Thermal Decomposition and Kinetic Parameters of Three Biomass Feedstocks for the Performance of the Gasification Process Using a Thermogravimetric Analyzer
Abstract
:1. Introduction
- The thermal behavior of selected biomass feedstocks (HW, SW, and RDF) will be experimentally investigated using a thermogravimetric analyzer (TGA);
- Several parameters such as characteristic reaction temperatures, devolatilization rates, and mass fractions will be introduced to describe the thermogravimetric data and will be evaluated for the three feedstocks listed above;
- A kinetic model, involving first-order independent parallel reactions, will be applied to data obtained from pyrolysis TGA experiments;
- Finally, a comparison will be provided between the TGA experimental measurements and model predictions.
2. Experimental Investigations and Measurements
2.1. Feedstock Materials
2.2. Apparatus and Instrument
2.3. TGA Method
2.4. Thermal Characterization of Samples
2.4.1. Proximate Analysis
2.4.2. Ultimate Analysis
2.4.3. Calorific Values
3. Mathematical Kinetics Model
4. Results and Discussion
4.1. Proximate and Ultimate Analyses
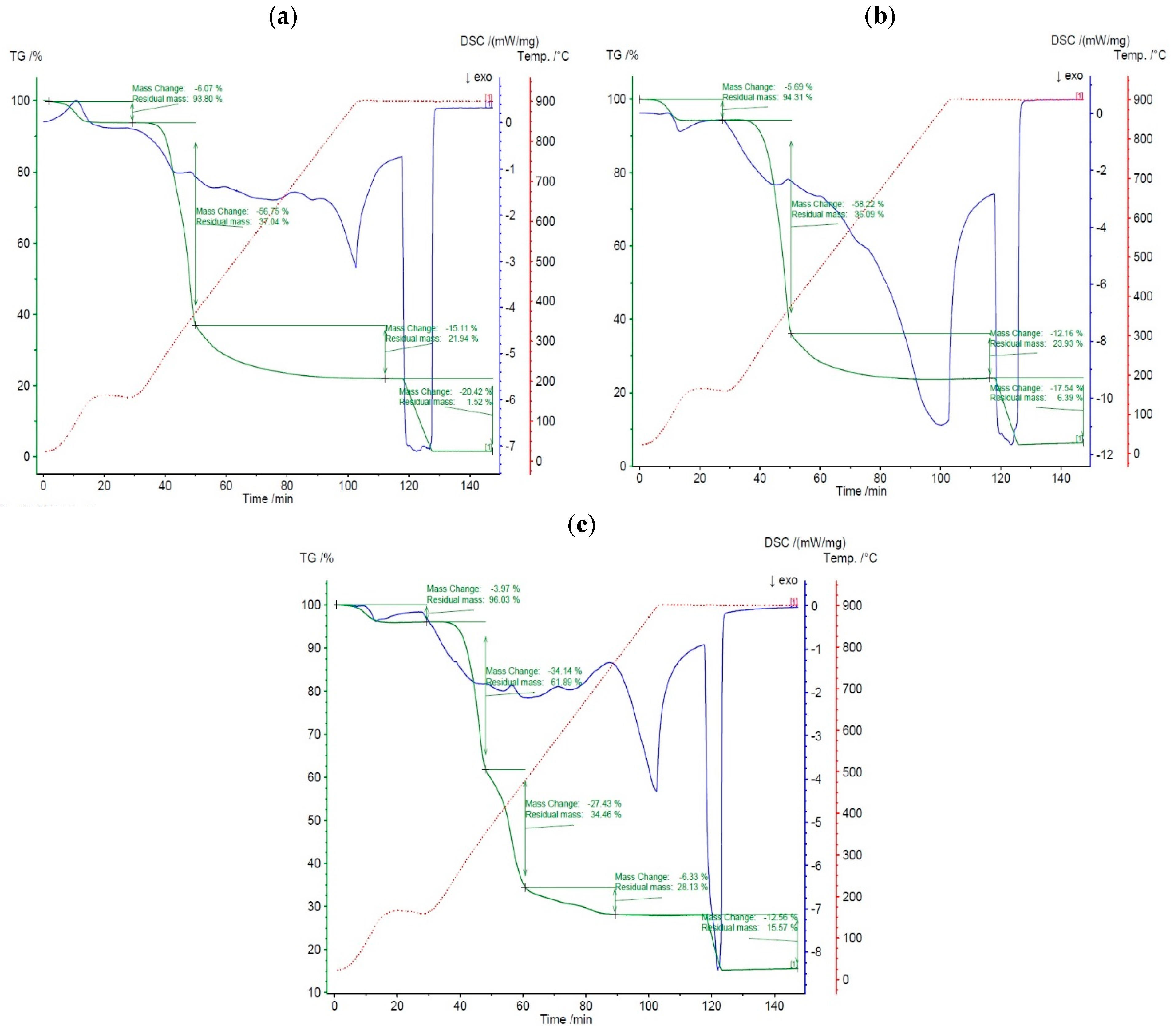
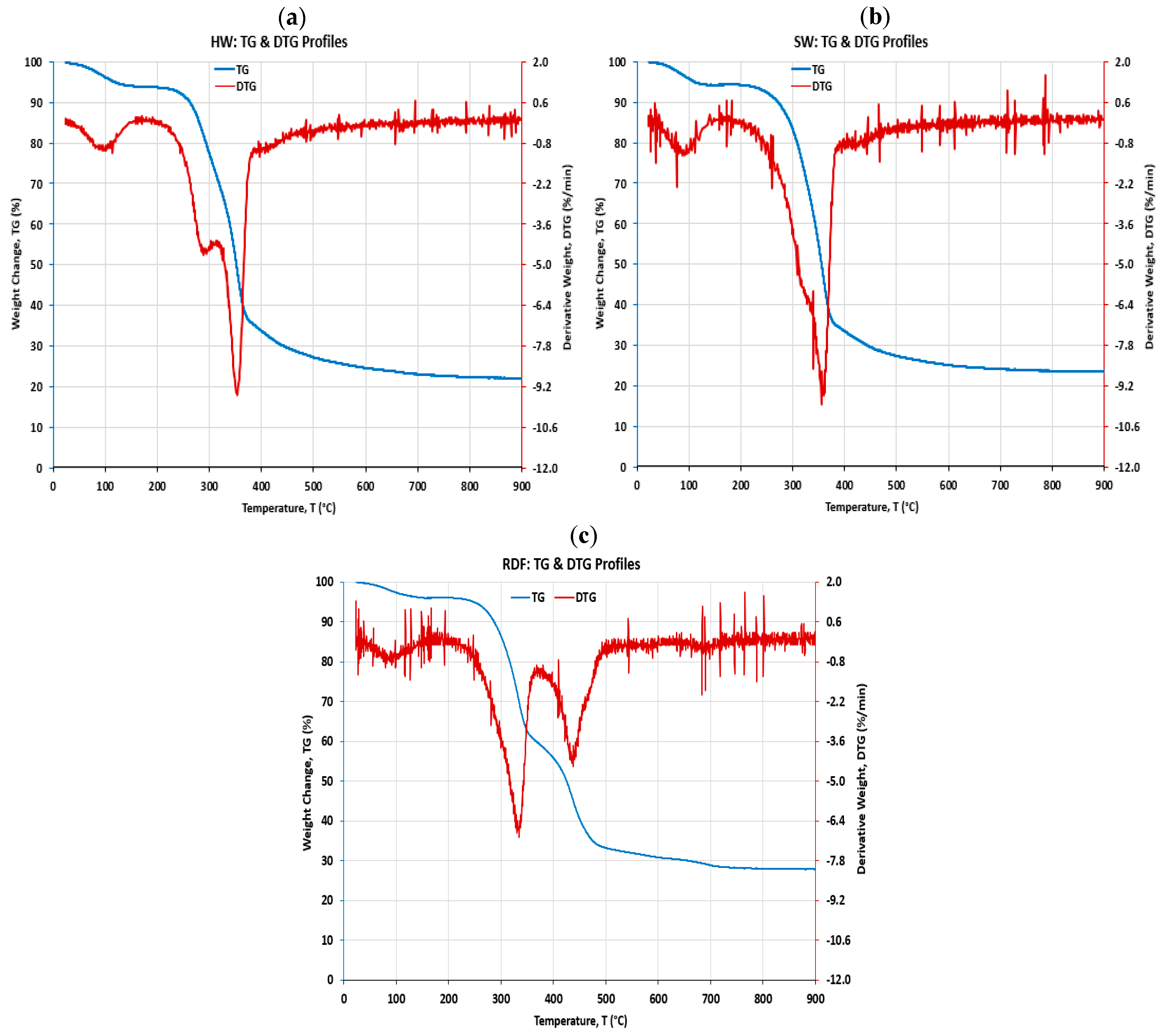
4.2. Thermal Decomposition Analysis
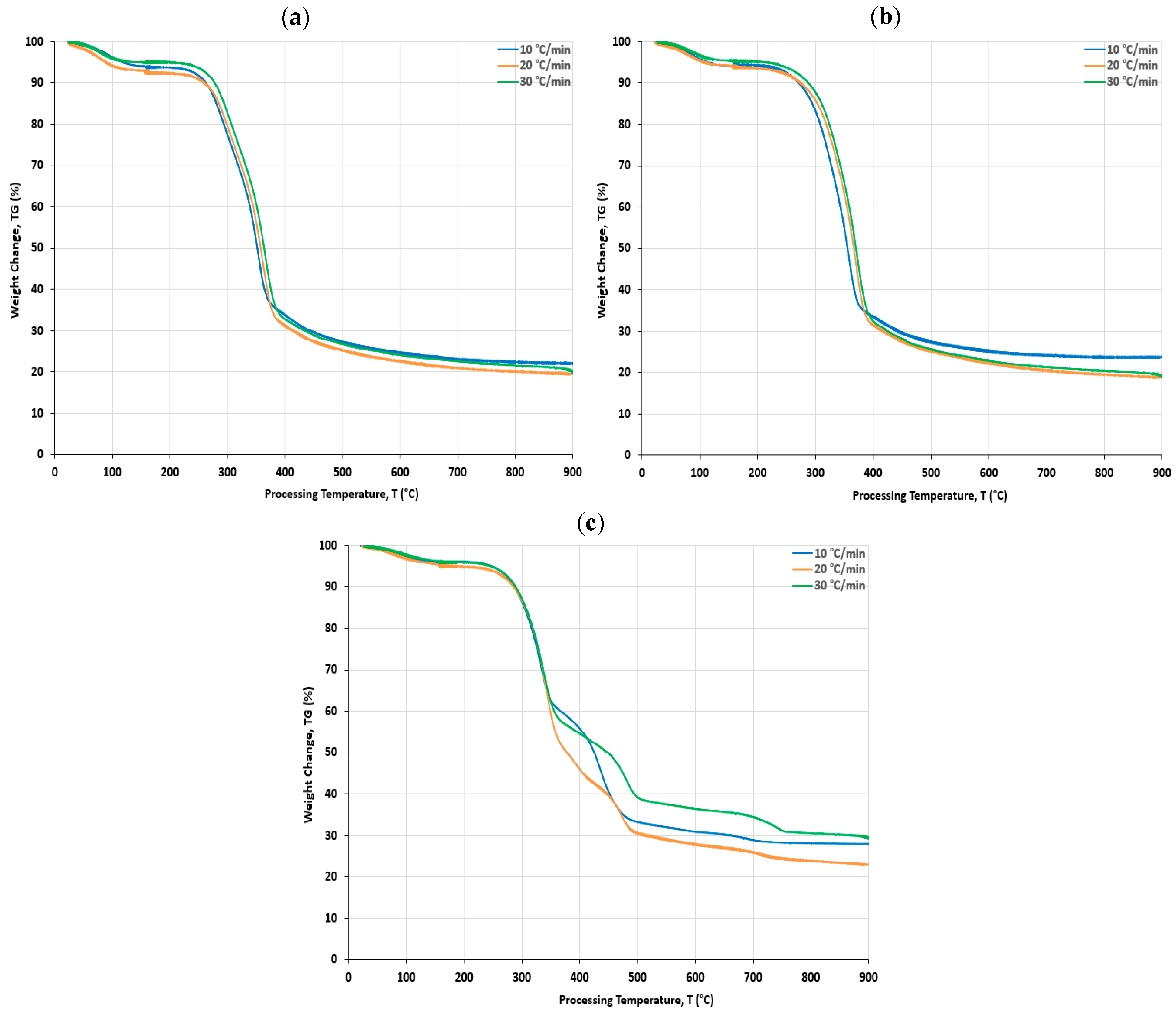
4.3. Thermogravimetric (TG) Analysis
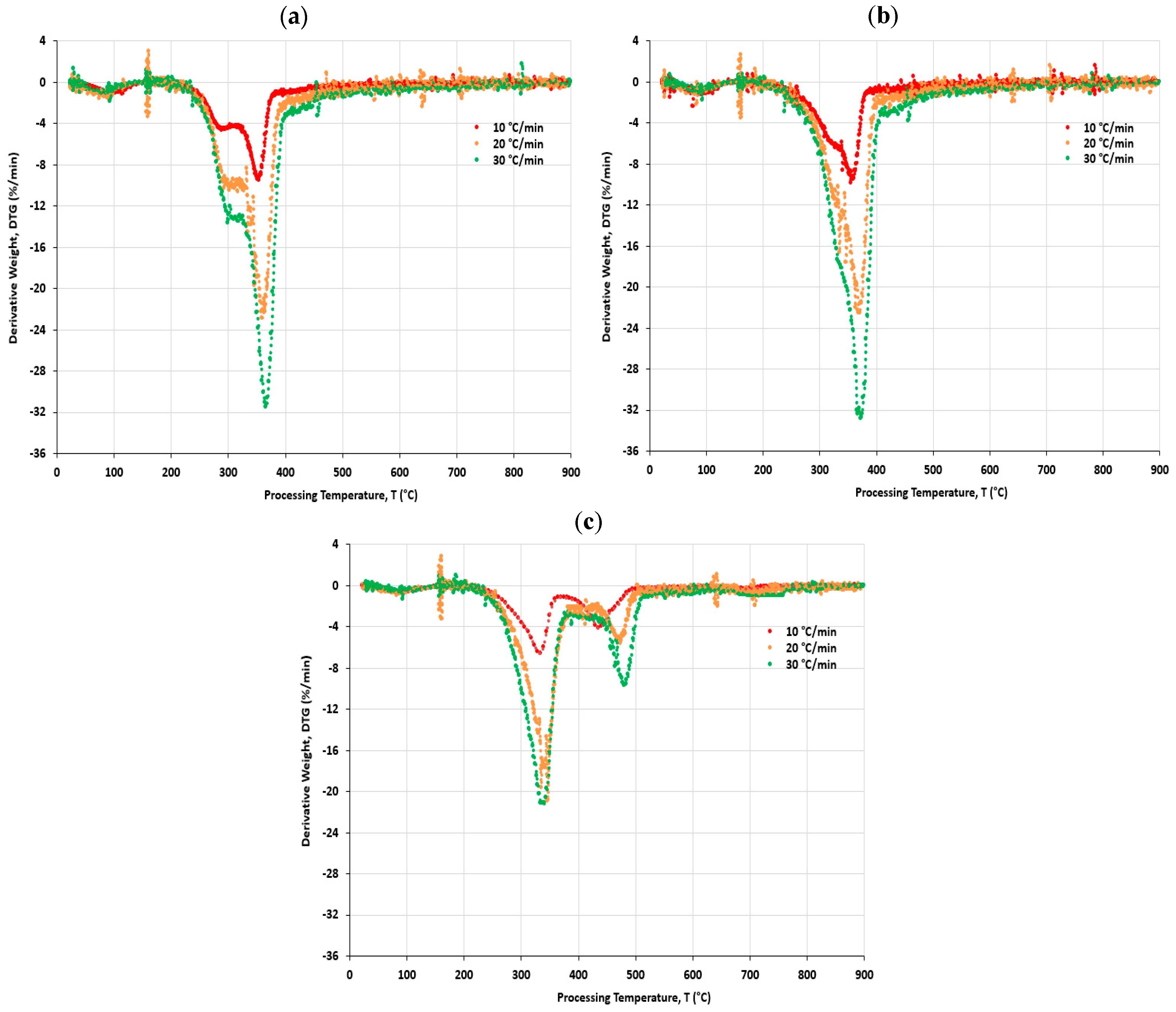
4.4. Differential Thermogravimetric (DTG) Analysis
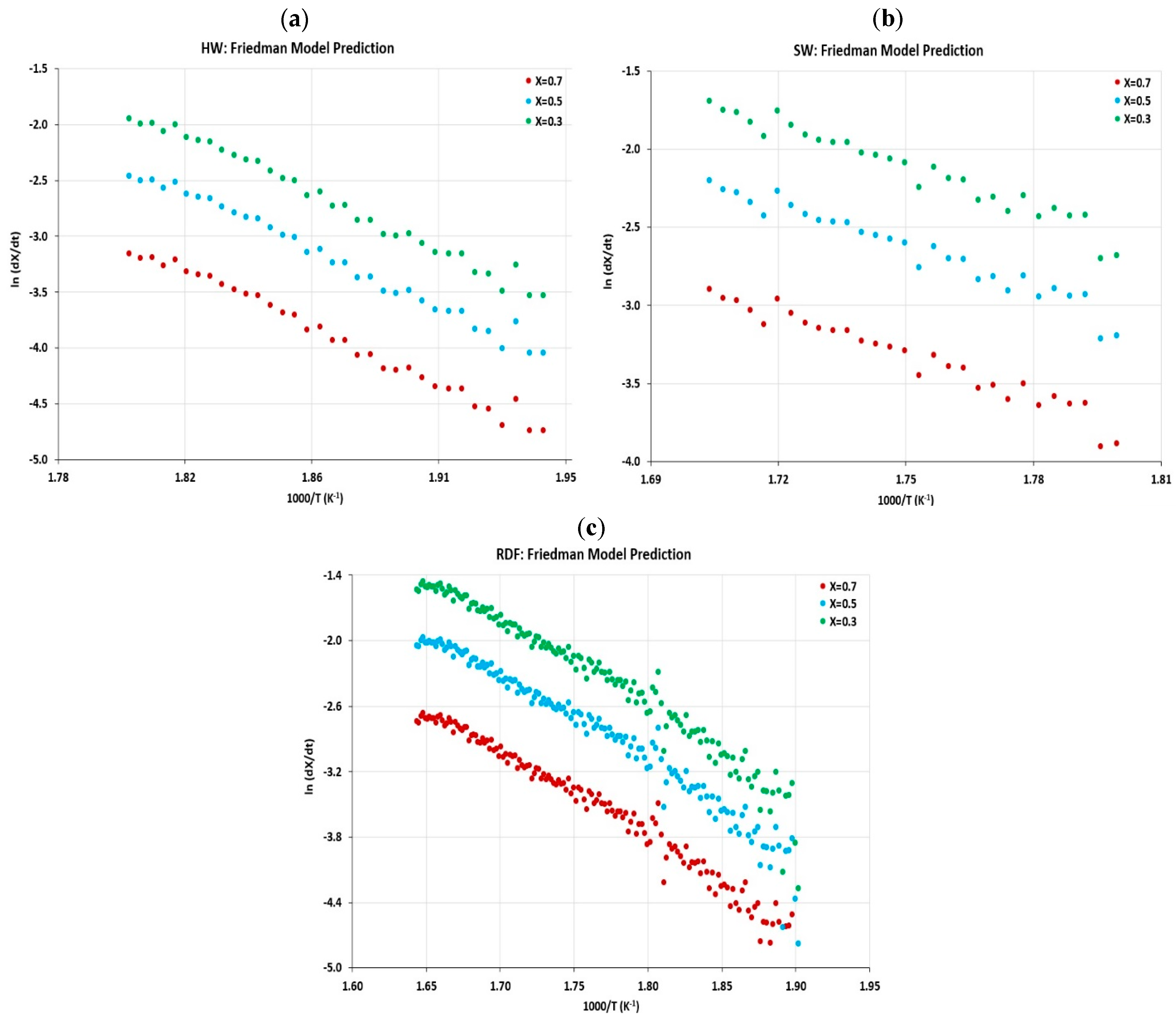
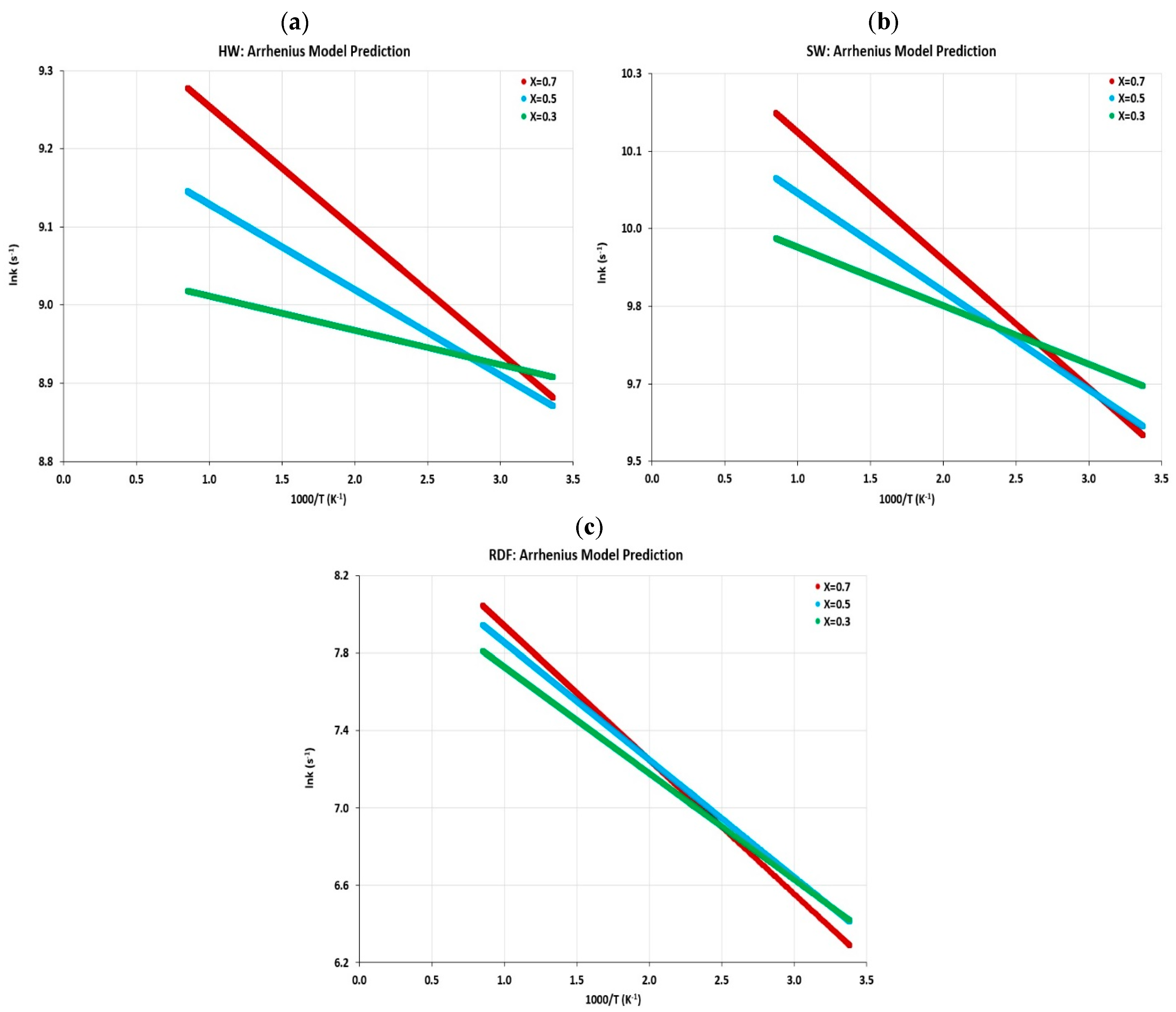
4.5. Kinetic Parameter Determination
4.5.1. Activation Energy and Frequency Factor
4.5.2. Reaction Rate Constant
5. Concluding Remarks
- The pyrolysis kinetics of three biomass feedstocks was carried out using a thermogravimetric analysis (TG) in the temperature range of 25–900 °C, at a heating rate of 10 °C/min, under a nitrogen atmosphere. It was found that the main pyrolysis process occurred in the temperature range of 200–600 °C for the three feedstock biomass samples;
- The first derivative thermogravimetry (DTG) of the mass change with temperature was plotted on the same TG graph to identify the points at which different mass changes occurred, where the decomposition reaction happened during the process of temperature ramp between 200 and 600 °C;
- The chemical components of the woody biomass (HW and SW) and refuse-derived fuel (RDF) materials were identified and distinguished based on their mass losses over the temperature range during the non-isothermal decomposition process;
- In this work, the kinetic parameters and thermal behavior of feedstocks were presented in terms of Arrhenius parameters and determined by using the Friedman method. The activation energy calculated as a function of conversion and re-exponential factor has been estimated for each biomass material;
- The present work provides insight into the decomposition characterization of biomass feedstocks, and the above results can provide useful information to predict the kinetic parameters of optimizing the gasification process conditions of downdraft gasifier systems in future research work.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| A | pre-exponential (frequency) factor (s−1) |
| Ea | apparent activation energy (J mol−1) |
| HHV | higher heating value (J/kg−1) |
| k | reaction rate (s−1) |
| n | reaction order (n = 1, first-order reaction) |
| R | universal gas constant (J mol−1 K−1) |
| R2 | correlation coefficient (--) |
| T | temperature (K or °C) |
| t | time (s) |
| X | mass fraction (g/g) |
| Greek letters | |
| β | heating rate (°C/min) |
| Abbreviations | |
| ASTM | American Society for Testing and Materials |
| DSC | differential scanning calorimetry |
| DTG | derivative or differential thermogravimetric |
| HW | hardwood |
| MSW | municipal solid waste |
| RDF | refuse-derived fuel |
| SRF | solid recovered fuel |
| SW | softwood |
| TG | thermogravimetric |
| TGA | thermogravimetric analysis |
| Subscripts | |
| 0 | initial condition |
| f | final condition |
| g | gas |
References
- Matúš, M.; Križan, P.; Šooš, Ľ.; Beniak, J. The Effect of Papermaking Sludge as an Additive to Biomass Pellets on the Final Quality of the Fuel. Fuel 2018, 219, 196–204. [Google Scholar] [CrossRef]
- Liu, Z.; Fei, B.; Jiang, Z.; Liu, X. Combustion Characteristics of Bamboo-Biochars. Bioresour. Technol. 2014, 167, 94–99. [Google Scholar] [CrossRef] [PubMed]
- IEA. Bioenergy; IEA: Paris, France, 2022; Available online: https://www.iea.org/reports/bioenergy (accessed on 22 January 2023).
- Saravanakumar, A.; Vijayakumar, P.; Hoang, A.T.; Kwon, E.E.; Chen, W.-H. Thermochemical Conversion of Large-Size Woody Biomass for Carbon Neutrality: Principles, applications, and issues. Bioresour. Technol. 2023, 370, 128562. [Google Scholar] [CrossRef] [PubMed]
- Hamzah, N.; Zandi, M.; Tokimatsu, K.; Yoshikawa, K. Wood Biomass Pellet Characterization for Solid Fuel Production in Power Generation. Int. J. Renew. Energy Sour. 2018, 3, 32–40. Available online: http://www.iaras.org/iaras/journals/ijres (accessed on 30 October 2023).
- Rada, E.; Andreottola, G. RDF/SRF: Which Perspective for Its Future in the EU. Waste Manag. 2012, 32, 1059–1060. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, A.; Carlos, M.; Bovea, M.D.; Colomer, F.J.; Albarrán, F. Analysis of Refuse-Derived Fuel from The Municipal Solid Waste Reject Fraction and Its Compliance with Quality Standards. J. Clean. Prod. 2014, 83, 118–125. [Google Scholar] [CrossRef]
- Nasrullah, M.; Vainikka, P.; Hannula, J.; Hurme, M.; Kärki, J. Mass, Energy, and Material Balances of SRF Production Process. Part 3, Solid Recovered Fuel Produced from Municipal Solid Waste. Waste Manag. Res. 2015, 33, 146–156. [Google Scholar] [CrossRef]
- Rada, E.C.; Ragazzi, M. Selective Collection as A Pretreatment for Indirect Solid Recovered Fuel Generation. Waste Manag. 2014, 34, 291–297. [Google Scholar] [CrossRef]
- Gug, J.; Cacciola, D.; Sobkowicz, M.J. Processing and Properties of Solid Energy Fuel from Municipal Solid Waste (MSW) and Recycled Plastics. Waste Manag. 2015, 35, 283–292. [Google Scholar] [CrossRef]
- Patel, C.; Lettieri, P.; Germanà, A. Techno-Economic Performance Analysis and Environmental Impact Assessment of Small to Medium Scale SRF Combustion Plants for Energy Production in the UK. Process. Saf. Environ. 2012, 90, 255–262. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Q.; Arnold, L.; Yang, W.; Blasiak, W. A Study of the Pyrolysis Behaviors of Pelletized Recovered Municipal Solid Waste Fuels. Appl. Energy 2013, 107, 173–182. [Google Scholar] [CrossRef]
- Garg, A.; Smith, R.; Hill, D.; Simms, N.; Pollard, S. Wastes as Co-Fuels: The Policy Framework for Solid Recovered Fuel (SRF) in Europe, with UK Implications. Environ. Sci. Technol. 2007, 41, 4868–4874. [Google Scholar] [CrossRef] [PubMed]
- Bosmans, A.; De Dobbelaere, C.; Lieve, H. Pyrolysis Characteristics of Excavated Waste Material Processed into Solid Fuel. Fuel 2014, 122, 198–205. [Google Scholar] [CrossRef]
- Robinson, T.; Bronson, B.; Gogolek, P.; Mehrani, P. Sample Preparation for Thermo-Gravimetric Determination and Thermo-Gravimetric Characterization of Refuse Derived Fuel. Waste Manag. 2016, 48, 265–274. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Chen, W.; Chang, N.-B. Comparative Evaluation of RDF and MSW Incineration. J. Hazard. Mater. 1998, 58, 33–45. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee Recommendations for Performing Kinetic Computations on Thermal Analysis Data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- White, J.E.; Catallo, W.J.; Legendre, B.L. Biomass Pyrolysis Kinetics: A Comparative Critical Review with Relevant Agricultural Residue Case Studies. J. Anal. Appl. Pyrolysis 2011, 91, 1–33. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Chrissafis, K.; Di Lorenzo, M.L.; Koga, N.; Pijolat, M.; Roduit, B.; Sbirrazzuoli, N.; Suñol, J.J. ICTAC Kinetics Committee Recommendations for Collecting Experimental Thermal Analysis Data for Kinetic Computations. Thermochim. Acta 2014, 590, 1–23. [Google Scholar] [CrossRef]
- Dhaundiyal, A.; Hanon, M.M. Calculation of Kinetic Parameters of the Thermal Decomposition of Residual Waste of Coniferous Species: Cedrus Deodara. Acta Technol. Agric. 2018, 21, 75–80. [Google Scholar] [CrossRef]
- Heydari, M.; Rahman, M.; Gupta, R. Kinetic Study, and Thermal Decomposition Behavior of Lignite Coal. Int. J. Chem. Eng. 2015, 2015, 481739. [Google Scholar] [CrossRef]
- Flynn, H. A General Differential Technique for the Determination of Parameters. J. Therm. Anal. 1991, 37, 293–305. [Google Scholar] [CrossRef]
- Kissinger, H. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Friedman, H. Kinetics of Thermal Degradation of Char-Forming Plastics from Thermogravimetry. Application to phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Flynn, J.; Wall, L.A. A Quick, Direct Method for The Determination of Activation Energy from Thermogravimetric Data. J. Polym. Sci. Part B Polym. Lett. 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Akahira, T.; Sunose, T.T. Joint Convention of Four Electrical Institutes, Report of Research. Chiba Inst. Technol. 1971, 16, 22–31. [Google Scholar]
- Khawam, A. Application of Solid-State Kinetics to Desolvation Reactions. Ph.D. Thesis, University of Iowa, Iowa City, IA, USA, 2007. [Google Scholar]
- Vyazovkin, S.; Sbirrazzuoli, N. Iso-Conversional Kinetic Analysis of Thermally Stimulated Processes in Polymers. Macromol. Rapid Commun. 2006, 27, 1515–1532. [Google Scholar] [CrossRef]
- Wu, W.; Cai, J.; Liu, R. Iso-Conversional Kinetic Analysis of Distributed Activation Energy Model Processes for Pyrolysis of Solid Fuels. Ind. Eng. Chem. Res. 2013, 52, 14376–14383. [Google Scholar] [CrossRef]
- Williams, B.; McIlveen-Wright, D.; Rezvani, S. Gasification of Waste Medium Density Fibreboard as a Route to Power Generation. Dev. Chem. Eng. Miner. Process. 2003, 11, 55–66. [Google Scholar] [CrossRef]
| Sample ID | Moisture Content (%) | Volatile Matter (%) | Fixed Carbon (%) | Ash (%) |
|---|---|---|---|---|
| HW | 6.06 | 78.96 | 14.02 | 0.96 |
| SW | 6.11 | 78.61 | 14.37 | 0.91 |
| RDF | 5.45 | 71.03 | 9.61 | 13.91 |
| ID | Carbon (%) | Hydrogen (%) | Oxygen (%) | Nitrogen (%) | Sulfur (%) | Calorific Value (kJ/kg) |
|---|---|---|---|---|---|---|
| HW | 51.93 | 5.61 | 42.00 | 0.42 | 0.04 | 19,230 |
| SW | 50.71 | 5.92 | 43.00 | 0.35 | 0.02 | 18,301 |
| RDF | 56.30 | 6.93 | 36.07 | 0.51 | 0.19 | 20,900 |
| X (%) | HW | SW | RDF | |||
|---|---|---|---|---|---|---|
| (kJmol−1) | A (s−1) | (kJmol−1) | A (s−1) | (kJmol−1) | A (s−1) | |
| 30 | 78.92 | 32,316.41 | 61.01 | 12,291.18 | 54.98 | 5621.37 |
| 50 | 93.62 | 27,141.05 | 69.20 | 10,280.94 | 60.62 | 4725.85 |
| 70 | 99.62 | 22,620.87 | 77.17 | 8560.15 | 69.33 | 3934.85 |
| Mean | 90.72 | 27,359.44 | 69.13 | 10,377.42 | 61.64 | 4760.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almusafir, R.; Smith, J.D. Thermal Decomposition and Kinetic Parameters of Three Biomass Feedstocks for the Performance of the Gasification Process Using a Thermogravimetric Analyzer. Energies 2024, 17, 396. https://doi.org/10.3390/en17020396
Almusafir R, Smith JD. Thermal Decomposition and Kinetic Parameters of Three Biomass Feedstocks for the Performance of the Gasification Process Using a Thermogravimetric Analyzer. Energies. 2024; 17(2):396. https://doi.org/10.3390/en17020396
Chicago/Turabian StyleAlmusafir, Rania, and Joseph D. Smith. 2024. "Thermal Decomposition and Kinetic Parameters of Three Biomass Feedstocks for the Performance of the Gasification Process Using a Thermogravimetric Analyzer" Energies 17, no. 2: 396. https://doi.org/10.3390/en17020396
APA StyleAlmusafir, R., & Smith, J. D. (2024). Thermal Decomposition and Kinetic Parameters of Three Biomass Feedstocks for the Performance of the Gasification Process Using a Thermogravimetric Analyzer. Energies, 17(2), 396. https://doi.org/10.3390/en17020396







