Enhanced Electrochemical Performance of Aqueous Zinc-Ion Batteries Using MnSO4 Electrolyte Additive and α-MnO2 Cathode
Abstract
:1. Introduction
2. Experimental Section
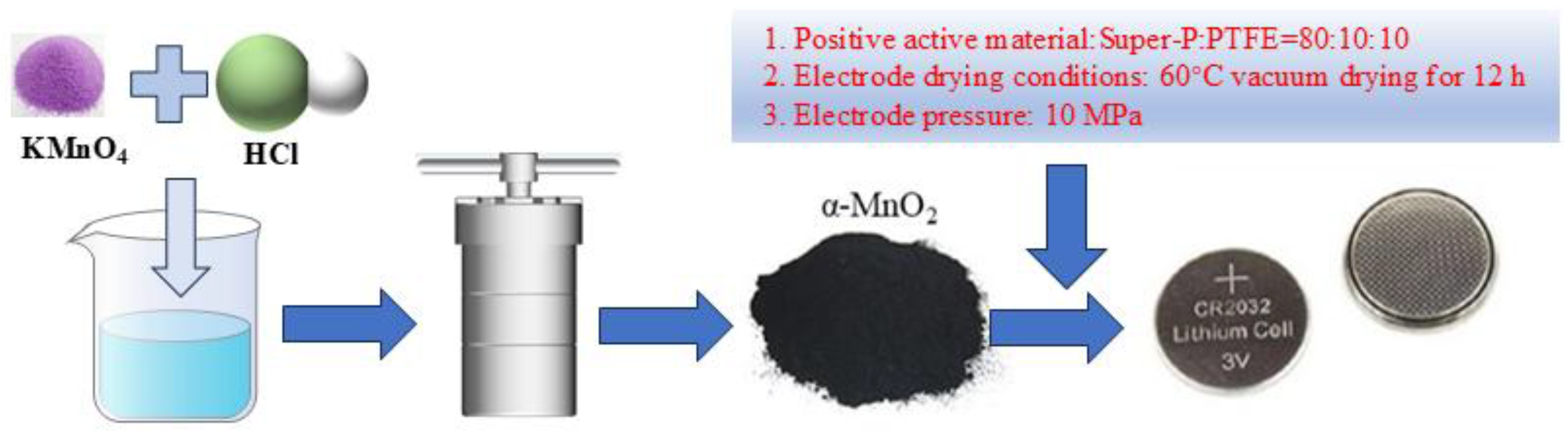
2.1. Materials Synthesis
2.2. Material Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
3.1. Characterization
3.2. Electrochemical Testing of MnO2-Based Cathode Materials
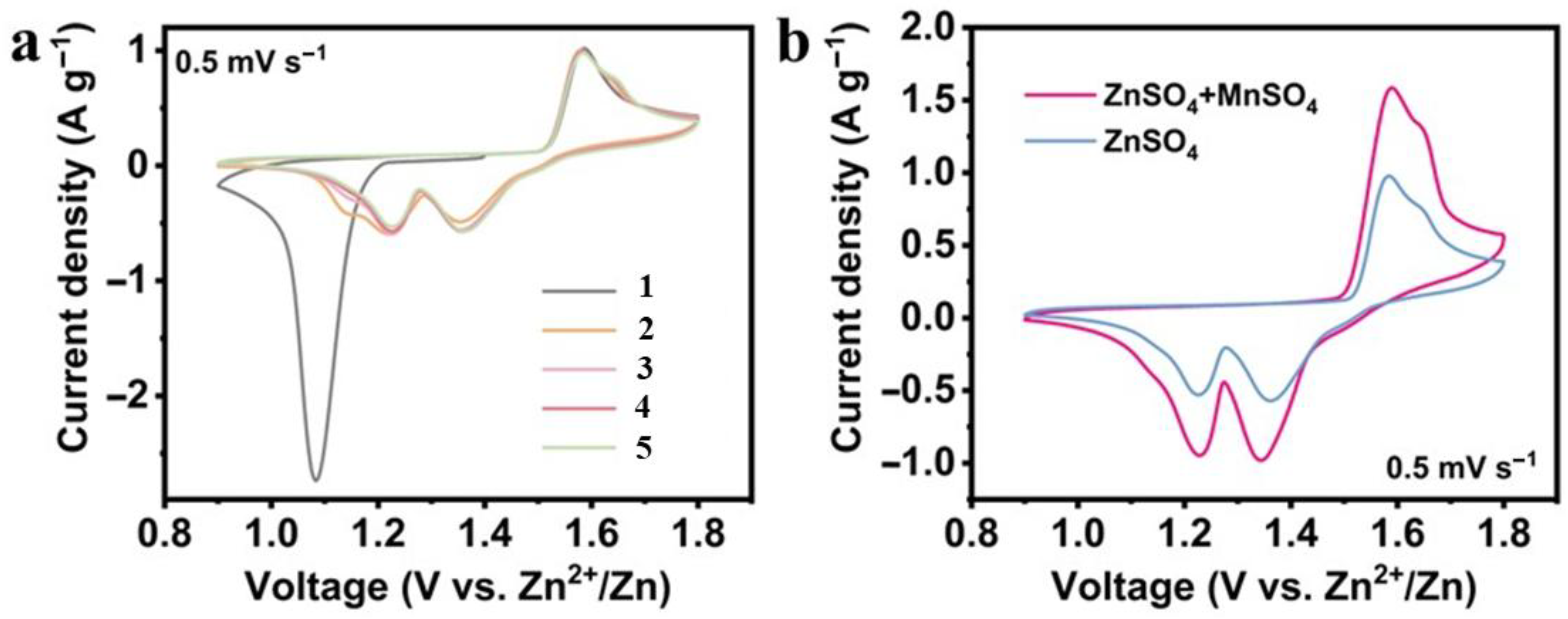
3.2.1. Cyclic Voltammetry
3.2.2. Ratio Test
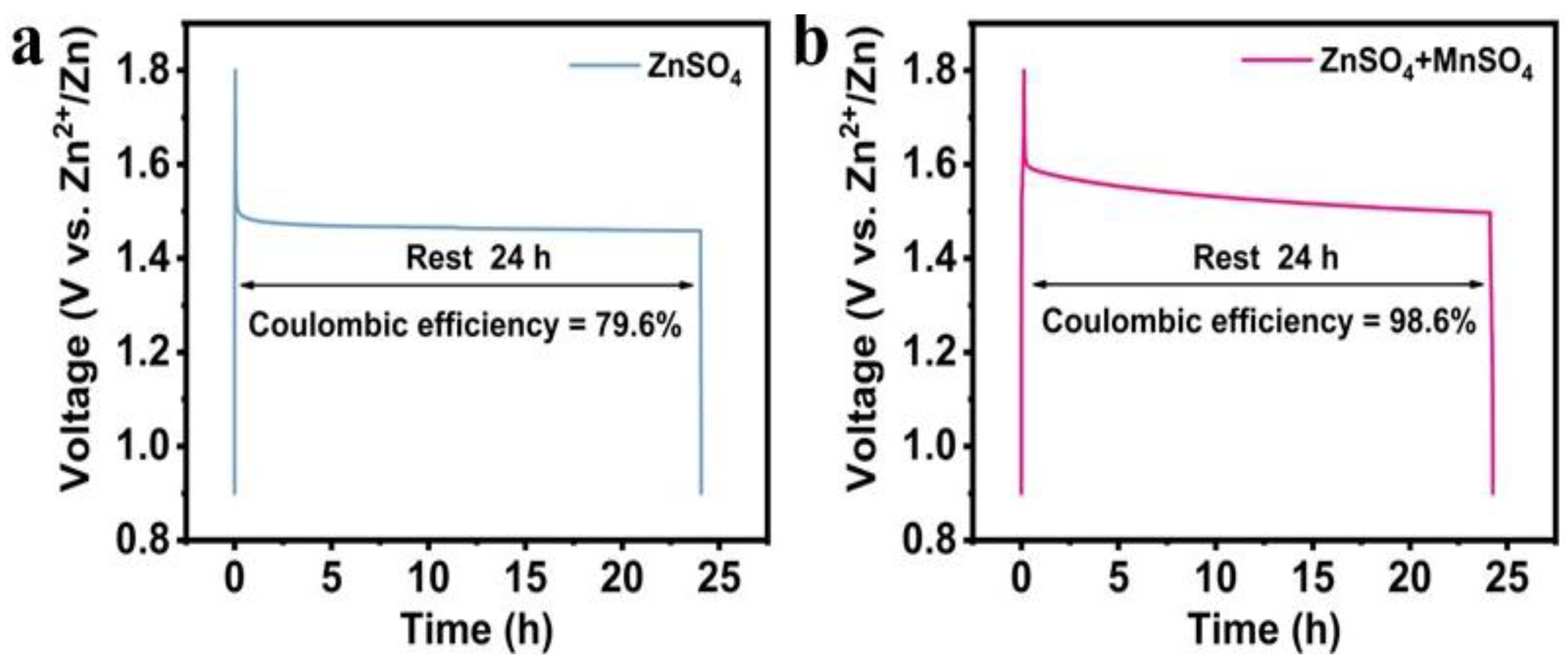
3.2.3. Self-Discharge Performance
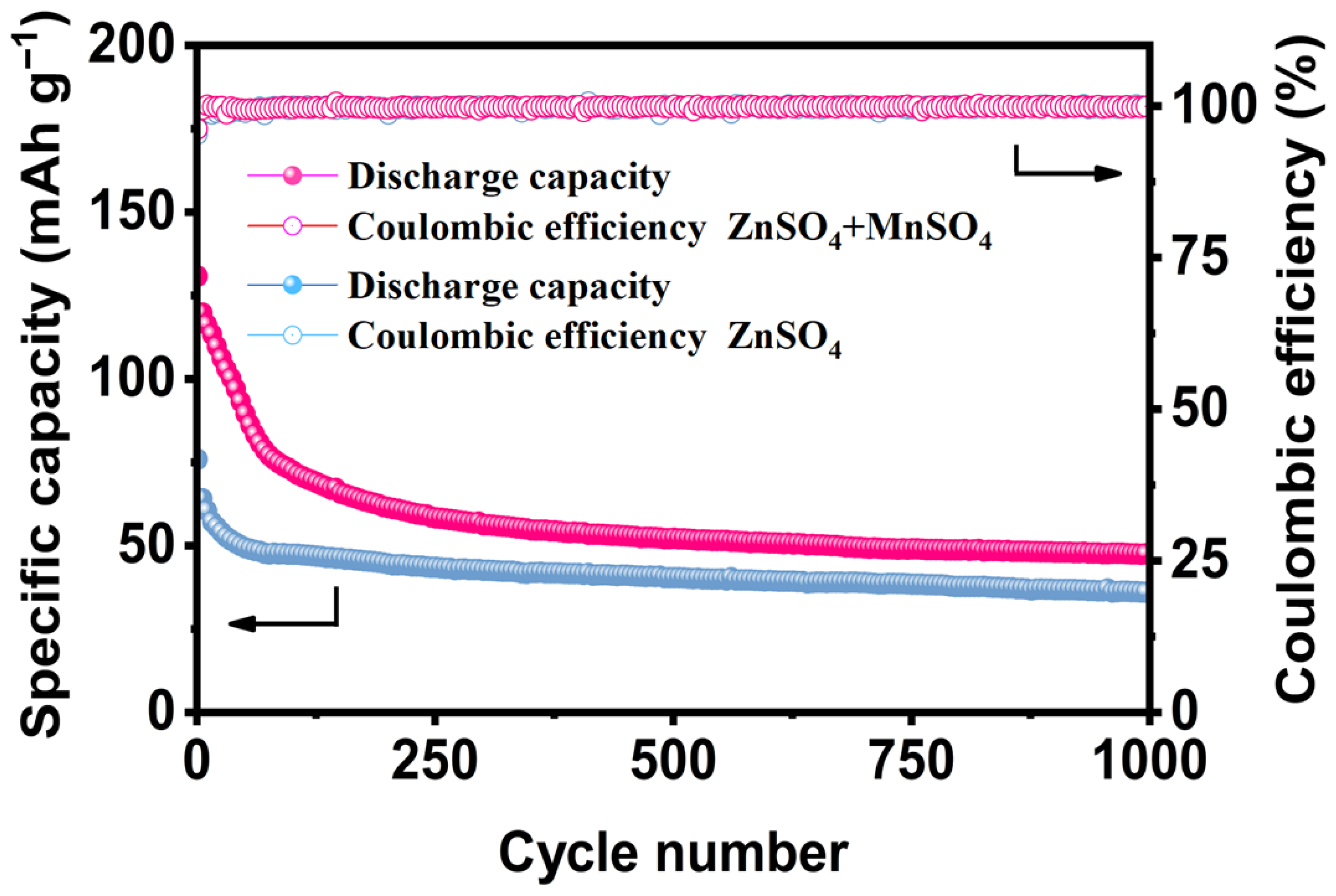
3.2.4. Cycle Performance
3.3. Application
4. Conclusions
- The α-MnO2 cathode material with a 2 × 2 tunnel structure (4.6 Å × 4.6 Å) was successfully synthesized using a modified hydrothermal method based on previous reports. XRD, SEM, and BET characterizations revealed a highly crystalline structure, uniform nanorod morphology, and rich mesoporous characteristics, which provide more active sites and excellent channels for ion transport and electrochemical reactions. The optimized synthesis conditions ensured the formation of a stable tunnel structure, which is critical for the high performance of the material;
- Electrochemical results show that the MnSO4-modified electrolyte increases the discharge capacity of the α-MnO2 cathode at 0.1 A g⁻1 from 172.9 mAh g⁻1 to 263.2 mAh g⁻1 (an increase of 52.2%); the capacity retention rate of the system containing the MnSO4 electrolyte reaches 47.4% after 1000 cycles, which is significantly improved compared with the pure ZnSO4 system (36.2%); the MnSO4 additive increases the coulombic efficiency of the battery from 79.6% to 98.6% after standing for 24 h. At the same time, the introduction of MnSO4 effectively inhibits the dissolution of manganese in α-MnO2, optimizes the dissolution–deposition balance, and improves the rate performance and self-discharge performance;
- The zinc-ion battery based on α-MnO2 exhibits excellent power supply performance in practical applications, which verifies the practical application potential of zinc-ion batteries in powering portable electronic devices.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Gao, T.; Olguin, M.; Ho, J.; Fan, X.; Xu, K. “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 2015, 350, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, X.; Lai, F.; Liu, T.; Shearing, P.R.; Parkin, I.P.; Brett, D.J. Rechargeable aqueous Zn-based energy storage devices. Joule 2021, 5, 2845–2903. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, W.; Luo, J.; Zhang, H.; Liu, J.; Liu, X.; Lu, X. A high-energy-density aqueous zinc–manganese battery with a La–Ca co-doped ε-MnO2 cathode. J. Mater. Chem. A 2020, 8, 11642–11648. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Neumann, J.; Petranikova, M.; Meeus, M.; Gamarra, J.D.; Younesi, R.; Winter, M.; Nowak, S. Recycling of lithium-ion batteries—Current state of the art, circular economy, and next-generation recycling. Adv. Energy Mater. 2022, 12, 2102917. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Zhang, S.; He, Y.; Hou, L.; Yuan, C. Two-pronged approach to achieving high-capacity and long stable-life aqueous Zn-ion batteries. Chem. Eng. J. 2024, 479, 147422. [Google Scholar] [CrossRef]
- Ma, K.; Yang, G.; Wang, C. Towards storable and durable Zn–MnO2 batteries with hydrous tetraglyme electrolyte. J. Energy Chem. 2023, 80, 432–441. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, C.; Yu, Y. A review on covalent organic frameworks for rechargeable zinc-ion batteries. Chin. Chem. Lett. 2024, 35, 108865. [Google Scholar] [CrossRef]
- Lee, B.; Yoon, C.S.; Lee, H.R.; Chung, K.Y.; Cho, B.W.; Oh, S.H. Electrochemically-induced reversible transition from the tunneled to layered polymorphs of manganese dioxide. Sci. Rep. 2014, 4, 6066. [Google Scholar] [CrossRef]
- Zhang, A.; Zhao, R.; Wang, Y.; Yang, J.; Wu, C.; Bai, Y. Regulating the electronic structure of manganese-based materials to optimize the performance of zinc-ion batteries. Energy Environ. Sci. 2023, 16, 3240–3301. [Google Scholar] [CrossRef]
- Dai, X.; Wan, F.; Zhang, L.; Cao, H.; Niu, Z. Freestanding graphene/VO2 composite films for highly stable aqueous Zn-ion batteries with superior rate performance. Energy Storage Mater. 2019, 17, 143–150. [Google Scholar] [CrossRef]
- Shchegolkov, A.V.; Lipkin, M.S.; Shchegolkov, A.V. Preparation of WO3 films on titanium and graphite foil for fuel cell and supercapacitor applications by electrochemical (cathodic) deposition method. Russ. J. Gen. Chem. 2022, 92, 1161–1167. [Google Scholar] [CrossRef]
- Li, M.; Maisuradze, M.; Sciacca, R.; Hasa, I.; Giorgetti, M. A structural perspective on Prussian blue analogues for aqueous zinc-ion batteries. Batter. Supercaps 2023, 6, e202300340. [Google Scholar] [CrossRef]
- Ali, S.; Ahmad Shah, S.S.; Sufyan Javed, M.; Najam, T.; Parkash, A.; Khan, S.; Qi, J. Recent advances of transition metal dichalcogenides-based materials for energy storage devices, in view of monovalent to divalent ions. Chem. Rec. 2024, 24, e202300145. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Zhang, X.; Javed, M.S.; Shah, H.U.; Ahmad, A.; Albaqami, M.D.; Qi, J. MoS2/Ti3CO2 heterostructure-based ceramics as promising electrode material for high-performance monovalent energy storage devices. Ceram. Int. 2024, 50, 4782–4789. [Google Scholar] [CrossRef]
- Jia, X.; Liu, C.; Neale, Z.G.; Yang, J.; Cao, G. Active materials for aqueous zinc ion batteries: Synthesis, crystal structure, morphology, and electrochemistry. Chem. Rev. 2020, 120, 7795–7866. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Sun, L.; Zhang, S.; Zhang, C.; Jin, H.; Davey, K.; Guo, Z. Developing cathode materials for aqueous zinc ion batteries: Challenges and practical prospects. Adv. Funct. Mater. 2024, 34, 2301291. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, Y.; Li, Z. Construction of novel hierarchical honeycomb-like Mn3O4−MnO2 core-shell architecture with high voltage for advanced aqueous zinc-ion batteries. J. Electrochem. Soc. 2022, 169, 040519. [Google Scholar] [CrossRef]
- Ko, W.Y.; Lubis, A.L.; Wang, H.Y.; Wu, T.C.; Lin, S.T.; Lin, K.J. Facile construction of Zn-doped Mn3O4−MnO2 vertical nanosheets for aqueous zinc-ion battery cathodes. ChemElectroChem 2022, 9, e202200750. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Rossouw, M.H.; Gummow, R.J.; Liles, D.C.; Pearce, K.; De Kock, A.; David, W.I.F.; Hull, S. Ramsdellite-MnO2 for Lithium Batteries: The Ramsdellite to Spinel Transformation. Electrochim. Acta 1993, 38, 1259–1267. [Google Scholar] [CrossRef]
- Feng, X.; Xu, K.; Wei, Z.; Du, W.; Zhang, H.; Liang, C.; Han, C. A novel improvement strategy and a comprehensive mechanism insight for α-MnO2 energy storage in rechargeable aqueous zinc-ion batteries. Carbon Energy 2024, 6, e536. [Google Scholar] [CrossRef]
- Wu, L.; Li, Z.; Xiang, Y.; Dong, W.; Wu, H.; Xu, Y.; Zhang, X. Unraveling the charge storage mechanism of β-MnO2 in aqueous zinc electrolytes. ACS Energy Lett. 2024, 9, 5801–5809. [Google Scholar] [CrossRef]
- Yao, H.; Yu, H.; Zheng, Y.; Li, N.W.; Li, S.; Luan, D.; Yu, L. Pre-intercalation of ammonium ions in layered δ-MnO2 nanosheets for high-performance aqueous zinc-ion batteries. Angew. Chem. Int. Ed. 2023, 62, e202315257. [Google Scholar] [CrossRef] [PubMed]
- Davoglio, R.A.; Cabello, G.; Marco, J.F.; Biaggio, S.R. Synthesis and characterization of α-MnO2 nanoneedles for electrochemical supercapacitors. Electrochim. Acta 2018, 261, 428–435. [Google Scholar] [CrossRef]
- Khalid, M.U.; Al Huwayz, M.; Zulfiqar, S.; Cochran, E.W.; Alrowaili, Z.A.; Al-Buriahi, M.S.; Shahid, M. Phase transformation of α-MnO2 to β-MnO2 induced by Cu doping: Improved electrochemical performance for next generation supercapacitor. Mater. Sci. Eng. B 2023, 295, 116580. [Google Scholar] [CrossRef]
- Fenta, F.W.; Olbasa, B.W.; Tsai, M.C.; Temesgen, N.T.; Huang, W.H.; Tekaligne, T.M.; Hwang, B.J. Structural engineering of α-MnO2 cathode by Ag+ incorporation for high capacity aqueous zinc-ion batteries. J. Power Sources 2022, 548, 232010. [Google Scholar] [CrossRef]
- Liu, Y.; Chi, X.; Han, Q.; Du, Y.; Huang, J.; Liu, Y.; Yang, J. α-MnO2 nanofibers/carbon nanotubes hierarchically assembled microspheres: Approaching practical applications of high-performance aqueous Zn-ion batteries. J. Power Sources 2019, 443, 227244. [Google Scholar] [CrossRef]
- Niu, T.; Li, J.; Qi, Y.; Huang, X.; Ren, Y. Preparation and electrochemical properties of α-MnO2/rGO-PPy composite as cathode material for zinc-ion battery. J. Mater. Sci. 2021, 56, 16582–16590. [Google Scholar] [CrossRef]
- Lee, S.; Je, Y.; Seok, B.; Kim, H.T.; Jo, Y.R.; Oh, S.J.; An, G.H. Anode surface engineering of zinc-ion batteries using tellurium nanobelt as a protective layer for enhancing energy storage performance. J. Energy Chem. 2024, 92, 113–123. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, J.; Feng, J.; Wang, Y.; Wu, X.; Ma, P.; Yan, X. A rechargeable aqueous zinc/sodium manganese oxides battery with robust performance enabled by Na2SO4 electrolyte additive. Energy Storage Mater. 2021, 38, 299–308. [Google Scholar] [CrossRef]
- Kong, J.; Guo, H.; Li, Y.; Gong, M.; Lin, X.; Zhang, L.; Wang, D. Highly improved aqueous Zn|LiMn2O4 hybrid-ion batteries using poly(ethylene glycol) and manganese sulfate as electrolyte additives. Sustain. Energy Fuels 2024, 8, 826–836. [Google Scholar] [CrossRef]
- Park, S.; An, G.H. Improvement of structural stability of cathode by manganese additive in electrolyte for zinc-ion batteries. Int. J. Energy Res. 2022, 46, 8464–8470. [Google Scholar] [CrossRef]
- Toupin, M.; Brousse, T.; Bélanger, D. Influence of microstructure on the charge storage properties of chemically synthesized manganese dioxide. Chem. Mater. 2002, 14, 3946–3952. [Google Scholar] [CrossRef]
- Sawangphruk, M.; Srimuk, P.; Chiochan, P.; Krittayavathananon, A.; Luanwuthi, S.; Limtrakul, J. High-performance supercapacitor of manganese oxide/reduced graphene oxide nanocomposite coated on flexible carbon fiber paper. Carbon 2013, 60, 109–116. [Google Scholar] [CrossRef]
- Su, X.; Yu, L.; Cheng, G.; Zhang, H.; Sun, M.; Zhang, X. High-performance α-MnO2 nanowire electrode for supercapacitors. Appl. Energy 2015, 153, 94–100. [Google Scholar] [CrossRef]
- Guo, C.; Liu, H.; Li, J.; Hou, Z.; Liang, J.; Zhou, J.; Qian, Y. Ultrathin δ-MnO2 nanosheets as cathode for aqueous rechargeable zinc-ion battery. Electrochim. Acta 2019, 304, 370–377. [Google Scholar] [CrossRef]
- Liu, N.; Wu, X.; Yin, Y.; Chen, A.; Zhao, C.; Guo, Z.; Zhang, N. Constructing the efficient ion diffusion pathway by introducing oxygen defects in Mn2O3 for high-performance aqueous zinc-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 28199–28205. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Wang, B.; Jian, J.; Wu, F.; Peng, L.; Wang, D. Stress-release design for high-capacity and long-time lifespan aqueous zinc-ion batteries. Mater. Today Energy 2021, 21, 100799. [Google Scholar] [CrossRef]
- Soundharrajan, V.; Sambandam, B.; Kim, S.; Islam, S.; Jo, J.; Kim, S.; Kim, J. The dominant role of Mn2+ additive on the electrochemical reaction in ZnMn2O4 cathode for aqueous zinc-ion batteries. Energy Storage Mater. 2020, 28, 407–417. [Google Scholar] [CrossRef]
- Huang, Z.; Duan, Y.; Jing, Q.; Sun, M.; Tang, B.; Shi, S. Assembly of Mn3O4 nanoparticles at low temperature on graphene with enhanced electrochemical property for zinc-ion battery. J. Alloys Compd. 2021, 864, 158316. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Ji, C.; Wan, L.; Zhang, X.; Wang, H.; Xie, L.; Gao, J. Enhanced Electrochemical Performance of Aqueous Zinc-Ion Batteries Using MnSO4 Electrolyte Additive and α-MnO2 Cathode. Energies 2025, 18, 1420. https://doi.org/10.3390/en18061420
Zhou X, Ji C, Wan L, Zhang X, Wang H, Xie L, Gao J. Enhanced Electrochemical Performance of Aqueous Zinc-Ion Batteries Using MnSO4 Electrolyte Additive and α-MnO2 Cathode. Energies. 2025; 18(6):1420. https://doi.org/10.3390/en18061420
Chicago/Turabian StyleZhou, Xinfeng, Chenchen Ji, Lingyun Wan, Xiaohui Zhang, Haopeng Wang, Longfei Xie, and Jie Gao. 2025. "Enhanced Electrochemical Performance of Aqueous Zinc-Ion Batteries Using MnSO4 Electrolyte Additive and α-MnO2 Cathode" Energies 18, no. 6: 1420. https://doi.org/10.3390/en18061420
APA StyleZhou, X., Ji, C., Wan, L., Zhang, X., Wang, H., Xie, L., & Gao, J. (2025). Enhanced Electrochemical Performance of Aqueous Zinc-Ion Batteries Using MnSO4 Electrolyte Additive and α-MnO2 Cathode. Energies, 18(6), 1420. https://doi.org/10.3390/en18061420






