zhang, X.P.; Zhou, J.; Ye, J.L.; Zhu, Y.S. An ultrathin natural cellulose based hydrogel membrane for the high-performance quasi-solid-state zinc-ion batteries. Chem. Eng. J. 2023, 475, 146314. [Google Scholar] [CrossRef]
- Yue, X.; Wang, Q.H.; Ao, K.L.; Shi, J.H.; Zhang, X.Y.; Zhao, H.; Uyanga, K.; Yang, Y.; Daoud, W.A. A novel 3D bacterial cellulose network as cathodic scaffold and hydrogel electrolyte for zinc-ion batteries. J. Power Sources 2023, 557, 232553. [Google Scholar] [CrossRef]
- Cao, G.H.; Zhao, L.; Ji, X.W.; Peng, Y.Y.; Yu, M.M.; Wang, X.Y.; Li, X.Y.; Ran, F. “Salting out” in Hofmeister Effect Enhancing Mechanical and Electrochemical Performance of Amide-based Hydrogel Electrolytes for Flexible Zinc-Ion Battery. Small 2023, 19, e2207610. [Google Scholar] [CrossRef]
- Xu, L.; Meng, T.T.; Zheng, X.Y.; Li, T.Y.; Brozena, A.H.; Mao, Y.M.; Zhang, Q.; Clifford, B.C.; Rao, J.C.; Hu, L.B. Nanocellulose-Carboxymethylcellulose Electrolyte for Stable, High-Rate Zinc-Ion Batteries. Adv. Funct. Mater. 2023, 33, 2302098. [Google Scholar] [CrossRef]
- Mittal, N.; Ojanguren, A.; Kundu, D.; Lizundia, E.; Niederberger, M. Bottom-Up Design of a Green and Transient Zinc-Ion Battery with Ultralong Lifespan. Small 2023, 19, e2206249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.C.; Li, C.W.; Li, Q.L.; Pan, Z.H.; Sun, J.; Zhou, Z.Y.; He, B.; Man, P.; Xie, L.Y.; Kang, L.X.; et al. Flexible and High-Voltage Coaxial-Fiber Aqueous Rechargeable Zinc-Ion Battery. Nano Lett. 2019, 19, 4035–4042. [Google Scholar] [CrossRef]
- Chen, M.F.; Chen, J.Z.; Zhou, W.J.; Han, X.; Yao, Y.G.; Wong, C.P. Realizing an All-Round Hydrogel Electrolyte toward Environmentally Adaptive Dendrite-Free Aqueous Zn-MnO2 Batteries. Adv. Mater. 2021, 33, 2007559. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
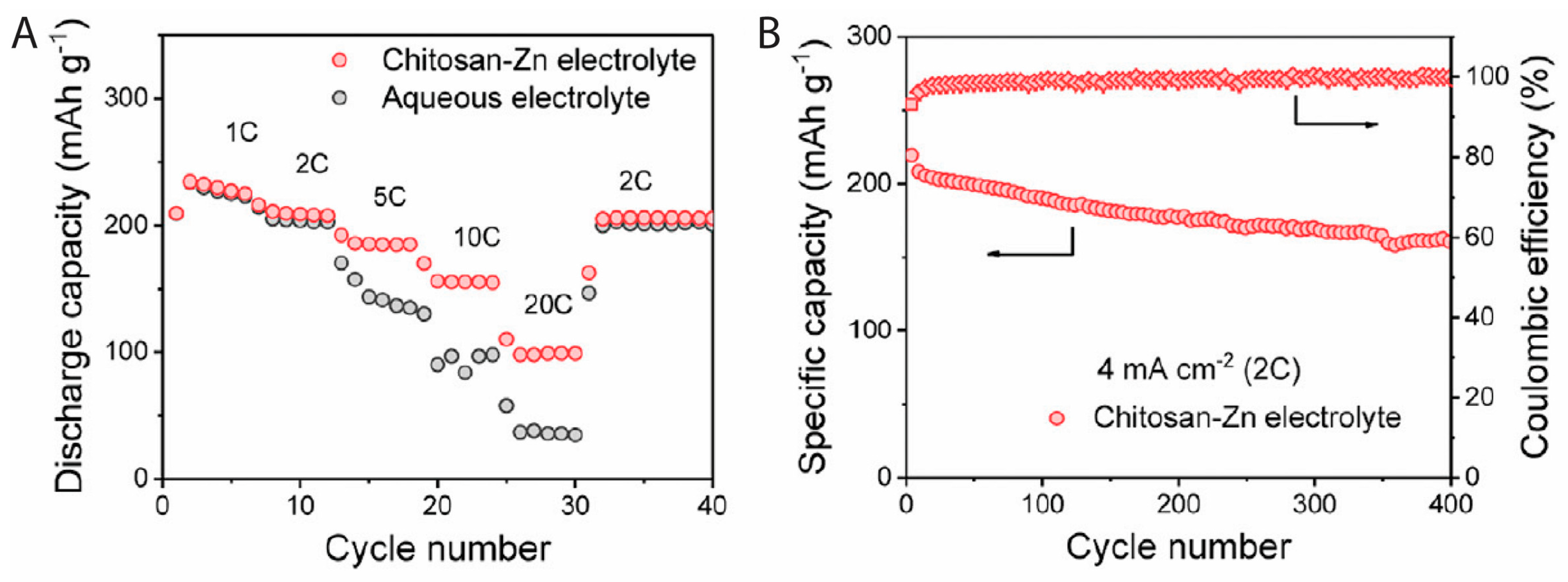
- Wu, M.L.; Zhang, Y.; Xu, L.; Yang, C.P.; Hong, M.; Cui, M.J.; Clifford, B.C.; He, S.M.; Jing, S.S.; Yao, Y.; et al. A sustainable chitosan-zinc electrolyte for high-rate zinc-metal batteries. Matter 2022, 5, 3402–3416. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.J.; Li, S.H.; Meng, Y.S.; Qin, Y.Y.; Jiang, L.T.; Huang, H.; Shen, L.D. Regulating zinc deposition for dendrite-free aqueous zinc-ion batteries: A zincophilic chitosan polyelectrolyte hydrogel modified glass fiber separator. J. Alloys Compd. 2023, 967, 171708. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.J.; Li, S.H.; Qin, Y.Y.; Meng, Y.S.; Jiang, L.T.; Huang, H.; Shen, L.D. Chitosan hydrogel polyelectrolyte-modified cotton pad as dendrite-inhibiting separators for stable zinc anodes in aqueous zinc-ion batteries. J. Power Sources 2023, 580, 233392. [Google Scholar] [CrossRef]
- Lu, H.Y.; Hu, J.S.; Wei, X.J.; Zhang, K.Q.; Xiao, X.; Zhao, J.X.; Hu, Q.; Yu, J.; Zhou, G.M.; Xu, B.A. A recyclable biomass electrolyte towards green zinc-ion batteries. Nat. Commun. 2023, 14, 4435. [Google Scholar] [CrossRef]
- Wang, F.F.; Zhang, J.P.; Lu, H.T.; Zhu, H.B.; Chen, Z.H.; Wang, L.; Yu, J.Y.; You, C.H.; Li, W.H.; Song, J.W.; et al. Production of gas-releasing electrolyte-replenishing Ah-scale zinc metal pouch cells with aqueous gel electrolyte. Nat. Commun. 2023, 14, 4211. [Google Scholar] [CrossRef]
- Zhan, H.J.; Chen, Y.Z.; Huang, L.; Yang, C.; Zhong, Y.L.; Wang, L.H.; Jiang, X.L. Synthesis and characterization of reversible thermosensitive methylated chitin and its application in zinc-ion battery thermal runaway. J. Polym. Sci. 2023. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, C.X.; Zhu, H.R.; Xie, X.S.; Gao, J.W.; Deng, C.B.; Han, M.M.; Liang, S.Q.; Zhou, J. Ion-confinement effect enabled by gel electrolyte for highly reversible dendrite-free zinc metal anode. Energy Storage Mater. 2020, 27, 109–116. [Google Scholar] [CrossRef]
- Zheng, Z.Y.; Cao, H.C.; Shi, W.H.; She, C.L.; Zhou, X.L.; Liu, L.L.; Zhu, Y.S. Low-Cost Zinc-Alginate-Based Hydrogel-Polymer Electrolytes for Dendrite-Free Zinc-Ion Batteries with High Performances and Prolonged Lifetimes. Polymers 2023, 15, 212. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Wu, X.M.; Liu, Y.S.; Yu, C.Y.; Liu, Y.C.; Sun, K.X.; Shen, C.Y.; Huang, W.; Zhou, Y.F.; Chen, J.S.; et al. Self-Adapting and Self-Healing Hydrogel Interface with Fast Zn2+ Transport Kinetics for Highly Reversible Zn Anodes. Adv. Funct. Mater. 2023, 33, 2300952. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Qin, L.P.; Fang, Y.; Chai, Y.Z.; Xie, X.S.; Lu, B.A.; Liang, S.Q.; Zhou, J. Tuning Zn2+ coordination tunnel by hierarchical gel electrolyte for dendrite-free zinc anode. Sci. Bull. 2022, 67, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Huang, Y.; Liu, B.B.; Li, Z.X.; Zhang, J.Y.; Yang, G.S.; Hiralal, P.; Jin, S.Y.; Zhou, H. Flexible and anti-freezing zinc-ion batteries using a guar-gum/sodium-alginate/ethylene-glycol hydrogel electrolyte. Energy Storage Mater. 2021, 41, 599–605. [Google Scholar] [CrossRef]
- Fernández-Benito, A.; Martinez-López, J.C.; Jafari, M.J.; Solin, N.; Martinez, J.G.; Garcia-Gimenez, D.; Ederth, T.; Inganaes, O.; Carretero-González, J. Green and Scalable Biopolymer-Based Aqueous Polyelectrolyte Complexes for Zinc-Ion Charge Storage Devices. ChemElectroChem 2023, 10, e202300327. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Su, K.; Wang, C.M. Recent advances in the use of gelatin in biomedical research. Biotechnol. Lett. 2015, 37, 2139–2145. [Google Scholar] [CrossRef]
- Han, Q.; Chi, X.W.; Liu, Y.Z.; Wang, L.; Du, Y.X.; Ren, Y.; Liu, Y. An inorganic salt reinforced Zn2+-conducting solid-state electrolyte for ultra-stable Zn metal batteries. J. Mater. Chem. A 2019, 7, 22287–22295. [Google Scholar] [CrossRef]
- García-Ochoa, F.; Santos, V.E.; Casas, J.A.; Gómez, E. Xanthan gum:: Production, recovery, and properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar gum: Processing, properties and food applications—A Review. J. Food Sci. Technol.-Mysore 2014, 51, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Ziada, A.; Blunden, G. Biological effects of gum arabic: A review of some recent research. Food Chem. Toxicol. 2009, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Yu, N.S.; Zeng, S.; Zhou, S.S.; Chen, M.H.; Di, J.T.; Li, Q.W. An adaptive and stable bio-electrolyte for rechargeable Zn-ion batteries. J. Mater. Chem. A 2018, 6, 12237–12243. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.H. A flexible zinc-ion battery based on the optimized concentrated hydrogel electrolyte for enhanced performance at subzero temperature. Electrochim. Acta 2021, 395, 139178. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, J.Y.; Liu, J.W.; Li, Z.X.; Jin, S.Y.; Li, Z.G.; Zhang, S.D.; Zhou, H. Flexible and stable quasi-solid-state zinc ion battery with conductive guar gum electrolyte. Mater. Today Energy 2019, 14, 100349. [Google Scholar] [CrossRef]
- Wu, K.; Cui, J.; Yi, J.; Liu, X.Y.; Ning, F.H.; Liu, Y.Y.; Zhang, J.J. Biodegradable Gel Electrolyte Suppressing Water-Induced Issues for Long-Life Zinc Metal Anodes. ACS Appl. Mater. Interfaces 2022, 14, 34612–34619. [Google Scholar] [CrossRef]
- Xu, W.W.; Liu, C.Z.; Wu, Q.L.; Xie, W.W.; Kim, W.Y.; Lee, S.Y.; Gwon, J. A stretchable solid-state zinc ion battery based on a cellulose nanofiber-polyacrylamide hydrogel electrolyte and a Mg0.23V2O5·1.0H2O cathode. J. Mater. Chem. A 2020, 8, 18327–18337. [Google Scholar] [CrossRef]
- Li, Z.L.; Zhou, G.Q.; Ye, L.; He, J.Y.; Xu, W.W.; Hong, S.; Chen, W.M.; Li, M.C.; Liu, C.Z.; Mei, C.T. High-energy and dendrite-free solid-state zinc metal battery enabled by a dual-network and water-confinement hydrogel electrolyte. Chem. Eng. J. 2023, 472, 144992. [Google Scholar] [CrossRef]
- Mao, Y.C.; Ren, H.Z.; Zhang, J.C.; Luo, T.; Liu, N.N.; Wang, B.; Le, S.R.; Zhang, N.Q. Modifying hydrogel electrolyte to induce zinc deposition for dendrite-free zinc metal anode. Electrochim. Acta 2021, 393, 139094. [Google Scholar] [CrossRef]
- Zhang, H.D.; Gan, X.T.; Song, Z.P.; Zhou, J.P. Amphoteric Cellulose-Based Double-Network Hydrogel Electrolyte Toward Ultra-Stable Zn Anode. Angew. Chem.-Int. Ed. 2023, 62, e202217833. [Google Scholar] [CrossRef]
- Gomez, I.; Alesanco, Y.; Blazquez, J.A.; Viñuales, A.; Colmenares, L.C. Room-Temperature Self-Standing Cellulose-Based Hydrogel Electrolytes for Electrochemical Devices. Polymers 2020, 12, 2686. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.E.; Zhang, X.S.; Liang, S.J.; Fu, Y.C.; Liu, W.T.; Chen, J.Z.; Liu, X.Y.; Zhang, L.L. Environmentally adaptable hydrogel electrolyte with the triple interpenetrating network in the flexible zinc-ion battery with ultralong stability. J. Power Sources 2022, 548, 232072. [Google Scholar] [CrossRef]
- Lin, P.X.; Cong, J.L.; Li, J.Y.; Zhang, M.H.; Lai, P.B.; Zeng, J.; Yang, Y.; Zhao, J.B. Achieving ultra-long lifespan Zn metal anodes by manipulating desolvation effect and Zn deposition orientation in a multiple cross-linked hydrogel electrolyte. Energy Storage Mater. 2022, 49, 172–180. [Google Scholar] [CrossRef]
- Xu, W.W.; Liu, C.Z.; Ren, S.X.; Lee, D.; Gwon, J.; Flake, J.C.; Lei, T.Z.; Baisakh, N.; Wu, Q.L. A cellulose nanofiber-polyacrylamide hydrogel based on a co-electrolyte system for solid-state zinc ion batteries to operate at extremely cold temperatures. J. Mater. Chem. A 2021, 9, 25651–25662. [Google Scholar] [CrossRef]
- Wu, T.; Zhou, W.J.; Quan, Y.H.; Chen, M.F.; Tian, Q.H.; Han, X.; Xu, J.L.; Chen, J.Z. Facile and green synthesis of nanocellulose with the assistance of ultraviolet light irradiation for high-performance quasi-solid-state zinc-ion batteries. J. Colloid Interfaces Sci. 2022, 628, 1–9. [Google Scholar] [CrossRef]
- Liu, Q.J.; Ji, Z.Y.; Mo, F.N.; Ling, W.; Wang, J.Q.; Lei, H.; Cui, M.W.; Zhang, Z.S.; Liu, Y.; Cheng, L.K.; et al. Stable Thermochromic Hydrogel for a Flexible and Wearable Zinc-Ion Yarn Battery with High-Temperature Warning Function. ACS Appl. Energy Mater. 2022, 5, 12448–12455. [Google Scholar] [CrossRef]
- Li, Y.Q.; Miao, R.T.; Yang, Y.; Han, L.; Han, Q.S. A zinc-ion battery-type self-powered strain sensing system by using a high-performance ionic hydrogel. Soft Matter 2023, 19, 8022–8032. [Google Scholar] [CrossRef]
- Liu, C.Z.; Xu, W.W.; Mei, C.T.; Li, M.C.; Chen, W.M.; Hong, S.; Kim, W.Y.; Lee, S.Y.; Wu, Q.L. A Chemically Self-Charging Flexible Solid-State Zinc-Ion Battery Based on VO2 Cathode and Polyacrylamide-Chitin Nanofiber Hydrogel Electrolyte. Adv. Energy Mater. 2021, 11, 2003902. [Google Scholar] [CrossRef]
- Li, Y.Q.; Yang, Y.; Liu, X.H.; Yang, Y.W.; Wu, Y.Y.; Han, L.; Han, Q.S. Flexible self-powered integrated sensing system based on a rechargeable zinc-ion battery by using a multifunctional polyacrylamide/carboxymethyl chitosan/LiCl ionic hydrogel. Colloids Surf. A-Physicochem. Eng. Asp. 2022, 648, 129254. [Google Scholar] [CrossRef]
- Dong, H.B.; Li, J.W.; Zhao, S.Y.; Jiao, Y.D.; Chen, J.T.; Tan, Y.S.; Brett, D.J.L.; He, G.J.; Parkin, I.P. Investigation of a Biomass Hydrogel Electrolyte Naturally Stabilizing Cathodes for Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 745–754. [Google Scholar] [CrossRef]
- Qiu, M.H.; Liu, H.Q.; Tawiah, B.; Jia, H.; Fu, S.H. Zwitterionic triple-network hydrogel electrolyte for advanced flexible zinc ion batteries. Compos. Commun. 2021, 28, 100942. [Google Scholar] [CrossRef]
- Wang, B.J.; Li, J.M.; Hou, C.Y.; Zhang, Q.H.; Li, Y.G.; Wang, H.Z. Stable Hydrogel Electrolytes for Flexible and Submarine-Use Zn-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 46005–46014. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, F.S.; Zaeim, D. Agar-based edible films for food packaging applications—A review. Int. J. Biol. Macromol. 2020, 159, 1165–1176. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.Y.; Shi, W.H.; Zhou, X.L.; Zhang, X.P.; Guo, W.L.; Shi, X.Y.; Xiong, Y.; Zhu, Y.S. Agar-based hydrogel polymer electrolyte for high-performance zinc-ion batteries at all climatic temperatures. Iscience 2023, 26, 106437. [Google Scholar] [CrossRef]
- Jiang, D.; Lu, N.; Li, L.B.; Zhang, H.Q.; Luan, J.S.; Wang, G.B. A highly compressible hydrogel electrolyte for flexible Zn-Mno2 battery. J. Colloid Interfacesf Sci. 2022, 608, 1619–1626. [Google Scholar] [CrossRef]
- Cao, Y.P.; Mezzenga, R. Design principles of food gels. Nat. Food 2020, 1, 106–118. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; Xie, L.; Xu, R.; Zhang, R.; Gao, M.; Tian, W.; Li, D.; Qiao, L.; Wang, T.; et al. Humidity-sensitive, shape-controllable, and transient zinc-ion batteries based on plasticizing gelatin-silk protein electrolytes. Mater. Today Energy 2021, 21, 100712. [Google Scholar] [CrossRef]
- Shi, G.; Peng, X.W.; Zeng, J.M.; Zhong, L.X.; Sun, Y.; Yang, W.; Zhong, Y.L.; Zhu, Y.X.; Zou, R.; Admassie, S.; et al. A Liquid Metal Microdroplets Initialized Hemicellulose Composite for 3D Printing Anode Host in Zn-Ion Battery. Adv. Mater. 2023, 35, e2300109. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Wang, Z.H.; Li, M.; Li, Z.Y.; Hu, X.Y.; Xu, Q.J.; Wang, Y.H.; Liu, H.M.; Wang, Y.G. 3D Printed Flexible Zinc Ion Micro-Batteries with High Areal Capacity toward Practical Application. Adv. Funct. Mater. 2023, 2310966. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vandeginste, V.; Wang, J. A Review of the Synthesis of Biopolymer Hydrogel Electrolytes for Improved Electrode–Electrolyte Interfaces in Zinc-Ion Batteries. Energies 2024, 17, 310. https://doi.org/10.3390/en17020310
Vandeginste V, Wang J. A Review of the Synthesis of Biopolymer Hydrogel Electrolytes for Improved Electrode–Electrolyte Interfaces in Zinc-Ion Batteries. Energies. 2024; 17(2):310. https://doi.org/10.3390/en17020310
Chicago/Turabian StyleVandeginste, Veerle, and Junru Wang. 2024. "A Review of the Synthesis of Biopolymer Hydrogel Electrolytes for Improved Electrode–Electrolyte Interfaces in Zinc-Ion Batteries" Energies 17, no. 2: 310. https://doi.org/10.3390/en17020310
APA StyleVandeginste, V., & Wang, J. (2024). A Review of the Synthesis of Biopolymer Hydrogel Electrolytes for Improved Electrode–Electrolyte Interfaces in Zinc-Ion Batteries. Energies, 17(2), 310. https://doi.org/10.3390/en17020310







