Optimizing Hydrogen-Rich Biofuel Production: Syngas Generation from Wood Chips and Corn Cobs
Abstract
1. Introduction
2. Materials and Methods
Simulation and Strategy Modeling
| Process | No. | Stoichiometric Chemical Equations | Kinetics | Ref. |
|---|---|---|---|---|
| R-3 | 1 | Partial oxidation of C: | [33,34] | |
| 2 | : | [35,36] | ||
| 3 | : | [37] | ||
| 4 | Hydrogen oxidation | [35,36] | ||
| 5 | Partial oxidation of phenol | [37] | ||
| 6 | Partial oxidation of benzene: | [38] | ||
| R-4 | 7 | Water gas: | [39,40] | |
| 8 | Water–gas shift: | [41] | ||
| 9 | [40,41] | |||
| 10 | Boudouard: | [34,40] | ||
| 11 | [42,43] | |||
| 12 | [43] | |||
| 13 | [40,44] |
| Simulation Parameters | Value | |
|---|---|---|
| Reaction | K | Ea (cal/mol) |
| (1) | 35826.8449 | |
| (2) | 42992.26139 | |
| (3) | 48246.87112 | |
| (4) | 2579.53568 | |
| (5) | 19155.44091 | |
| (6) | 30094.58298 | |
| (7) | 11918.41024 | |
| (8) | 3009.45829 | |
| (9) | 29855.73708 | |
| (10) | 32244.19604 | |
| (11) | 23884.58966 | |
| (12) | 23884.58966 | |
| (13) | 83596.06382 |
3. Results and Discussion
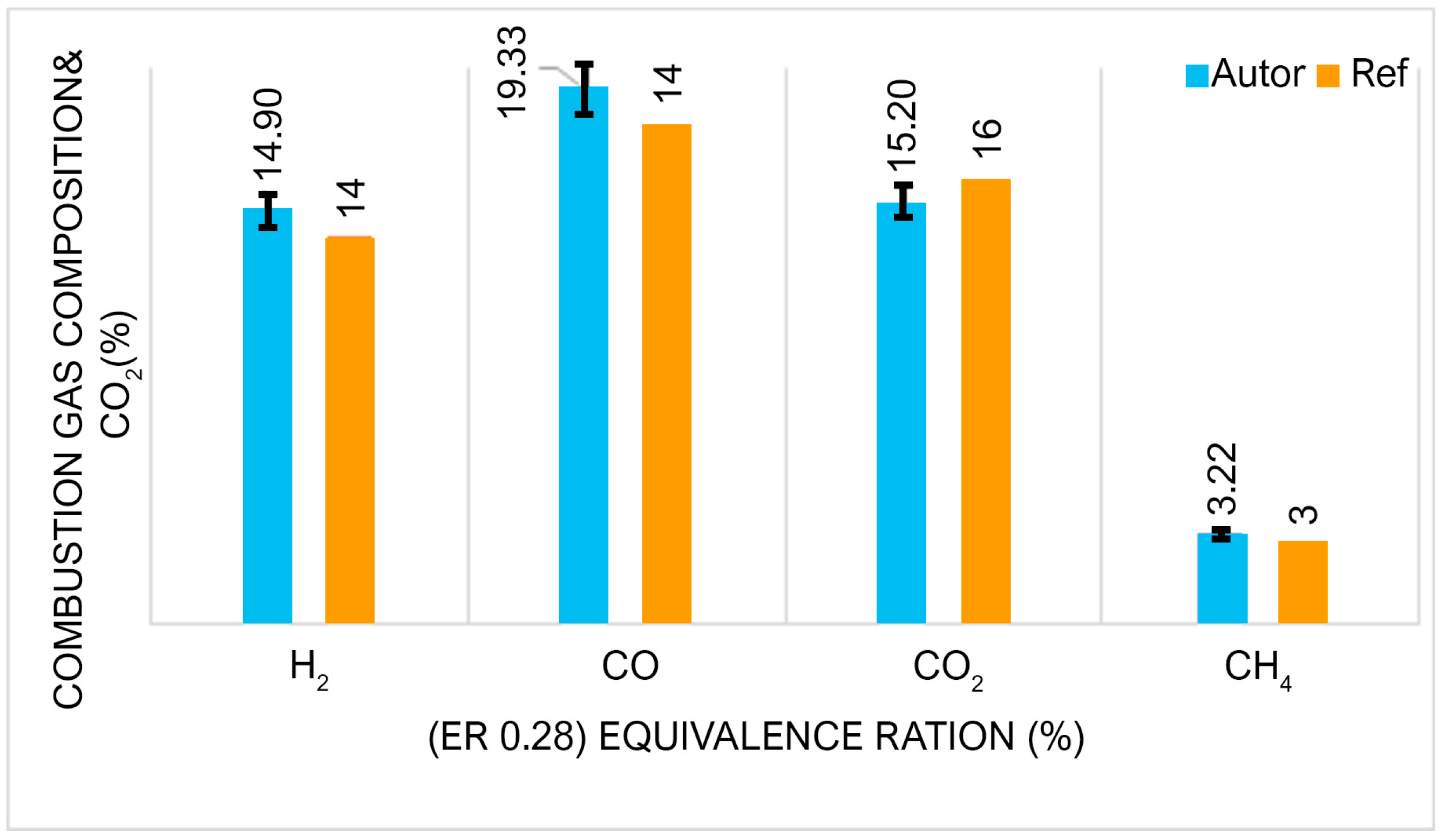
Effect of the Gasifying Agent and Moisture on Syngas Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Monge, M.; Lazcano, A. Commodity Prices after COVID-19: Persistence and Time Trends. Risks 2022, 10, 128. [Google Scholar] [CrossRef]
- Mohideen, M.M.; Ramakrishna, S.; Prabu, S.; Liu, Y. Advancing green energy solution with the impetus of COVID-19 pandemic. J. Energy Chem. 2021, 59, 688–705. [Google Scholar] [CrossRef]
- Fang, Y.; Shao, Z. The Russia-Ukraine Conflict and Volatility Risk of Commodity Markets. Financ. Res. Lett. 2022, 50, 103264. [Google Scholar] [CrossRef]
- Aleluia Reis, L.; Tavoni, M. Glasgow to Paris—The impact of the Glasgow commitments for the Paris climate agreement. iScience 2023, 26, 105933. [Google Scholar] [CrossRef] [PubMed]
- Couto, N.D.; Silva, V.B.; Rouboa, A. Assessment on steam gasification of municipal solid waste against biomass substrates. Energy Convers. Manag. 2016, 124, 92–103. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, P.; Liang, S.; Liu, B.; Shuai, Y.; Li, B. Exergy analysis of hydrogen production from steam gasification of biomass: A review. Int. J. Hydrogen Energy 2019, 44, 14290–14302. [Google Scholar] [CrossRef]
- Gabbrielli, R.; Seggiani, M.; Frigo, S.; Puccini, M.; Vitolo, S.; Raggio, G.; Puccioni, F. Validation of a small scale woody biomass downdraft gasification plant coupled with gas engine. Chem. Eng. Trans. 2016, 50, 241–246. [Google Scholar]
- Cecílio, D.M.; Gonçalves, J.R.M.; Correia, M.J.N.; Mateus, M.M. Aspen Plus® Modeling and Simulation of an Industrial Biomass Direct Liquefaction Process. Fuels 2023, 4, 221–242. [Google Scholar] [CrossRef]
- Jiamin, S.; Chengcheng, Y.; Lijing, Z.; Gang, T. Aspen plus Simulation and Analysis of Methanol Synthesis Process. In Proceedings of the 8th International Symposium on Energy Science and Chemical Engineering (ISESCE 2023), Guangzhou, China, 24–26 March 2023; p. 04009. [Google Scholar]
- Ramos, A.; Afonso Teixeira, C.; Rouboa, A. Environmental analysis of waste-to-energy—A Portuguese case study. Energies 2018, 11, 548. [Google Scholar] [CrossRef]
- Zhiznin, S.Z.; Shvets, N.N.; Timokhov, V.M.; Gusev, A.L. Economics of hydrogen energy of green transition in the world and Russia. Part I. Int. J. Hydrogen Energy 2023, 48, 21544–21567. [Google Scholar] [CrossRef]
- Ahmed, A.; Salmiaton, A.; Choong, T.; Azlina, W.W. Review of kinetic and equilibrium concepts for biomass tar modeling by using Aspen Plus. Renew. Sustain. Energy Rev. 2015, 52, 1623–1644. [Google Scholar] [CrossRef]
- Gomez, E.; Rani, D.A.; Cheeseman, C.; Deegan, D.; Wise, M.; Boccaccini, A. Thermal plasma technology for the treatment of wastes: A critical review. J. Hazard. Mater. 2009, 161, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Couto, N.; Silva, V.; Monteiro, E.; Brito, P.; Rouboa, A. Experimental and numerical analysis of coffee husks biomass gasification in a fluidized bed reactor. Energy Procedia 2013, 36, 591–595. [Google Scholar] [CrossRef]
- Mallick, D.; Mahanta, P.; Moholkar, V.S. Co–gasification of coal/biomass blends in 50 kWe circulating fluidized bed gasifier. J. Energy Inst. 2020, 93, 99–111. [Google Scholar] [CrossRef]
- Wiatowski, M. An Experimental Study on the Quantitative and Qualitative Characteristics of Tar Formed during Ex Situ Coal Gasification. Energies 2023, 16, 2777. [Google Scholar] [CrossRef]
- Kopetz, H. Build a biomass energy market. Nature 2013, 494, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Pio, D.; Tarelho, L. Empirical and chemical equilibrium modelling for prediction of biomass gasification products in bubbling fluidized beds. Energy 2020, 202, 117654. [Google Scholar] [CrossRef]
- Vaquerizo, L.; Cocero, M.J. CFD–Aspen Plus interconnection method. Improving thermodynamic modeling in computational fluid dynamic simulations. Comput. Chem. Eng. 2018, 113, 152–161. [Google Scholar] [CrossRef]
- Monteiro, E.; Ismail, T.M.; Ramos, A.; Abd El-Salam, M.; Brito, P.; Rouboa, A. Assessment of the miscanthus gasification in a semi-industrial gasifier using a CFD model. Appl. Therm. Eng. 2017, 123, 448–457. [Google Scholar] [CrossRef]
- Couto, N.; Silva, V.; Rouboa, A.J.E. Municipal solid waste gasification in semi-industrial conditions using air-CO2 mixtures. Energy 2016, 104, 42–52. [Google Scholar] [CrossRef]
- Marinho, D.A.; Silva, A.J.; Reis, V.M.; Barbosa, T.M.; Vilas-Boas, J.P.; Alves, F.B.; Machado, L.; Rouboa, A.I. Three-dimensional CFD analysis of the hand and forearm in swimming. J. Appl. Biomech. 2011, 27, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Rouboa, A. Optimizing the gasification operating conditions of forest residues by coupling a two-stage equilibrium model with a response surface methodology. Fuel Process. Technol. 2014, 122, 163–169. [Google Scholar] [CrossRef]
- Abuadala, A.; Dincer, I.; Naterer, G.F. Exergy analysis of hydrogen production from biomass gasification. Int. J. Hydrogen Energy 2010, 35, 4981–4990. [Google Scholar] [CrossRef]
- Gao, N.; Li, A.; Quan, C.; Gao, F. Hydrogen-rich gas production from biomass steam gasification in an updraft fixed-bed gasifier combined with a porous ceramic reformer. Int. J. Hydrogen Energy 2008, 33, 5430–5438. [Google Scholar] [CrossRef]
- Awais, M.; Omar, M.M.; Munir, A.; Ajmal, M.; Hussain, S.; Ahmad, S.A.; Ali, A. Co-gasification of different biomass feedstock in a pilot-scale (24 kWe) downdraft gasifier: An experimental approach. Energy 2022, 238, 121821. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Zawawi, N.A.; Kasim, F.H.; Inayat, A.; Khasri, A. Assessing the gasification performance of biomass: A review on biomass gasification process conditions, optimization and economic evaluation. Renew. Sustain. Energy Rev. 2016, 53, 1333–1347. [Google Scholar] [CrossRef]
- Silva, V.B.; Rouboa, A. In situ activation procedures applied to a DMFC: Analysis and optimization study. Fuel 2012, 93, 677–683. [Google Scholar] [CrossRef]
- Sousa, P.; Soares, A.; Monteiro, E.; Rouboa, A. A CFD study of the hydrodynamics in a desalination membrane filled with spacers. Desalination 2014, 349, 22–30. [Google Scholar] [CrossRef]
- Haydary, J. Chemical Process Design and Simulation: Aspen Plus and Aspen Hysys Applications; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Aguilar-Jiménez, J.A.; Hernández-Callejo, L.; Alonso-Gómez, V.; Velázquez, N.; López-Zavala, R.; Acuña, A.; Mariano-Hernández, D. Techno-economic analysis of hybrid PV/T systems under different climate scenarios and energy tariffs. Sol. Energy 2020, 212, 191–202. [Google Scholar] [CrossRef]
- Puig-Gamero, M.; Pio, D.; Tarelho, L.; Sánchez, P.; Sanchez-Silva, L. Simulation of biomass gasification in bubbling fluidized bed reactor using aspen plus®. Energy Convers. Manag. 2021, 235, 113981. [Google Scholar] [CrossRef]
- Champion, W.M.; Cooper, C.D.; Mackie, K.R.; Cairney, P. Development of a chemical kinetic model for a biosolids fluidized-bed gasifier and the effects of operating parameters on syngas quality. J. Air Waste Manag. Assoc. 2014, 64, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.C.; Li, X.Y.; Park, T.J.; Kim, J.H.; Lee, J.G. Numerical study on the coal gasification characteristics in an entrained flow coal gasifier. Fuel 2001, 80, 2193–2201. [Google Scholar] [CrossRef]
- Groppi, G.; Tronconi, E.; Forzatti, P.; Berg, M. Mathematical modelling of catalytic combustors fuelled by gasified biomasses. Catal. Today 2000, 59, 151–162. [Google Scholar] [CrossRef]
- Yan, W.-C.; Shen, Y.; You, S.; Sim, S.H.; Luo, Z.-H.; Tong, Y.W.; Wang, C.-H. Model-based downdraft biomass gasifier operation and design for synthetic gas production. J. Clean. Prod. 2018, 178, 476–493. [Google Scholar] [CrossRef]
- Smoot, L.D.; Smith, P.J. Coal Gasification and Combustion; Plenum Press: New York, NY, USA, 1985. [Google Scholar]
- Westbrook, C.K.; Dryer, F.L. Chemical kinetic modeling of hydrocarbon combustion. Prog. Energy Combust. Sci. 1984, 10, 1–57. [Google Scholar] [CrossRef]
- Corella, J.; Sanz, A. Modeling circulating fluidized bed biomass gasifiers. A pseudo-rigorous model for stationary state. Fuel Process. Technol. 2005, 86, 1021–1053. [Google Scholar] [CrossRef]
- Gonzalez, A.M.; Lora, E.E.S.; Palacio, J.C.E.; del Olmo, O.A.A. Hydrogen production from oil sludge gasification/biomass mixtures and potential use in hydrotreatment processes. Int. J. Hydrogen Energy 2018, 43, 7808–7822. [Google Scholar] [CrossRef]
- Gómez-Barea, A.; Leckner, B. Modeling of biomass gasification in fluidized bed. Prog. Energy Combust. Sci. 2010, 36, 444–509. [Google Scholar] [CrossRef]
- Umeki, K.; Namioka, T.; Yoshikawa, K. Analysis of an updraft biomass gasifier with high temperature steam using a numerical model. Appl. Energy 2012, 90, 38–45. [Google Scholar] [CrossRef]
- Morf, P.; Hasler, P.; Nussbaumer, T. Mechanisms and kinetics of homogeneous secondary reactions of tar from continuous pyrolysis of wood chips. Fuel 2002, 81, 843–853. [Google Scholar] [CrossRef]
- Sreejith, C.C.; Muraleedharan, C.; Arun, P. Performance prediction of steam gasification of wood using an ASPEN PLUS thermodynamic equilibrium model. Int. J. Sustain. Energy 2014, 33, 416–434. [Google Scholar] [CrossRef]
- Ji, P.; Feng, W.; Chen, B. Production of ultrapure hydrogen from biomass gasification with air. Chem. Eng. Sci. 2009, 64, 582–592. [Google Scholar] [CrossRef]
- Puig-Gamero, M.; Argudo-Santamaria, J.; Valverde, J.L.; Sánchez, P.; Sanchez-Silva, L. Three integrated process simulation using aspen plus®: Pine gasification, syngas cleaning and methanol synthesis. Energy Convers. Manag. 2018, 177, 416–427. [Google Scholar] [CrossRef]
- Islamova, S.; Tartygasheva, A.; Karaeva, J.; Panchenko, V.; Litti, Y. A Comprehensive Study on the Combustion of Sunflower Husk Pellets by Thermogravimetric and Kinetic Analysis, Kriging Method. Agriculture 2023, 13, 840. [Google Scholar] [CrossRef]
- Luo, S.; Xiao, B.; Guo, X.; Hu, Z.; Liu, S.; He, M. Hydrogen-rich gas from catalytic steam gasification of biomass in a fixed bed reactor: Influence of particle size on gasification performance. Int. J. Hydrogen Energy 2009, 34, 1260–1264. [Google Scholar] [CrossRef]
- Luo, S.; Xiao, B.; Hu, Z.; Liu, S.; Guo, X.; He, M. Hydrogen-rich gas from catalytic steam gasification of biomass in a fixed bed reactor: Influence of temperature and steam on gasification performance. Int. J. Hydrogen Energy 2009, 34, 2191–2194. [Google Scholar] [CrossRef]
- Herguido, J.; Corella, J.; Gonzalez-Saiz, J. Steam gasification of lignocellulosic residues in a fluidized bed at a small pilot scale. Effect of the type of feedstock. Ind. Eng. Chem. Res. 1992, 31, 1274–1282. [Google Scholar] [CrossRef]
- Turn, S.; Kinoshita, C.; Zhang, Z.; Ishimura, D.; Zhou, J. An experimental investigation of hydrogen production from biomass gasification. Int. J. Hydrogen Energy 1998, 23, 641–648. [Google Scholar] [CrossRef]





| Ultimate Analysis (wt%) | Proximate Analysis 1 (wt%) | LHV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Biomass | C | H | O | N | S | Ash | VM | FC 2 | Moisture | (MJ/Nm3) |
| Wood chips | 48.12 | 6.12 | 45.74 | 0.08 | 0 | 3.5 | 63 | 14.5 | 7.5 | 3.36 |
| Corn cobs | 44 | 6.9 | 38.65 | 1.8 | 0.09 | 2.5 | 69 | 17 | 12 | 3.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, M.; Monteiro, E.; Rouboa, A. Optimizing Hydrogen-Rich Biofuel Production: Syngas Generation from Wood Chips and Corn Cobs. Energies 2024, 17, 1859. https://doi.org/10.3390/en17081859
Oliveira M, Monteiro E, Rouboa A. Optimizing Hydrogen-Rich Biofuel Production: Syngas Generation from Wood Chips and Corn Cobs. Energies. 2024; 17(8):1859. https://doi.org/10.3390/en17081859
Chicago/Turabian StyleOliveira, Matheus, Eliseu Monteiro, and Abel Rouboa. 2024. "Optimizing Hydrogen-Rich Biofuel Production: Syngas Generation from Wood Chips and Corn Cobs" Energies 17, no. 8: 1859. https://doi.org/10.3390/en17081859
APA StyleOliveira, M., Monteiro, E., & Rouboa, A. (2024). Optimizing Hydrogen-Rich Biofuel Production: Syngas Generation from Wood Chips and Corn Cobs. Energies, 17(8), 1859. https://doi.org/10.3390/en17081859








