Phosphorus Removal Rate and Efficiency in an Electrochemical Sequencing Reactor for the Treatment of Wastewater with Low Organic Carbon Content
Abstract
1. Introduction
2. Materials and Methods
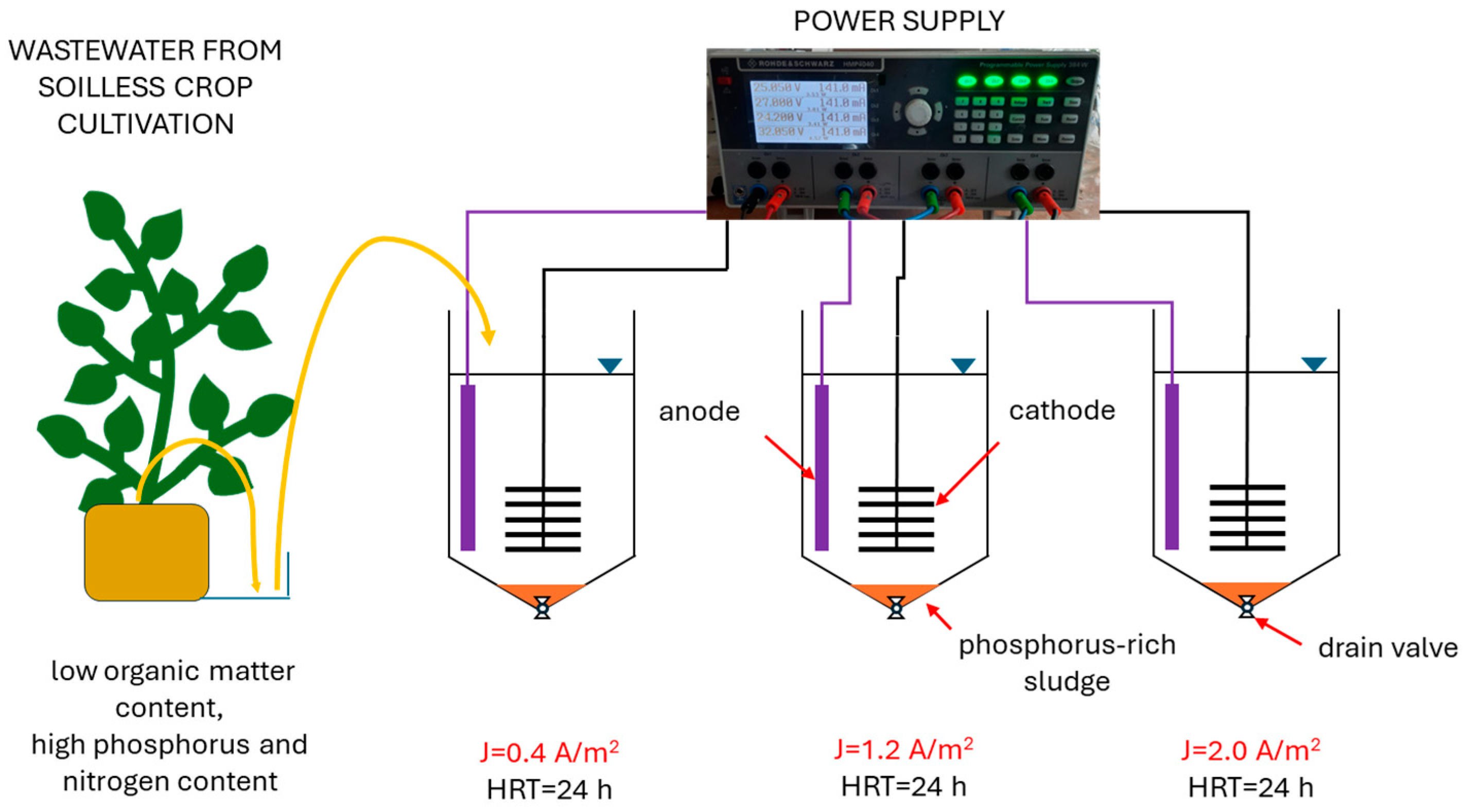
2.1. Experimental Setup and Research Organization
2.2. Wastewater
2.3. Physicochemical Analysis of Wastewater
2.4. Quantity and Quality of Sludge
3. Results and Discussion
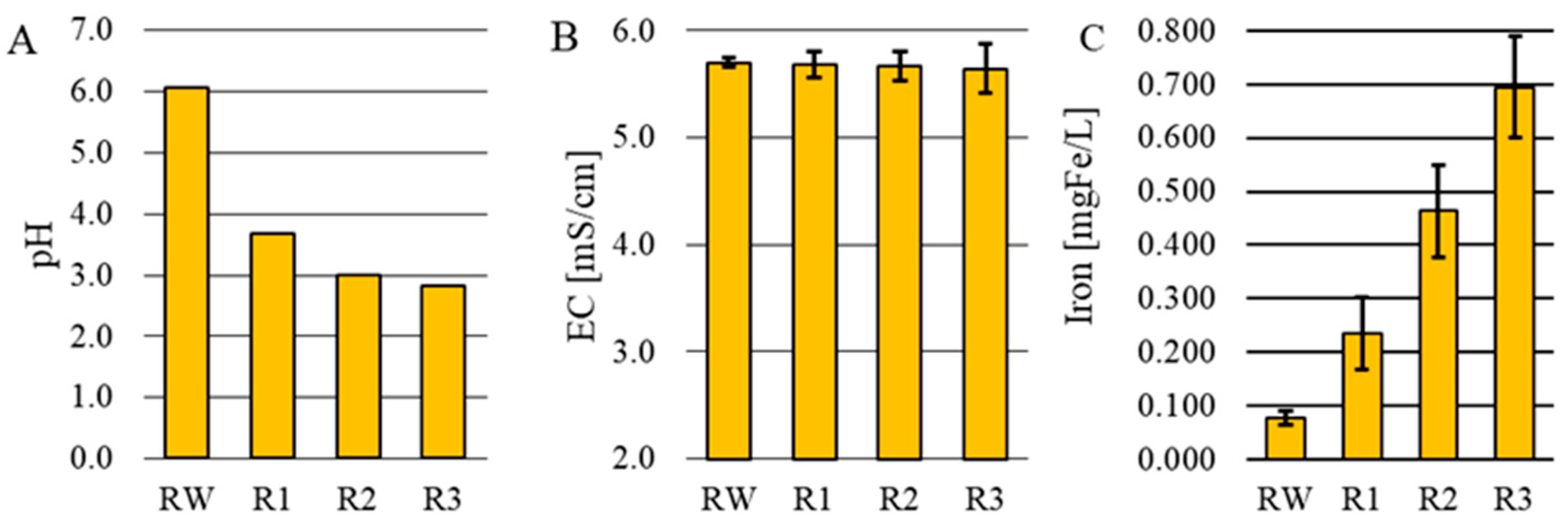
3.1. pH, Electrolytic Conductivity, Iron Content
3.2. Removal of Phosphorus Compounds, Nitrogen and Organic Compounds
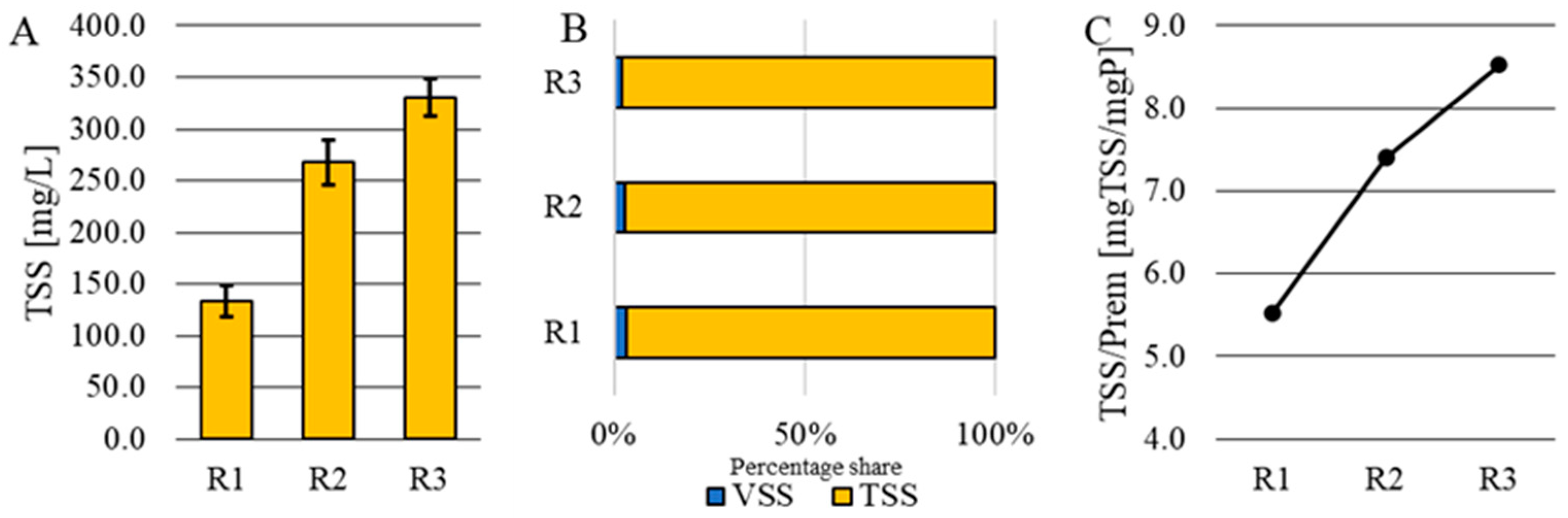
3.3. Quantity and Quality of Sludge
4. Conclusions
- A threefold increase in electrical current density (from 0.4 to 1.2 A/m2) resulted in a 16% increase in dephosphatation rate, while a fivefold increase in electrical current density (from 0.4 to 2.0 A/m2) resulted in a 32% increase.
- The rate of TP removal ranged from 26.45 to 34.79 mg/L·h, TN from 2.07 to 6.58 mg/L·h, and organic compounds from 0.44 to 1.50 mg/L·h, respectively, for electrical current densities of 0.4 and 2.0 A/m2.
- Increasing the electrical current density above 1.2 A/m2 did not significantly increase the efficiency of TP removal. The observed effectiveness was 71.5 ± 6.0%, 88.0 ± 4.9%, and 88.6 ± 2.5%, respectively, for electrical current densities of 0.4, 1.2 and 2.0 A/m2.
- Due to the low pH of the treated wastewater, phosphorus was likely removed through electrostatic adsorption on the surface of iron oxides via surface complexation.
- The use of DC and iron electrodes also allowed for the removal of a maximum of 7.4 ± 2.5% TN and 51.1 ± 8.3% organic compounds for an electrical current density of 2.0 A/m2.
- The amount of sludge resulting from electrocoagulation using an iron electrode and HRT = 24h increased with the increase in electrical current density, ranging from 347 ± 38 mg/L (J = 0.4 A/m2) to 665 ± 36 mg/L (J = 2.0 A/m2). This sludge was characterized by a high percentage of the mineral fraction, ranging from 96.7% to 97.8%.
- With the increase in applied electrical current density and the increase in the amount of generated sludge, the percentage of phosphorus in the sludge decreased, ranging from 18.1% (J = 0.4 A/m2) to 11.7% (J = 2.0 A/m2).
- Electrocoagulation led to a decrease in the pH of the treated wastewater from 6.1 to 2.8 (J = 2.0 A/m2), while no significant change in electrolytic conductivity was observed.
- Dissolving the iron electrode led to an increase in the iron concentration in the treated wastewater from 0.077 ± 0.012 mg Fe/L to 0.695 ± 0.095 mg Fe/L (J = 2.0 A/m2).
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vermeulen, T.; van Os, E.A.; van der Linden, A.M.A.; Wipfler, E.L. Need for Clean Water and Recirculation to Reduce Emissions of Plant Protection Products from Soilless Cultivation. Acta Hortic. 2017, 1176, 87–94. [Google Scholar] [CrossRef]
- Elvanidi, A.; Benitez Reascos, C.; Gourzoulidou, E.; Kunze, A.; Max, J.; Katsoulas, N. Implementation of the Circular Economy Concept in Greenhouse Hydroponics for Ultimate Use of Water and Nutrients. Horticulturae 2020, 6, 83. [Google Scholar] [CrossRef]
- Mielcarek, A.; Rodziewicz, J.; Janczukowicz, W.; Dobrowolski, A. Analysis of Wastewater Generated in Greenhouse Soilless Tomato Cultivation in Central Europe. Water 2019, 11, 2538. [Google Scholar] [CrossRef]
- Mielcarek, A.; Jóźwiak, T.; Rodziewicz, J.; Bryszewski, K.; Janczukowicz, W.; Kalisz, B.; Tavares, J.M.R. Recovery of Phosphorus and Other Minerals from Greenhouse Wastewater Generated during Soilless Tomato Cultivation by Means of Alkalizing Agents. Sci. Total Environ. 2023, 892, 164757. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Llanderal, A.; Maksimovic, I.; Lao, M. Cascade Cropping System with Horticultural and Ornamental Plants under Greenhouse Conditions. Water 2018, 10, 125. [Google Scholar] [CrossRef]
- Karatsivou, E.; Elvanidi, A.; Faliagka, S.; Naounoulis, I.; Katsoulas, N. Performance Evaluation of a Cascade Cropping System. Horticulturae 2023, 9, 802. [Google Scholar] [CrossRef]
- Richa, A.; Fizir, M.; Touil, S. Advanced Monitoring of Hydroponic Solutions Using Ion-Selective Electrodes and the Internet of Things: A Review. Environ. Chem. Lett. 2021, 19, 3445–3463. [Google Scholar] [CrossRef]
- Thompson, R.B.; Incrocci, L.; van Ruijven, J.; Massa, D. Reducing Contamination of Water Bodies from European Vegetable Production Systems. Agric. Water Manag. 2020, 240, 106258. [Google Scholar] [CrossRef]
- Mielcarek, A.; Kłobukowska, K.; Rodziewicz, J.; Janczukowicz, W.; Bryszewski, K.Ł. Water Nutrient Management in Soilless Plant Cultivation versus Sustainability. Sustainability 2023, 16, 152. [Google Scholar] [CrossRef]
- Tong, S.; Zhang, B.; Feng, C.; Zhao, Y.; Chen, N.; Hao, C.; Pu, J.; Zhao, L. Characteristics of Heterotrophic/Biofilm-Electrode Autotrophic Denitrification for Nitrate Removal from Groundwater. Bioresour. Technol. 2013, 148, 121–127. [Google Scholar] [CrossRef]
- Mousset, E.; Trellu, C.; Olvera-Vargas, H.; Pechaud, Y.; Fourcade, F.; Oturan, M.A. Electrochemical Technologies Coupled with Biological Treatments. Curr. Opin. Electrochem. 2021, 26, 100668. [Google Scholar] [CrossRef]
- Rodziewicz, J.; Mielcarek, A.; Janczukowicz, W.; Jóźwiak, T.; Struk–Sokołowska, J.; Bryszewski, K. The Share of Electrochemical Reduction, Hydrogenotrophic and Heterotrophic Denitrification in Nitrogen Removal in Rotating Electrobiological Contactor (REBC) Treating Wastewater from Soilless Cultivation Systems. Sci. Total Environ. 2019, 683, 21–28. [Google Scholar] [CrossRef]
- Bryszewski, K.Ł.; Rodziewicz, J.; Mielcarek, A.; Janczukowicz, W.; Jóźwiakowski, K. Investigation on the Improved Electrochemical and Bio-Electrochemical Treatment Processes of Soilless Cultivation Drainage (SCD). Sci. Total Environ. 2021, 783, 146846. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, S.; Arıman, S. Domestic Wastewater Treatment by Real-Scale Electrocoagulation Process. Water Sci. Technol. 2020, 81, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Han, Y.; Xu, C.; Han, H.; Zhong, D.; Zheng, M.; Ma, W. Effect of Low-Intensity Direct Current Electric Field on Microbial Nitrate Removal in Coal Pyrolysis Wastewater with Low COD to Nitrogen Ratio. Bioresour. Technol. 2019, 287, 121465. [Google Scholar] [CrossRef] [PubMed]
- Mousazadeh, M.; Niaragh, E.K.; Usman, M.; Khan, S.U.; Sandoval, M.A.; Al-Qodah, Z.; Khalid, Z.B.; Gilhotra, V.; Emamjomeh, M.M. A Critical Review of State-of-the-Art Electrocoagulation Technique Applied to COD-Rich Industrial Wastewaters. Environ. Sci. Pollut. Res. 2021, 28, 43143–43172. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Zhao, Y.; Cotterill, S. Examining Current and Future Applications of Electrocoagulation in Wastewater Treatment. Water 2023, 15, 1455. [Google Scholar] [CrossRef]
- Chae, K.-J.; Kang, J. Estimating the Energy Independence of a Municipal Wastewater Treatment Plant Incorporating Green Energy Resources. Energy Convers. Manag. 2013, 75, 664–672. [Google Scholar] [CrossRef]
- Mohyudin, S.; Farooq, R.; Jubeen, F.; Rasheed, T.; Fatima, M.; Sher, F. Microbial Fuel Cells a State-of-the-Art Technology for Wastewater Treatment and Bioelectricity Generation. Environ. Res. 2022, 204, 112387. [Google Scholar] [CrossRef]
- Kłodowska, I.; Rodziewicz, J.; Janczukowicz, W. The Influence of Electrical Current Density and Type of the External Source of Carbon on Nitrogen and Phosphorus Efficiency Removal in the Sequencing Batch Biofilm Reactor. J. Ecol. Eng. 2018, 19, 172–179. [Google Scholar] [CrossRef]
- Tong, S.; Liu, H.; Feng, C.; Chen, N.; Zhao, Y.; Xu, B.; Zhao, J.; Zhu, M. Stimulation Impact of Electric Currents on Heterotrophic Denitrifying Microbial Viability and Denitrification Performance in High Concentration Nitrate-Contaminated Wastewater. J. Environ. Sci. 2019, 77, 363–371. [Google Scholar] [CrossRef]
- Tong, S.; Chen, N.; Wang, H.; Liu, H.; Tao, C.; Feng, C.; Zhang, B.; Hao, C.; Pu, J.; Zhao, J. Optimization of C/N and Current Density in a Heterotrophic/Biofilm-Electrode Autotrophic Denitrification Reactor (HAD-BER). Bioresour. Technol. 2014, 171, 389–395. [Google Scholar] [CrossRef]
- Moussa, D.T.; El-Naas, M.H.; Nasser, M.; Al-Marri, M.J. A Comprehensive Review of Electrocoagulation for Water Treatment: Potentials and Challenges. J. Environ. Manag. 2017, 186, 24–41. [Google Scholar] [CrossRef]
- Kabdaşlı, I.; Arslan-Alaton, I.; Ölmez-Hancı, T.; Tünay, O. Electrocoagulation Applications for Industrial Wastewaters: A Critical Review. Environ. Technol. Rev. 2012, 1, 2–45. [Google Scholar] [CrossRef]
- Almukdad, A.; Hafiz, M.; Yasir, A.T.; Alfahel, R.; Hawari, A.H. Unlocking the Application Potential of Electrocoagulation Process through Hybrid Processes. J. Water Process Eng. 2021, 40, 101956. [Google Scholar] [CrossRef]
- Ingelsson, M.; Yasri, N.; Roberts, E.P.L. Electrode Passivation, Faradaic Efficiency, and Performance Enhancement Strategies in Electrocoagulation—A Review. Water Res. 2020, 187, 116433. [Google Scholar] [CrossRef]
- Kwon, M.J.; Hwang, Y.; Lee, J.; Ham, B.; Rahman, A.; Azam, H.; Yang, J.-S. Waste Nutrient Solutions from Full-Scale Open Hydroponic Cultivation: Dynamics of Effluent Quality and Removal of Nitrogen and Phosphorus Using a Pilot-Scale Sequencing Batch Reactor. J. Environ. Manag. 2021, 281, 111893. [Google Scholar] [CrossRef]
- Nariyan, E.; Wolkersdorfer, C.; Sillanpää, M. Sulfate Removal from Acid Mine Water from the Deepest Active European Mine by Precipitation and Various Electrocoagulation Configurations. J. Environ. Manag. 2018, 227, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Hidayat, I.; Saakes, M.; van der Weijden, R.; Buisman, C.J.N. Fate of Calcium, Magnesium and Inorganic Carbon in Electrochemical Phosphorus Recovery from Domestic Wastewater. Chem. Eng. J. 2019, 362, 453–459. [Google Scholar] [CrossRef]
- Sahu, O.; Mazumdar, B.; Chaudhari, P.K. Treatment of Wastewater by Electrocoagulation: A Review. Environ. Sci. Pollut. Res. 2014, 21, 2397–2413. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.L.; Guerinot, M. Lou Minireview Iron Stress in Plants. Plant J. 2002, 3, reviews1024.1. [Google Scholar]
- Decree of the Minister of Maritime Affairs and Inland Navigation of 12 July 2019 on Substances Particularly Hazardous to Water and Conditions to Be Complied with When Discharging Waste Water into Water Bodies or into the Ground and When Discharging Rainwater or Snowmelt into Water Bodies or into Water Installations; Ministry of Maritime Affairs and Inland Navigation: Warsaw, Poland, 2019.
- Tahreen, A.; Jami, M.S.; Ali, F. Role of Electrocoagulation in Wastewater Treatment: A Developmental Review. J. Water Process Eng. 2020, 37, 101440. [Google Scholar] [CrossRef]
- Behbahani, M.; Alavi Moghaddam, M.R.; Arami, M. A Comparison between Aluminum and Iron Electrodes on Removal of Phosphate from Aqueous Solutions by Electrocoagulation Process. Int. J. Environ. Res. 2011, 5, 403–412. [Google Scholar]
- Wang, Y.; Kuntke, P.; Saakes, M.; van der Weijden, R.D.; Buisman, C.J.N.; Lei, Y. Electrochemically Mediated Precipitation of Phosphate Minerals for Phosphorus Removal and Recovery: Progress and Perspective. Water Res. 2022, 209, 117891. [Google Scholar] [CrossRef]
- Wang, Q.; Liao, Z.; Yao, D.; Yang, Z.; Wu, Y.; Tang, C. Phosphorus Immobilization in Water and Sediment Using Iron-Based Materials: A Review. Sci. Total Environ. 2021, 767, 144246. [Google Scholar] [CrossRef]
- Mao, Y.; Ninh Pham, A.; Xin, Y.; David Waite, T. Effects of PH, Floc Age and Organic Compounds on the Removal of Phosphate by Pre-Polymerized Hydrous Ferric Oxides. Sep. Purif. Technol. 2012, 91, 38–45. [Google Scholar] [CrossRef]
- Jang, M.; Min, S.-H.; Kim, T.-H.; Park, J.K. Removal of Arsenite and Arsenate Using Hydrous Ferric Oxide Incorporated into Naturally Occurring Porous Diatomite. Environ. Sci. Technol. 2006, 40, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Omwene, P.I.; Kobya, M.; Can, O.T. Phosphorus Removal from Domestic Wastewater in Electrocoagulation Reactor Using Aluminium and Iron Plate Hybrid Anodes. Ecol. Eng. 2018, 123, 65–73. [Google Scholar] [CrossRef]
- Reza, A.; Chen, L. Electrochemical Treatment of Livestock Waste Streams. A Review. Environ. Chem. Lett. 2022, 20, 1863–1895. [Google Scholar] [CrossRef]
- Gamshadzehi, E.; Nassiri, M.; Ershadifar, H. One-Pot Synthesis of Microporous Fe2O3/g-C3N4 and Its Application for Efficient Removal of Phosphate from Sewage and Polluted Seawater. Colloids Surf. A Physicochem. Eng. Asp. 2019, 567, 7–15. [Google Scholar] [CrossRef]
- Weng, Y.; Vekeman, J.; Zhang, H.; Chou, L.; Elskens, M.; Tielens, F. Unravelling Phosphate Adsorption on Hydrous Ferric Oxide Surfaces at the Molecular Level. Chemosphere 2020, 261, 127776. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, A.; Nassef, E.; Mubark, A.; Hussein, M. Phosphate Removal from Wastewater by Electrocoagulation Using Aluminium Electrodes. Am. J. Environ. Eng. Sci. 2014, 1, 90–98. [Google Scholar]
- Majlesi, M.; Mohseny, S.M.; Sardar, M.; Golmohammadi, S.; Sheikhmohammadi, A. Improvement of Aqueous Nitrate Removal by Using Continuous Electrocoagulation/Electroflotation Unit with Vertical Monopolar Electrodes. Sustain. Environ. Res. 2016, 26, 287–290. [Google Scholar] [CrossRef]
- Mook, W.T.; Chakrabarti, M.H.; Aroua, M.K.; Khan, G.M.A.; Ali, B.S.; Islam, M.S.; Abu Hassan, M.A. Removal of Total Ammonia Nitrogen (TAN), Nitrate and Total Organic Carbon (TOC) from Aquaculture Wastewater Using Electrochemical Technology: A Review. Desalination 2012, 285, 1–13. [Google Scholar] [CrossRef]
- Benekos, A.K.; Tziora, F.E.; Tekerlekopoulou, A.G.; Pavlou, S.; Qun, Y.; Katsaounis, A.; Vayenas, D.V. Nitrate Removal from Groundwater Using a Batch and Continuous Flow Hybrid Fe-Electrocoagulation and Electrooxidation System. J. Environ. Manag. 2021, 297, 113387. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, A.N.; Ajjam, S.K. Kinetic Modelling of Nitrate Removal from Aqueous Solution during Electrocoagulation. Civ. Environ. Res. 2013, 3, 64–73. [Google Scholar]
- Ghazouani, M.; Bousselmi, L.; Akrout, H. Combined Electrocoagulation and Electrochemical Treatment on BDD Electrodes for Simultaneous Removal of Nitrates and Phosphates. J. Environ. Chem. Eng. 2020, 8, 104509. [Google Scholar] [CrossRef]
- Puig, S.; Corominas, L.; Balaguer, M.D.; Colprim, J. Biological Nutrient Removal by Applying SBR Technology in Small Wastewater Treatment Plants: Carbon Source and C/N/P Ratio Effects. Water Sci. Technol. 2007, 55, 135–141. [Google Scholar] [CrossRef]
- Ryan, D.R.; Maher, E.K.; Heffron, J.; Mayer, B.K.; McNamara, P.J. Electrocoagulation-Electrooxidation for Mitigating Trace Organic Compounds in Model Drinking Water Sources. Chemosphere 2021, 273, 129377. [Google Scholar] [CrossRef]
- Erses, A.S.; Onay, T.T.; Yenigun, O. Comparison of Aerobic and Anaerobic Degradation of Municipal Solid Waste in Bioreactor Landfills. Bioresour. Technol. 2008, 99, 5418–5426. [Google Scholar] [CrossRef]
- Prystay, W.; Lo, K.V. Treatment of Greenhouse Wastewater Using Constructed Wetlands. J. Environ. Sci. Health Part B 2001, 36, 341–353. [Google Scholar] [CrossRef]
- Rodziewicz, J.; Mielcarek, A.; Janczukowicz, W.; Tavares, J.M.R.; Jóźwiakowski, K. Characteristics of Sludge from the Treatment of Soilless Plant Cultivation Wastewater in a Rotating Electrobiological Disc Contactor (REBDC). Energies 2023, 16, 1022. [Google Scholar] [CrossRef]
- Rajaniemi, K.; Tuomikoski, S.; Lassi, U. Electrocoagulation Sludge Valorization—A Review. Resources 2021, 10, 127. [Google Scholar] [CrossRef]
- Boinpally, S.; Kolla, A.; Kainthola, J.; Kodali, R.; Vemuri, J. A State-of-the-Art Review of the Electrocoagulation Technology for Wastewater Treatment. Water Cycle 2023, 4, 26–36. [Google Scholar] [CrossRef]
- Akyol, A. Treatment of Paint Manufacturing Wastewater by Electrocoagulation. Desalination 2012, 285, 91–99. [Google Scholar] [CrossRef]
- Mansoorian, H.J.; Mahvi, A.H.; Jafari, A.J. Removal of Lead and Zinc from Battery Industry Wastewater Using Electrocoagulation Process: Influence of Direct and Alternating Current by Using Iron and Stainless Steel Rod Electrodes. Sep. Purif. Technol. 2014, 135, 165–175. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Burton, F.L.; Stensel, D.H. Metcalf &Eddy: Wastewater Engineering: Treatment and Reuse; McGraw Hill Companies, Inc.: New York, NY, USA, 2014. [Google Scholar]
- Lin, X.; Han, Z.; Yu, H.; Ye, Z.; Zhu, S.; Zhu, J. Struvite Precipitation from Biogas Digestion Slurry Using a Two-Chamber Electrolysis Cell with a Magnesium Anode. J. Clean. Prod. 2018, 174, 1598–1607. [Google Scholar] [CrossRef]
- Golder, A.K.; Samanta, A.N.; Ray, S. Removal of Phosphate from Aqueous Solutions Using Calcined Metal Hydroxides Sludge Waste Generated from Electrocoagulation. Sep. Purif. Technol. 2006, 52, 102–109. [Google Scholar] [CrossRef]
- Tezcan Un, U.; Onpeker, S.E.; Ozel, E. The Treatment of Chromium Containing Wastewater Using Electrocoagulation and the Production of Ceramic Pigments from the Resulting Sludge. J. Environ. Manag. 2017, 200, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Joshi, H. Utilization of Electrocoagulation-Treated Spent Wash Sludge in Making Building Blocks. Int. J. Environ. Sci. Technol. 2016, 13, 349–358. [Google Scholar] [CrossRef]
- Tezcan Un, U.; Topal, S.; Ates, F. Electrocoagulation of Tissue Paper Wastewater and an Evaluation of Sludge for Pyrolysis. Desalination Water Treat. 2016, 57, 28724–28733. [Google Scholar] [CrossRef]
- Samy, M.; Alalm, M.G.; Mossad, M. Utilization of Iron Sludge Resulted from Electro-Coagulation in Heterogeneous Photo-Fenton Process. Water Pr. Technol. 2020, 15, 1228–1237. [Google Scholar] [CrossRef]

| Total Phosphorus | Total Nitrogen | Organic Compounds | |||||||
|---|---|---|---|---|---|---|---|---|---|
| k | r | R2 | k | r | R2 | k | r | R2 | |
| R1 | 0.421 | 26.45 | 0.996 | 0.137 | 2.07 | 0.998 | 0.130 | 0.44 | 0.988 |
| R2 | 0.396 | 30.65 | 0.982 | 0.109 | 2.78 | 0.980 | 0.125 | 0.61 | 0.983 |
| R3 | 0.448 | 34.79 | 0.992 | 0.190 | 6.58 | 0.969 | 0.262 | 1.50 | 0.999 |
| W | Average Elemental Content [%] | |||
|---|---|---|---|---|
| N | P | C | Fe | |
| R1 | 4.18 | 18.1 | 0.65 | 8.08 |
| R2 | 2.28 | 13.5 | 0.43 | 8.67 |
| R3 | 2.68 | 11.7 | 0.55 | 13.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mielcarek, A.; Bryszewski, K.Ł.; Rodziewicz, J.; Kłobukowska, K.; Janczukowicz, W. Phosphorus Removal Rate and Efficiency in an Electrochemical Sequencing Reactor for the Treatment of Wastewater with Low Organic Carbon Content. Energies 2024, 17, 1352. https://doi.org/10.3390/en17061352
Mielcarek A, Bryszewski KŁ, Rodziewicz J, Kłobukowska K, Janczukowicz W. Phosphorus Removal Rate and Efficiency in an Electrochemical Sequencing Reactor for the Treatment of Wastewater with Low Organic Carbon Content. Energies. 2024; 17(6):1352. https://doi.org/10.3390/en17061352
Chicago/Turabian StyleMielcarek, Artur, Kamil Łukasz Bryszewski, Joanna Rodziewicz, Karolina Kłobukowska, and Wojciech Janczukowicz. 2024. "Phosphorus Removal Rate and Efficiency in an Electrochemical Sequencing Reactor for the Treatment of Wastewater with Low Organic Carbon Content" Energies 17, no. 6: 1352. https://doi.org/10.3390/en17061352
APA StyleMielcarek, A., Bryszewski, K. Ł., Rodziewicz, J., Kłobukowska, K., & Janczukowicz, W. (2024). Phosphorus Removal Rate and Efficiency in an Electrochemical Sequencing Reactor for the Treatment of Wastewater with Low Organic Carbon Content. Energies, 17(6), 1352. https://doi.org/10.3390/en17061352










