Techno-Economic Assessment for the Best Flexible Operation of the CO2 Removal Section by Potassium Taurate Solvent in a Coal-Fired Power Plant
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Electricity Market in Italy
2.2. Details of the Considered Plant
2.3. Theory
- -
- Fixed operation at 90% CO2 removal;
- -
- Fixed operation at a given % ratio;
- -
- Flexible operation.
2.3.1. Profit Minimization
- -
- Wout, the net production of electrical power exiting from the power station (MW = MWh/h);
- -
- Cenergy, the price of electrical energy (EUR/MWh);
- -
- CCO2Tax, the carbon tax (EUR/tCO2);
- -
- FCO2, the amount of CO2 vented in one hour (tCO2/h);
- -
- Ffuel, the fuel consumption (kg/h);
- -
- Cfuel, the fuel cost (EUR/kg);
- -
- Cb,O&M, the cost of base plant operation and maintenance (EUR/h), excluding the cost of Wout.
2.3.2. Assumptions
- The electricity price is based on Italian historical electricity prices referring to 2015, set out by Gestore dei Mercati Energetici [48] (the methodology could be extended to other electricity markets, and also applied when considering the electricity prices of the following years, when available);
- The electricity demand was set with reference to the historical data by Terna [35], scaling for the considered power plant production;
- Carbon tax varies in the range of 5 EUR/tCO2 to 200 EUR/tCO2, in steps of 5 EUR/tCO2;
- Cold start-up and shut-down costs are neglected;
- Losses in efficiency due to variations in % ratio are considered with a ramp of 5%/min, as also done by Cohen et al. [49];
- In cases when additional power is produced in each hour (because of the production set defined on a provision based on the expected trend of electricity consumption) that is not required by the market, it is assumed to be lost;
- January (generally the coldest month in Italy) and July (when the highest variations in electricity demand and prices occur [50]) have been selected;
- The time step for the analysis is 1 h, corresponding to the time step at which data on the prices of electricity have been found.
- -
- Base case, i.e., 90% CO2 removal at fixed operation;
- -
- Fixed capture at 90%, 80%, 70% and 60% of the base case;
- -
- CLR flexible operation;
- -
- No capture.
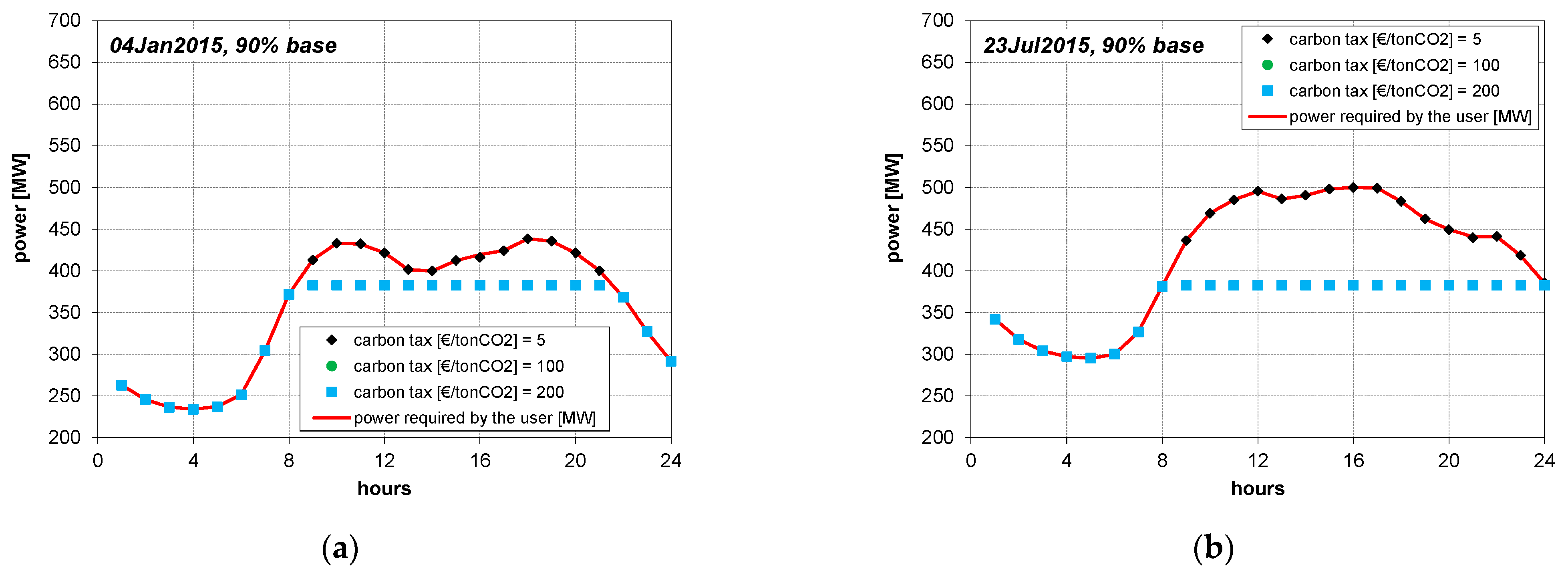
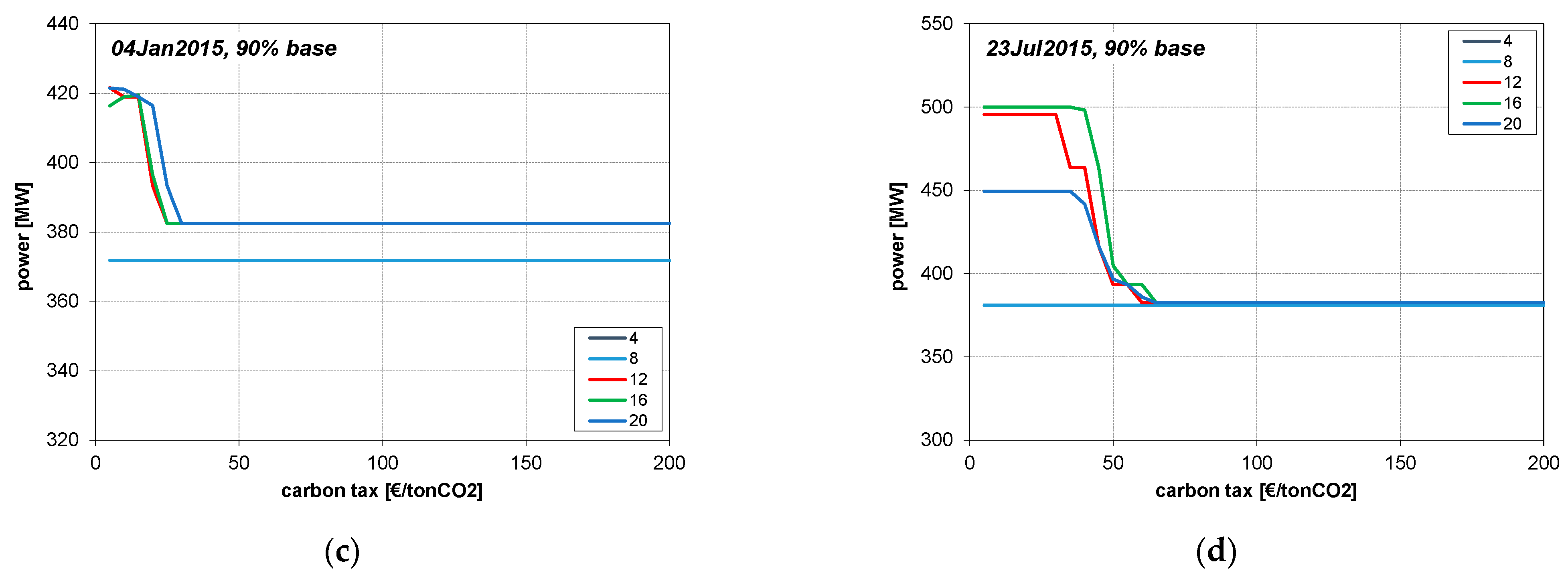
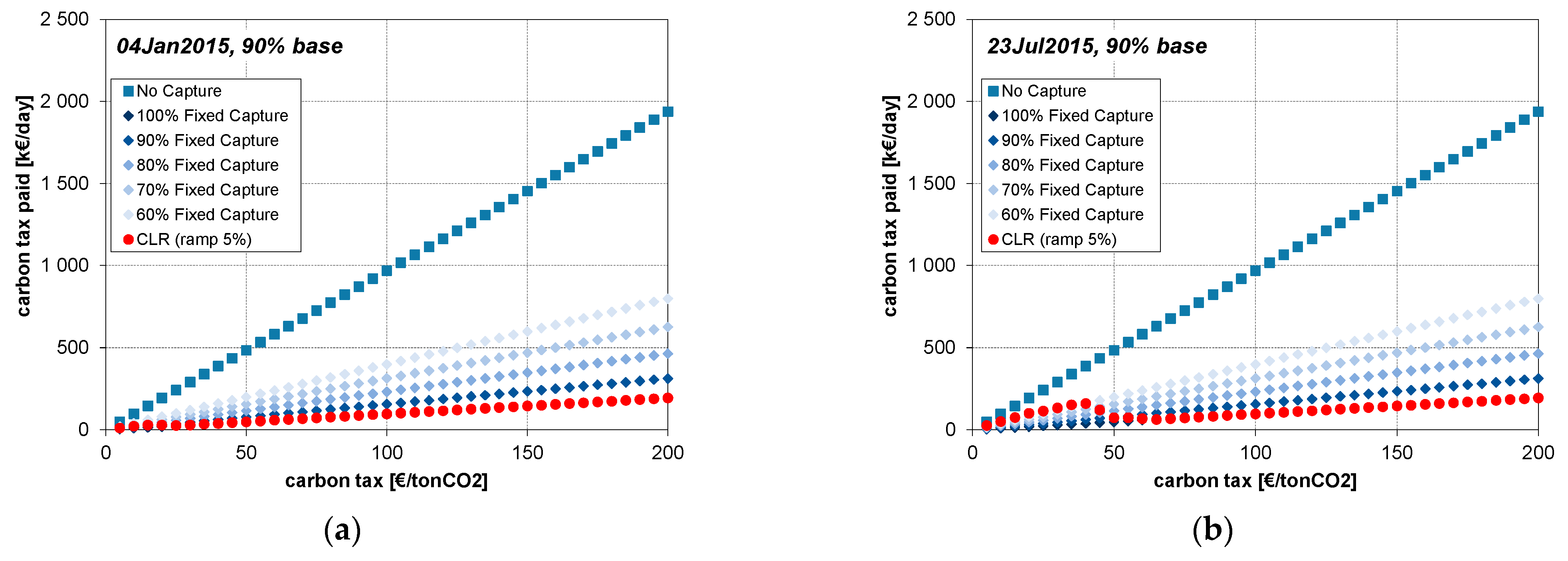
3. Results
4. Discussion
4.1. CLR Mode
4.2. Case of No CO2 Removal
4.3. Cases of Fixed Operation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IEA. Putting CO2 to Use; IEA: Paris, France, 2019. [Google Scholar]
- De Guido, G.; Pellegrini, L.A. Calculation of solid-vapor equilibria for cryogenic carbon capture. Comp. Chem. Eng. 2022, 156, 107569. [Google Scholar] [CrossRef]
- Pellegrini, L.A.; De Guido, G.; Ingrosso, S. Thermodynamic framework for cryogenic carbon capture. Comput. Aided Chem. Eng. 2020, 48, 475–480. [Google Scholar]
- Ebner, A.D.; Ritter, J.A. State-of-the-art Adsorption and Membrane Separation Processes for Carbon Dioxide Production from Carbon Dioxide Emitting Industries. Sep. Sci. Technol. 2009, 44, 1273–1421. [Google Scholar] [CrossRef]
- Feron, P.H.M.; Jansen, A.E.; Klaassen, R. Membrane technology in carbon dioxide removal. Energy Convers. Manag. 1992, 33, 421–428. [Google Scholar] [CrossRef]
- Yang, C.; Li, T.; Tantikhajorngosol, P.; Sema, T.; Xiao, M.; Tontiwachwuthikul, P. Evaluation of novel aqueous piperazine-based physical-chemical solutions as biphasic solvents for CO2 capture: Initial absorption rate, equilibrium solubility, phase separation and desorption rate. Chem. Eng. Sci. 2023, 277, 118852. [Google Scholar] [CrossRef]
- Pellegrini, L.A.; Gilardi, M.; Giudici, F.; Spatolisano, E. New Solvents for CO2 and H2S Removal from Gaseous Streams. Energies 2021, 14, 6687. [Google Scholar] [CrossRef]
- Lu, Y.; Nielsen, P.; Lu, H. Development and Bench Scale Testing of a Novel Biphasic Solvent Enabled Absorption Process for Post Combustion Carbon Capture DE FE0031600. In Proceedings of the 2022 Carbon Management Project Review Meeting, Pittsburgh, PA, USA, 15–19 August 2022. [Google Scholar]
- Wanderley, R.R.; Pinto, D.D.D.; Knuutila, H.K. From hybrid solvents to water-lean solvents—A critical and historical review. Sep. Purif. Technol. 2021, 260, 118193. [Google Scholar] [CrossRef]
- Schuur, B.; Brouwer, T.; Smink, D.; Sprakel, L.M.J. Green solvents for sustainable separation processes. Curr. Opin. Green Sustain. Chem. 2019, 18, 57–65. [Google Scholar] [CrossRef]
- Vanderveen, J.R.; Patiny, L.; Chalifoux, C.B.; Jessop, M.J.; Jessop, P.G. A virtual screening approach to identifying the greenest compound for a task: Application to switchable-hydrophilicity solvents. Green Chem. 2015, 17, 5182–5188. [Google Scholar] [CrossRef]
- Ramezani, R.; Mazinani, S.; Di Felice, R. State-of-the-art of CO2 capture with amino acid salt solutions. Rev. Chem. Eng. 2022, 38, 273–299. [Google Scholar] [CrossRef]
- Lerche, B.M. CO2 Capture from Flue Gas Using Amino Acid Salt Solutions. Ph.D. Thesis, Technical University of Denmark, Lyngby, Denmark, 2012. [Google Scholar]
- Majchrowicz, M.E. Amino Acid Salt Solutions for Carbon Dioxide Capture. Ph.D. Thesis, University of Twente, Twente, The Netherlands, 2014. [Google Scholar]
- Majchrowicz, M.E.; Brilman, D.W.F.W.; Groeneved, M.J. Precipitation regime for selected amino acid salts for CO2 capture from flue gases. Energy Procedia 2009, 1, 979–984. [Google Scholar] [CrossRef]
- Sanchez-Fernandez, E.; Goetheer, E.L.V. DECAB: Process development of a phase change absorption process. Energy Procedia 2011, 4, 868–875. [Google Scholar] [CrossRef]
- Sanchez Fernandez, E.; Goetheer, E.L.V.; Manzolini, G.; Macchi, E.; Rezvani, S.; Vlugt, T.J.H. Thermodynamic assessment of amine based CO2 capture technologies in power plants based on European Benchmarking Task Force methodology. Fuel 2014, 129, 318–329. [Google Scholar] [CrossRef]
- Sanchez Fernandez, E.; Heffernan, K.; van der Ham, L.V.; Linders, M.J.G.; Eggink, E.; Schrama, F.N.H.; Brilman, D.W.F.; Goetheer, E.L.V.; Vlugt, T.J.H. Conceptual Design of a Novel CO2 Capture Process Based on Precipitating Amino Acid Solvents. Ind. Eng. Chem. Res. 2013, 52, 12223–12235. [Google Scholar] [CrossRef]
- Brouwer, J.P.; Feron, P.H.M.; ten Asbroek, N.A.M. Amino-Acid Salts for CO2 Capture from Flue Gases. 2009. Available online: https://www.researchgate.net/publication/228408743_Amino-acid_salts_for_CO2_capture_from_flue_gases (accessed on 15 April 2023).
- Li, B.; Duan, Y.; Luebke, D.; Morreale, B. Advances in CO2 capture technology: A patent review. Appl. Energy 2013, 102, 1439–1447. [Google Scholar] [CrossRef]
- Domenichini, R.; Mancuso, L.; Ferrari, N.; Davison, J. Operating Flexibility of Power Plants with Carbon Capture and Storage (CCS). Enrgy Procedia 2013, 37, 2727–2737. [Google Scholar] [CrossRef]
- Bui, M.; Gunawan, I.; Verheyen, V.; Feron, P.; Meuleman, E.; Adeloju, S. Dynamic modelling and optimisation of flexible operation in post-combustion CO2 capture plants—A review. Comp. Chem. Eng. 2014, 61, 245–265. [Google Scholar] [CrossRef]
- Bui, M.; Tait, P.; Lucquiaud, M.; Mac Dowell, N. Dynamic operation and modelling of amine-based CO2 capture at pilot scale. Int. J. Greenh. Gas Control 2018, 79, 134–153. [Google Scholar] [CrossRef]
- Chalmers, H.; Gibbins, J. Initial evaluation of the impact of post-combustion capture of carbon dioxide on supercritical pulverised coal power plant part load performance. Fuel 2007, 86, 2109–2123. [Google Scholar] [CrossRef]
- Gibbins, J.R.; Crane, R.I. Scope for reductions in the cost of CO2 capture using flue gas scrubbing with amine solvents. Proc. Inst. Mech. Eng. A-J. Power Energy 2004, 218, 231–239. [Google Scholar] [CrossRef]
- Delarue, E.; Martens, P.; D’haeseleer, W. Market opportunities for power plants with post-combustion carbon capture. Int. J. Greenh. Gas Control 2012, 6, 12–20. [Google Scholar] [CrossRef]
- Haines, M.R.; Davison, J.E. Designing Carbon Capture power plants to assist in meeting peak power demand. Greenh. Gas Control Technol. 2009, 1, 1457–1464. [Google Scholar] [CrossRef]
- Moioli, S.; Pellegrini, L.A. Operating the CO2 absorption plant in a post-combustion unit in flexible mode for cost reduction. Chem. Eng. Res. Des. 2019, 147, 604–614. [Google Scholar] [CrossRef]
- Errey, O.; Chalmers, H.; Lucquiaud, M.; Gibbins, J. Valuing Responsive Operation of Post-combustion CCS Power Plants in Low Carbon Electricity Markets. Energy Procedia 2014, 63, 7471–7484. [Google Scholar] [CrossRef]
- Rubin, E.S.; Chen, C.; Rao, A.B. Cost and performance of fossil fuel power plants with CO2 capture and storage. Energy Policy 2007, 35, 4444–4454. [Google Scholar] [CrossRef]
- Mac Dowell, N.; Shah, N. Optimisation of Post-combustion CO2 Capture for Flexible Operation. Energy Procedia 2014, 63 (Suppl. C), 1525–1535. [Google Scholar] [CrossRef]
- Mechleri, E.; Fennell, P.S.; Dowell, N.M. Flexible Operation Strategies for Coal- and Gas-CCS Power Stations under the UK and USA Markets. Energy Procedia 2017, 114, 6543–6551. [Google Scholar] [CrossRef]
- Bui, M.; Flø, N.E.; de Cazenove, T.; Mac Dowell, N. Demonstrating flexible operation of the Technology Centre Mongstad (TCM) CO2 capture plant. Int. J. Greenh. Gas Control 2020, 93, 102879. [Google Scholar] [CrossRef]
- Bui, M.; Gunawan, I.; Verheyen, V.; Feron, P.; Meuleman, E. Flexible operation of CSIRO’s post-combustion CO2 capture pilot plant at the AGL Loy Yang power station. Int. J. Greenh. Gas Control 2016, 48, 188–203. [Google Scholar] [CrossRef]
- Terna, S.p.A. Available online: https://www.terna.it/it (accessed on 15 April 2023).
- Moioli, S.; Pellegrini, L.A. Fixed and Capture Level Reduction operating modes for carbon dioxide removal in a Natural Gas Combined Cycle power plant. J. Clean. Prod. 2020, 254, 120016. [Google Scholar] [CrossRef]
- Moioli, S.; Ho, M.H.; Wiley, D.E.; Pellegrini, L.A. Assessment of carbon dioxide capture by precipitating potassium taurate solvent. Int. J. Greenh. Gas Control 2019, 87, 159–169. [Google Scholar] [CrossRef]
- Baiguini, N. Flexible Operation in Post-Combustion CO2 Removal Plants by MEA Solvent and Potassium Taurate Solvent. Master’s Thesis, Politecnico di Milano, Milano, Italy, 2018. [Google Scholar]
- Fluor. Available online: http://www.fluor.com/econamine/pages/default.aspx (accessed on 15 April 2023).
- Just, P.-E. Advances in the development of CO2 capture solvents. Energy Procedia 2013, 37, 314–324. [Google Scholar] [CrossRef]
- Shell CANSOLV Carbon Dioxide (CO2) Capture System. Available online: http://www.shell.com/business-customers/global-solutions/gas-processing-licensing/licensed-technologies/shell-cansolv-gas-absorption-solutions/cansolv-co2-capture-system.html (accessed on 15 April 2023).
- Biliyok, C.; Canepa, R.; Wang, M.; Yeung, H. Techno-Economic Analysis of a Natural Gas Combined Cycle Power Plant with CO2 Capture. In Computer Aided Chemical Engineering; Kraslawski, A., Turunen, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 32, pp. 187–192. [Google Scholar]
- Aromada, S.A.; Eldrup, N.H.; Øi, L.E. Cost and Emissions Reduction in CO2 Capture Plant Dependent on Heat Exchanger Type and Different Process Configurations: Optimum Temperature Approach Analysis. Energies 2022, 15, 425. [Google Scholar] [CrossRef]
- Fout, T.; Zoelle, A.; Keairns, D.; Turner, M.; Woods, M.; Kuehn, N.; Shah, V.; Chou, V.; Pinkerton, L. Cost and Performance Baseline for Fossil Energy Plants Volume 1a: Bituminous Coal (PC) and Natural Gas to Electricity Revision 3. DOE/NETL-2015/1723; National Energy Technology Laboratory (NETL): Pittsburgh, PA, USA; Morgantown, WV, USA, 2015. [Google Scholar]
- Moioli, S.; Pellegrini, L.A. Comparison of CLR and SS Flexible Systems for Post-Combustion CO2 Removal in a NGCC Power Plant. Chem. Eng. Trans. 2019, 74, 829–834. [Google Scholar]
- Cohen, S.M.; Rochelle, G.T.; Webber, M.E. Optimal operation of flexible post-combustion CO2 capture in response to volatile electricity prices. Energy Procedia 2011, 4, 2604–2611. [Google Scholar] [CrossRef]
- Moioli, S.; Ho, M.H.; Wiley, D.E.; Pellegrini, L.A. Thermodynamic Modeling of the System of CO2 and Potassium Taurate Solution for Simulation of the Process of Carbon Dioxide Removal. Chem. Eng. Res. Des. 2018, 136, 834–845. [Google Scholar] [CrossRef]
- GME Gestore Mercati Energetici. Available online: http://www.mercatoelettrico.org/it/ (accessed on 15 April 2023).
- Cohen, S.M.; Rochelle, G.T.; Webber, M.E. Optimizing post-combustion CO2 capture in response to volatile electricity prices. Int. J. Greenh. Gas Control 2012, 8, 180–195. [Google Scholar] [CrossRef]
- Moioli, S.; Pellegrini, L.A. Optimal Operation of a CO2 Absorption Plant in a Post-Combustion Unit for Cost Reduction. Chem. Eng. Trans. 2018, 69, 151–156. [Google Scholar]
- Chalmers, H.; Leach, M.; Lucquiaud, M.; Gibbins, J. Valuing flexible operation of power plants with CO2 capture. Greenh. Gas Control Technol. 2009, 1, 4289–4296. [Google Scholar] [CrossRef]
- Chalmers, H.; Lucquiaud, M.; Gibbins, J.; Leach, M. Flexible Operation of Coal Fired Power Plants with Postcombustion Capture of Carbon Dioxide. J. Environ. Eng.-Asce 2009, 135, 449–458. [Google Scholar] [CrossRef]
- Lucquiaud, M.; Chalmers, H.; Gibbins, J. Capture-ready supercritical coal-fired power plants and flexible post-combustion CO2 capture. Greenh. Gas Control Technol. 2009, 1, 1411–1418. [Google Scholar] [CrossRef]
- Lucquiaud, M.; Fernandez, E.S.; Chalmers, H.; Mac Dowell, N.; Gibbins, J. Enhanced operating flexibility and optimised off-design operation of coal plants with post-combustion capture. Energy Procedia 2014, 63, 7494–7507. [Google Scholar] [CrossRef]




| Characteristic | Absorber | Regeneration Column |
|---|---|---|
| Number of columns | 3 | 1 |
| Diameter (m) | 12 | 12.7 |
| Packed height (m) | 30 | 17.6 |
| Packing type | Mellapak 250X | Mellapak 250X |
| Lean loading | 0.27 | |
| Rich loading | 0.44 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moioli, S.; Spatolisano, E.; Pellegrini, L.A. Techno-Economic Assessment for the Best Flexible Operation of the CO2 Removal Section by Potassium Taurate Solvent in a Coal-Fired Power Plant. Energies 2024, 17, 1736. https://doi.org/10.3390/en17071736
Moioli S, Spatolisano E, Pellegrini LA. Techno-Economic Assessment for the Best Flexible Operation of the CO2 Removal Section by Potassium Taurate Solvent in a Coal-Fired Power Plant. Energies. 2024; 17(7):1736. https://doi.org/10.3390/en17071736
Chicago/Turabian StyleMoioli, Stefania, Elvira Spatolisano, and Laura A. Pellegrini. 2024. "Techno-Economic Assessment for the Best Flexible Operation of the CO2 Removal Section by Potassium Taurate Solvent in a Coal-Fired Power Plant" Energies 17, no. 7: 1736. https://doi.org/10.3390/en17071736





