A Review of Proton Exchange Membrane Degradation Pathways, Mechanisms, and Mitigation Strategies in a Fuel Cell
Abstract
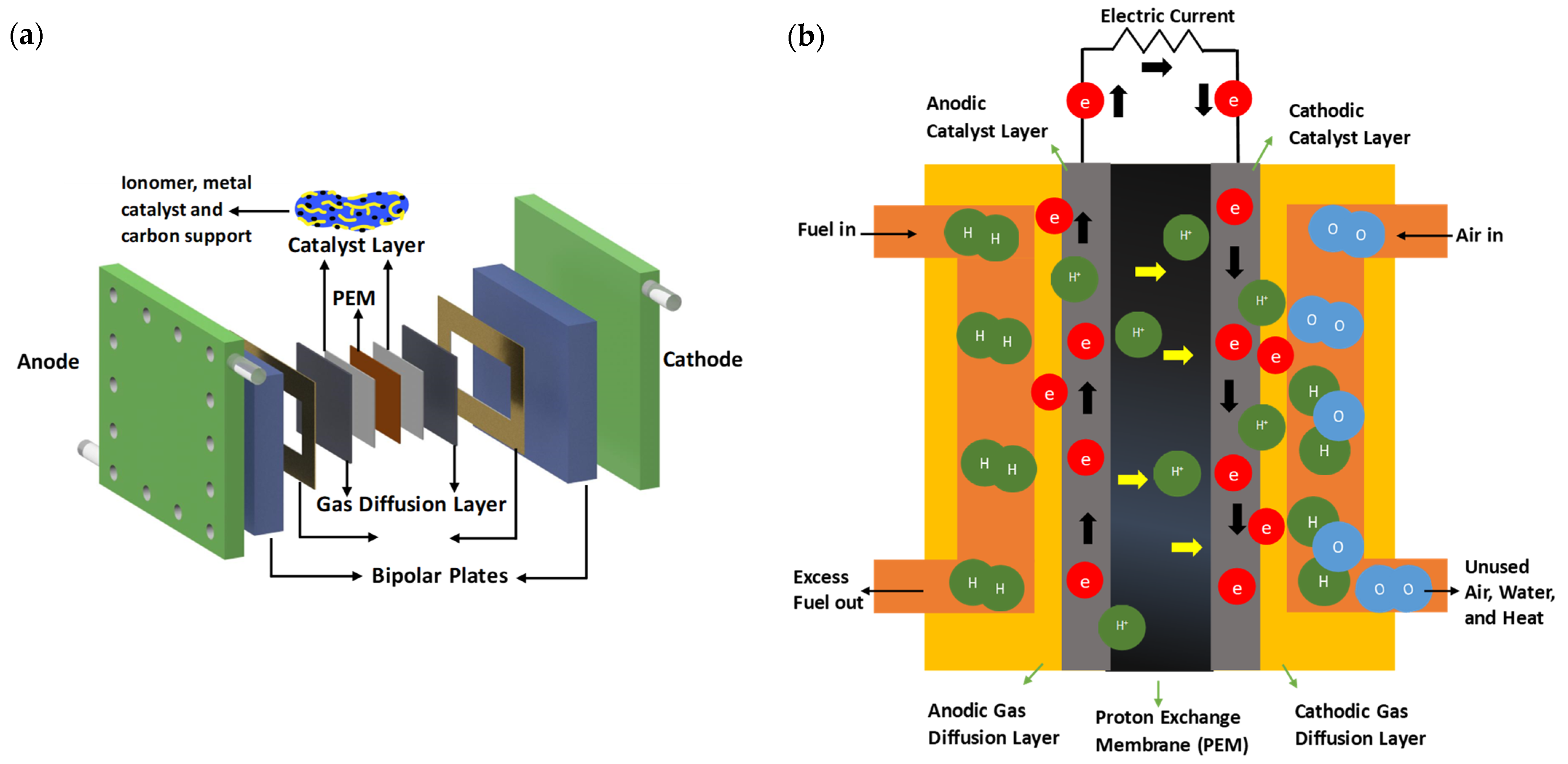
1. Introduction
2. Chemical Degradation
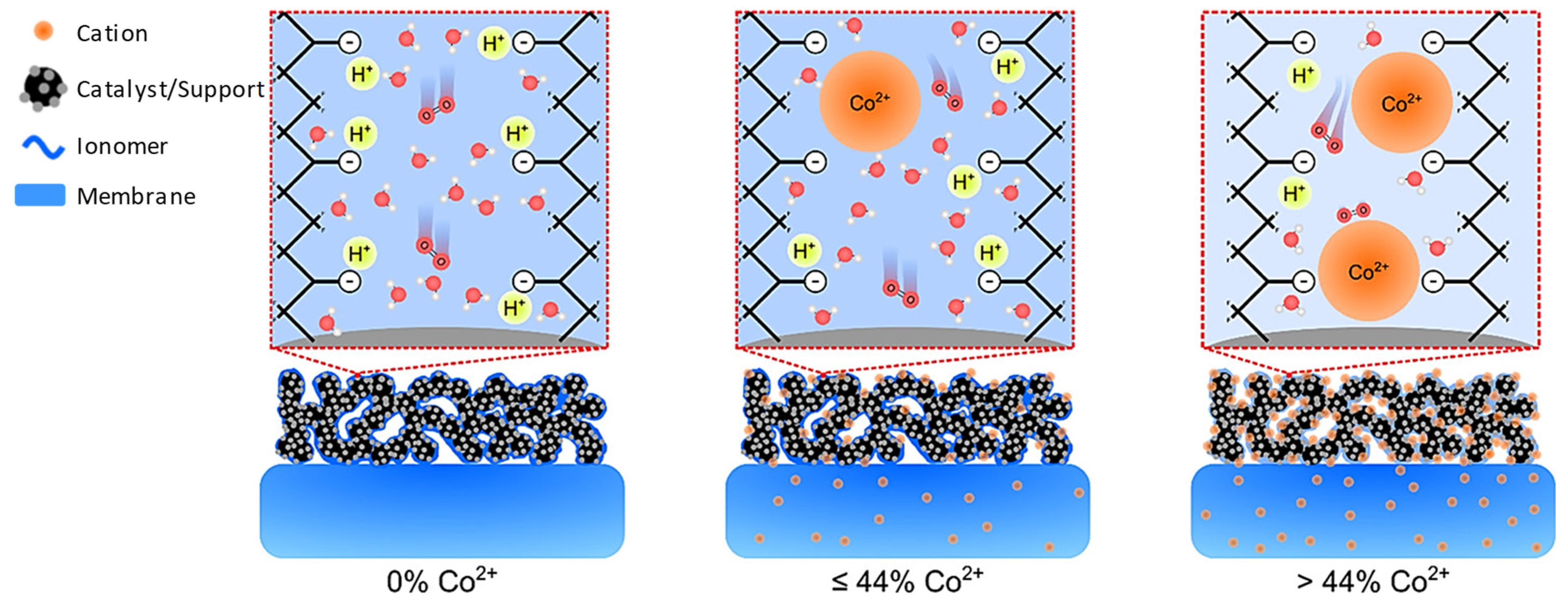
2.1. Metal Poisoning
2.2. Impurities in the Fuel and Air Streams
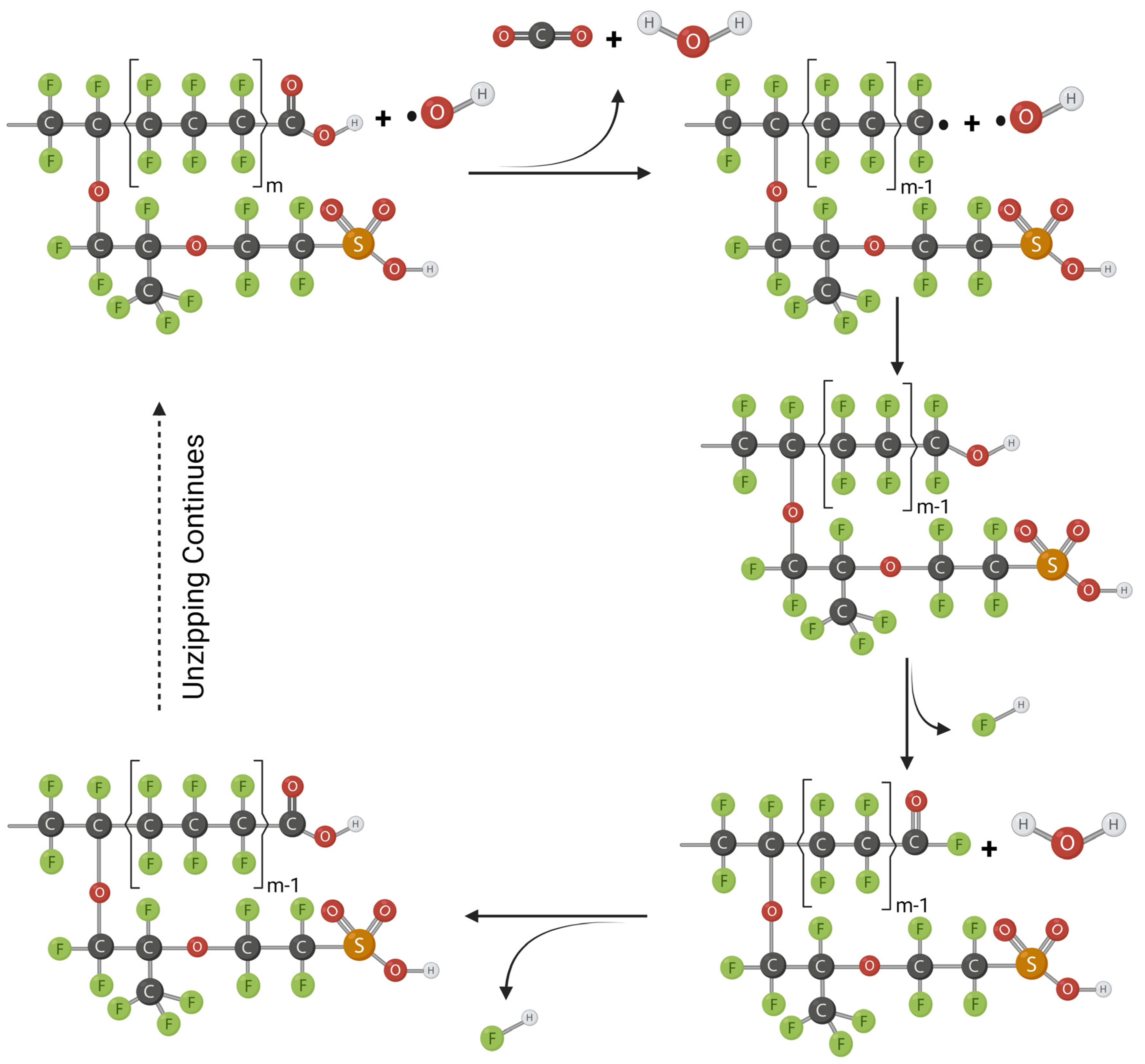
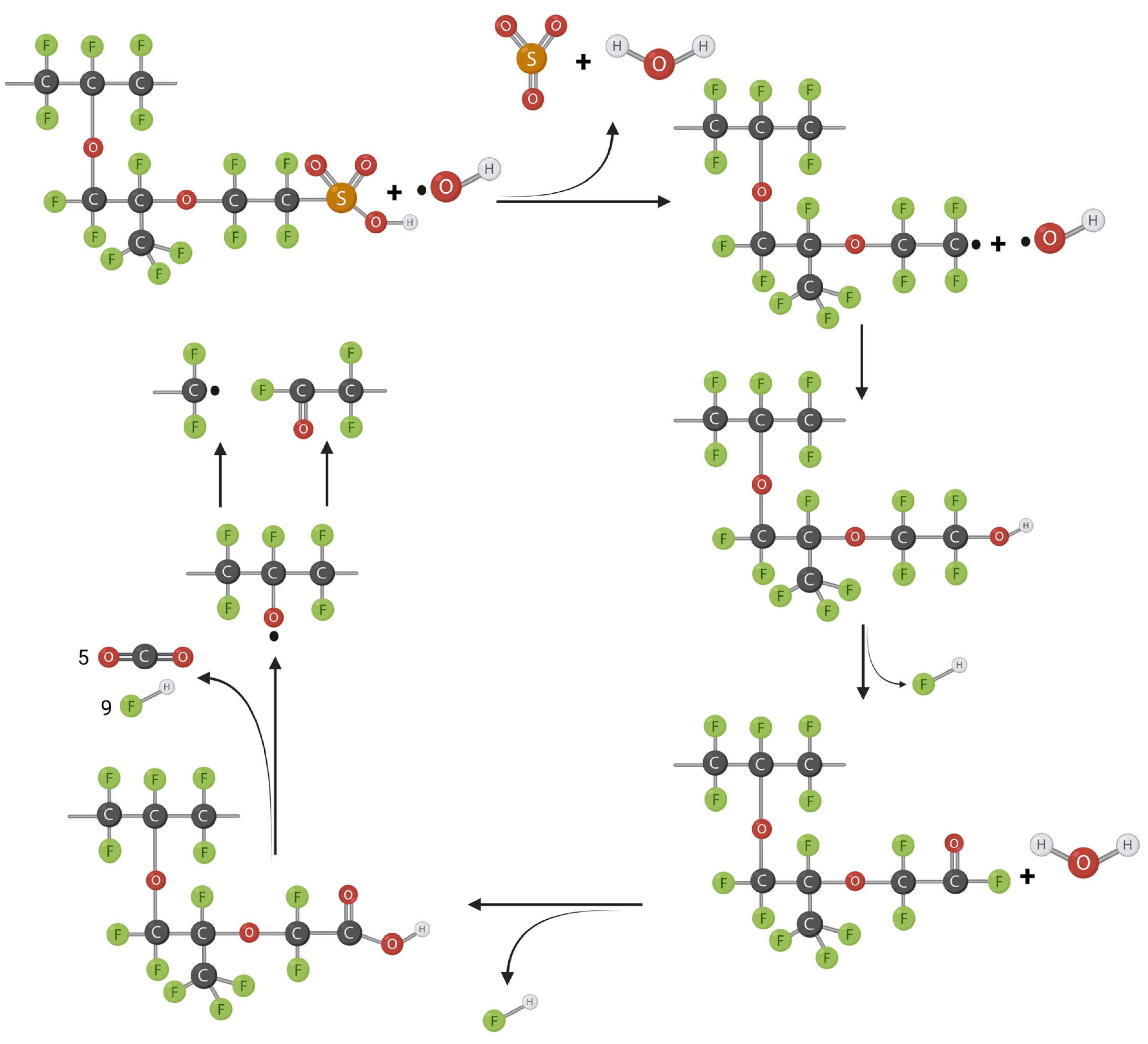
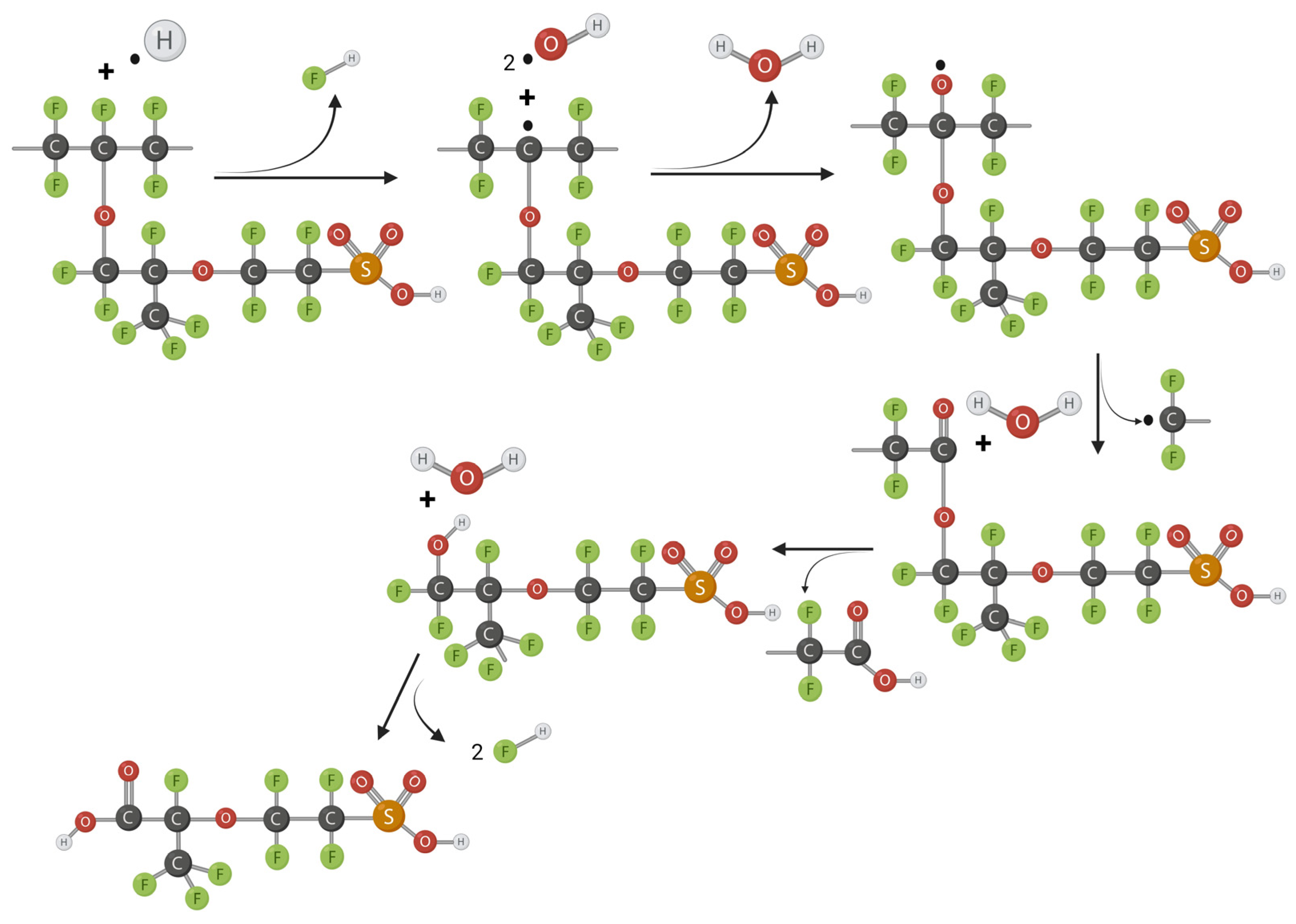
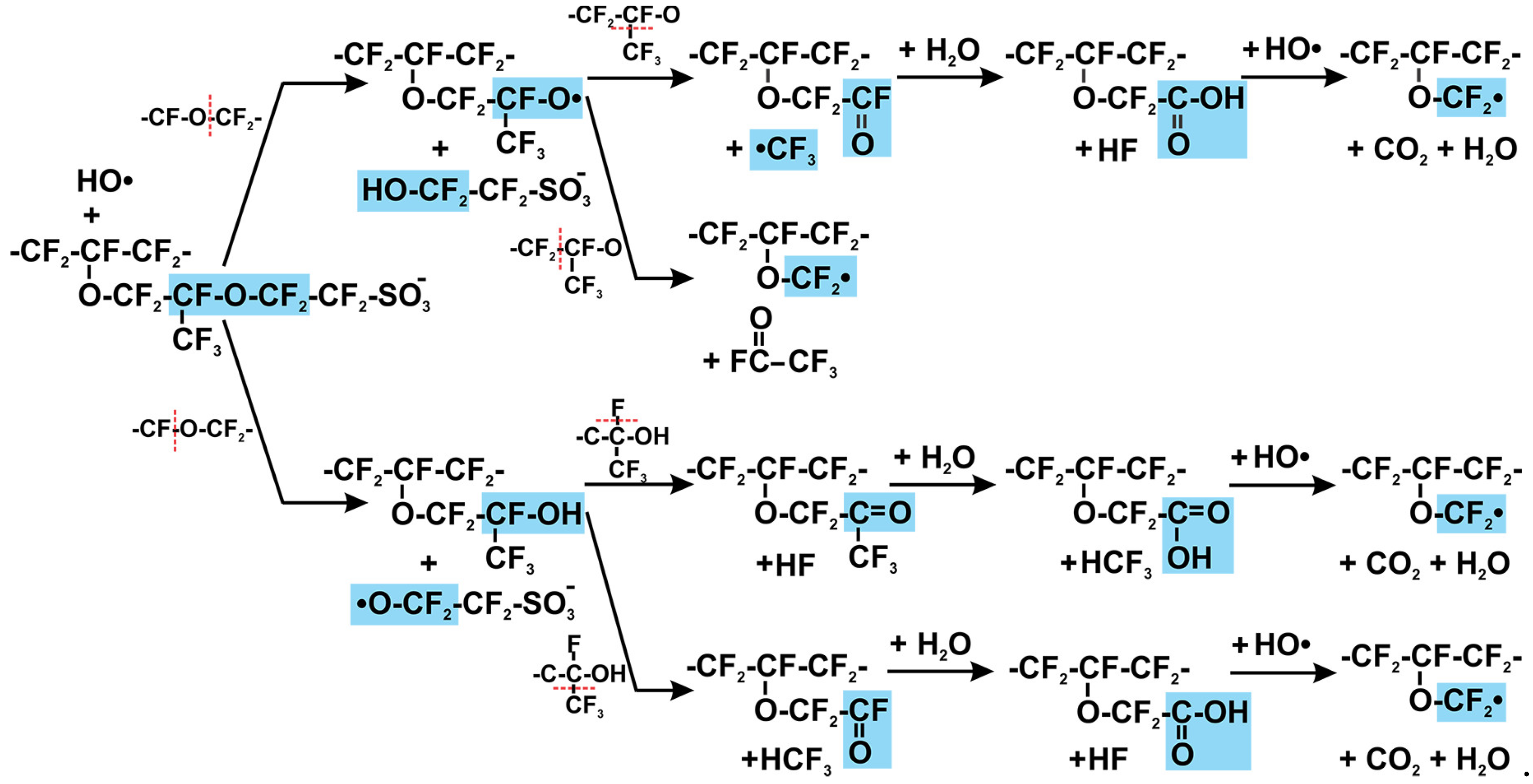
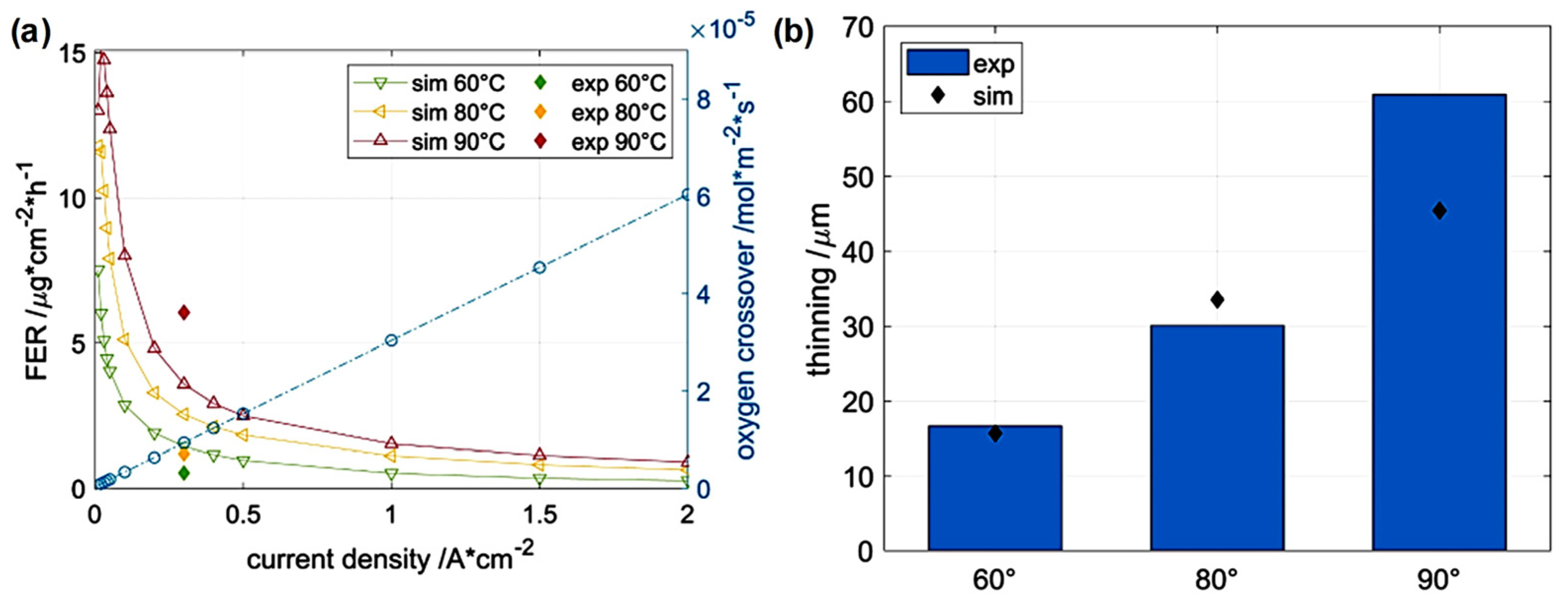
2.3. Radical Attack
2.4. Mitigation Strategies
3. Mechanical Degradation
3.1. Mechanical Stress
3.2. Hygrothermal Cycling
3.3. Freeze–Thaw Cycling
3.4. Mitigation Strategies
4. Thermal Degradation
4.1. Thermal Stress and Cycling
4.2. Drying
4.3. Mitigation Strategies
5. Progress in PEM Development for Fuel Cells
5.1. Chemically Modified PEMs
5.2. Composite PEMs
6. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stančin, H.; Mikulčić, H.; Wang, X.; Duić, N. A Review on Alternative Fuels in Future Energy System. Renew. Sustain. Energy Rev. 2020, 128, 109927. [Google Scholar] [CrossRef]
- Halkos, G.E.; Gkampoura, E.-C. Reviewing Usage, Potentials, and Limitations of Renewable Energy Sources. Energies 2020, 13, 2906. [Google Scholar] [CrossRef]
- Madhav, D.; Buffel, B.; Desplentere, F.; Moldenaers, P.; Vandeginste, V. Bio-Inspired Mineralization of CO2 into CaCO3: Single-Step Carbon Capture and Utilization with Controlled Crystallization. Fuel 2023, 345, 128157. [Google Scholar] [CrossRef]
- Madhav, D.; Coppitters, T.; Ji, Y.; Thielemans, W.; Desplentere, F.; Moldenaers, P.; Vandeginste, V. Amino Acid Promoted Single-Step Carbon Dioxide Capture and Mineralization Integrated with Polymer-Mediated Crystallization of Carbonates. J. Clean. Prod. 2023, 415, 137845. [Google Scholar] [CrossRef]
- Madhav, D.; Chergaoui, S.; Vandeginste, V. Carbon Capture by Amino Acid Materials. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2023; ISBN 978-0-12-409548-9. [Google Scholar]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef]
- Yatoo, M.A.; Habib, F.; Malik, A.H.; Qazi, M.J.; Ahmad, S.; Ganayee, M.A.; Ahmad, Z. Solid-Oxide Fuel Cells: A Critical Review of Materials for Cell Components. MRS Commun. 2023, 13, 378–384. [Google Scholar] [CrossRef]
- Borup, R.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D.; et al. Scientific Aspects of Polymer Electrolyte Fuel Cell Durability and Degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef] [PubMed]
- Shabani, B.; Andrews, J. Hydrogen and Fuel Cells. In Energy Sustainability Through Green Energy; Sharma, A., Kar, S.K., Eds.; Green Energy and Technology; Springer: New Delhi, India, 2015; pp. 453–491. ISBN 978-81-322-2337-5. [Google Scholar]
- Xing, Y.; Li, H.; Avgouropoulos, G. Research Progress of Proton Exchange Membrane Failure and Mitigation Strategies. Materials 2021, 14, 2591. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jensen, J.O.; Savinell, R.F.; Bjerrum, N.J. High Temperature Proton Exchange Membranes Based on Polybenzimidazoles for Fuel Cells. Prog. Polym. Sci. 2009, 34, 449–477. [Google Scholar] [CrossRef]
- Souzy, R.; Ameduri, B. Functional Fluoropolymers for Fuel Cell Membranes. Prog. Polym. Sci. 2005, 30, 644–687. [Google Scholar] [CrossRef]
- Miyake, J.; Miyatake, K. Fluorine-Free Sulfonated Aromatic Polymers as Proton Exchange Membranes. Polym. J. 2017, 49, 487–495. [Google Scholar] [CrossRef]
- Adamski, M.; Peressin, N.; Holdcroft, S. On the Evolution of Sulfonated Polyphenylenes as Proton Exchange Membranes for Fuel Cells. Mater. Adv. 2021, 2, 4966–5005. [Google Scholar] [CrossRef]
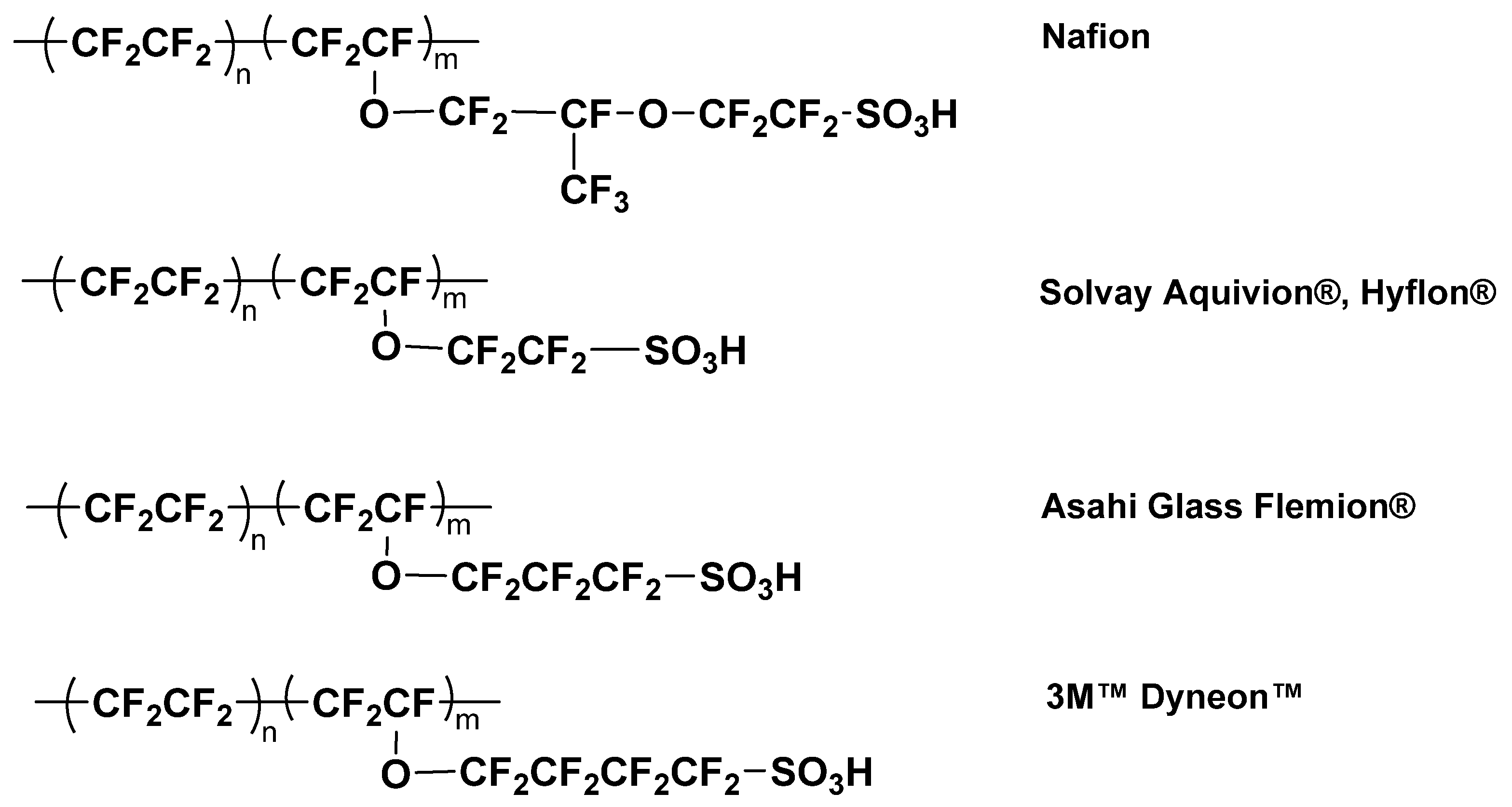
- Zhu, L.-Y.; Li, Y.-C.; Liu, J.; He, J.; Wang, L.-Y.; Lei, J.-D. Recent Developments in High-Performance Nafion Membranes for Hydrogen Fuel Cells Applications. Pet. Sci. 2022, 19, 1371–1381. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the Proton Exchange Membranes for Fuel Cell Applications. Int. J. Hydrogen Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Schiraldi, D.A. Perfluorinated Polymer Electrolyte Membrane Durability. J. Macromol. Sci. Part C 2006, 46, 315–327. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Wu, J.; Zhang, J. Chapter 11—Fuel Cell Degradation and Failure Analysis. In Pem Fuel Cell Testing and Diagnosis; Zhang, J., Zhang, H., Wu, J., Zhang, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 283–335. ISBN 978-0-444-53688-4. [Google Scholar]
- Yuan, X.-Z.; Nayoze-Coynel, C.; Shaigan, N.; Fisher, D.; Zhao, N.; Zamel, N.; Gazdzicki, P.; Ulsh, M.; Friedrich, K.A.; Girard, F.; et al. A Review of Functions, Attributes, Properties and Measurements for the Quality Control of Proton Exchange Membrane Fuel Cell Components. J. Power Sources 2021, 491, 229540. [Google Scholar] [CrossRef]
- Okada, T.; Møller-Holst, S.; Gorseth, O.; Kjelstrup, S. Transport and Equilibrium Properties of Nafion® Membranes with H+ and Na+ Ions. J. Electroanal. Chem. 1998, 442, 137–145. [Google Scholar] [CrossRef]
- Kelly, M.J.; Fafilek, G.; Besenhard, J.O.; Kronberger, H.; Nauer, G.E. Contaminant Absorption and Conductivity in Polymer Electrolyte Membranes. J. Power Sources 2005, 145, 249–252. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An Overview of Hydrogen Production Technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Zhao, Y.; Mao, Y.; Zhang, W.; Tang, Y.; Wang, P. Reviews on the Effects of Contaminations and Research Methodologies for PEMFC. Int. J. Hydrogen Energy 2020, 45, 23174–23200. [Google Scholar] [CrossRef]
- Madhav, D.; Shao, C.; Mus, J.; Buysschaert, F.; Vandeginste, V. The Effect of Salty Environments on the Degradation Behavior and Mechanical Properties of Nafion Membranes. Energies 2023, 16, 2256. [Google Scholar] [CrossRef]
- Gao, G.; Wang, S.; Xue, R.; Liu, D.; Ren, H.; Zhang, R. Uncovering the Characteristics of Air Pollutants Emission in Industrial Parks and Analyzing Emission Reduction Potential: Case Studies in Henan, China. Sci. Rep. 2021, 11, 23709. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Shi, Z.; Glass, N.; Zhang, L.; Zhang, J.; Song, D.; Liu, Z.-S.; Wang, H.; Shen, J. A Review of PEM Hydrogen Fuel Cell Contamination: Impacts, Mechanisms, and Mitigation. J. Power Sources 2007, 165, 739–756. [Google Scholar] [CrossRef]
- Novalin, T.; Eriksson, B.; Proch, S.; Bexell, U.; Moffatt, C.; Westlinder, J.; Lagergren, C.; Lindbergh, G.; Lindström, R.W. Trace-Metal Contamination in Proton Exchange Membrane Fuel Cells Caused by Laser-Cutting Stains on Carbon-Coated Metallic Bipolar Plates. Int. J. Hydrogen Energy 2021, 46, 13855–13864. [Google Scholar] [CrossRef]
- Li, H.; Tsay, K.; Wang, H.; Shen, J.; Wu, S.; Zhang, J.; Jia, N.; Wessel, S.; Abouatallah, R.; Joos, N.; et al. Durability of PEM Fuel Cell Cathode in the Presence of Fe3+ and Al3+. J. Power Sources 2010, 195, 8089–8093. [Google Scholar] [CrossRef]
- Sulek, M.; Adams, J.; Kaberline, S.; Ricketts, M.; Waldecker, J.R. In Situ Metal Ion Contamination and the Effects on Proton Exchange Membrane Fuel Cell Performance. J. Power Sources 2011, 196, 8967–8972. [Google Scholar] [CrossRef]
- Wang, X.; Qi, J.; Ozdemir, O.; Uddin, A.; Pasaogullari, U.; Bonville, L.J.; Molter, T. Ca2+ as an Air Impurity in Polymer Electrolyte Membrane Fuel Cells. J. Electrochem. Soc. 2014, 161, F1006. [Google Scholar] [CrossRef]
- Uddin, M.A.; Wang, X.; Park, J.; Pasaogullari, U.; Bonville, L. Distributed Effects of Calcium Ion Contaminant on Polymer Electrolyte Fuel Cell Performance. J. Power Sources 2015, 296, 64–69. [Google Scholar] [CrossRef]
- Qi, J.; Ge, J.; Uddin, M.A.; Zhai, Y.; Pasaogullari, U.; St-Pierre, J. Evaluation of Cathode Contamination with Ca2+ in Proton Exchange Membrane Fuel Cells. Electrochim. Acta 2018, 259, 510–516. [Google Scholar] [CrossRef]
- Zhu, J.; Tan, J.; Pan, Q.; Liu, Z.; Hou, Q. Effects of Mg2+ Contamination on the Performance of Proton Exchange Membrane Fuel Cell. Energy 2019, 189, 116135. [Google Scholar] [CrossRef]
- Li, G.; Tan, J.; Gong, J.; Zhang, X.; Xin, Y.; Hu, X. Performance of Proton Exchange Membrane in the Presence of Mg2+. J. Fuel Cell Sci. Technol. 2014, 11, 044501. [Google Scholar] [CrossRef]
- Qi, J.; Wang, X.; Ozdemir, M.O.; Uddin, M.A.; Bonville, L.; Pasaogullari, U.; Molter, T. Effect of Cationic Contaminants on Polymer Electrolyte Fuel Cell Performance. J. Power Sources 2015, 286, 18–24. [Google Scholar] [CrossRef]
- Hongsirikarn, K.; Goodwin, J.G.; Greenway, S.; Creager, S. Effect of Cations (Na+, Ca2+, Fe3+) on the Conductivity of a Nafion Membrane. J. Power Sources 2010, 195, 7213–7220. [Google Scholar] [CrossRef]
- Okada, T.; Satou, H.; Okuno, M.; Yuasa, M. Ion and Water Transport Characteristics of Perfluorosulfonated Ionomer Membranes with H+ and Alkali Metal Cations. J. Phys. Chem. B 2002, 106, 1267–1273. [Google Scholar] [CrossRef]
- Lee, C.; Wang, X.; Peng, J.-K.; Katzenberg, A.; Ahluwalia, R.K.; Kusoglu, A.; Komini Babu, S.; Spendelow, J.S.; Mukundan, R.; Borup, R.L. Toward a Comprehensive Understanding of Cation Effects in Proton Exchange Membrane Fuel Cells. ACS Appl. Mater. Interfaces 2022, 14, 35555–35568. [Google Scholar] [CrossRef]
- Li, H.; You, J.; Cheng, X.; Yan, X.; Shen, S.; Zhang, J. New Insight into the Effect of Co2+ Contamination on Local Oxygen Transport in PEMFCs. Chem. Eng. J. 2023, 453, 139945. [Google Scholar] [CrossRef]
- Kienitz, B.L.; Baskaran, H.; Zawodzinski, T.A. Modeling the Steady-State Effects of Cationic Contamination on Polymer Electrolyte Membranes. Electrochim. Acta 2009, 54, 1671–1679. [Google Scholar] [CrossRef]
- Zhu, F.; Wu, A.; Luo, L.; Wang, C.; Yang, F.; Wei, G.; Xia, G.; Yin, J.; Zhang, J. The Asymmetric Effects of Cu2+ Contamination in a Proton Exchange Membrane Fuel Cell (PEMFC). Fuel Cells 2020, 20, 196–202. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, G.; Shen, Q.; Li, S.; Li, Z.; Liao, J.; Jiang, Z.; Wang, H.; Zhang, H.; Ye, W. Study on the Transport Performance Degradation of Nafion Membrane Due to the Presence of Na+ and Ca2+ Using Molecular Dynamics Simulations. J. Power Sources 2022, 542, 231740. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, Q.; Yang, D.; Chu, T.; Li, B.; Ming, P.; Zhang, C. The Synergetic Effect of Air Pollutants and Metal Ions on Performance of a 5 kW Proton-Exchange Membrane Fuel Cell Stack. Int. J. Energy Res. 2021, 45, 7974–7986. [Google Scholar] [CrossRef]
- Valdés-López, V.F.; Mason, T.; Shearing, P.R.; Brett, D.J.L. Carbon Monoxide Poisoning and Mitigation Strategies for Polymer Electrolyte Membrane Fuel Cells—A Review. Prog. Energy Combust. Sci. 2020, 79, 100842. [Google Scholar] [CrossRef]
- Baschuk, J.J.; Li, X. Carbon Monoxide Poisoning of Proton Exchange Membrane Fuel Cells. Int. J. Energy Res. 2001, 25, 695–713. [Google Scholar] [CrossRef]
- Sethuraman, V.A.; Weidner, J.W. Analysis of Sulfur Poisoning on a PEM Fuel Cell Electrode. Electrochim. Acta 2010, 55, 5683–5694. [Google Scholar] [CrossRef]
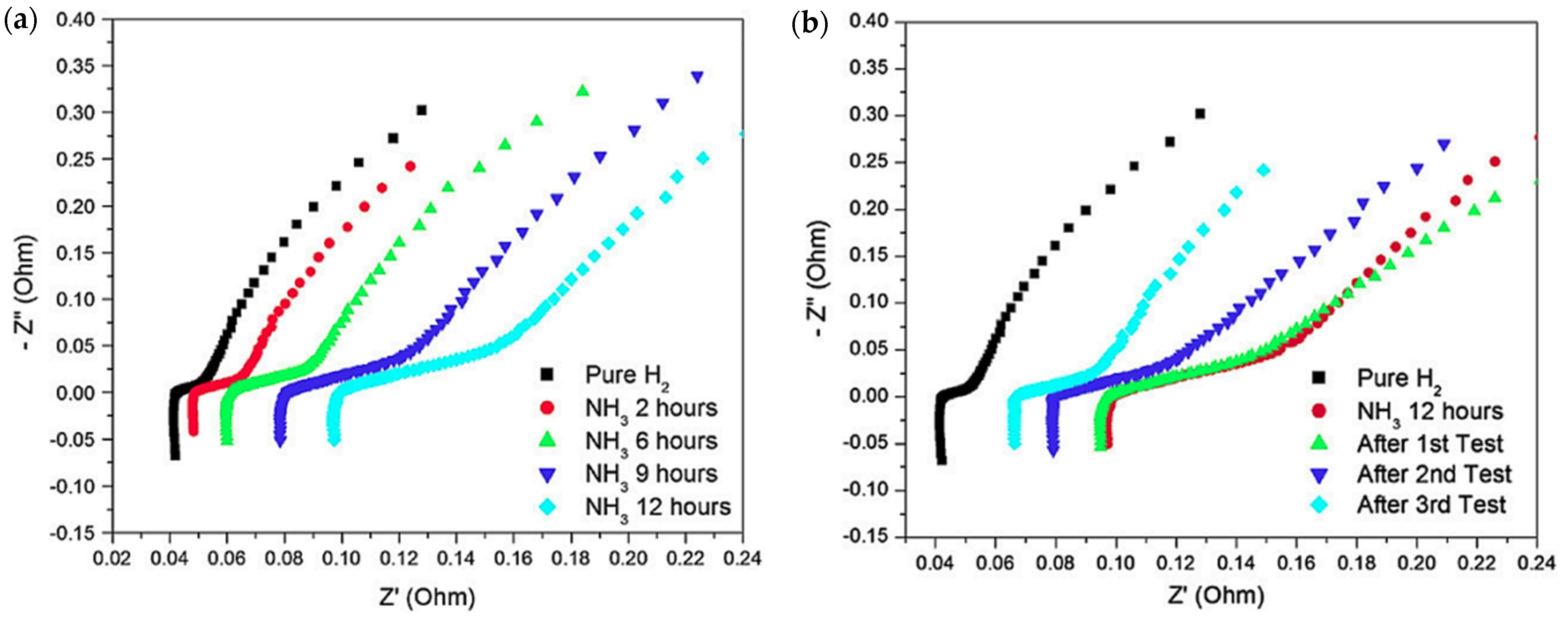
- Gomez, Y.A.; Oyarce, A.; Lindbergh, G.; Lagergren, C. Ammonia Contamination of a Proton Exchange Membrane Fuel Cell. J. Electrochem. Soc. 2018, 165, F189. [Google Scholar] [CrossRef]
- Zhang, X.; Pasaogullari, U.; Molter, T. Influence of Ammonia on Membrane-Electrode Assemblies in Polymer Electrolyte Fuel Cells. Int. J. Hydrogen Energy 2009, 34, 9188–9194. [Google Scholar] [CrossRef]
- Uribe, F.A.; Gottesfeld, S.; Zawodzinski, T.A. Effect of Ammonia as Potential Fuel Impurity on Proton Exchange Membrane Fuel Cell Performance. J. Electrochem. Soc. 2002, 149, A293. [Google Scholar] [CrossRef]
- Narusawa, K.; Hayashida, M.; Kamiya, Y.; Roppongi, H.; Kurashima, D.; Wakabayashi, K. Deterioration in Fuel Cell Performance Resulting from Hydrogen Fuel Containing Impurities: Poisoning Effects by CO, CH4, HCHO and HCOOH. JSAE Rev. 2003, 24, 41–46. [Google Scholar] [CrossRef]
- Nachiappan, N.; Kalaignan, G.P.; Sasikumar, G. Influence of Methanol Impurity in Hydrogen on PEMFC Performance. Ionics 2013, 19, 517–522. [Google Scholar] [CrossRef]
- Wang, X.; Baker, P.; Zhang, X.; Garces, H.F.; Bonville, L.J.; Pasaogullari, U.; Molter, T.M. An Experimental Overview of the Effects of Hydrogen Impurities on Polymer Electrolyte Membrane Fuel Cell Performance. Int. J. Hydrogen Energy 2014, 39, 19701–19713. [Google Scholar] [CrossRef]
- Shabani, B.; Hafttananian, M.; Khamani, S.; Ramiar, A.; Ranjbar, A.A. Poisoning of Proton Exchange Membrane Fuel Cells by Contaminants and Impurities: Review of Mechanisms, Effects, and Mitigation Strategies. J. Power Sources 2019, 427, 21–48. [Google Scholar] [CrossRef]
- Talke, A.; Misz, U.; Konrad, G.; Heinzel, A. Impact of Air Contaminants on Subscale Single Fuel Cells and an Automotive Short Stack. JEE 2015, 3, 70–79. [Google Scholar] [CrossRef][Green Version]
- Lemaire, O.; Barthe, B.; Rouillon, L.; Franco, A.A. Mechanistic Investigations of NO2 Impact on ORR in PEM Fuel Cells: A Coupled Experimental and Multi-Scale Modeling Approach. ECS Trans. 2009, 25, 1595. [Google Scholar] [CrossRef]
- Misz, U.; Talke, A.; Heinzel, A.; Konrad, G. Sensitivity Analyses on the Impact of Air Contaminants on Automotive Fuel Cells. Fuel Cells 2016, 16, 444–462. [Google Scholar] [CrossRef]
- Talke, A.; Misz, U.; Konrad, G.; Heinzel, A.; Klemp, D.; Wegener, R. Influence of Urban Air on Proton Exchange Membrane Fuel Cell Vehicles—Long Term Effects of Air Contaminants in an Authentic Driving Cycle. J. Power Sources 2018, 400, 556–565. [Google Scholar] [CrossRef]
- Hongsirikarn, K.; Goodwin, J.G.; Greenway, S.; Creager, S. Influence of Ammonia on the Conductivity of Nafion Membranes. J. Power Sources 2010, 195, 30–38. [Google Scholar] [CrossRef]
- Doyle, M.; Lewittes, M.E.; Roelofs, M.G.; Perusich, S.A.; Lowrey, R.E. Relationship between Ionic Conductivity of Perfluorinated Ionomeric Membranes and Nonaqueous Solvent Properties. J. Membr. Sci. 2001, 184, 257–273. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, C.; Cheng, J.; Xu, W.; Sun, Y.; Li, H.; Xu, L. Development of Hydrogen Fuel Cell Technology and Prospect for Its Military Application. In Proceedings of the Communications, Signal Processing, and Systems, Changbaishan, China, 24–25 July 2021; Liang, Q., Wang, W., Liu, X., Na, Z., Li, X., Zhang, B., Eds.; Springer: Singapore, 2021; pp. 1722–1730. [Google Scholar]
- Moore, J.M.; Adcock, P.L.; Lakeman, J.B.; Mepsted, G.O. The Effects of Battlefield Contaminants on PEMFC Performance. J. Power Sources 2000, 85, 254–260. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Ben Belgacem, I.; Emori, W.; Uzoma, P.C. Nafion Degradation Mechanisms in Proton Exchange Membrane Fuel Cell (PEMFC) System: A Review. Int. J. Hydrogen Energy 2021, 46, 27956–27973. [Google Scholar] [CrossRef]
- Robert, M.; El Kaddouri, A.; Perrin, J.-C.; Mozet, K.; Daoudi, M.; Dillet, J.; Morel, J.-Y.; André, S.; Lottin, O. Effects of Conjoint Mechanical and Chemical Stress on Perfluorosulfonic-Acid Membranes for Fuel Cells. J. Power Sources 2020, 476, 228662. [Google Scholar] [CrossRef]
- Fang, X.; Shen, P.K.; Song, S.; Stergiopoulos, V.; Tsiakaras, P. Degradation of Perfluorinated Sulfonic Acid Films: An in-Situ Infrared Spectro-Electrochemical Study. Polym. Degrad. Stab. 2009, 94, 1707–1713. [Google Scholar] [CrossRef]
- Gubler, L.; Dockheer, S.M.; Koppenol, W.H. Radical (HO•, H• and HOO•) Formation and Ionomer Degradation in Polymer Electrolyte Fuel Cells. J. Electrochem. Soc. 2011, 158, B755. [Google Scholar] [CrossRef]
- Zhao, M.; Shi, W.; Wu, B.; Liu, W.; Liu, J.; Xing, D.; Yao, Y.; Hou, Z.; Ming, P.; Zou, Z. Influence of Membrane Thickness on Membrane Degradation and Platinum Agglomeration under Long-Term Open Circuit Voltage Conditions. Electrochim. Acta 2015, 153, 254–262. [Google Scholar] [CrossRef]
- Frühwirt, P.; Kregar, A.; Törring, J.T.; Katrašnik, T.; Gescheidt, G. Holistic Approach to Chemical Degradation of Nafion Membranes in Fuel Cells: Modelling and Predictions. Phys. Chem. Chem. Phys. 2020, 22, 5647–5666. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Ralph, T.R.; Walsh, F.C. Modeling and Simulation of the Degradation of Perfluorinated Ion-Exchange Membranes in PEM Fuel Cells. J. Electrochem. Soc. 2009, 156, B465. [Google Scholar] [CrossRef]
- Curtin, D.E.; Lousenberg, R.D.; Henry, T.J.; Tangeman, P.C.; Tisack, M.E. Advanced Materials for Improved PEMFC Performance and Life. J. Power Sources 2004, 131, 41–48. [Google Scholar] [CrossRef]
- Chen, C.; Fuller, T.F. The Effect of Humidity on the Degradation of Nafion® Membrane. Polym. Degrad. Stab. 2009, 94, 1436–1447. [Google Scholar] [CrossRef]
- Xie, T.; Hayden, C.A. A Kinetic Model for the Chemical Degradation of Perfluorinated Sulfonic Acid Ionomers: Weak End Groups versus Side Chain Cleavage. Polymer 2007, 48, 5497–5506. [Google Scholar] [CrossRef]
- Robert, M.; El Kaddouri, A.; Perrin, J.-C.; Raya, J.; Lottin, O. Time-Resolved Monitoring of Composite NafionTM XL Membrane Degradation Induced by Fenton’s Reaction. J. Membr. Sci. 2021, 621, 118977. [Google Scholar] [CrossRef]
- Mittal, V.O.; Kunz, H.R.; Fenton, J.M. Membrane Degradation Mechanisms in PEMFCs. J. Electrochem. Soc. 2007, 154, B652. [Google Scholar] [CrossRef]
- Ghassemzadeh, L.; Kreuer, K.-D.; Maier, J.; Müller, K. Chemical Degradation of Nafion Membranes under Mimic Fuel Cell Conditions as Investigated by Solid-State NMR Spectroscopy. J. Phys. Chem. C 2010, 114, 14635–14645. [Google Scholar] [CrossRef]
- Kadirov, M.K.; Bosnjakovic, A.; Schlick, S. Membrane-Derived Fluorinated Radicals Detected by Electron Spin Resonance in UV-Irradiated Nafion and Dow Ionomers: Effect of Counterions and H2O2. J. Phys. Chem. B 2005, 109, 7664–7670. [Google Scholar] [CrossRef]
- Coms, F.D. The Chemistry of Fuel Cell Membrane Chemical Degradation. ECS Trans. 2008, 16, 235. [Google Scholar] [CrossRef]
- Ghassemzadeh, L.; Peckham, T.J.; Weissbach, T.; Luo, X.; Holdcroft, S. Selective Formation of Hydrogen and Hydroxyl Radicals by Electron Beam Irradiation and Their Reactivity with Perfluorosulfonated Acid Ionomer. J. Am. Chem. Soc. 2013, 135, 15923–15932. [Google Scholar] [CrossRef]
- Ghassemzadeh, L.; Holdcroft, S. Quantifying the Structural Changes of Perfluorosulfonated Acid Ionomer upon Reaction with Hydroxyl Radicals. J. Am. Chem. Soc. 2013, 135, 8181–8184. [Google Scholar] [CrossRef]
- Long, H.; Larson, C.; Coms, F.; Pivovar, B.; Dahlke, G.; Yandrasits, M. Role of H3O· Radical in the Degradation of Fuel Cell Proton-Exchange Membranes. ACS Phys. Chem. Au 2022, 2, 527–534. [Google Scholar] [CrossRef]
- Ding, F.; Zou, T.; Wei, T.; Chen, L.; Qin, X.; Shao, Z.; Yang, J. The Pinhole Effect on Proton Exchange Membrane Fuel Cell (PEMFC) Current Density Distribution and Temperature Distribution. Appl. Energy 2023, 342, 121136. [Google Scholar] [CrossRef]
- Jeong, K.I.; Oh, J.; Song, S.A.; Lee, D.; Lee, D.G.; Kim, S.S. A Review of Composite Bipolar Plates in Proton Exchange Membrane Fuel Cells: Electrical Properties and Gas Permeability. Compos. Struct. 2021, 262, 113617. [Google Scholar] [CrossRef]
- Özyalcin, C.; Mauermann, P.; Dirkes, S.; Thiele, P.; Sterlepper, S.; Pischinger, S. Investigation of Filtration Phenomena of Air Pollutants on Cathode Air Filters for PEM Fuel Cells. Catalysts 2021, 11, 1339. [Google Scholar] [CrossRef]
- Yang, D.; Ma, X.; Lv, H.; Li, B.; Zhang, C. NO Adsorption and Temperature Programmed Desorption on K2CO3 Modified Activated Carbons. J. Cent. South Univ. 2018, 25, 2339–2348. [Google Scholar] [CrossRef]
- Oesch, S.; Faller, M. Environmental Effects on Materials: The Effect of the Air Pollutants SO2, NO2, NO and O3 on the Corrosion of Copper, Zinc and Aluminium. A Short Literature Survey and Results of Laboratory Exposures. Corros. Sci. 1997, 39, 1505–1530. [Google Scholar] [CrossRef]
- Molochas, C.; Tsiakaras, P. Carbon Monoxide Tolerant Pt-Based Electrocatalysts for H2-PEMFC Applications: Current Progress and Challenges. Catalysts 2021, 11, 1127. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, S.; Zhuo, L.; Wang, Z.; Wang, C.; Sun, F.; Niu, K.; Xu, F.; Che, X.; Zhang, J.; et al. Advanced Proton Exchange Membrane Prepared from N-Heterocyclic Poly(Aryl Ether Ketone)s with Pendant Benzenesulfonic Moieties and Performing Enhanced Radical Tolerance and Fuel Cell Properties. J. Membr. Sci. 2023, 681, 121767. [Google Scholar] [CrossRef]
- Wallnöfer-Ogris, E.; Poimer, F.; Köll, R.; Macherhammer, M.-G.; Trattner, A. Main Degradation Mechanisms of Polymer Electrolyte Membrane Fuel Cell Stacks—Mechanisms, Influencing Factors, Consequences, and Mitigation Strategies. Int. J. Hydrogen Energy 2024, 50, 1159–1182. [Google Scholar] [CrossRef]
- Zatoń, M.; Rozière, J.; Jones, D.J. Current Understanding of Chemical Degradation Mechanisms of Perfluorosulfonic Acid Membranes and Their Mitigation Strategies: A Review. Sustain. Energy Fuels 2017, 1, 409–438. [Google Scholar] [CrossRef]
- Liu, W.; Qiu, D.; Peng, L.; Lai, X. Study on the Nonuniform Mechanical Degradation of Membranes Considering Temperature and Relative Humidity Distribution in Proton Exchange Membrane Fuel Cells. Fuel Cells 2023, 23, 170–180. [Google Scholar] [CrossRef]
- De Bruijn, F.A.; Dam, V.A.T.; Janssen, G.J.M. Review: Durability and Degradation Issues of PEM Fuel Cell Components. Fuel Cells 2008, 8, 3–22. [Google Scholar] [CrossRef]
- Qiu, D.; Peng, L.; Liang, P.; Yi, P.; Lai, X. Mechanical Degradation of Proton Exchange Membrane along the MEA Frame in Proton Exchange Membrane Fuel Cells. Energy 2018, 165, 210–222. [Google Scholar] [CrossRef]
- Jouin, M.; Gouriveau, R.; Hissel, D.; Péra, M.-C.; Zerhouni, N. Degradations Analysis and Aging Modeling for Health Assessment and Prognostics of PEMFC. Reliab. Eng. Syst. Saf. 2016, 148, 78–95. [Google Scholar] [CrossRef]
- Patil, Y.P.; Jarrett, W.L.; Mauritz, K.A. Deterioration of Mechanical Properties: A Cause for Fuel Cell Membrane Failure. J. Membr. Sci. 2010, 356, 7–13. [Google Scholar] [CrossRef]
- Shi, S.; Sun, X.; Lin, Q.; Chen, J.; Fu, Y.; Hong, X.; Li, C.; Guo, X.; Chen, G.; Chen, X. Fatigue Crack Propagation Behavior of Fuel Cell Membranes after Chemical Degradation. Int. J. Hydrogen Energy 2020, 45, 27653–27664. [Google Scholar] [CrossRef]
- Kundu, S.; Simon, L.C.; Fowler, M.; Grot, S. Mechanical Properties of NafionTM Electrolyte Membranes under Hydrated Conditions. Polymer 2005, 46, 11707–11715. [Google Scholar] [CrossRef]
- Tang, H.; Peikang, S.; Jiang, S.P.; Wang, F.; Pan, M. A Degradation Study of Nafion Proton Exchange Membrane of PEM Fuel Cells. J. Power Sources 2007, 170, 85–92. [Google Scholar] [CrossRef]
- Dafalla, A.M.; Jiang, F. Stresses and Their Impacts on Proton Exchange Membrane Fuel Cells: A Review. Int. J. Hydrogen Energy 2018, 43, 2327–2348. [Google Scholar] [CrossRef]
- McDonald, R.C.; Mittelsteadt, C.K.; Thompson, E.L. Effects of Deep Temperature Cycling on Nafion® 112 Membranes and Membrane Electrode Assemblies. Fuel Cells 2004, 4, 208–213. [Google Scholar] [CrossRef]
- Taylor, A.K.; Smith, C.; Neyerlin, K.C. Mitigation and Diagnosis of Pin-Hole Formation in Polymer Electrolyte Membrane Fuel Cells. J. Power Sources 2023, 571, 232971. [Google Scholar] [CrossRef]
- Qiu, D.; Peng, L.; Lai, X.; Ni, M.; Lehnert, W. Mechanical Failure and Mitigation Strategies for the Membrane in a Proton Exchange Membrane Fuel Cell. Renew. Sustain. Energy Rev. 2019, 113, 109289. [Google Scholar] [CrossRef]
- Jiang, F.; Fang, W.; Wang, C.-Y. Non-Isothermal Cold Start of Polymer Electrolyte Fuel Cells. Electrochim. Acta 2007, 53, 610–621. [Google Scholar] [CrossRef]
- Cho, E.; Ko, J.-J.; Ha, H.Y.; Hong, S.-A.; Lee, K.-Y.; Lim, T.-W.; Oh, I.-H. Effects of Water Removal on the Performance Degradation of PEMFCs Repetitively Brought to < 0 °C. J. Electrochem. Soc. 2004, 151, A661. [Google Scholar] [CrossRef]
- Cho, E.; Ko, J.-J.; Ha, H.Y.; Hong, S.-A.; Lee, K.-Y.; Lim, T.-W.; Oh, I.-H. Characteristics of the PEMFC Repetitively Brought to Temperatures below 0 °C. J. Electrochem. Soc. 2003, 150, A1667. [Google Scholar] [CrossRef]
- Frensch, S.H.; Serre, G.; Fouda-Onana, F.; Jensen, H.C.; Christensen, M.L.; Araya, S.S.; Kær, S.K. Impact of Iron and Hydrogen Peroxide on Membrane Degradation for Polymer Electrolyte Membrane Water Electrolysis: Computational and Experimental Investigation on Fluoride Emission. J. Power Sources 2019, 420, 54–62. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X. A Review of Polymer Electrolyte Membrane Fuel Cell Durability for Vehicular Applications: Degradation Modes and Experimental Techniques. Energy Convers. Manag. 2019, 199, 112022. [Google Scholar] [CrossRef]
- Wilkie, C.A.; Thomsen, J.R.; Mittleman, M.L. Interaction of Poly(Methyl Methacrylate) and Nafions. J. Appl. Polym. Sci. 1991, 42, 901–909. [Google Scholar] [CrossRef]
- Pan, M.; Pan, C.; Li, C.; Zhao, J. A Review of Membranes in Proton Exchange Membrane Fuel Cells: Transport Phenomena, Performance and Durability. Renew. Sustain. Energy Rev. 2021, 141, 110771. [Google Scholar] [CrossRef]
- Dafalla, A.M.; Wei, L.; Habte, B.T.; Guo, J.; Jiang, F. Membrane Electrode Assembly Degradation Modeling of Proton Exchange Membrane Fuel Cells: A Review. Energies 2022, 15, 9247. [Google Scholar] [CrossRef]
- Okada, T.; Satou, H.; Yuasa, M. Effects of Additives on Oxygen Reduction Kinetics at the Interface between Platinum and Perfluorinated Ionomer. Langmuir 2003, 19, 2325–2332. [Google Scholar] [CrossRef]
- Collier, A.; Wang, H.; Zi Yuan, X.; Zhang, J.; Wilkinson, D.P. Degradation of Polymer Electrolyte Membranes. Int. J. Hydrogen Energy 2006, 31, 1838–1854. [Google Scholar] [CrossRef]
- Collette, F.M.; Lorentz, C.; Gebel, G.; Thominette, F. Hygrothermal Aging of Nafion®. J. Membr. Sci. 2009, 330, 21–29. [Google Scholar] [CrossRef]
- Ergun, D.; Devrim, Y.; Bac, N.; Eroglu, I. Phosphoric Acid Doped Polybenzimidazole Membrane for High Temperature PEM Fuel Cell. J. Appl. Polym. Sci. 2012, 124, E267–E277. [Google Scholar] [CrossRef]
- Haider, R.; Wen, Y.; Ma, Z.F.; Wilkinson, D.P.; Zhang, L.; Yuan, X.; Song, S.; Zhang, J. High Temperature Proton Exchange Membrane Fuel Cells: Progress in Advanced Materials and Key Technologies. Chem. Soc. Rev. 2021, 50, 1138–1187. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, G.; Zhang, X.; Sun, C.; Jiao, K.; Wang, Y. Thermal Management of Polymer Electrolyte Membrane Fuel Cells: A Review of Cooling Methods, Material Properties, and Durability. Appl. Energy 2021, 286, 116496. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, X.; Kang, H.; Lv, J.; Zeng, R.; Shen, J. Thermal Management of Polymer Electrolyte Membrane Fuel Cells: A Critical Review of Heat Transfer Mechanisms, Cooling Approaches, and Advanced Cooling Techniques Analysis. Energy Convers. Manag. 2022, 254, 115221. [Google Scholar] [CrossRef]
- Peng, M.; Chen, L.; Zhang, R.; Xu, W.; Tao, W.-Q. Improvement of Thermal and Water Management of Air-Cooled Polymer Electrolyte Membrane Fuel Cells by Adding Porous Media into the Cathode Gas Channel. Electrochim. Acta 2022, 412, 140154. [Google Scholar] [CrossRef]
- Raguman, A.; Vedagiri, P. A Review on Recent Approaches in Thermal and Water Management of Polymer Electrolyte Membrane Fuel Cells. Mater. Today Proc. 2022, 68, 1975–1979. [Google Scholar] [CrossRef]
- Hooshyari, K.; Amini Horri, B.; Abdoli, H.; Fallah Vostakola, M.; Kakavand, P.; Salarizadeh, P. A Review of Recent Developments and Advanced Applications of High-Temperature Polymer Electrolyte Membranes for PEM Fuel Cells. Energies 2021, 14, 5440. [Google Scholar] [CrossRef]
- Primachenko, O.N.; Marinenko, E.A.; Odinokov, A.S.; Kononova, S.V.; Kulvelis, Y.V.; Lebedev, V.T. State of the Art and Prospects in the Development of Proton-Conducting Perfluorinated Membranes with Short Side Chains: A Review. Polym. Adv. Technol. 2021, 32, 1386–1408. [Google Scholar] [CrossRef]
- Ju, Q.; Chao, G.; Wang, Y.; Lv, Z.; Geng, K.; Li, N. An Antioxidant Polybenzimidazole with Naphthalene Group for High-Temperature Proton Exchange Membrane Fuel Cells. J. Membr. Sci. 2023, 686, 121970. [Google Scholar] [CrossRef]
- Harun, N.A.M.; Shaari, N.; Nik Zaiman, N.F.H. A Review of Alternative Polymer Electrolyte Membrane for Fuel Cell Application Based on Sulfonated Poly(Ether Ether Ketone). Int. J. Energy Res. 2021, 45, 19671–19708. [Google Scholar] [CrossRef]
- Mohamad Nor, N.A.; Mohamed, M.A.; Jaafar, J. Modified Sulfonated Polyphenylsulfone Proton Exchange Membrane with Enhanced Fuel Cell Performance: A Review. J. Ind. Eng. Chem. 2022, 116, 32–59. [Google Scholar] [CrossRef]
- Xu, M.; Xue, H.; Wang, Q.; Jia, L. Sulfonated Poly(Arylene Ether)s Based Proton Exchange Membranes for Fuel Cells. Int. J. Hydrogen Energy 2021, 46, 31727–31753. [Google Scholar] [CrossRef]
- Liu, F.; Miyatake, K. Well-Designed Polyphenylene PEMs with High Proton Conductivity and Chemical and Mechanical Durability for Fuel Cells. J. Mater. Chem. A 2022, 10, 7660–7667. [Google Scholar] [CrossRef]
- Wijaya, F.; Woo, S.; Lee, H.; Nugraha, A.F.; Shin, D.; Bae, B. Sulfonated Poly(Phenylene-Co-Arylene Ether Sulfone) Multiblock Membranes for Application in High-Performance Fuel Cells. J. Membr. Sci. 2022, 645, 120203. [Google Scholar] [CrossRef]
- Karimi, A.; Mirfarsi, S.H.; Rowshanzamir, S.; Beyraghi, F.; Lester, D. Vicious Cycle during Chemical Degradation of Sulfonated Aromatic Proton Exchange Membranes in the Fuel Cell Application. Int. J. Energy Res. 2020, 44, 8877–8891. [Google Scholar] [CrossRef]
- Dahe, G.J.; Singh, R.P.; Dudeck, K.W.; Yang, D.; Berchtold, K.A. Influence of Non-Solvent Chemistry on Polybenzimidazole Hollow Fiber Membrane Preparation. J. Membr. Sci. 2019, 577, 91–103. [Google Scholar] [CrossRef]
- Qu, E.; Hao, X.; Xiao, M.; Han, D.; Huang, S.; Huang, Z.; Wang, S.; Meng, Y. Proton Exchange Membranes for High Temperature Proton Exchange Membrane Fuel Cells: Challenges and Perspectives. J. Power Sources 2022, 533, 231386. [Google Scholar] [CrossRef]
- Guo, Z.; Perez-Page, M.; Chen, J.; Ji, Z.; Holmes, S.M. Recent Advances in Phosphoric Acid–Based Membranes for High–Temperature Proton Exchange Membrane Fuel Cells. J. Energy Chem. 2021, 63, 393–429. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, T.; Che, X.; Dong, J.; Li, Q.; Yang, J. Poly(Arylene Pyridine)s: New Alternative Materials for High Temperature Polymer Electrolyte Fuel Cells. J. Power Sources 2022, 526, 231131. [Google Scholar] [CrossRef]
- Hu, X.; Ao, Y.; Gao, Y.; Liu, B.; Zhao, C. Facile Preparation of Triazole-Functionalized Poly(Arylene Perfluorophenyl) High Temperature Proton Exchange Membranes via Para-Fluoro-Thiol Click Reaction with High Radical Resistance. J. Membr. Sci. 2023, 687, 122102. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Wang, J.; Liu, X.; Yang, Y.; Yang, F.; He, R. Terphenyl Pyridine Based Polymers for Superior Conductivity and Excellent Chemical Stability of High Temperature Proton Exchange Membranes. Eur. Polym. J. 2022, 173, 111295. [Google Scholar] [CrossRef]
- Huang, H.; Zeng, X.; Zhang, X.; Fan, J.; Li, H. Proton Exchange Membrane Based on Interpenetrating Polymer Network Structure for Excellent Cell Performance and Chemical Stability. J. Power Sources 2023, 558, 232602. [Google Scholar] [CrossRef]
- Wang, J.; Dai, Y.; Wan, R.; Wei, W.; Xu, S.; Zhai, F.; He, R. Grafting Free Radical Scavengers onto Polyarylethersulfone Backbones for Superior Chemical Stability of High Temperature Polymer Membrane Electrolytes. Chem. Eng. J. 2021, 413, 127541. [Google Scholar] [CrossRef]
- Agarwal, T.; Adhikari, S.; Kim, Y.S.; Babu, S.K.; Tian, D.; Bae, C.; Pham, N.N.T.; Lee, S.G.; Prasad, A.K.; Advani, S.G.; et al. Fluoroalkyl Phosphonic Acid Radical Scavengers for Proton Exchange Membrane Fuel Cells. J. Mater. Chem. A 2023, 11, 9748–9754. [Google Scholar] [CrossRef]
- Teixeira, F.C.; Teixeira, A.P.S.; Rangel, C.M. Chemical Stability of New Nafion Membranes Doped with Bisphosphonic Acids under Fenton Oxidative Conditions. Int. J. Hydrogen Energy 2023, 48, 37489–37499. [Google Scholar] [CrossRef]
- Son, B.; Oh, K.; Park, S.; Lee, T.-G.; Lee, D.H.; Kwon, O. Study of Morphological Characteristics on Hydrophilicity-Enhanced SiO2/Nafion Composite Membranes by Using Multimode Atomic Force Microscopy. Int. J. Energy Res. 2019, 43, 4157–4169. [Google Scholar] [CrossRef]
- Maiti, T.K.; Singh, J.; Dixit, P.; Majhi, J.; Bhushan, S.; Bandyopadhyay, A.; Chattopadhyay, S. Advances in Perfluorosulfonic Acid-Based Proton Exchange Membranes for Fuel Cell Applications: A Review. Chem. Eng. J. Adv. 2022, 12, 100372. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, S.; Zhu, Q.; Zhao, L. Enhanced Anhydrous Proton Conductivity of SPEEK/IL Composite Membrane Embedded with Amino Functionalized Mesoporous Silica. Int. J. Hydrogen Energy 2019, 44, 6148–6159. [Google Scholar] [CrossRef]
- Zhao, D.; Yi, B.L.; Zhang, H.M.; Yu, H.M. MnO2/SiO2–SO3H Nanocomposite as Hydrogen Peroxide Scavenger for Durability Improvement in Proton Exchange Membranes. J. Membr. Sci. 2010, 346, 143–151. [Google Scholar] [CrossRef]
- Gashoul, F.; Parnian, M.J.; Rowshanzamir, S. A New Study on Improving the Physicochemical and Electrochemical Properties of SPEEK Nanocomposite Membranes for Medium Temperature Proton Exchange Membrane Fuel Cells Using Different Loading of Zirconium Oxide Nanoparticles. Int. J. Hydrogen Energy 2017, 42, 590–602. [Google Scholar] [CrossRef]
- Khalifa, R.E.; Omer, A.M.; Abd Elmageed, M.H.; Mohy Eldin, M.S. Titanium Dioxide/Phosphorous-Functionalized Cellulose Acetate Nanocomposite Membranes for DMFC Applications: Enhancing Properties and Performance. ACS Omega 2021, 6, 17194–17202. [Google Scholar] [CrossRef]
- Chandra Kishore, S.; Perumal, S.; Atchudan, R.; Alagan, M.; Wadaan, M.A.; Baabbad, A.; Manoj, D. Recent Advanced Synthesis Strategies for the Nanomaterial-Modified Proton Exchange Membrane in Fuel Cells. Membranes 2023, 13, 590. [Google Scholar] [CrossRef]
- Vandeginste, V.; Madhav, D. Interface Modification and Characterization of PVC Based Composites and Nanocomposites. In Poly (Vinyl Chloride) Based Composites and Nanocomposites; Engineering Materials; H, A., Sabu, T., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 55–86. ISBN 978-3-031-45375-5. [Google Scholar]
- Vinothkannan, M.; Hariprasad, R.; Ramakrishnan, S.; Kim, A.R.; Yoo, D.J. Potential Bifunctional Filler (CeO2–ACNTs) for Nafion Matrix toward Extended Electrochemical Power Density and Durability in Proton-Exchange Membrane Fuel Cells Operating at Reduced Relative Humidity. ACS Sustain. Chem. Eng. 2019, 7, 12847–12857. [Google Scholar] [CrossRef]
- Maiti, T.K.; Singh, J.; Maiti, S.K.; Majhi, J.; Ahuja, A.; Singh, M.; Bandyopadhyay, A.; Manik, G.; Chattopadhyay, S. Molecular Dynamics Simulations and Experimental Studies of the Perfluorosulfonic Acid-Based Composite Membranes Containing Sulfonated Graphene Oxide for Fuel Cell Applications. Eur. Polym. J. 2022, 174, 111345. [Google Scholar] [CrossRef]
- Rambabu, G.; Nagaraju, N.; Bhat, S.D. Functionalized Fullerene Embedded in Nafion Matrix: A Modified Composite Membrane Electrolyte for Direct Methanol Fuel Cells. Chem. Eng. J. 2016, 306, 43–52. [Google Scholar] [CrossRef]
- Postnov, V.N.; Mel’nikova, N.A.; Shul’meister, G.A.; Novikov, A.G.; Murin, I.V.; Zhukov, A.N. Nafion- and Aquivion-Based Nanocomposites Containing Detonation Nanodiamonds. Russ J Gen Chem 2017, 87, 2754–2755. [Google Scholar] [CrossRef]
- Madhav, D.; Malankowska, M.; Coronas, J. Synthesis of Nanoparticles of Zeolitic Imidazolate Framework ZIF-94 Using Inorganic Deprotonators. New J. Chem. 2020, 44, 20449–20457. [Google Scholar] [CrossRef]
- Li, X.-M.; Gao, J. Recent Advances of Metal–Organic Frameworks-Based Proton Exchange Membranes in Fuel Cell Applications. SusMat 2022, 2, 504–534. [Google Scholar] [CrossRef]
- Khoo, K.S.; Chia, W.Y.; Wang, K.; Chang, C.-K.; Leong, H.Y.; Maaris, M.N.B.; Show, P.L. Development of Proton-Exchange Membrane Fuel Cell with Ionic Liquid Technology. Sci. Total Environ. 2021, 793, 148705. [Google Scholar] [CrossRef] [PubMed]
- Alashkar, A.; Al-Othman, A.; Tawalbeh, M.; Qasim, M. A Critical Review on the Use of Ionic Liquids in Proton Exchange Membrane Fuel Cells. Membranes 2022, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Rui, Z.; Liu, J. Understanding of Free Radical Scavengers Used in Highly Durable Proton Exchange Membranes. Prog. Nat. Sci. Mater. Int. 2020, 30, 732–742. [Google Scholar] [CrossRef]
- Liu, L.; Xing, Y.; Li, Y.; Fu, Z.; Li, Z.; Li, H. Double-Layer Expanded Polytetrafluoroethylene Reinforced Membranes with Cerium Oxide Radical Scavengers for Highly Stable Proton Exchange Membrane Fuel Cells. ACS Appl. Energy Mater. 2022, 5, 8743–8755. [Google Scholar] [CrossRef]
- Ram, F.; Velayutham, P.; Sahu, A.K.; Lele, A.K.; Shanmuganathan, K. Enhancing Thermomechanical and Chemical Stability of Polymer Electrolyte Membranes Using Polydopamine Coated Nanocellulose. ACS Appl. Energy Mater. 2020, 3, 1988–1999. [Google Scholar] [CrossRef]
- Tinh, V.D.C.; Thuc, V.D.; Kim, D. Chemically Sustainable Fuel Cells via Layer-by-Layer Fabrication of Sulfonated Poly(Arylene Ether Sulfone) Membranes Containing Cerium Oxide Nanoparticles. J. Membr. Sci. 2021, 634, 119430. [Google Scholar] [CrossRef]
- Xu, K.; Pei, S.; Zhang, W.; Han, Z.; Liu, G.; Xu, X.; Ma, J.; Zhang, Y.; Liu, F.; Zhang, Y.; et al. Chemical Stability of Proton Exchange Membranes Synergistically Promoted by Organic Antioxidant and Inorganic Radical Scavengers. J. Membr. Sci. 2022, 655, 120594. [Google Scholar] [CrossRef]
- Agarwal, T.; Sievert, A.C.; Komini Babu, S.; Adhikari, S.; Park, E.J.; Prasad, A.K.; Advani, S.G.; Hopkins, T.E.; Park, A.M.; Kim, Y.S.; et al. Enhancing Durability of Polymer Electrolyte Membrane Using Cation Size Selective Agents. J. Power Sources 2023, 580, 233362. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, X.; Shen, X.; Yu, W.; Yang, X.; Li, Q.; Ge, X.; Wu, L.; Xu, T. In Situ Construction of Ultra-Thin PANI/CeOx Layer on Proton Exchange Membrane for Enhanced Oxidation Resistance in PEMFC. J. Membr. Sci. 2024, 689, 122167. [Google Scholar] [CrossRef]
- Zhiyan, R.; Qingbing, L.; Youxiu, H.; Rui, D.; Jia, L.; Jia, L.; Jianguo, L. Ceria Nanorods as Highly Stable Free Radical Scavengers for Highly Durable Proton Exchange Membranes. RSC Adv. 2021, 11, 32012–32021. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Li, M.; Xie, N.; Zhang, J.; Qin, Y.; Yin, Y.; Guiver, M.D. Durability Enhancement of Proton Exchange Membrane Fuel Cells by Ferrocyanide or Ferricyanide Additives. J. Membr. Sci. 2021, 629, 119282. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Liu, X.; Zhang, J.; Yin, Y.; Guiver, M.D. A Paradigm Shift for a New Class of Proton Exchange Membranes with Ferrocyanide Proton-Conducting Groups Providing Enhanced Oxidative Stability. J. Membr. Sci. 2020, 616, 118536. [Google Scholar] [CrossRef]
- Shen, X.; Liang, X.; Xu, Y.; Yu, W.; Li, Q.; Ge, X.; Wu, L.; Xu, T. In-Situ Growth of PPy/MnOx Radical Quenching Layer for Durability Enhancement of Proton Exchange Membrane in PEMFCs. J. Membr. Sci. 2023, 675, 121556. [Google Scholar] [CrossRef]

| Typical Pollutant | Sources |
|---|---|
| Ions of Fe, Cr, Ni, Mo, Mn, Cu | Metal bipolar plates |
| Ions of Ca, Na | Membrane, gaskets, fuel |
| Ions of Si, Ca, Mg, K | Gaskets |
| Ions of Si, Na, Ca, Mg, Al, S, K, Fe, Cu, V, Cr | Coolants, DI water |
| Typical Pollutants in Hydrogen | Hydrogen Sources |
|---|---|
| CO, NH3, H2S, HCN | Crude oil, natural gas |
| Hydrocarbons, aldehydes | Gasolines |
| Mercaptans | Diesels |
| CO, odorants, alcohols | Methanol/dimethyl ether |
| Cations, aldehydes, alcohols, formic acid, NH3, H2S, HCN | Biomass |
| CO, CO2, SO2, NH3, H2S, benzene | Industrial by-products |
| Ions of Ca, Na, Cl | Water |
| Typical Pollutants in Air | Sources |
|---|---|
| SOx, NOx, COx, O3, hydrocarbons, soot, particulates | Fuel combustion, pollution |
| NH3 | Ambient air, farming |
| Cations and anions from ocean salts, dust | Natural sources |
| Ions of Na, Ca, Mg, Al, K, Fe, Cu, V, Cr, Cl | De-icers, humidifiers |
| SO2, NO2, CO, propane, benzene | Battlefield pollutants |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madhav, D.; Wang, J.; Keloth, R.; Mus, J.; Buysschaert, F.; Vandeginste, V. A Review of Proton Exchange Membrane Degradation Pathways, Mechanisms, and Mitigation Strategies in a Fuel Cell. Energies 2024, 17, 998. https://doi.org/10.3390/en17050998
Madhav D, Wang J, Keloth R, Mus J, Buysschaert F, Vandeginste V. A Review of Proton Exchange Membrane Degradation Pathways, Mechanisms, and Mitigation Strategies in a Fuel Cell. Energies. 2024; 17(5):998. https://doi.org/10.3390/en17050998
Chicago/Turabian StyleMadhav, Dharmjeet, Junru Wang, Rajesh Keloth, Jorben Mus, Frank Buysschaert, and Veerle Vandeginste. 2024. "A Review of Proton Exchange Membrane Degradation Pathways, Mechanisms, and Mitigation Strategies in a Fuel Cell" Energies 17, no. 5: 998. https://doi.org/10.3390/en17050998
APA StyleMadhav, D., Wang, J., Keloth, R., Mus, J., Buysschaert, F., & Vandeginste, V. (2024). A Review of Proton Exchange Membrane Degradation Pathways, Mechanisms, and Mitigation Strategies in a Fuel Cell. Energies, 17(5), 998. https://doi.org/10.3390/en17050998










