Electrochemical Mechanism Underlying Lithium Plating in Batteries: Non-Invasive Detection and Mitigation
Abstract
1. Introduction
| Physical and Chemical Mechanism of Damage | Effect on Battery Performance |
|---|---|
| Continuous growth of SEI | Loss of cyclable lithium resulting in capacity fade and impedance rise [33]. |
| Lithium plating and electrolyte decomposition | Loss of cyclable lithium ions results in rapid capacity loss [24,26]. |
| Particle cracking and solvent intercalation | Loss of active electrode material and lithium ions showing rapid capacity fade, increase in overpotential [24,34]. |
| Loss in porosity due to irreversible salt deposition | Impedance rises in the cell [24,35]. |
| Delamination and dissolution of electrode material | Loss of active material and capacity fade [36,37]. |
| Decomposition of electrolyte and binder and gas evolution | Loss of cyclable lithium, impedance rise, and capacity fade of the cell [24]. |
| Corrosion of current collector | Impedance rises and inhomogeneous current-voltage distribution [24,38]. |

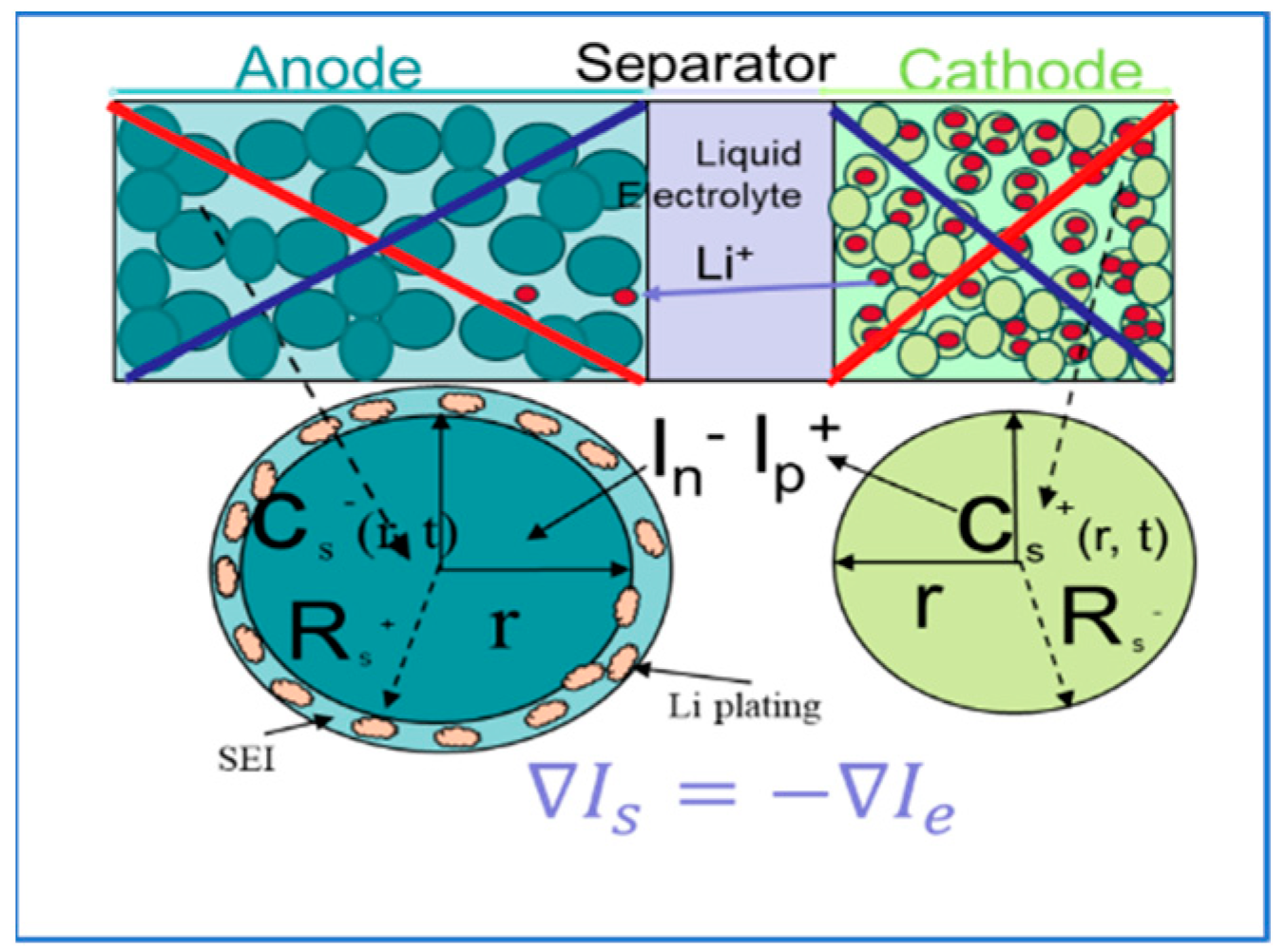
2. Fundamental Mechanism Behind Lithium Deposition
3. Reason for Lithium Plating
3.1. Internal and External Factors Affecting Lithium Plating
3.2. Internal Variables
3.2.1. Manufacturing Conditions and Local Defects
3.2.2. Aging Condition
3.3. External Variable
3.3.1. Effect of High Charging Rate Protocol
3.3.2. Operating Temperature
Low Temperature
Non-Uniform Temperature Gradient
3.3.3. Effect of Applied Loads
3.3.4. Overcharging and High State of Charge Condition
4. Plating Characterization and Estimation
4.1. Electrochemical Impedance Spectroscopy (EIS)
4.2. Voltage Plateau Analysis (VRP)
| Characterization Types | Ex Situ | In Situ | Advantages | Disadvantages | Ref |
|---|---|---|---|---|---|
| Electrochemical Impedance Spectroscopy | X | It can detect and quantify the trend of degradation mechanisms. | It required a controlled and stable electrochemical system with specialized equipment. | [57,114,115,116,117,123] | |
| Voltage Plateau (VRP) Analysis | X | VRP detects phase change in reversible lithium during stripping. | It cannot quantify the actual plating and stripping capacity. | [33,46,68,128,129,130] | |
| Incremental Capacity (IC) and Differential Voltage (DV) Curve | X | IC and DV can detect the stripping lithium and its mass during discharging. | It does not provide information on the total plating loss and the stripping mass during CV charging. | [31,55,133] | |
| Coulombic Efficiency (CE) and Discharge Capacity | X | Most of the battery testers collect these data as a primary measurement. A significant drop in it signifies lithium plating. | It does not differentiate between different losses and fails to quantify them individually. | [33,134,135] | |
| Dynamic Capacity Measurement | X | DCM can dynamically indicate the onset of lithium plating and stripping in real time. It can also monitor the change in the capacitance of the electrical double layer. | DCM requires a specific frequency. Otherwise, its measured reading will be noisy and overlap with the cathodic background signature. | [131,132] | |
| Physics-based and Data-driven Modeling | X | Physics-based electrochemical modeling provides insights such as plating onset time, degradation quantification, and its behavior under different conditions. | It requires specialized domain knowledge and higher computing power. | [46,136,137,138,139,140,141,142] |
4.3. Incremental Capacity and Differential Voltage Curve
4.4. Discharge Capacity and Coulombic Efficiency (CE)
4.5. Dynamic Capacity Measurement and Differential Current Analysis
4.6. Physics-Based and Data-Driven Modeling
5. Mitigation and Countermeasures
5.1. Electrolyte Engineering
5.1.1. Solvation Behavior Modification

5.1.2. Additive Addition
5.2. SEI Layer Modification
| Type of Factor | Mechanism | Effectivity and Limitation | Ref |
|---|---|---|---|
| Solvation Behavior Modification | Changing the chemical composition of the electrolyte by adding methyl acetate, fluorobenzene-based electrolyte, and acetonitrile solvents to form a stable SEI layer that can transport lithium quicker and enhance the desolvation process. | Ensured better-charged transfer at low temperatures and fast charging rates. The higher cost of production limits its application. It is also challenging to find strongly bound solvents for lithium ions. | [148,150,151] |
| Additive Addition to the Electrolyte | Additives (LIBFEP, LPSE) act as a deoxidizing agent in the electrolyte, and they (a) accelerate lithium-ion transport at the interface and (b) suppress its dendritic growth. In addition, additives also help (c) to reduce viscosity and (d) improve the transference number of the electrolyte. | It improves the coulombic efficiency and, eventually, higher capacity retention after fast cycling. It also (a) improves high charging rate performance, (b) increases ionic conductivity, and (c) enhances low-temperature performance. This eventually replicates into higher capacity retention, increased power densities, and reduced anodic interface resistance. However, this process is costly and not suitable for larger scales. Adulterated electrolytes sometimes react with the current collector and generate gases. It still needs to be commercially used and needs proper optimization. | [158,159,160,161,162,163,164,166,167,168,169,170] |
| SEI Layer Modification | Artificially formed LIF-rich, thin SEI layer controls the lithium flux and mechanically inhibits dendritic plating. | It improves cycling efficiency during fast charging. More literature is needed in this direction. | [153,165] |
| AC Pulse Charging | Application of the alternating pulse current relaxes charge polarization, which ultimately reduces plating. Pulse heating generates heat and provides an optimum temperature for charging. | Pulse charging has the potential for rapid adaptability. However, limitations included the unavailability of low-cost ICs for switching high currents. Pulse heating is still limited in lab-scale studies. | [43,155,171,172,173,174] |
| Adaptive Charging Strategy | Variable charging rates in real time using a feedback controller. | Adaptive charging has great potential, but the current application is limited due to the unavailability of a cheap, fast, and intelligent feedback controller. | [126,145,175] |
| Externally Applied Field | External magnetic field affected the movement of charged lithium ion due to Lorentz force and reduced the non-uniformity in lithium plating. | Research shows it can result in significant capacity gain. More literature is needed in this direction. | [135,176,177] |
| Surface Engineering | Engineered pore increases anode porosity. Sometimes, making a flexible ionic transport channel reduces the charge overpotential and limits the plating. | This method is still far from commercial use due to its customization. | [156,157,178,179,180,181] |
| Internal Heating Arrangement | Adding an external heating element into the battery enhances the electrolyte mobility and electrode kinetics. | It shows minimal degradation. The main drawback of this design is the addition of an extra heating element to the cell design, which increases the cost and weight. Cell fabrication also has a critical role to play. | [42,182] |
5.3. Alternating Current Pulse Charging and Pulse Heating
5.4. Adaptive Charging Strategy
5.5. External Magnetic Field
5.6. Surface Engineering
5.6.1. Surface Channeling
5.6.2. Gradient Porosity Distribution
5.7. Internal Heating Arrangement
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| I = Current density |
| k = Kinetic constant |
| c = Lithium concentration |
| F = Faraday number |
| T = Temperature |
| UOCP = Open circuit potential |
| m = Mass of plated material |
| suffix |
| e = Electrolyte |
| s = Solid |
| n = Negative electrode |
| pl = Plating |
| st = Stripping |
| el = Intercalating |
| surf = Surface |
References
- Delmotte, V.M.; Zhai, P.; Pirani, A.; Connors, S.L.; Pean, C.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; Mathews, J.B.R.; Berger, S.; et al. Climate Change 2021: The Physical Science Basis; Contribution of Working Group I to the IPCC Sixth Assessment Report; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- UN Climate Press Release. COP28 Agreement Signals “Beginning of the End” of the Fossil Fuel Era. 2023. Available online: https://unfccc.int/news/cop28-agreement-signals-beginning-of-the-end-of-the-fossil-fuel-era (accessed on 8 April 2024).
- Xiong, R.; Sun, F.; He, H.; Nguyen, T.D. A data-driven adaptive state of charge and power capability joint estimator of lithium-ion polymer battery used in electric vehicles. Energy 2013, 63, 295–308. [Google Scholar] [CrossRef]
- Pathak, P.K.; Gupta, A.R. Battery Energy Storage System. In Proceedings of the 2018 4th International Conference on Computational Intelligence & Communication Technology (CICT), Ghaziabad, India, 9–10 February 2018. [Google Scholar]
- Diouf, B.; Pode, R. Potential of lithium-ion batteries in renewable energy. Renew. Energy 2015, 76, 375–380. [Google Scholar] [CrossRef]
- Das, S.; Banerjee, T.; Mondal, N. A Comparative CFD Study to Analyze the Performance of NACA 0018 and S1210 Darrieus Wind Turbine Blade. In Fluid Mechanics and Fluid Power (Vol. 2); Springer Nature: Singapore, 2023; pp. 279–284. [Google Scholar]
- Slinger, K. Electrical Batteries for Renewable Energy. 2015. Available online: https://sites.tufts.edu/eeseniordesignhandbook/2015/electrical-batteries-for-renewable-energy/ (accessed on 10 April 2024).
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Abraham, K.M. How Comparable Are Sodium-Ion Batteries to Lithium-Ion Counterparts? ACS Energy Lett. 2020, 5, 3544–3547. [Google Scholar] [CrossRef]
- Masias, A.; Marcicki, J.; Paxton, W.A. Opportunities and Challenges of Lithium Ion Batteries in Automotive Applications. ACS Energy Lett. 2021, 6, 621–630. [Google Scholar] [CrossRef]
- Frith, J.T.; Lacey, M.J.; Ulissi, U. A non-academic perspective on the future of lithium-based batteries. Nat. Commun. 2023, 14, 420. [Google Scholar] [CrossRef]
- Belgibayeva, A.; Rakhmetova, A.; Rakhatkyzy, M.; Kairova, M.; Mukushev, I.; Issatayev, N.; Kalimuldina, G.; Nurpeissova, A.; Sun, Y.-K.; Bakenov, Z. Lithium-ion batteries for low-temperature applications: Limiting factors and solutions. J. Power Sources 2023, 557, 232550. [Google Scholar]
- El Haj Assad, M.; Khosravi, A.; Malekan, M.; Rosen, M.A.; Nazari, M.A. Chapter 14—Energy storage. In Design and Performance Optimization of Renewable Energy Systems; Assad, M.E.H., Rosen, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 205–219. [Google Scholar]
- Yin, Y.; Shen, C.; Yturriaga, S.; Zheng, J. The Power-Energy Coupling Effect of Mixed Hard-Carbon/Graphite Anode. J. Mater. Sci. Chem. Eng. 2021, 9, 16–31. [Google Scholar] [CrossRef]
- Birrozzi, A.; Copley, M.; Zamory, J.V.; Pasqualini, M.; Calcaterra, S.; Nobili, F.; Cicco, A.D.; Rajantie, H.; Briceno, M.; Bilbe, E.; et al. Scaling up “Nano” Li4Ti5O12 for High-Power Lithium-Ion Anodes Using Large Scale Flame Spray Pyrolysis. J. Electrochem. Soc. 2015, 162, A2331. [Google Scholar] [CrossRef]
- Toigo, C.; Frankenberger, M.; Billot, N.; Pscherer, C.; Stumper, B.; Distelrath, F.; Schubert, J.; Pettinger, K.H.; Arbizzani, A. Improved Li4Ti5O12 electrodes by modified current collector surface. Electrochim. Acta 2021, 392, 138978. [Google Scholar] [CrossRef]
- Xiong, Z.; Yun, Y.S.; Jin, H.-J. Applications of Carbon Nanotubes for Lithium Ion Battery Anodes. Materials 2013, 6, 1138. [Google Scholar] [CrossRef]
- Manthiram, A. An Outlook on Lithium Ion Battery Technology. ACS Cent. Sci. 2017, 3, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Klett, M.; Eriksson, R.; Groot, J.; Svens, C.; Högström, K.; Lindström, R.W.; Berg, H.; Gustafson, T.; Lindbergh, G.; Edström, K. Non-uniform aging of cycled commercial LiFePO4//graphite cylindrical cells revealed by post-mortem analysis. J. Power Sources 2014, 257, 126–137. [Google Scholar] [CrossRef]
- Janakiraman, U.; Garrick, T.R.; Fortier, M.E. Review—Lithium Plating Detection Methods in Li-Ion Batteries. J. Electrochem. Soc. 2020, 167, 160552. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, X.; Usubelli, C.; Mayer, D.; Linder, C.; Christensen, J. Understanding thermal and mechanical effects on lithium plating in lithium-ion batteries. J. Power Sources 2022, 541, 231632. [Google Scholar] [CrossRef]
- Bach, T.C.; Schuster, S.F.; Fleder, E.; Müller, J.; Brand, M.J.; Lorrmann, H.; Jossen, A.; Sextl, G. Nonlinear aging of cylindrical lithium-ion cells linked to heterogeneous compression. J. Energy Storage 2016, 5, 212–223. [Google Scholar] [CrossRef]
- Broussely, M.; Herreyre, S.; Biensan, P.; Kasztejna, P.; Nechev, K.; Staniewicz, R.J. Aging mechanism in Li ion cells and calendar life predictions. J. Power Sources 2001, 97, 13–21. [Google Scholar] [CrossRef]
- Zhou, J.; Notten, P.H.L. Studies on the degradation of Li-ion batteries by the use of microreference electrodes. J. Power Sources 2008, 177, 553–560. [Google Scholar] [CrossRef]
- Grolleau, S.; Delaille, A.; Gualous, H.; Gyan, P.; Revel, R.; Bernard, J.; Redondo-Iglesias, E.; Peter, J. Calendar aging of commercial graphite/LiFePO4 cell—Predicting capacity fade under time dependent storage conditions. J. Power Sources 2014, 255, 450–458. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Bai, J.; Zhou, L.; Wang, Z. Influence of lithium plating on lithium-ion battery aging at high temperature. Electrochim. Acta 2023, 454, 142362. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, X.; Li, Z.; Mei, Y.; Zhang, Y.; Liu, L.; Wang, K.; Gu, H.; Li, L. Preventing Sudden Death of High-Energy Lithium-Ion Batteries at Elevated Temperature Through Interfacial Ion-Flux Rectification. Adv. Funct. Mater. 2023, 33, 2208329. [Google Scholar] [CrossRef]
- Choi, J.; Manthiram, A. Role of Chemical and Structural Stabilities on the Electrochemical Properties of Layered LiNi1 ∕ 3Mn1 ∕ 3Co1 ∕ 3O2 Cathodes. J. Electrochem. Soc. 2005, 152, A1714. [Google Scholar] [CrossRef]
- Choi, N.-S.; Yeon, J.-T.; Lee, Y.-W.; Han, J.-G.; Lee, K.T.; Kim, S.-S. Degradation of spinel lithium manganese oxides by low oxidation durability of LiPF6-based electrolyte at 60 °C. Solid State Ion. 2012, 219, 41–48. [Google Scholar] [CrossRef]
- Downie, L.E.; Krause, L.J.; Burns, J.C.; Jensen, L.D.; Chevrier, V.L.; Dahn, J.R. In Situ Detection of Lithium Plating on Graphite Electrodes by Electrochemical Calorimetry. J. Electrochem. Soc. 2013, 160, A588–A594. [Google Scholar] [CrossRef]
- Petzl, M.; Kasper, M.; Danzer, M.A. Lithium plating in a commercial lithium-ion battery A low-temperature aging study. J. Power Sources 2015, 275, 799–807. [Google Scholar] [CrossRef]
- Cannarella, J.; Arnold, C.B. Stress evolution and capacity fade in constrained lithium-ion pouch cells. J. Power Sources 2014, 245, 745–751. [Google Scholar] [CrossRef]
- Sarkar, A.; Shrotriya, P.; Nlebedim, I.C. Anodic interfacial evolution in extremely fast charged lithium-ion batteries. ACS Appl. Energy Mater. 2022, 5, 3179–3188. [Google Scholar] [CrossRef]
- Ruess, R.; Schweidler, S.; Hemmelmann, H.; Conforto, G.; Bielefeld, A.; Weber, D.A.; Sann, J.; Elm, M.T.; Janek, J. Influence of NCM Particle Cracking on Kinetics of Lithium-Ion Batteries with Liquid or Solid Electrolyte. J. Electrochem. Soc. 2020, 167, 100532. [Google Scholar] [CrossRef]
- Aurbach, D.; Markovsky, B.; Rodkin, A.; Cojocaru, M.; Levi, E.; Kim, H.-J. An analysis of rechargeable lithium-ion batteries after prolonged cycling. Electrochim. Acta 2002, 47, 1899–1911. [Google Scholar] [CrossRef]
- Amatucci, G.G.; Tarascon, J.M.; Klein, L.C. Cobalt dissolution in LiCoO2-based non-aqueous rechargeable batteries. Solid State Ion. 1996, 83, 167–173. [Google Scholar] [CrossRef]
- Arora, P.; White, R.E.; Doyle, M. Capacity Fade Mechanisms and Side Reactions in Lithium-Ion Batteries. J. Electrochem. Soc. 1998, 145, 3647. [Google Scholar] [CrossRef]
- Guo, L.; Thornton, D.B.; Koronfel, M.A.; Stephens, I.E.L.; Ryan, M.P. Degradation in lithium ion battery current collectors. J. Phys. Energy 2021, 3, 032015. [Google Scholar]
- Vetter, J.; Novak, P.; Wagner, M.R.; Veit, C.; Moller, K.C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Lin, X.K.; Khosravinia, K.; Hu, X.S.; Li, J.; Lu, W. Lithium Plating Mechanism, Detection, and Mitigation in Lithium-Ion Batteries. Prog. Energy Combust. Sci. 2021, 87, 100953. [Google Scholar] [CrossRef]
- Burns, J.C.; Stevens, D.A.; Dahn, J.R. In-situ detection of lithium plating using high precision coulometry. J. Electrochem. Soc. 2015, 162, A959–A964. [Google Scholar] [CrossRef]
- Yang, X.-G.; Zhang, G.; Ge, S.; Wang, C.-Y. Fast charging of lithium-ion batteries at all temperatures. Proc. Natl. Acad. Sci. USA. 2018, 115, 7266–7271. [Google Scholar] [CrossRef]
- Purushothaman, B.K.; Landau, U. Rapid Charging of Lithium-Ion Batteries Using Pulsed Currents: A Theoretical Analysis. J. Electrochem. Soc. 2006, 153, A533. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, K.-H.; Sanchez, A.J.; Kazyak, E.; Goel, V.; Gorlin, Y.; Christensen, J.; Thornton, K.; Dasgupta, N.P. Operando video microscopy of Li plating and re-intercalation on graphite anodes during fast charging. J. Mater. Chem. A 2021, 9, 23522–23536. [Google Scholar] [CrossRef]
- Gao, T.; Han, Y.; Fraggedakis, D.; Das, S.; Zhou, T.; Yeh, C.-N.; Xu, S.; Chueh, W.C.; Li, J.; Bazant, M.Z. Interplay of Lithium Intercalation and Plating on a Single Graphite Particle. Joule 2021, 5, 393–414. [Google Scholar] [CrossRef]
- Sarkar, A.; Nlebedim, I.C.; Shrotriya, P. Performance degradation due to anodic failure mechanisms in lithium-ion batteries. J. Power Sources 2021, 502, 229145. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, S.; Li, R.; Ren, D.; Yi, M.; Xu, C.; Han, X.; Lu, L.; Friess, B.; Offer, G.; et al. Inhomogeneous degradation induced by lithium plating in a large-format lithium-ion battery. J. Power Sources 2022, 542, 231753. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Bai, J. Influences of multi factors on thermal runaway induced by overcharging of lithium-ion battery. J. Energy Chem. 2022, 70, 531–541. [Google Scholar] [CrossRef]
- Lyu, P.; Liu, X.; Qu, J.; Zhao, J.; Huo, Y.; Qu, Z.; Rao, Z. Recent advances of thermal safety of lithium ion battery for energy storage. Energy Storage Mater. 2020, 31, 195–220. [Google Scholar] [CrossRef]
- Wang, B.; Le Fevre, L.W.; Brookfield, A.; McInnes, E.J.L.; Dryfe, R.A.W. Resolution of Lithium Deposition versus Intercalation of Graphite Anodes in Lithium Ion Batteries: An In Situ Electron Paramagnetic Resonance Study. Angew. Chem. Int. Ed. 2021, 60, 21860–21867. [Google Scholar] [CrossRef]
- Wang, C.; Yang, C.; Zheng, Z. Toward Practical High-Energy and High-Power Lithium Battery Anodes: Present and Future. Adv. Sci. 2022, 9, 2105213. [Google Scholar] [CrossRef]
- Rangarajan, S.P.; Fear, C.; Adhikary, T.; Barsukov, Y.; Dadheech, G.; Mukherjee, P.P. Dynamics of lithium stripping on graphite electrodes after fast charging. Cell Rep. Phys. Sci. 2023, 4, 101740. [Google Scholar] [CrossRef]
- Keyser, M.; Pesaran, A.; Li, Q.; Santhanagopalan, S.; Smith, K.; Wood, E.; Ahmed, S.; Bloom, I.; Dufek, E.; Shirk, M.; et al. Enabling fast charging—Battery thermal considerations. J. Power Sources 2017, 367, 228–236. [Google Scholar] [CrossRef]
- Gargh, P.P.; Sarkar, A.; Nlebedim, I.C.; Shrotriya, P. Lithium plating induced degradation during fast charging of batteries subjected to compressive loading. J. Energy Storage 2024, 84, 110701. [Google Scholar] [CrossRef]
- Kurzweil, P.; Scheuerpflug, W.; Frenzel, B.; Schell, C.; Schottenbauer, J. Differential Capacity as a Tool for SOC and SOH Estimation of Lithium Ion Batteries Using Charge/Discharge Curves, Cyclic Voltammetry, Impedance Spectroscopy, and Heat Events: A Tutorial. Energies 2022, 15, 4520. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Y.; Chen, L.; Zhang, S.; Zhu, X.; Shen, T.; Ren, D.; He, X.; Qiu, J.; Wang, L.; et al. New Insight on Graphite Anode Degradation Induced by Li-Plating. Energy Environ. Mater. 2022, 5, 872–876. [Google Scholar] [CrossRef]
- Li, Y.; Feng, X.; Ren, D.; Ouyang, M.; Lu, L.; Han, Z. Thermal Runaway Triggered by Plated Lithium on the Anode after Fast Charging. ACS Appl. Mater. Interfaces 2019, 11, 46839–46850. [Google Scholar] [CrossRef]
- Gargh, P.; Sarkar, A.; Lui, Y.H.; Shen, S.; Hu, C.; Hu, S.; Nlebedim, I.C.; Shrotriya, P. Correlating capacity fade with film resistance loss in fast charging of lithium-ion battery. J. Power Sources 2021, 485, 229360. [Google Scholar] [CrossRef]
- Birkenmaier, C.; Bitzer, B.; Harzheim, M.; Hintennach, A.; Schleid, T. Lithium Plating on Graphite Negative Electrodes: Innovative Qualitative and Quantitative Investigation Methods. J. Electrochem. Soc. 2015, 162, A2646. [Google Scholar] [CrossRef]
- Fonseca Rodrigues, M.-T.; Maroni, V.A.; Gosztola, D.J.; Yao, K.P.C.; Kalaga, K.; Shkrob, I.A.; Abraham, D.P. Lithium Acetylide: A Spectroscopic Marker for Lithium Deposition During Fast Charging of Li-Ion Cells. ACS Appl. Energy Mater. 2019, 2, 873–881. [Google Scholar] [CrossRef]
- Tang, Y.; Shen, K.; Lv, Z.; Xu, X.; Hou, G.; Cao, H.; Wu, L.; Zheng, G.; Deng, Y. Three-dimensional ordered macroporous Cu current collector for lithium metal anode: Uniform nucleation by seed crystal. J. Power Sources 2018, 403, 82–89. [Google Scholar] [CrossRef]
- Xie, W.; Liu, X.; He, R.; Li, Y.; Gao, X.; Li, X.; Peng, Z.; Feng, S.; Feng, X.; Yang, S. Challenges and opportunities toward fast-charging of lithium-ion batteries. J. Energy Storage 2020, 32, 101837. [Google Scholar] [CrossRef]
- Honbo, H.; Takei, K.; Ishii, Y.; Nishida, T. Electrochemical properties and Li deposition morphologies of surface modified graphite after grinding. J. Power Sources 2009, 189, 337–343. [Google Scholar] [CrossRef]
- Lin, H.P.; Chua, D.; Salomon, M.; Shiao, H.C.; Hendrickson, M.; Plichta, E.; Slane, S. Low-Temperature Behavior of Li-Ion Cells. Electrochem. Solid-State Lett. 2001, 4, A71. [Google Scholar] [CrossRef]
- Sheng, L.; Wang, Q.; Liu, X.; Cui, H.; Wang, X.; Xu, Y.; Li, Z.; Wang, L.; Chen, Z.; Xu, G.-L.; et al. Suppressing electrolyte-lithium metal reactivity via Li+-desolvation in uniform nano-porous separator. Nat. Commun. 2022, 13, 172. [Google Scholar] [CrossRef]
- Hogrefe, C.; Waldmann, T.; Hölzle, M.; Wohlfahrt-Mehrens, M. Direct observation of internal short circuits by lithium dendrites in cross-sectional lithium-ion in situ full cells. J. Power Sources 2023, 556, 232391. [Google Scholar] [CrossRef]
- Schuster, S.F.; Bach, T.; Fleder, E.; Müller, J.; Brand, M.; Sextl, G.; Jossen, A. Nonlinear aging characteristics of lithium-ion cells under different operational conditions. J. Energy Storage 2015, 1, 44–53. [Google Scholar] [CrossRef]
- Meyer, J.M.; Harrison, K.L.; Mukherjee, P.P.; Roberts, S.A. Developing a model for the impact of non-conformal lithium contact on electro-chemo-mechanics and dendrite growth. Cell Rep. Phys. Sci. 2023, 4, 101364. [Google Scholar] [CrossRef]
- Sarkar, A.; Shrotriya, P.; Nlebedim, I.C. Parametric analysis of anodic degradation mechanisms for fast charging lithium batteries with graphite anode. Comput. Mater. Sci. 2022, 202, 110979. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Y.; Kim, S.C.; Pei, A.; Li, Y.; Boyle, D.T.; Wang, H.; Zhang, Z.; Ye, Y.; Huang, W.; et al. Underpotential lithium plating on graphite anodes caused by temperature heterogeneity. Proc. Natl. Acad. Sci. USA 2020, 117, 29453–29461. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Bloom, I.; Jansen, A.N.; Tanim, T.; Dufek, E.J.; Pesaran, A.; Burnham, A.; Carlson, R.B.; Dias, F.; Hardy, K.; et al. Enabling fast charging—A battery technology gap assessment. J. Power Sources 2017, 367, 250–262. [Google Scholar] [CrossRef]
- Hein, S.; Latz, A. Influence of local lithium metal deposition in 3D microstructures on local and global behavior of Lithium-ion batteries. Electrochim. Acta 2016, 201, 354–365. [Google Scholar] [CrossRef]
- Swiderska-Mocek, A.; Rudnicka, E.; Lewandowski, A. Temperature coefficients of Li-ion battery single electrode potentials and related entropy changes—Revisited. Phys. Chem. Chem. Phys. 2019, 21, 2115–2120. [Google Scholar] [CrossRef]
- Bazinski, S.J.; Wang, X. The Influence of Cell Temperature on the Entropic Coefficient of a Lithium Iron Phosphate (LFP) Pouch Cell. J. Electrochem. Soc. 2014, 161, A168. [Google Scholar] [CrossRef]
- Takano, K.; Saito, Y.; Kanari, K.; Nozaki, K.; Kato, K.; Negishi, A.; Kato, T. Entropy change in lithium-ion cells on charge and discharge. J. Appl. Electrochem. 2002, 32, 251–258. [Google Scholar] [CrossRef]
- Bai, P.; Li, J.; Brushett, F.R.; Bazant, M.Z. Transition of lithium growth mechanisms in liquid electrolytes. Energy Environ. Sci. 2016, 9, 3221–3229. [Google Scholar] [CrossRef]
- Legrand, N.; Knosp, B.; Desprez, P.; Lapicque, F.; Rael, S. Physical characterization of the charging process of a Li-ion battery and prediction of Li plating by electrochemical modelling. J. Power Sources 2014, 245, 208–216. [Google Scholar] [CrossRef]
- Bazant, M.Z. Thermodynamic stability of driven open systems and control of phase separation by electro-autocatalysis. Faraday Discus. 2017, 199, 423–463. [Google Scholar] [CrossRef] [PubMed]
- Bazant, M.Z. Theory of Chemical Kinetics and Charge Transfer based on Nonequilibrium Thermodynamics. Acc. Chem. Res. 2013, 46, 1144–1160. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.-N.; Yang, S.-J.; Liu, H.; Cheng, X.-B.; Liu, L.; Xiang, R.; Zhang, Q.; Kaskel, S.; Huang, J.-Q. Mechanism understanding for stripping electrochemistry of Li metal anode. SusMat 2021, 1, 506–536. [Google Scholar] [CrossRef]
- Park, G.; Gunawardhana, N.; Nakamura, H.; Lee, Y.S.; Yoshio, M. The study of electrochemical properties and lithium deposition of graphite at low temperature. J. Power Sources 2012, 199, 293–299. [Google Scholar] [CrossRef]
- Gao, X.; Li, S.; Xue, J.; Hu, D.; Xu, J. A Mechanistic and Quantitative Understanding of the Interactions between SiO and Graphite Particles. Adv. Energy Mater. 2023, 13, 2202584. [Google Scholar] [CrossRef]
- Son, Y.; Cha, H.; Lee, T.; Kim, Y.; Boies, A.; Cho, J.; De Volder, M. Analysis of Differences in Electrochemical Performance Between Coin and Pouch Cells for Lithium-Ion Battery Applications. Energy Environ. Mater. 2023, 7, e12615. [Google Scholar] [CrossRef]
- Spingler, F.B.; Friedrich, S.; Kücher, S.; Schmid, S.; López-Cruz, D.; Jossen, A. The Effects of Non-Uniform Mechanical Compression of Lithium-Ion Cells on Local Current Densities and Lithium Plating. J. Electrochem. Soc. 2021, 168, 110515. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Hu, J.; Zhang, P.; Zhang, L.; Lao, L. Investigation on calendar experiment and failure mechanism of lithium-ion battery electrolyte leakage. J. Energy Storage 2022, 54, 105286. [Google Scholar] [CrossRef]
- Deichmann, E.; Torres-Castro, L.; Lamb, J.; Karulkar, M.; Ivanov, S.; Grosso, C.; Gray, L.; Langendorf, J.; Garzon, F. Investigating the Effects of Lithium Deposition on the Abuse Response of Lithium-Ion Batteries. J. Electrochem. Soc. 2020, 167, 090552. [Google Scholar] [CrossRef]
- Smith, A.J.; Fang, Y.; Mikheenkova, A.; Ekström, H.; Svens, P.; Ahmed, I.; Lacey, M.J.; Lindbergh, G.; Furó, I.; Lindström, R.W. Localized lithium plating under mild cycling conditions in high-energy lithium-ion batteries. J. Power Sources 2023, 573, 233118. [Google Scholar] [CrossRef]
- Attia, P.M.; Bills, A.; Planella, F.B.; Dechent, P.; dos Reis, G.; Dubarry, M.; Gasper, P.; Gilchrist, R.; Greenbank, S.; Howey, D.; et al. Review—“Knees” in Lithium-Ion Battery Aging Trajectories. J. Electrochem. Soc. 2022, 169, 060517. [Google Scholar] [CrossRef]
- Ecker, M.; Sabet, P.S.; Sauer, D.U. Influence of operational condition on lithium plating for commercial lithium-ion batteries—Electrochemical experiments and post-mortem-analysis. Appl. Energy 2017, 206, 934–946. [Google Scholar] [CrossRef]
- Cannarella, J.; Arnold, C.B. The Effects of Defects on Localized Plating in Lithium-Ion Batteries. J. Electrochem. Soc. 2015, 162, A1365–A1373. [Google Scholar] [CrossRef]
- Peabody, C.; Arnold, C.B. The role of mechanically induced separator creep in lithium-ion battery capacity fade. J. Power Sources 2011, 196, 8147–8153. [Google Scholar] [CrossRef]
- Matasso, A.; Wetz, D.; Liu, F. The Effects of Internal Pressure Evolution on the Aging of Commercial Li-Ion Cells. ECS Trans. 2014, 58, 37. [Google Scholar] [CrossRef]
- Pan, Y.; Zhong, Z. Modeling the Ion Transport Restriction in Mechanically Strained Separator Membranes. J. Electrochem. Soc. 2014, 161, A583. [Google Scholar] [CrossRef]
- Antartis, D.; Dillon, S.; Chasiotis, I. Effect of porosity on electrochemical and mechanical properties of composite Li-ion anodes. J. Compos. Mater. 2015, 49, 1849–1862. [Google Scholar] [CrossRef]
- Yao, F.; Güneş, F.; Ta, H.Q.; Lee, S.M.; Chae, S.J.; Sheem, K.Y.; Cojocaru, C.S.; Xie, S.S.; Lee, Y.H. Diffusion Mechanism of Lithium Ion through Basal Plane of Layered Graphene. J. Am. Chem. Soc. 2012, 134, 8646–8654. [Google Scholar] [CrossRef]
- McShane, E.J.; Colclasure, A.M.; Brown, D.E.; Konz, Z.M.; Smith, K.; McCloskey, B.D. Quantification of Inactive Lithium and Solid–Electrolyte Interphase Species on Graphite Electrodes after Fast Charging. ACS Energy Lett. 2020, 5, 2045–2051. [Google Scholar] [CrossRef]
- Tang, M.; Albertus, P.; Newman, J. Two-Dimensional Modeling of Lithium Deposition during Cell Charging. J. Electrochem. Soc. 2009, 156, A390. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Yang, S.-J.; Yan, C.; Huang, J.-Q. Electrolyte inhomogeneity induced lithium plating in fast charging lithium-ion batteries. J. Energy Chem. 2022, 73, 394–399. [Google Scholar] [CrossRef]
- Dubarry, M.; Truchot, C.; Liaw, B.Y. Synthesize battery degradation modes via a diagnostic and prognostic model. J. Power Sources 2012, 219, 204–216. [Google Scholar] [CrossRef]
- Zhou, H.; Fear, C.; Jeevarajan, J.A.; Mukherjee, P.P. State-of-electrode (SOE) analytics of lithium-ion cells under overdischarge extremes. Energy Storage Mater. 2023, 54, 60–74. [Google Scholar] [CrossRef]
- Yin, T.; Jia, L.; Li, X.; Zheng, L.; Dai, Z. Effect of High-Rate Cycle Aging and Over-Discharge on NCM811 (LiNi0.8Co0.1Mn0.1O2) Batteries. Energies 2022, 15, 2862. [Google Scholar] [CrossRef]
- Wang, L.; Xie, L.; Song, Y.; Liu, X.; Zhang, H.; He, X. Identifying cathode and anode polarizations during practical high-rate charging/discharging in different Li-ion pouch batteries. Battery Energy 2023, 2, 20220025. [Google Scholar] [CrossRef]
- Mei, W.; Zhang, L.; Sun, J.; Wang, Q. Experimental and numerical methods to investigate the overcharge caused lithium plating for lithium ion battery. Energy Storage Mater. 2020, 32, 91–104. [Google Scholar] [CrossRef]
- Louli, A.J.; Genovese, M.; Weber, R.; Hames, S.G.; Logan, E.R.; Dahn, J.R. Exploring the Impact of Mechanical Pressure on the Performance of Anode-Free Lithium Metal Cells. J. Electrochem. Soc. 2019, 166, A1291–A1299. [Google Scholar] [CrossRef]
- Jaguemont, J.; Boulon, L.; Dube, Y.; Poudrier, D. Low Temperature Discharge Cycle Tests for a Lithium Ion Cell. In Proceedings of the 2014 IEEE Vehicle Power and Propulsion Conference (VPPC), Coimbra, Portugal, 27–30 October 2014. [Google Scholar]
- Cho, H.-M.; Choi, W.-S.; Go, J.-Y.; Bae, S.-E.; Shin, H.-C. A study on time-dependent low temperature power performance of a lithium-ion battery. J. Power Sources 2012, 198, 273–280. [Google Scholar] [CrossRef]
- Carter, R.; Kingston, T.A.; Atkinson, R.W.; Parmananda, M.; Dubarry, M.; Fear, C.; Mukherjee, P.P.; Love, C.T. Directionality of thermal gradients in lithium-ion batteries dictates diverging degradation modes. Cell Rep. Phys. Sci. 2021, 2, 100351. [Google Scholar] [CrossRef]
- Zhang, G.; Shen, W.; Wei, X. Lithium-ion battery thermal safety evolution during high-temperature nonlinear aging. Fuel 2024, 362, 130845. [Google Scholar] [CrossRef]
- Yuan, W.; Liang, D.; Chu, Y.; Wang, Q. Aging effect delays overcharge-induced thermal runaway of lithium-ion batteries. J. Loss Prev. Process Ind. 2022, 79, 104830. [Google Scholar] [CrossRef]
- Zhao, L.; Zheng, M.; Zhang, J.; Liu, H.; Li, W.; Chen, M. Numerical modeling of thermal runaway for low temperature cycling lithium-ion batteries. J. Energy Storage 2023, 63, 107053. [Google Scholar] [CrossRef]
- Aufschläger, A.; Durdel, A.; Kraft, L.; Jossen, A. Optimizing mechanical compression for cycle life and irreversible swelling of high energy and high power lithium-ion pouch cells. J. Energy Storage 2024, 76, 109883. [Google Scholar] [CrossRef]
- Fang, C.; Lu, B.; Pawar, G.; Zhang, M.; Cheng, D.; Chen, S.; Ceja, M.; Doux, J.-M.; Musrock, H.; Cai, M.; et al. Pressure-tailored lithium deposition and dissolution in lithium metal batteries. Nat. Energy 2021, 6, 987–994. [Google Scholar] [CrossRef]
- Lee, Y.K.; Shin, H. Modeling and simulation of a composite solid-state battery: The effects of stack pressure on electrochemical and mechanical behavior. J. Energy Storage 2024, 78, 110051. [Google Scholar] [CrossRef]
- Cao, C.; Steinrück, H.-G.; Paul, P.P.; Dunlop, A.R.; Trask, S.E.; Jansen, A.N.; Kasse, R.M.; Thampy, V.; Yusuf, M.; Weker, J.N.; et al. Conformal Pressure and Fast-Charging Li-Ion Batteries. J. Electrochem. Soc. 2022, 169, 040540. [Google Scholar] [CrossRef]
- Petzl, M.; Danzer, M.A. Nondestructive detection, characterization, and quantification of lithium plating in commercial lithium-ion batteries. J. Power Sources 2014, 254, 80–87. [Google Scholar] [CrossRef]
- Waldmann, T.; Iturrondobeitia, A.; Kasper, M.; Ghanbari, N.; Aguesse, F.; Bekaert, E.; Daniel, L.; Genies, S.; Gordon, I.J.; Löble, M.W.; et al. Review—Post-Mortem Analysis of Aged Lithium-Ion Batteries: Disassembly Methodology and Physico-Chemical Analysis Techniques. J. Electrochem. Soc. 2016, 163, A2149. [Google Scholar] [CrossRef]
- Harris, S.J.; Timmons, A.; Baker, D.R.; Monroe, C. Direct in situ measurements of Li transport in Li-ion battery negative electrodes. Chem. Phys. Lett. 2010, 485, 265–274. [Google Scholar] [CrossRef]
- Zinth, V.; Von Lüders, C.; Hofmann, M.; Hattendorff, J.; Buchberger, I.; Erhard, S.; Rebelo-Kornmeier, J.; Jossen, A.; Gilles, R. Lithium plating in lithium-ion batteries at sub-ambient temperatures investigated by in situ neutron diffraction. J. Power Sources 2014, 271, 152–159. [Google Scholar] [CrossRef]
- Niemoller, A.; Jakes, P.; Eichel, R.A.; Granwehr, J. EPR Imaging of Metallic Lithium and its Application to Dendrite Localisation in Battery Separators. Sci. Rep. 2018, 8, 14331. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, H.; Au, M.; Chen, N.; Heiden, P.A.; Yassar, R.S. Real-time observation of lithium fibers growth inside a nanoscale lithium-ion battery. Appl. Phys. Lett. 2011, 99, 123113. [Google Scholar] [CrossRef]
- Chen, G.; Zhuang, G.V.; Richardson, T.J.; Liu, G.; Ross, P.N. Anodic Polymerization of Vinyl Ethylene Carbonate in Li-Ion Battery Electrolyte. Electrochem. Solid-State Lett. 2005, 8, A344. [Google Scholar] [CrossRef]
- Waldmann, T.; Ghanbari, N.; Kasper, M.; Wohlfahrt-Mehrens, M. Correlations between Electrochemical Data and Results from Post-Mortem Analysis of Aged Lithium-Ion Batteries. J. Electrochem. Soc. 2015, 162, A1500. [Google Scholar] [CrossRef]
- Andre, D.; Meiler, M.; Steiner, K.; Walz, H.; Soczka-Guth, T.; Sauer, D.U. Characterization of high-power lithium-ion batteries by electrochemical impedance spectroscopy. II: Modelling. J. Power Sources 2011, 196, 5349–5356. [Google Scholar] [CrossRef]
- Pastor-Fernández, C.; Widanage, W.D.; Marco, J.; Gama-Valdez, M.Á.; Chouchelamane, G.H. Identification and quantification of ageing mechanisms in Lithium-ion batteries using the EIS technique. In Proceedings of the 2016 IEEE Transportation Electrification Conference and Expo (ITEC), Dearborn, MI, USA, 27–29 June 2016. [Google Scholar]
- Pastor-Fernandez, C.; Uddin, K.; Chouchelamane, G.H.; Widanage, W.D.; Marco, J. A Comparison between Electrochemical Impedance Spectroscopy and Incremental Capacity-Differential Voltage as Li-ion Diagnostic Techniques to Identify and Quantify the Effects of Degradation Modes within Battery Management Systems. J. Power Sources 2017, 360, 301–318. [Google Scholar] [CrossRef]
- Koseoglou, M.; Tsioumas, E.; Ferentinou, D.; Jabbour, N.; Papagiannis, D.; Mademlis, C. Lithium plating detection using dynamic electrochemical impedance spectroscopy in lithium-ion batteries. J. Power Sources 2021, 512, 230508. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.Y.; Liu, M.M.; Huang, T.; Yu, A.S. Detection of lithium plating in lithium-ion batteries by distribution of relaxation times. J. Power Sources 2021, 496, 229867. [Google Scholar] [CrossRef]
- Koleti, U.R.; Zhang, C.; Malik, R.; Dinh, T.Q.; Marco, J. The development of optimal charging strategies for lithium-ion batteries to prevent the onset of lithium plating at low ambient temperatures. J. Energy Storage 2019, 24, 100798. [Google Scholar] [CrossRef]
- Yang, X.G.; Ge, S.; Liu, T.; Leng, Y.; Wang, C.Y. A look into the voltage plateau signal for detection and quantification of lithium plating in lithium-ion cells. J. Power Sources 2018, 395, 251–261. [Google Scholar] [CrossRef]
- Tian, Y.; Lin, C.; Li, H.L.; Du, J.Y.; Xiong, R. Detecting undesired lithium plating on anodes for lithium-ion batteries-A review on the in-situ methods. Appl. Energy 2021, 300, 117386. [Google Scholar] [CrossRef]
- Xu, L.; Xiao, Y.; Yang, Y.; Yang, S.-J.; Chen, X.-R.; Xu, R.; Yao, Y.-X.; Cai, W.-L.; Yan, C.; Huang, J.-Q.; et al. Operando Quantified Lithium Plating Determination Enabled by Dynamic Capacitance Measurement in Working Li-Ion Batteries. Angew. Chem. Int. Ed. 2022, 61, e202210365. [Google Scholar] [CrossRef] [PubMed]
- Koseoglou, M.; Tsioumas, E.; Ferentinou, D.; Panagiotidis, I.; Jabbour, N.; Papagiannis, D.; Mademlis, C. Lithium plating detection using differential charging current analysis in lithium-ion batteries. J. Energy Storage 2022, 54, 105345. [Google Scholar] [CrossRef]
- Safari, M.; Delacourt, C. Aging of a Commercial Graphite/LiFePO4 Cell. J. Electrochem. Soc. 2011, 158, A1123. [Google Scholar] [CrossRef]
- Fan, J.; Tan, S. Studies on charging lithium-ion cells at low temperatures. J. Electrochem. Soc. 2006, 153, A1081–A1092. [Google Scholar] [CrossRef]
- Sarkar, A.; Shrotriya, P.; Nlebedim, I.C. Magnetohydrodynamic Control of Interfacial Degradation in Lithium-Ion Batteries for Fast Charging Applications. ACS Appl. Mater. Interfaces 2021, 13, 43606–43614. [Google Scholar] [CrossRef]
- Doyle, M.; Fuller, T.F.; Newman, J. Modeling of Galvanostatic Charge and Discharge of the Lithium/Polymer/Insertion Cell. J. Electrochem. Soc. 1993, 140, 1526. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, L.; Zhang, Z.; Yu, H.; Wang, W.; Ouyang, M.; Zhang, C.; Sun, Q.; Yan, X.; Yang, S.; et al. Battery State of Health Estimate Strategies: From Data Analysis to End-Cloud Collaborative Framework. Batteries 2023, 9, 351. [Google Scholar] [CrossRef]
- Arora, P.; Doyle, M.; White, R.E. Mathematical Modeling of the Lithium Deposition Overcharge Reaction in Lithium-Ion Batteries Using Carbon-Based Negative Electrodes. J. Electrochem. Soc. 1999, 146, 3543. [Google Scholar] [CrossRef]
- Gao, Z.H.; Xie, H.C.; Yang, X.B.; Niu, W.F.; Li, S.; Chen, S.Y. The Dilemma of C-Rate and Cycle Life for Lithium-Ion Batteries under Low Temperature Fast Charging. Batteries 2022, 8, 234. [Google Scholar] [CrossRef]
- Li, W.; Xie, Y.; Hu, X.; Tran, M.K.; Fowler, M.; Panchal, S.; Zheng, J.; Liu, K. An Internal Heating Strategy for Lithium-Ion Batteries Without Lithium Plating Based on Self-Adaptive Alternating Current Pulse. IEEE Trans. Veh. Technol. 2023, 72, 5809–5823. [Google Scholar] [CrossRef]
- Liang, J.; Gan, Y.; Yao, M. Numerical analysis on the aging characteristics of a LiFePO4 battery: Effect of active particle sizes in electrodes. J. Energy Storage 2023, 67, 107546. [Google Scholar] [CrossRef]
- Parmananda, M.; Vishnugopi, B.S.; Garg, H.; Mukherjee, P.P. Underpinnings of Multiscale Interactions and Heterogeneities in Li-Ion Batteries: Electrode Microstructure to Cell Format. Energy Technol. 2023, 11, 2200691. [Google Scholar] [CrossRef]
- Yang, X.G.; Wang, C.Y. Understanding the trilemma of fast charging, energy density and cycle life of lithium-ion batteries. J. Power Sources 2018, 402, 489–498. [Google Scholar] [CrossRef]
- Duan, X.; Li, B.; Li, J.; Gao, X.; Wang, L.; Xu, J. Quantitative Understanding of Lithium Deposition-Stripping Process on Graphite Anodes of Lithium-Ion Batteries. Adv. Energy Mater. 2023, 13, 2203767. [Google Scholar] [CrossRef]
- Zoerr, C.; Sturm, J.J.; Solchenbach, S.; Erhard, S.V.; Latz, A. Electrochemical polarization-based fast charging of lithium-ion batteries in embedded systems. J. Energy Storage 2023, 72, 108234. [Google Scholar] [CrossRef]
- Chen, B.-R.; Kunz, M.R.; Tanim, T.R.; Dufek, E.J. A machine learning framework for early detection of lithium plating combining multiple physics-based electrochemical signatures. Cell Rep. Phys. Sci. 2021, 2, 100352. [Google Scholar] [CrossRef]
- Weddle, P.J.; Kim, S.; Chen, B.-R.; Yi, Z.; Gasper, P.; Colclasure, A.M.; Smith, K.; Gering, K.L.; Tanim, T.R.; Dufek, E.J. Battery state-of-health diagnostics during fast cycling using physics-informed deep-learning. J. Power Sources 2023, 585, 233582. [Google Scholar] [CrossRef]
- Lei, S.; Zeng, Z.; Liu, M.; Zhang, H.; Cheng, S.; Xie, J. Balanced solvation/de-solvation of electrolyte facilitates Li-ion intercalation for fast charging and low-temperature Li-ion batteries. Nano Energy 2022, 98, 107265. [Google Scholar] [CrossRef]
- Persson, K.; Hinuma, Y.; Meng, Y.S.; der Van Ven, A.; Ceder, G. Thermodynamic and kinetic properties of the Li-graphite system from first-principles calculations. Phys. Rev. B 2010, 82, 125416. [Google Scholar] [CrossRef]
- Chen, X.; Yao, N.; Zeng, B.-S.; Zhang, Q. Ion–solvent chemistry in lithium battery electrolytes: From mono-solvent to multi-solvent complexes. Fundam. Res. 2021, 1, 393–398. [Google Scholar] [CrossRef]
- Yamada, Y.; Furukawa, K.; Sodeyama, K.; Kikuchi, K.; Yaegashi, M.; Tateyama, Y.; Yamada, A. Unusual Stability of Acetonitrile-Based Superconcentrated Electrolytes for Fast-Charging Lithium-Ion Batteries. J. Am. Chem. Soc. 2014, 136, 5039–5046. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yan, Q.; Holoubek, J.; Yin, Y.; Bao, W.; Liu, H.; Baskin, A.; Li, M.; Cai, G.; Li, W.; et al. Enhanced Electrolyte Transport and Kinetics Mitigate Graphite Exfoliation and Li Plating in Fast-Charging Li-Ion Batteries. Adv. Energy Mater. 2023, 13, 2202906. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, J.; Dong, Y.; Chen, Y.; Shi, Z.; Xu, X.; Li, X.; Liang, Z. Reversible Li Plating on Graphite Anodes through Electrolyte Engineering for Fast-Charging Batteries. Angew. Chem. Int. Ed. 2023, 62, e202302285. [Google Scholar] [CrossRef]
- Shuai, Y.; Zhang, Z.; Chen, K.; Lou, J.; Wang, Y. Highly stable lithium plating by a multifunctional electrolyte additive in a lithium-sulfurized polyacrylonitrile battery. Chem. Commun. 2019, 55, 2376–2379. [Google Scholar] [CrossRef]
- Jeong, Y.T.; Shin, H.R.; Lee, J.; Ryu, M.-H.; Choi, S.; Kim, H.; Jung, K.-N.; Lee, J.-W. Insight into pulse-charging for lithium plating-free fast-charging lithium-ion batteries. Electrochim. Acta 2023, 462, 142761. [Google Scholar] [CrossRef]
- An, J.; Zhang, H.; Qi, L.; Li, G.; Li, Y. Self-Expanding Ion-Transport Channels on Anodes for Fast-Charging Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2022, 61, e202113313. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Mijailovic, A.; Wang, G.; Xiong, J.; Mathew, K.; Lu, W.; Sheldon, B.W.; Wu, Q. Gradient porosity electrodes for fast charging lithium-ion batteries. J. Mater. Chem. A 2022, 10, 12114–12124. [Google Scholar] [CrossRef]
- Yim, T.; Woo, S.-G.; Lim, S.H.; Cho, W.; Song, J.H.; Han, Y.-K.; Kim, Y.-J. 5V-class high-voltage batteries with over-lithiated oxide and a multi-functional additive. J. Mater. Chem. A 2015, 3, 6157–6167. [Google Scholar] [CrossRef]
- Yao, Y.-X.; Chen, X.; Yao, N.; Gao, J.-H.; Xu, G.; Ding, J.-F.; Song, C.-L.; Cai, W.-L.; Yan, C.; Zhang, Q. Unlocking Charge Transfer Limitations for Extreme Fast Charging of Li-Ion Batteries. Angew. Chem. Int. Ed. 2023, 62, e202214828. [Google Scholar] [CrossRef]
- Nan, B.; Chen, L.; Rodrigo, N.D.; Borodin, O.; Piao, N.; Xia, J.; Pollard, T.; Hou, S.; Zhang, J.; Ji, X.; et al. Enhancing Li+ Transport in NMC811||Graphite Lithium-Ion Batteries at Low Temperatures by Using Low-Polarity-Solvent Electrolytes. Angew. Chem. 2022, 134, e202205967. [Google Scholar] [CrossRef]
- Logan, E.R.; Dahn, J.R. Electrolyte Design for Fast-Charging Li-Ion Batteries. Trends Chem. 2020, 2, 354–366. [Google Scholar] [CrossRef]
- Lei, S.; Zeng, Z.; Cheng, S.; Xie, J. Fast-charging of lithium-ion batteries: A review of electrolyte design aspects. Battery Energy 2023, 2, 20230018. [Google Scholar] [CrossRef]
- Berhaut, C.L.; Porion, P.; Timperman, L.; Schmidt, G.; Lemordant, D.; Anouti, M. LiTDI as electrolyte salt for Li-ion batteries: Transport properties in EC/DMC. Electrochim. Acta 2015, 180, 778–787. [Google Scholar] [CrossRef]
- Hall, D.S.; Eldesoky, A.; Logan, E.R.; Tonita, E.M.; Ma, X.; Dahn, J.R. Exploring Classes of Co-Solvents for Fast-Charging Lithium-Ion Cells. J. Electrochem. Soc. 2018, 165, A2365. [Google Scholar] [CrossRef]
- He, D.; Lu, J.; He, G.; Chen, H. Recent Advances in Solid-Electrolyte Interphase for Li Metal Anode. Front. Chem. 2022, 10, 916132. [Google Scholar] [CrossRef]
- Barlowz, C.G. Reaction of Water with Hexafluorophosphates and with Li Bis(perfluoroethylsulfonyl)imide Salt. Electrochem. Solid-State Lett. 1999, 2, 362. [Google Scholar] [CrossRef]
- Freunberger, S.A.; Chen, Y.; Peng, Z.; Griffin, J.M.; Hardwick, L.J.; Bardé, F.; Novák, P.; Bruce, P.G. Reactions in the Rechargeable Lithium–O2 Battery with Alkyl Carbonate Electrolytes. J. Am. Chem. Soc. 2011, 133, 8040–8047. [Google Scholar] [CrossRef]
- Milien, M.S.; Beyer, H.; Beichel, W.; Klose, P.; Gasteiger, H.A.; Lucht, B.L.; Krossing, I. Lithium Bis(2,2,2-trifluoroethyl)phosphate Li[O2P(OCH2CF3)2]: A High Voltage Additive for LNMO/Graphite Cells. J. Electrochem. Soc. 2018, 165, A2569. [Google Scholar] [CrossRef]
- Diederichsen, K.M.; McShane, E.J.; McCloskey, B.D. Promising Routes to a High Li+ Transference Number Electrolyte for Lithium Ion Batteries. ACS Energy Lett. 2017, 2, 2563–2575. [Google Scholar] [CrossRef]
- Logan, E.R.; Tonita, E.M.; Gering, K.L.; Li, J.; Ma, X.; Beaulieu, L.Y.; Dahn, J.R. A Study of the Physical Properties of Li-Ion Battery Electrolytes Containing Esters. J. Electrochem. Soc. 2018, 165, A21. [Google Scholar] [CrossRef]
- Qu, Z.G.; Jiang, Z.Y.; Wang, Q. Experimental study on pulse self–heating of lithium–ion battery at low temperature. Int. J. Heat Mass Transf. 2019, 135, 696–705. [Google Scholar] [CrossRef]
- Zhang, G.; Wei, X.; Han, G.; Dai, H.; Zhu, J.; Wang, X.; Tang, X.; Ye, J. Lithium plating on the anode for lithium-ion batteries during long-term low temperature cycling. J. Power Sources 2021, 484, 229312. [Google Scholar] [CrossRef]
- Qin, Y.; Zuo, P.; Chen, X.; Yuan, W.; Huang, R.; Yang, X.; Du, J.; Lu, L.; Han, X.; Ouyang, M. An ultra-fast charging strategy for lithium-ion battery at low temperature without lithium plating. J. Energy Chem. 2022, 72, 442–452. [Google Scholar] [CrossRef]
- Wu, Y.; Long, X.; Lu, J.; Wu, Y.; Zhou, R.; Liu, L. Effect of temperature on the high-rate pulse charging of lithium-ion batteries. J. Electroanal. Chem. 2022, 922, 116773. [Google Scholar] [CrossRef]
- Katzer, F.; Mößle, P.; Schamel, M.; Danzer, M.A. Adaptive fast charging control using impedance-based detection of lithium deposition. J. Power Sources 2023, 555, 232354. [Google Scholar] [CrossRef]
- Shen, K.; Wang, Z.; Bi, X.; Ying, Y.; Zhang, D.; Jin, C.; Hou, G.; Cao, H.; Wu, L.; Zheng, G.; et al. Magnetic Field–Suppressed Lithium Dendrite Growth for Stable Lithium-Metal Batteries. Adv. Energy Mater. 2019, 9, 1900260. [Google Scholar] [CrossRef]
- Dong, J.; Dai, H.; Wang, C.; Lai, C. Uniform lithium deposition driven by vertical magnetic field for stable lithium anodes. Solid State Ion. 2019, 341, 115033. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material—Fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Sander, J.S.; Erb, R.M.; Li, L.; Gurijala, A.; Chiang, Y.M. High-performance battery electrodes via magnetic templating. Nat. Energy 2016, 1, 16099. [Google Scholar] [CrossRef]
- Parikh, D.; Li, J. Bilayer hybrid graphite anodes via freeze tape casting for extreme fast charging applications. Carbon 2022, 196, 525–531. [Google Scholar] [CrossRef]
- Kalnaus, S.; Livingston, K.; Hawley, W.B.; Wang, H.; Li, J. Design and processing for high performance Li ion battery electrodes with double-layer structure. J. Energy Storage 2021, 44, 103582. [Google Scholar] [CrossRef]
- Ruan, H.; Jiang, J.; Sun, B.; Su, X.; He, X.; Zhao, K. An optimal internal-heating strategy for lithium-ion batteries at low temperature considering both heating time and lifetime reduction. Appl. Energy 2019, 256, 113797. [Google Scholar] [CrossRef]
- Das, S.; Mohammad, B.; Navidi, S.; Sarkar, A.; Hu, C.; Shrotriya, P. Physics-Based State of Health Prediction of Lithium-Ion Battery during Fast Charging. ECS Meet. Abstr. 2024, MA2024-01, 469. [Google Scholar] [CrossRef]
- Goel, V.; Chen, K.-H.; Dasgupta, N.P.; Thornton, K. Optimization of laser-patterned electrode architectures for fast charging of Li-ion batteries using simulations parameterized by machine learning. Energy Storage Mater. 2023, 57, 44–58. [Google Scholar] [CrossRef]
- Karati, A.; Gargh, P.P.; Paul, S.; Das, S.; Shrotriya, P.; Nlebedim, I.C. Materials recovery from NMC batteries with water as the sole solvent. J. Environ. Manag. 2024, 366, 121710. [Google Scholar] [CrossRef]
- Sarkar, A.; Shrotriya, P.; Nlebedim, I.C. Electrochemical-driven green recovery of lithium, graphite and cathode from lithium-ion batteries using water. Waste Manag. 2022, 150, 320–327. [Google Scholar] [CrossRef]
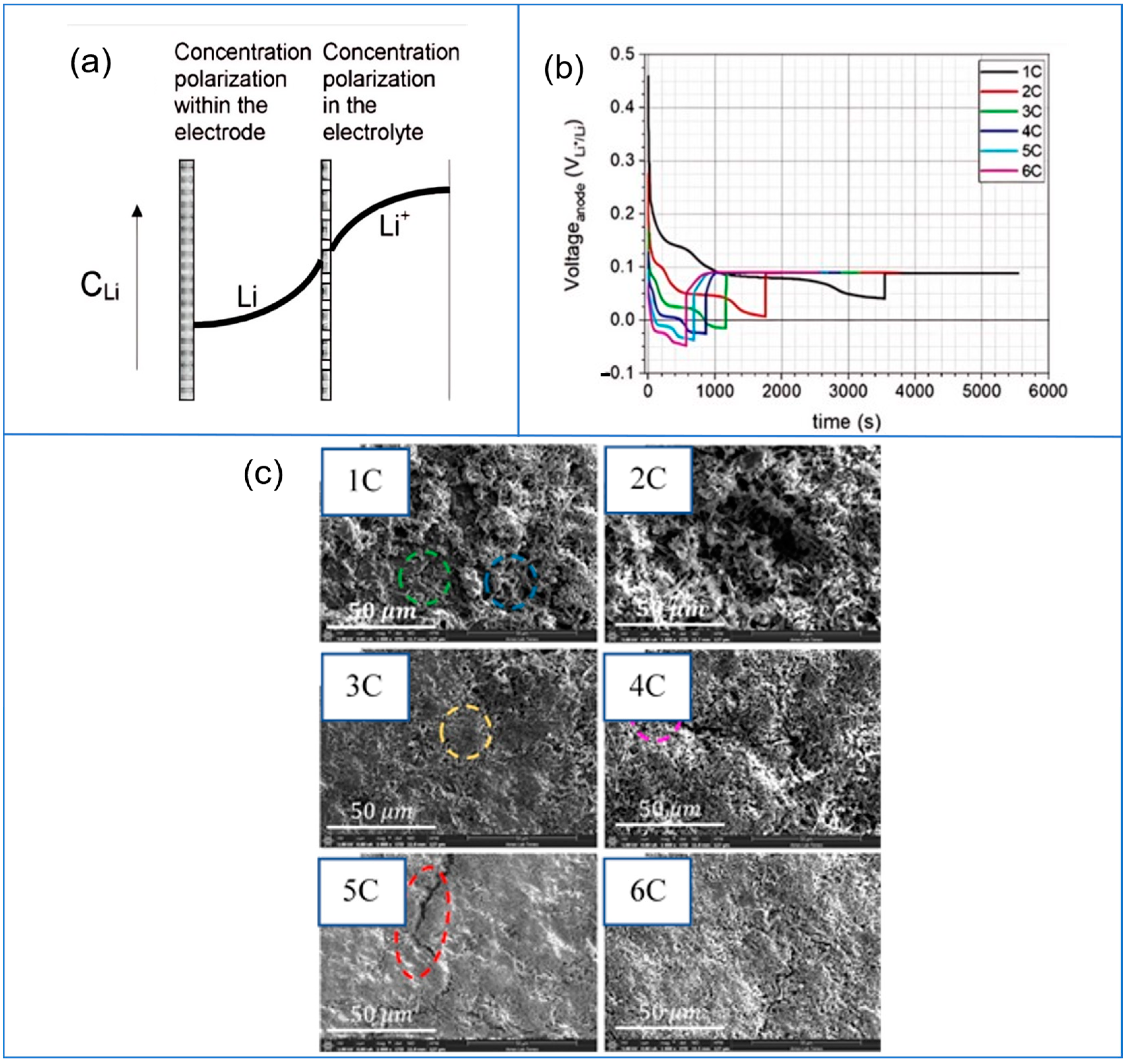

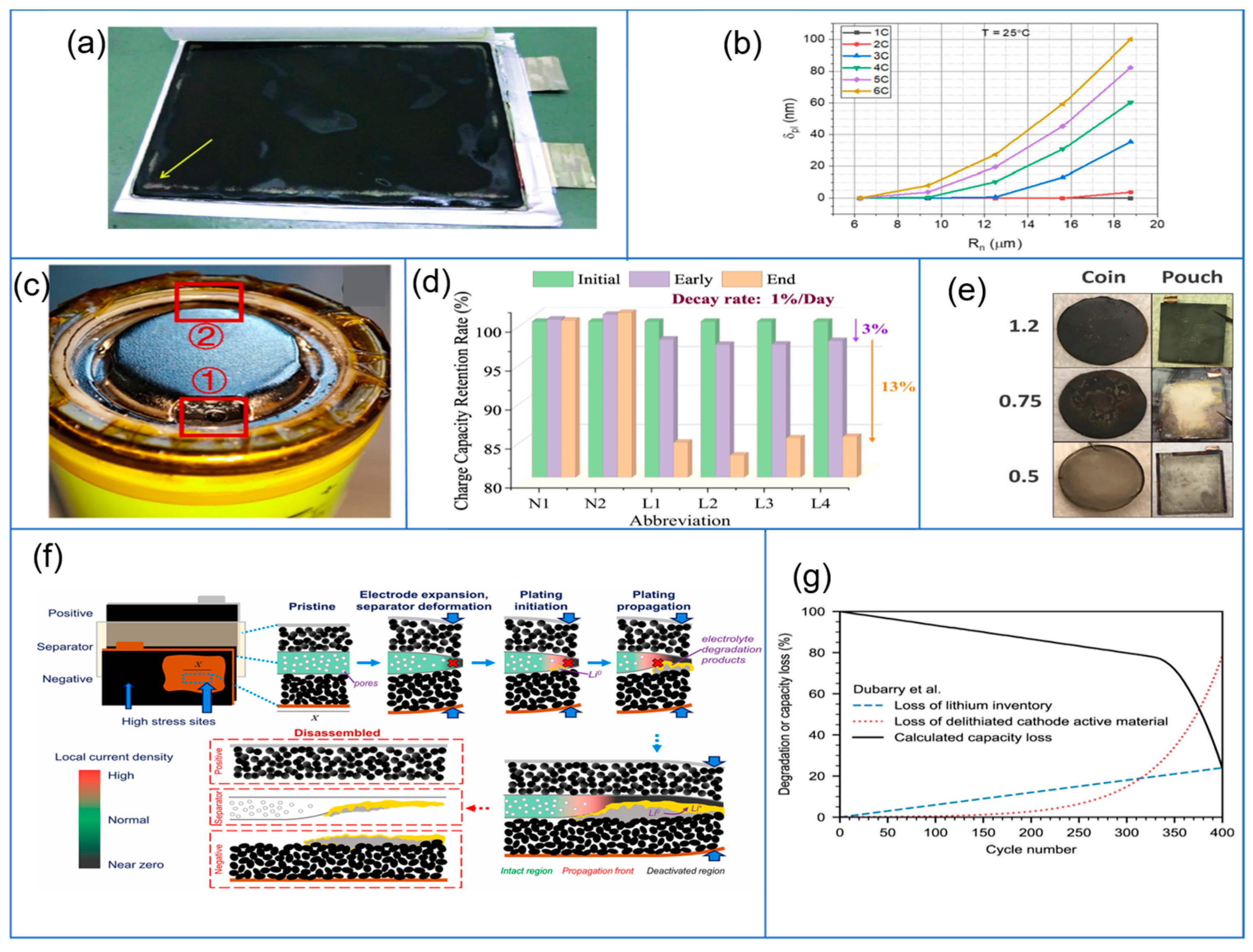


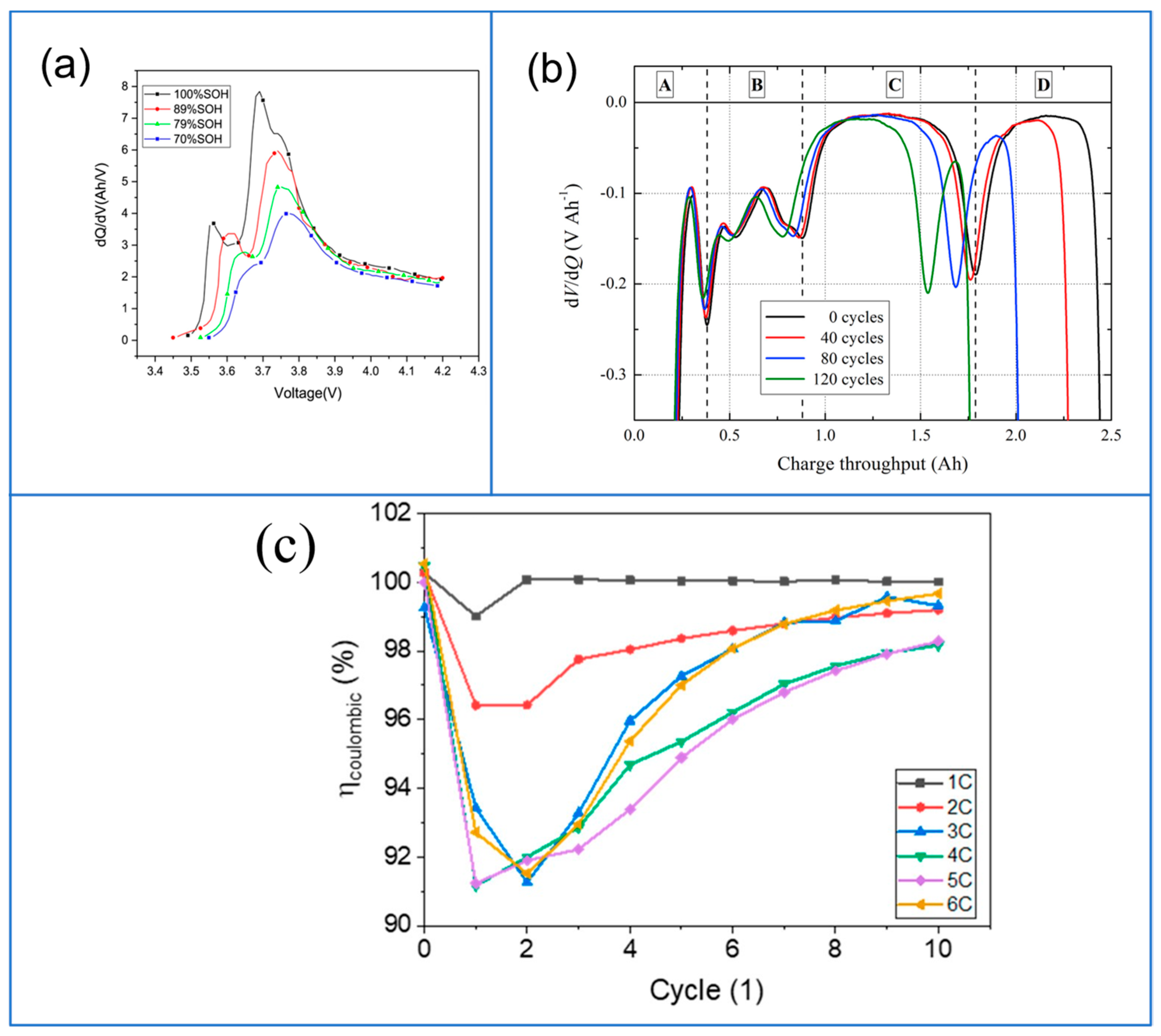

| Plating Condition Type | Description | Causes and Condition | Ref |
|---|---|---|---|
| Internal | Manufacturing and Shape Defect | Localized separator defects due to inconsistent manufacturing or non-uniform electrolyte distribution create current non-uniformity, significantly influencing plating. | [15,85,90,91,96,97,98] |
| Internal | Aging | Aging drives the delamination of anodic material and the corresponding reduction in active surface area and resulting lithium plating. | [86,87,88,99] |
| External | High CC-CV Charging Rates | Fast charging generates large lithium-ion flux toward the anode, but the anode solid intercalation remains slower. This leads to lithium-ion accumulation at the particle surface. | [46,69,89,100,101,102] |
| External | Operating Temperature | Low temperature reduces electrode diffusivity and induces slower kinetics, generating charge polarization at the anode–electrolyte interface. | [31,43,86,101] |
| External | Overcharging | Overcharging the cell around 4.5 V forces more lithium into the anode, causing accumulation at the anodic interface. | [31,63,89,103] |
| External/Internal | Load | Pressure clogs the separator pore, increasing localized current flux and resulting in lithium plating. In contrast, limited external pressure causes stable plating growth. | [90,91,104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, S.; Shrotriya, P. Electrochemical Mechanism Underlying Lithium Plating in Batteries: Non-Invasive Detection and Mitigation. Energies 2024, 17, 5930. https://doi.org/10.3390/en17235930
Das S, Shrotriya P. Electrochemical Mechanism Underlying Lithium Plating in Batteries: Non-Invasive Detection and Mitigation. Energies. 2024; 17(23):5930. https://doi.org/10.3390/en17235930
Chicago/Turabian StyleDas, Sourav, and Pranav Shrotriya. 2024. "Electrochemical Mechanism Underlying Lithium Plating in Batteries: Non-Invasive Detection and Mitigation" Energies 17, no. 23: 5930. https://doi.org/10.3390/en17235930
APA StyleDas, S., & Shrotriya, P. (2024). Electrochemical Mechanism Underlying Lithium Plating in Batteries: Non-Invasive Detection and Mitigation. Energies, 17(23), 5930. https://doi.org/10.3390/en17235930








