Performance and Kinetics of Anaerobic Digestion of Sewage Sludge Amended with Zero-Valent Iron Nanoparticles, Analyzed Using Sigmoidal Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Setup
2.3. Analytical Methods and Performance Parameters
2.4. Kinetic Simulation
| Model | Equation Form and Parameter Definition | Reference |
|---|---|---|
| Gompertz | , where α: decay constant or growth rate parameter (1/d); r0: initial rate of biogas production (mL/g-VSadded/d). | [55] |
| Modified Gompertz | , where P(t): cumulative biogas/CH4 production at time t (mL/g-VSadded); Pmax: maximum production potential of biogas/CH4 (mL/g-VSadded); Rmax: maximum biogas/CH4 production rate (ml/g-VSadded/d); λ: duration of the lag phase (d); t: time of anaerobic digestion (d); e: Euler’s constant, which is equal to 2.7183. | [58] |
| Richards | , where ν: shape factor (dimensionless). | [59] |
| Modified Richards | , where P0: initial biogas/CH4 production (mL/g-VSadded). | [56,60] |
| Logistic | , where k: specific growth rate constant or reaction rate coefficient (1/d); t0: time at which production rate is the highest (d). | [61] |
| Modified logistic | [59] | |
| Cone | , where n: shape factor (dimensionless); k: hydrolysis rate constant (1/d). | [62] |
| Schnute | , where β: scaling parameter controlling the curve shape (dimensionless). | [63] |
2.5. Kinetic Model Accuracy Evaluation
2.6. Statistical Analysis
3. Results
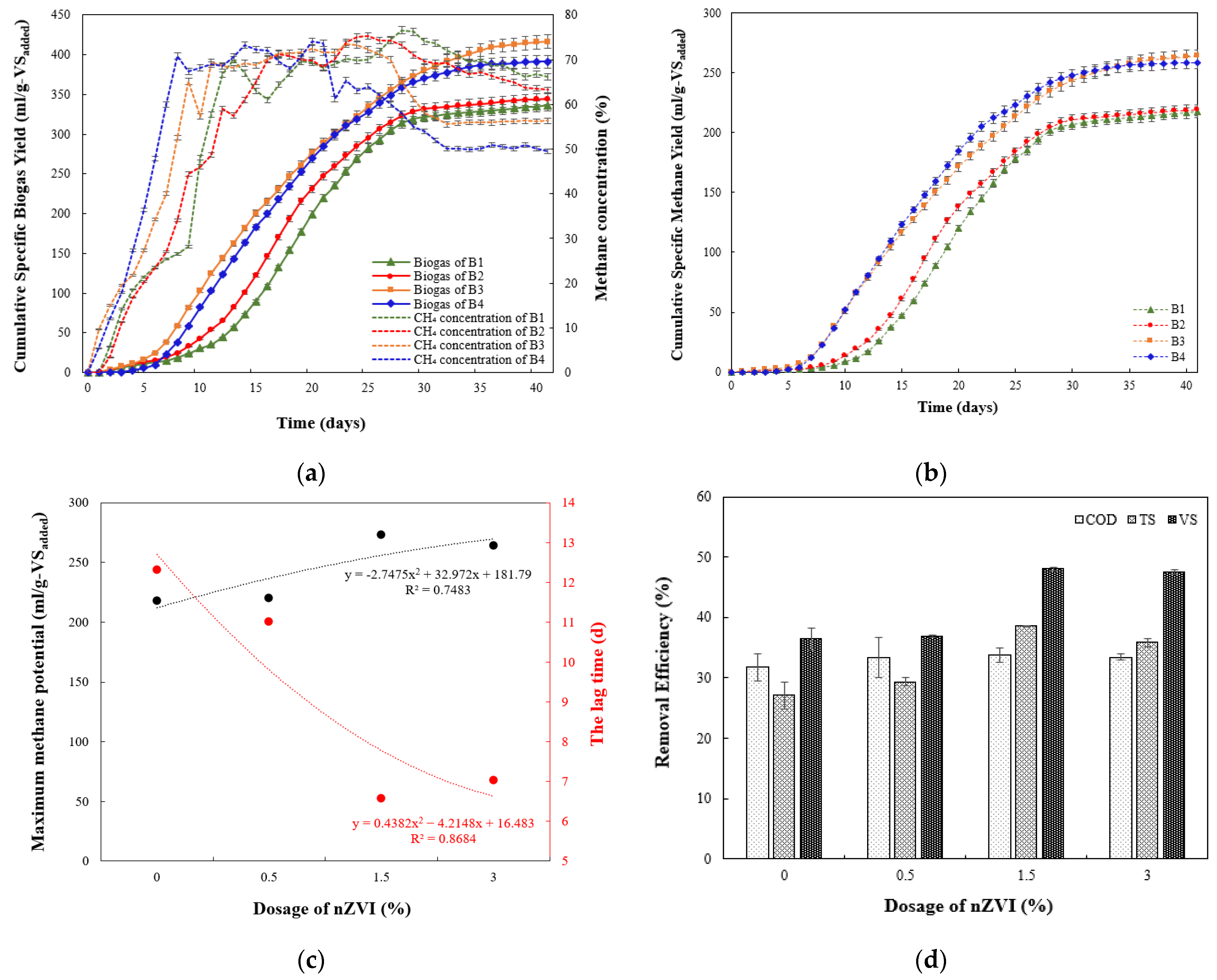
3.1. Effect of nZVI on Cumulative Biogas/Methane Generation and AD Performance
3.2. Biogas Production and Kinetics
3.3. Methane Production and Kinetics
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marguti, A.L.; Ferreira Filho, S.S.; Piveli, R.P. Full-scale effects of addition of sludge from water treatment stations into processes of sewage treatment by conventional activated sludge. J. Environ. Manag. 2018, 215, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Gulhan, H.; Dizaji, R.F.; Hamidi, M.N.; Abdelrahman, A.M.; Basa, S.; Kurt, E.S.; Koyuncu, I.; Guven, H.; Ozgun, H.; Ersahin, M.E.; et al. Use of water treatment plant sludge in high-rate activated sludge systems: A techno-economic investigation. Sci. Total Environ. 2023, 901, 66431. [Google Scholar] [CrossRef] [PubMed]
- Lasaki, B.A.; Maurer, P.; Schönberger, H. Uncovering the reasons behind high-performing primary sedimentation tanks for municipal wastewater treatment: An in-depth analysis of key factors. J. Environ. Chem. Eng. 2024, 12, 112460. [Google Scholar] [CrossRef]
- James, O.O.; Cao, J.-S.; Kabo-Bah, A.T.; Wang, G. Assessing the impact of Solids Retention Time (SRT) on the secondary clarifier capacity using the State Point Analysis. KSCE J. Civ. Eng. 2015, 19, 1265–1270. [Google Scholar] [CrossRef]
- Romero-Güiza, M.S.; Flotats, X.; Asiain-Mira, R.; Palatsi, J. Enhancement of sewage sludge thickening and energy self-sufficiency with advanced process control tools in a full-scale wastewater treatment plant. Water Res. 2022, 222, 118924. [Google Scholar] [CrossRef]
- Siddiqua, A.; Hahladakis, J.N.; Al-Attiya, W.A.K.A. An overview of the environmental pollution and health effects associated with waste landfilling and open dumping. Environ. Sci. Pollut. Res. Int. 2022, 29, 58514–58536. [Google Scholar] [CrossRef]
- Mabrouk, O.; Hamdi, H.; Sayadi, S.; Al-Ghouti, M.A.; Abu-Dieyeh, M.H.; Zouari, N. Reuse of Sludge as Organic Soil Amendment: Insights into the Current Situation and Potential Challenges. Sustainability 2023, 15, 6773. [Google Scholar] [CrossRef]
- Giwa, A.S.; Maurice, N.J.; Luoyan, A.; Liu, X.; Yunlong, Y.; Hong, Z. Advances in sewage sludge application and treatment: Process integration of plasma pyrolysis and anaerobic digestion with the resource recovery. Heliyon 2023, 9, e19765. [Google Scholar] [CrossRef]
- Chojnacka, K.; Moustakas, K. Anaerobic digestate management for carbon neutrality and fertilizer use: A review of current practices and future opportunities. Biomass Bioenergy 2024, 180, 106991. [Google Scholar] [CrossRef]
- Enebe, N.L.; Chigor, C.B.; Obileke, K.; Lawal, M.S.; Enebe, M.C. Biogas and Syngas Production from Sewage Sludge: A Sustainable Source of Energy Generation. Methane 2023, 2, 192–217. [Google Scholar] [CrossRef]
- Harirchi, S.; Wainaina, S.; Sar, T.; Nojoumi, S.A.; Parchami, M.; Parchami, M.; Varjani, S.; Khanal, S.K.; Wong, J.; Awasthi, M.K.; et al. Microbiological insights into anaerobic digestion for biogas, hydrogen or volatile fatty acids (VFAs): A review. Bioengineered 2022, 13, 6521–6557. [Google Scholar] [CrossRef] [PubMed]
- Bezirgiannidis, A.; Chatzopoulos, P.; Tsakali, A.; Ntougias, S.; Melidis, P. Renewable energy recovery from sewage sludge derived from chemically enhanced precipitation. Renew. Energy 2020, 162, 1811–1818. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Mousavi, S.A.; Darvishi, P. Performance and recent development in sewage sludge-to-bioenergy using microbial fuel cells: A comprehensive review. Int. J. Hydrogen Energy 2024, 50, 1432–1455. [Google Scholar] [CrossRef]
- Cucina, M. Integrating anaerobic digestion and composting to boost energy and material recovery from organic wastes in the Circular Economy framework in Europe: A review. Bioresour. Technol. Reports 2023, 24, 101642. [Google Scholar] [CrossRef]
- Nayeri, D.; Mohammadi, P.; Bashardoust, P.; Eshtiaghi, N. A comprehensive review on the recent development of anaerobic sludge digestions: Performance, mechanism, operational factors, and future challenges. Results Eng. 2024, 22, 102292. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Lues, R. Anaerobic Digestion of Lignocellulosic Biomass: Substrate Characteristics (Challenge) and Innovation. Fermentation 2023, 9, 755. [Google Scholar] [CrossRef]
- Liao, X.; Li, H.; Zhang, Y.; Liu, C.; Chen, Q. Accelerated high-solids anaerobic digestion of sewage sludge using low-temperature thermal pretreatment. Int. Biodeter. Biodegr. 2016, 106, 141–149. [Google Scholar] [CrossRef]
- Jung, H.; Kim, D.; Choi, H.; Lee, C. A review of technologies for in-situ sulfide control in anaerobic digestion. Renew. Sustain. Energy Rev. 2022, 157, 112068. [Google Scholar] [CrossRef]
- Sihlangu, E.; Luseba, D.; Regnier, T.; Magama, P.; Chiyanzu, I.; Nephawe, K.A. Investigating Methane, Carbon Dioxide, Ammonia, and Hydrogen Sulphide Content in Agricultural Waste during Biogas Production. Sustainability 2024, 16, 5145. [Google Scholar] [CrossRef]
- Vu, H.P.; Nguyen, L.N.; Wang, Q.; Ngo, H.H.; Liu, Q.; Zhang, X.; Nghiem, L.D. Hydrogen sulphide management in anaerobic digestion: A critical review on input control, process regulation, and post-treatment. Bioresour. Technol. 2022, 346, 126634. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Chaudhary, D.K.; Dahal, R.H.; Trinh, N.H.; Kim, J.; Chang, S.W.; Hong, Y.; La, D.D.; Nguyen, X.C.; Ngo, H.H.; et al. Review on pretreatment techniques to improve anaerobic digestion of sewage sludge. Fuel 2021, 285, 119105. [Google Scholar] [CrossRef]
- Martín, M.A.; Serrano, A.; Rincón, B.; Gutiérrez, M.C.; Amil-Ruiz, F.; Barbudo-Lunar, M.; Alhama, J.; Michán, C.; Siles, J.A. Biomethanisation of sewage sludge: Sonication pretreatment and monitoring of microbial communities. Environ. Technol. Innov. 2024, 36, 103750. [Google Scholar] [CrossRef]
- Zielinski, M.; Zielinska, M.; Cydzik-Kwiatkowska, A.; Rusanowska, P.; Debowski, M. Effect of static magnetic field on microbial community during anaerobic digestion. Bioresour. Technol. 2021, 323, 124600. [Google Scholar] [CrossRef] [PubMed]
- Di Costanzo, N.; Di Capua, F.; Cesaro, A.; Mascolo, M.C.; Pirozzi, F.; Esposito, G. Impact of high-intenisty static magnetic field on chemical properties and anaerobic digestion of sewage sludge. Waste Biomass Valori. 2023, 14, 2469–2479. [Google Scholar] [CrossRef]
- Sarker, S.; Lamb, J.J.; Hjelme, D.R.; Lien, K.M. A Review of the Role of Critical Parameters in the Design and Operation of Biogas Production Plants. Appl. Sci. 2019, 9, 1915. [Google Scholar] [CrossRef]
- Issahaku, M.; Derkyi, N.S.A.; Kemausuor, F. A systematic review of the design considerations for the operation and maintenance of small-scale biogas digesters. Heliyon 2024, 10, e24019. [Google Scholar] [CrossRef]
- Hajji, A.; Rhachi, M.; Garoum, M.; Laaroussi, N. The effects of pH, temperature and agitation on biogas production under mesophilic regime. In Proceedings of the 2016 3rd International Conference on Renewable Energies for Developing Countries (REDEC), Zouk Mosbeh, Lebanon; 2016; pp. 1–4. [Google Scholar] [CrossRef]
- Elsayed, A.; Kakar, F.L.; Abdelrahman, M.A.; Ahmed, N.; AlSayed, A.; Zagloul, M.S.; Muller, C.; Bell, K.Y.; Santoro, D.; Norton, J.; et al. Enhancing anaerobic digestion Efficiency: A comprehensive review on innovative intensification technologies. Energy Convers. Manag. 2024, 320, 118979. [Google Scholar] [CrossRef]
- Mutegoa, E.; Sahini, M.G. Approaches to mitigation of hydrogen sulfide during anaerobic digestion process—A review. Heliyon 2023, 9, e19768. [Google Scholar] [CrossRef]
- Wang, R.; Al-Dhabi, N.A.; Jiang, Y.; Dai, X.; Li, R.; Tang, W.; Guo, R.; Fu, S. Effect of nano zero-valent iron on the anaerobic digestion of food waste: Performance and mechanism. Fuel 2024, 366, 131342. [Google Scholar] [CrossRef]
- He, C.-S.; He, P.-P.; Yang, H.-Y.; Li, L.-L.; Lin, Y.; Mu, Y.; Yu, H.-Q. Impact of zero-valent iron nanoparticles on the activity of anaerobic granular sludge: From macroscopic to microscopic investigation. Water Res. 2017, 127, 32–40. [Google Scholar] [CrossRef]
- Xiang, Y.; Yang, Z.; Zhang, Y.; Xu, R.; Zheng, Y.; Hu, J.; Li, X.; Jia, M.; Xiong, W.; Cao, J. Influence of nanoscale zero-valent iron and magnetite nanoparticles on aanaerobic digestion performance and macrolide, aminoglycoside, β-lactam resistance genes reduction. Bioresour. Technol. 2019, 294, 122139. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, D.; Fang, H. Comparison of enhancement of anaerobic digestion of waste activated sludge through adding nano-zero valent iron and zero valent iron. RSC Adv. 2018, 48, 27181–27190. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, J.; Hu, Z. Impact of nano zero valent iron (NZVI) on methanogenic activity and population dynamics in anaerobic digestion. Water Res. 2013, 47, 6790–6800. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Quan, X.; Chen, S. Enhanced anaerobic digestion of waste activated sludge digestion by the addition of zero valent iron. Water Res. 2014, 52, 242–250. [Google Scholar] [CrossRef]
- Dauknys, R.; Mažeikienė, A. Process Improvement of Biogas Production from Sewage Sludge Applying Iron Oxides-Based Additives. Energies 2023, 16, 3285. [Google Scholar] [CrossRef]
- Elagroudy, S.; Radwan, A.G.; Banadda, N.; Mostafa, N.G.; Owusu, P.A.; Janajreh, I. Mathematical models comparison of biogas production from anaerobic digestion of microwave pretreated mixed sludge. Renew. Energy 2020, 155, 1009–1020. [Google Scholar] [CrossRef]
- Mihi, M.; Ouhammou, B.; Aggour, M.; Daouchi, B.; Naaim, S.; Kousksou, T. Modeling and forecasting biogas production from anaerobic digestion process for sustainable resource energy recovery. Heliyon 2024, 10, e38472. [Google Scholar] [CrossRef]
- Panaro, D.B.; Mattei, M.R.; Esposito, G.; Steyer, J.P.; Capone, F.; Frunzo, L. A modelling and simulation study of anaerobic digestion in plug-flow reactors. Commun. Nonlinear Sci. Numer. 2022, 105, 106062. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhu, J.; Xiao, Y.; Schrader, L.C.; Wu, S.X.; Robinson, N.A., Jr.; Wang, Z. Employing micro-aeration in anaerobic digestion of poultry litter and wheat straw: Batch kinetics and continuous performance. Bioresour. Technol. 2023, 368, 128351. [Google Scholar] [CrossRef]
- Zhang, L.; Lim, E.Y.; Loh, K.-C.; Ok, Y.S.; Lee, J.T.E.; Shen, Y.; Wang, C.-H.; Dai, Y.; Tong, Y.W. Biochar enhanced thermophilic anaerobic digestion of food waste and pilot-scale application. Energy Convers. Manag. 2020, 209, 112654. [Google Scholar] [CrossRef]
- Lizama, A.C.; Figueiras, C.C.; Pedreguera, A.Z.; Espinoza, J.E.R. Enhancing the performance and stability of the anaerobic digestion of sewage sludge by zero valent iron nanoparticles dosage. Bioresour. Technol. 2019, 275, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Cao, Z.; Zhang, M.; Ying, Z.; Ma, L. Zero-valent iron enhanced in-situ advanced anaerobic digestion for the removal of antibiotics and antibiotic resistance genes in sewage sludge. Sci. Total Environ. 2021, 754, 142077. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhou, Y.; You, X.; Zhang, H.; Gong, L.; Wang, J.; Zuo, T. Potential promotion of activated carbon supported nano zero-valent iron on anaerobic digestion of waste activated sludge. Environ. Technol. 2022, 43, 3538–3551. [Google Scholar] [CrossRef] [PubMed]
- Suanon, F.; Sun, Q.; Mama, D.; Li, J.; Dimon, B.; Yu, C.-P. Effect of nanoscale zero-valent iron and magnetite (Fe3O4) on the fate of metals during anaerobic digestion of sludge. Water Res. 2016, 88, 897–903. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 1995. [Google Scholar]
- Men, Y.; Zheng, L.; Zhang, L.; Li, Z.; Wang, X.; Zhou, X.; Cheng, S.; Bao, W. Effects of adding zero valent iron on the anaerobic digestion of cow manure and lignocellulose. Front. Bioeng. Biotechnol. 2020, 8, 590200. [Google Scholar] [CrossRef]
- Sapkaite, I.; Barrado, E.; Fdz-Polanco, F.; Perez-Elvira, S.I. Optimization of thermal hydrolysis process for sludge pre-treatment. J. Environ. Manag. 2017, 192, 25–30. [Google Scholar] [CrossRef]
- Lim, Y.F.; Chan, Y.J.; Hue, F.S.; Ng, S.C.; Hashma, H. Anaerobic co-digestion of palm oil mill effluent (POME) with decanter cake (DC): Effect of mixing ratio and kinetic study. Bioresour. Tech. Rep. 2021, 15, 100736. [Google Scholar] [CrossRef]
- Phan, H.N.Q.; Leu, J.H.; Nguyen, V.N.D. The combination of anaerobic digestion and electro-oxidation for efficient COD removal in beverage wastewater: Investigation of electrolytic cells. Sustainability 2023, 15, 5551. [Google Scholar] [CrossRef]
- Ovi, D.; Chang, S.W.; Wong, J.W.C.; Johravindar, D.; Varjani, S.; Jeung, J.H.; Chung, W.J.; Thirupathi, A. Effect of rice husk and palm tree-based biochar addition on the anaerobic digestion of food waste/sludge. Fuel 2022, 315, 123188. [Google Scholar] [CrossRef]
- Leite, V.D.; Ramos, R.O.; Lopes, W.S.; Araujo, M.C.U.; Almeida, V.E.; Oliveira, N.M.S.; Viriato, C.L. Kinetic modeling of anaerobic co-digestion of plant solid waste with sewage sludge: Synergistic influences of total solids and substrate particle size in biogas generation. Bioenergy Res. 2024, 17, 744–755. [Google Scholar] [CrossRef]
- Emebu, S.; Pecha, J.; Janacova, D. Review on anaerobic digestion models: Model classification & elaboration of process phenomena. Renew. Sustain. Energy Rev. 2022, 160, 112288. [Google Scholar] [CrossRef]
- Basinas, P.; Chamradova, K.; Rusin, J.; Kaldis, S.P. Anaerobic digestion performance and kinetics of biomass pretreated with various strains utilizing exponential and sigmoidal equation models. Renew. Eenrgy 2024, 235, 121390. [Google Scholar] [CrossRef]
- Ali, M.M.; Dia, N.; Bilal, B.; Ndongo, M. Theoretical models for prediction of methane production from anaerobic digestion: A critical review. Int. J. Phys. Sci. 2018, 13, 206–216. [Google Scholar] [CrossRef]
- Zhang, H.; An, D.; Cao, Y.; Tian, Y.; He, J. Modeling the methane production kinetics of anaerobic co-digestion of agricultural wastes using sigmoidal functions. Energies 2021, 14, 258. [Google Scholar] [CrossRef]
- Zahan, Z.; Othman, M.Z.; Muster, T.H. Anaerobic digestion/co-digestion kinetic potentials of different agro-industrial wastes: A comprehensive batch study for C/N optimisation. Waste Manag. 2018, 71, 663–674. [Google Scholar] [CrossRef]
- Pramanik, S.K.; Suja, F.B.; Porhemmat, M.; Pramanik, B.K. Performance and kinetic model of a single-stage anaerobic digestion system operated at different successive operating stages for the treatment of food waste. Processes 2019, 7, 600. [Google Scholar] [CrossRef]
- Roberts, S.; Mathaka, N.; Zeleke, M.A.; Nwaigwe, K.N. Comparative analysis of five kinetic models for prediction of methane yield. J. Inst. Eng. A 2023, 104, 335–342. [Google Scholar] [CrossRef]
- Murunga, S.I.; Were, F. Predicting microbial growth in anaerobic digester using Gompertz and logistic models. IRE J. 2019, 3, 2456–8880. [Google Scholar]
- Altaş, L. Inhibitory effect of heavy metals on methane-producing anaerobic granular sludge. J. Hazard. Mater. 2009, 162, 1551–1556. [Google Scholar] [CrossRef]
- Bedoić, R.; Špehar, A.; Puljko, J.; Čuček, L.; Ćosić, B.; Pukšec, T.; Duić, N. Opportunities and challanges: Experimental and kinetic analysis of anaerobic co-digestion of food waste and rendering industry streams for biogas production. Renew. Sustain. Energy Rev. 2020, 130, 109951. [Google Scholar] [CrossRef]
- Jijai, S.; Siripatana, C. Kinetic model of biogas production from co-digestion of thai rice noodle wastewater (khanomjeen) with chicken manure. Energy Procedia 2017, 138, 386–392. [Google Scholar] [CrossRef]
- Alrawashdeh, K.A.B.; Al-Sameraie, L.; Bsoul, A.A.; Khasawneh, A.; Al-Tabbal, J. Correlation between kinetic parameters, reactor performance, and biogas and methane potential of co-digestion and mono-digestion of active sludge and olive mill wastewater. Int. J. Low Carbon Technol. 2024, 19, 1501–1515. [Google Scholar] [CrossRef]
- Arora, V.; Mahla, S.K.; Leekha, R.S.; Dhir, A.; Lee, K.; Ko, H. Intervention of artificial neural network with an improved activation function to predict the performance and emission characteristics of a biogas powered dual fuel engine. Electronics 2021, 10, 584. [Google Scholar] [CrossRef]
- Lima, D.R.S.; Adarme, O.F.H.; Baêta, B.E.L.; Gurgel, L.V.A.; Aquino, S.F. Influence of different thermal pretreatments and inoculum selection on the biomethanation of sugarcane bagasse by solid-state anaerobic digestion: A kinetic analysis. Ind. Crops Prod. 2018, 111, 684–693. [Google Scholar] [CrossRef]
- Zaidi, A.A.; RuiZhe, F.; Shi, Y.; Khan, S.Z.; Mushtaq, K. Nanoparticles augmentation on biogas yield from microalgal biomass anaerobic digestion. Int. J. Hydrogen Energy 2018, 31, 14202–14213. [Google Scholar] [CrossRef]
- Xu, R.; Xu, S.; Zhang, L.; Florentino, A.P.; Yang, Z.; Liu, Y. Impact of zero valent iron on blackwater anaerobic digestion. Bioresour. Technol. 2019, 285, 121351. [Google Scholar] [CrossRef]
- Lizama, A.C.; Figueiras, C.C.; Herrera, R.R.; Pedreguera, A.Z.; Espinoza, J.E.R. Effects of ultrasonic pretreatment on the solubilization and kinetic study of biogas production from anaerobic digestion of waste activated sludge. Int. Biodeterior. Biodegrad. 2017, 123, 1–9. [Google Scholar] [CrossRef]
- Jia, T.; Wang, Z.; Shan, H.; Liu, Y.; Gong, L. Effect of nanoscale zero-valent iron on sludge anaerobic digestion. Resour. Conserv. Recy. 2017, 127, 190–195. [Google Scholar] [CrossRef]
- Zhou, J.; You, X.; Jis, T.; Niu, B.; Gong, L.; Yang, X.; Zhou, Y. Effect of nanoscale zero-valent iron on the change of sludge anaerobic digestion process. Environ. Technol. 2020, 41, 3199–3209. [Google Scholar] [CrossRef]
- Su, L.; Shi, X.; Guo, G.; Zhao, A.; Zhao, Y. Stabilization of sewage sludge in the presence of nanoscale zero-valent iron (nZVI): Abatement of odor and improvement of biogas production. J. Mater. Cycles Waste Manag. 2013, 15, 461–468. [Google Scholar] [CrossRef]
- Eljamal, O.; Eljamal, R.; Falyouna, O.; Maamoun, I.; Thompson, I.P. Exceptional contribution of iron nanoparticle and aloe vera biomass additives to biogas production from anaerobic digestion of waste sludge. Energy 2024, 302, 131761. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Quan, X. Zero-valent iron enhanced methanogenic activity in anaerobic digestion of waste activated sludge after heat and alkali pretreatment. Waste Manag. 2015, 38, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, Y.; Wang, X.; Zhang, G.; Zhao, Z. Synergic role of potassium ferrate pretreatment and zero valent iron for enhancement of waste activated sludge anaerobic digestion. Renew. Energy 2024, 235, 121387. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, L.; Wang, T.; Ren, J.; Cao, Y.; Zhou, S. Impacts of ferric chloride, ferrous chloride and solid retention time on the methane-producing and physicochemical characterization in high-solids sludge anaerobic digestion. Renew. Energy 2019, 139, 1290–1298. [Google Scholar] [CrossRef]
- Suanon, F.; Sun, Q.; Li, M.; Cai, X.; Zhang, Y.; Yan, Y.; Yu, C. Application of nanoscale zero valent iron and iron powder during sludge anaerobic digestion: Impact on methane yield and pharmaceutical and personal care products generation. J. Hazard. Mater. 2017, 321, 47–53. [Google Scholar] [CrossRef]
- Syaichurrozi, I.; Sumardiono, B.S. Predicting kinetic model of biogas production and biodegradability organic materials: Biogas production from vinasse at variation of COD/N ratio. Bioresour. Technol. 2013, 149, 390–397. [Google Scholar] [CrossRef]
- Gaur, R.Z.; Suthar, S. Anaerobic digestion of activated sludge, anaerobic granular sludge and cow dung with food waste for enhanced methane production. J. Clean. Prod. 2017, 164, 557–566. [Google Scholar] [CrossRef]
- D’Silva, T.C.; Isha, A.; Verma, S.; Shirsath, G.; Chandra, R.; Vijay, V.K.; Subbarao, P.M.V.; Kovacs, K.L. Anaerobic co-digestion of dry fallen leaves, fruit/vegetable wastes and cow dung without an active inoculum—a biomethane potential study. Bioresour. Technol. Rep. 2022, 19, 101189. [Google Scholar] [CrossRef]
- Erden, G.; Demir, O.; Filibeli, A. Disintegration of biological sludge: Effect of ozone oxidation and ultrasonic treatment on aerobic digestibility. Bioresour. Technol. 2010, 101, 8093–8098. [Google Scholar] [CrossRef]
- Park, B.; Ahn, J.H.; Kim, J.; Hwang, S. Use of microwave pretreatment for enhanced anaerobiosis of secondary sludge. Water Sci. Technol. 2004, 50, 17–23. [Google Scholar] [CrossRef]
- Deepanraj, B.; Sivasubramanian, V.; Jayaraj, S. Experimental and kinetic study on anaerobic digestion of food waste: The effect of total solids and pH. J. Renew. Sustain. Energy 2015, 7, 063104. [Google Scholar] [CrossRef]
- Shitophyta, L.M.; Arnita, A.; Wulansari, H.D. Evaluation and modelling of biogas production from batch anaerobic digestion of corn stover with oxalic acid. J. Agr. Eng. 2023, 69, 151–157. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Xu, R.; Xiang, Y.; Jis, M.; Hu, J.; Zheng, Y.; Xiong, W.; Cao, J. Enhanced mesophilic anaerobic digestion of waste sludge with the iron nanoparticles addition and kinetic analysis. Sci. Total Environ. 2019, 683, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.M.M.; Dastyar, W.; Meshref, M.N.A.; Maal-Bared, R.; Dhar, B.R. Low-temperature thermal hydrolysis of anaerobic digestion facility in wastewater treatment plant with primary sludge fermentation. Chem. Eng. J. 2021, 426, 130485. [Google Scholar] [CrossRef]
- Farghali, M.; Mohamed, I.M.A.; Hassan, D.; Iwasaki, M.; Yoshida, G.; Umetsu, K.; Ihara, I. Kinetic modeling of anaerobic co-digestion with glycerol: Implications for process stability and organic overloads. Biochem. Eng. J. 2023, 199, 109061. [Google Scholar] [CrossRef]
- Bakraoui, M.; Karouach, F.; Ouhammou, B.; Lahboubi, N.; Gnaoui, Y.E.; Kerrou, O.; Aggour, M.; Bari, H.E. Kinetics study of methane production from anaerobic digestion of sludge and wastewater recycled pulp and paper. IOP Conf. Ser. Mater. Sci. Eng. 2020, 946, 012009. [Google Scholar] [CrossRef]
- Bakhov, Z.K.; Korazbekov, K.U.; Lakhanova, K.M. The kinetics of methane production from co-digestion of cattle manure. Pak. J. Biol. Sci. 2014, 17, 1023–1029. [Google Scholar] [CrossRef]
- Grosser, A.; Grobelak, A.; Rorat, A.; Courtois, P.; Vandelbulcke, F.; Lemiere, S.; Guyoneaud, R.; Attard, E.; Celary, P. Effects of silver nanoparticles on performance of anaerobic digestion of sewage lsudge and asociated microbial communities. Renew. Energy 2021, 171, 1014–1025. [Google Scholar] [CrossRef]
- Pan, Y.; Zhi, Z.; Zhen, G.; Lu, X.; Bakonyi, P.; Li, Y.-Y.; Zhao, Y.; Banu, J.B. Synergistic effect and biodegradation of sewage sludge and food waste mesophilic anaerobic co-digestion and the underlying stimulation mechanisms. Fuel 2019, 253, 40–49. [Google Scholar] [CrossRef]
- Bakari, O.; Njau, K.N.; Noubactep, C. Effects of zero-valent iron on sludge and methane production in anaerobic digestion of domestic wastewater. Case Stud. Chem. Environ. Eng. 2023, 8, 100377. [Google Scholar] [CrossRef]
- Dong, D.; Aleta, P.; Zhao, X.; Choi, O.K.; Kim, S.; Lee, J.W. Effects of nanoscale zero valent iron (nZVI) concentration on the biochemical conversion of gaseous carbon dioxide (CO2) into methane (CH4). Bioresour. Technol. 2019, 275, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Mohammadrezaei, R.; Zareei, S.; Behroozi-Khazaei, N. Optimum mixing rate in biogas reactors: Energy balance calculations and computational fluid dynamics simulation. Energy 2018, 159, 54–60. [Google Scholar] [CrossRef]
- Stafford, D.A. The effects of mixing and volatile fatty acid concentrations on anaerobic digester performance. Biomass 1982, 2, 43–55. [Google Scholar] [CrossRef]
- Ghanimesh, S.; Fadel, M.E.; Saikaly, P. Mixing effect on thermophilic anaerobic digestion of source-sorted organic fraction of municipal solid waste. Bioresour. Technol. 2012, 117, 63–71. [Google Scholar] [CrossRef]
- Neuner, T.; Meister, M.; Pillei, M.; Senfter, T.; Draxl-Weiskopf, S.; Ebner, C.; Winkler, J.; Rauch, W. Impact of design and mixing strategies on biogas production in anaerobic digesters. Water 2024, 16, 2205. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, G.; Yu, L.; Siddhu, M.A.H.; Gao, M.; Abdeltawab, A.A.; Al-Deyab, S.S.; Chen, X. Computational fluid dynamics study on mixing mode and power concumption in anaerobic mono- and co-digestion. Bioresour. Technol. 2016, 203, 166–172. [Google Scholar] [CrossRef]
- Terashima, M.; Goel, R.; Komatsu, K.; Yasui, H.; Takahashi, H.; Li, Y.Y.; Noike, T. CFD simulation of mixing in anaerobic digesters. Bioresour. Technol. 2009, 100, 2228–2233. [Google Scholar] [CrossRef]
- Gebreeyessus, G.D.; Jenicek, P. Thermophilic versus mesophilic anaerobic digestion of sewage sludge: A comparative review. Bioengineered 2016, 3, 15. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.; Pang, L.; Chatzisymeon, E.; Lu, Y.; Yang, P. Using metagenomics to reveal the effects of zero-valent iron with different sizes on the mesophilic and thermophilic anaerobic digestion of sludge. Biochem. Eng. J. 2025, 217, 109688. [Google Scholar] [CrossRef]
- Song, Y.-C.; Kwon, S.-J.; Woo, J.H. Mesophilic and thermophilic temperature co-phase anaerobic digestion compared with single-stage mesophilic- and thermophilic digestion of sewage sludge. Water Res. 2004, 38, 1653–1662. [Google Scholar] [CrossRef]
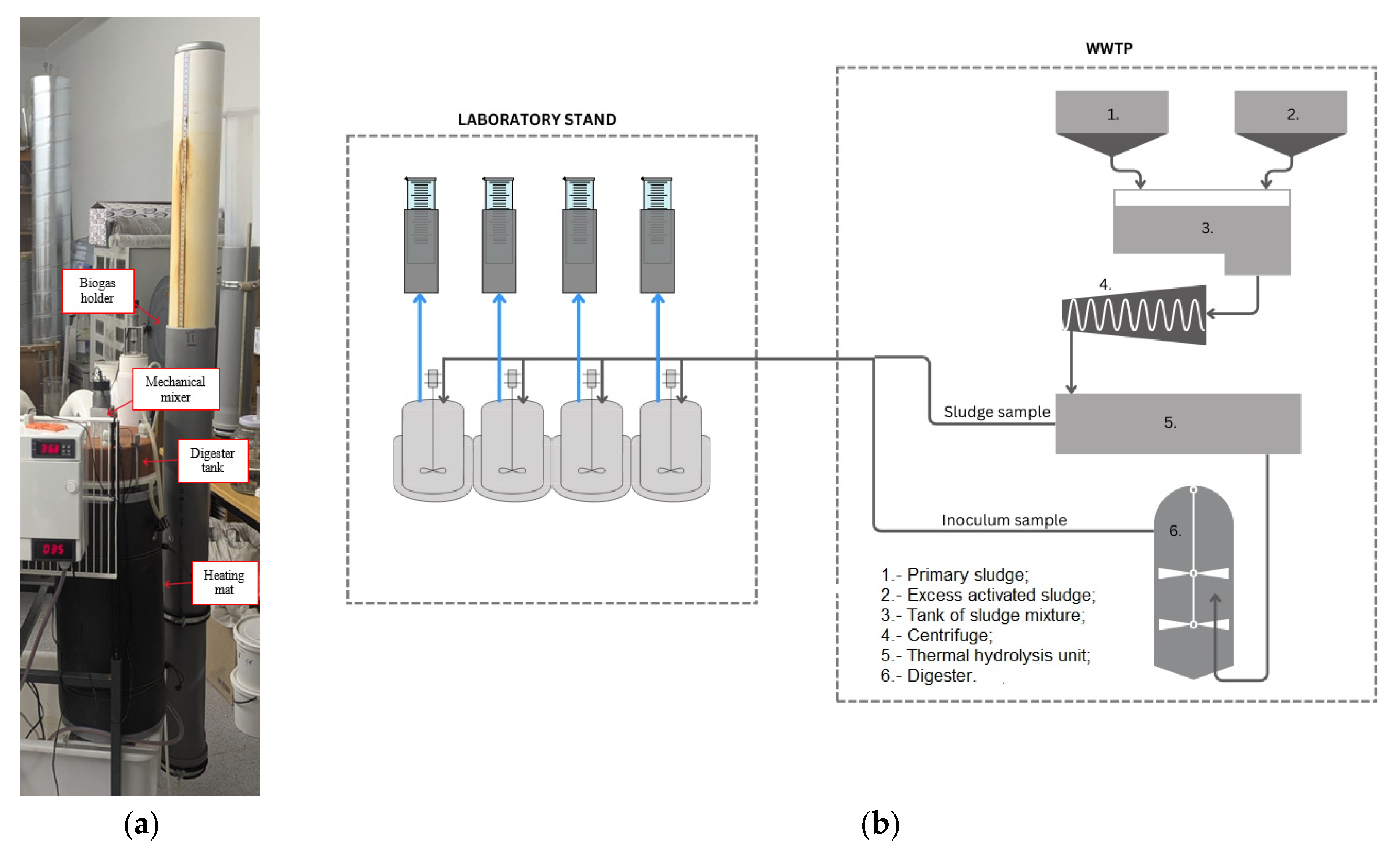


| Parameters | Substrate | Inoculum | Mixed Sludge (SS + Inoc.) |
|---|---|---|---|
| Total solids (TS, %) | 8.52 ± 0.03 | 4.29 ± 0.03 | 7.33 ± 0.06 |
| Volatile solids (VS, %) | 5.14 ± 0.02 | 2.53 ± 0.02 | 4.39 ± 0.04 |
| VS/TS (%) | 60.27 | 58.96 | 59.86 |
| pH | 5.41 ± 0.11 | 7.69 ± 0.15 | 6.96 ± 0.003 |
| Chemical oxygen demand (COD, g/L) | 82.967 ± 0.586 | 35.533 ± 0.208 | 70.967 ± 0.252 |
| Reactor | Added Substrate Amount (kg) | Added Inoculum Amount (kg) | Added nZVI Amount (g) |
|---|---|---|---|
| B1 | 10.5 | 3.5 | 0 |
| B2 | 5 | ||
| B3 | 15.1 | ||
| B4 | 30.2 |
| Parameter | B1 | B2 | B3 | B4 |
|---|---|---|---|---|
| Max. CH4 content (%) | 76.6 (day 28th) | 75.3 (day 25th) | 73.5 (day 23rd) | 74.05 (day 20th) |
| Avg. CH4 content during steady period (%) | 69.91 | 71.48 | 71.75 | 71.57 |
| Cum. biogas yield (mL) | 206,317 | 211,493 | 266,412 | 256,428 |
| Cum. CH4 yield (mL) | 133,106 | 134,204 | 168,614 | 169,579 |
| Cum. specific biogas yield (mL/g-VSadded) | 336.08 | 344.51 | 416.42 | 391.24 |
| Cum. specific CH4 yield (mL/g-VSadded) | 217.19 | 218.97 | 263.99 | 258.73 |
| Biogas increase respect to the control (%) | - | 2.5083 | 23.905 | 16.413 |
| Model | Parameter | Treatment | |||
|---|---|---|---|---|---|
| Control | 0.5% nZVI | 1.5% nZVI | 3% nZVI | ||
| Gompertz | Pmax | 345.83 | 353.27 | 430.13 | 401.67 |
| r0 | 3.0782 | 2.0577 | 0.6374 | 1.0339 | |
| α | 0.1746 | 0.1676 | 0.1239 | 0.1454 | |
| R2 | 0.9979 | 0.9986 | 0.9991 | 0.9995 | |
| RMSE | 7.278 | 5.6728 | 5.6195 | 4.1385 | |
| NRMSE | 2.1656 | 1.6467 | 1.3495 | 1.0578 | |
| AIC | 172.73 | 151.79 | 151 | 125.31 | |
| Akaike’s weight | 5.5752 × 10−25 | 1.0729 × 10−16 | 7.59875 × 10−9 | 0.0001 | |
| Modified Gompertz | Pmax | 345.83 | 353.26 | 430.128 | 401.67 |
| Rmax | 22.22 | 21.784 | 19.602 | 21.484 | |
| λ | 10.704 | 8.9965 | 5.1534 | 6.6156 | |
| R2 | 0.9979 | 0.9986 | 0.9991 | 0.9995 | |
| RMSE | 7.278 | 5.6728 | 5.6195 | 4.1385 | |
| NRMSE | 2.1656 | 1.6467 | 1.3495 | 1.0578 | |
| AIC | 172.73 | 151.79 | 151 | 125.31 | |
| Akaike’s weight | 5.5752 × 10−25 | 1.0729 × 10−16 | 7.59875 × 10−9 | 0.0001 | |
| Richards | Pmax | 344.17 | 334.82 | 430.09 | 401.56 |
| Rmax | 1.831 | 18.688 | 0.0674 | 0.0625 | |
| v | 0.0311 | 0.6511 | 0.0013 | 0.0011 | |
| λ | 10.859 | 9.3603 | 5.1548 | 6.6159 | |
| R2 | 0.9979 | 0.9999 | 0.9991 | 0.9995 | |
| RMSE | 7.0951 | 2.3556 | 5.6317 | 4.1456 | |
| NRMSE | 2.1112 | 0.6838 | 1.3524 | 1.0596 | |
| AIC | 172.59 | 79.972 | 153.19 | 127.45 | |
| Akaike’s weight | 5.9794 × 10−25 | 0.4223 | 2.54208 × 10−9 | 4.40391 × 10−5 | |
| Modified Richards | Pmax | 334.57 | 334.82 | 431.33 | 401.53 |
| P0 | 20.198 | 16.197 | 0.4236 | 0.3667 | |
| Rmax | 1.0109 | 0.6509 | 0.0044 | 0.0041 | |
| ν | 3.1259 | 3.3468 | 0.1027 | 0.0916 | |
| λ | 11.296 | 9.3602 | 5.1192 | 6.6182 | |
| R2 | 0.9999 | 0.9999 | 0.9989 | 0.9995 | |
| RMSE | 1.9359 | 2.3556 | 5.6777 | 4.1649 | |
| NRMSE | 0.5854 | 0.6838 | 1.3634 | 1.0645 | |
| AIC | 66.84 | 81.972 | 155.87 | 129.84 | |
| Akaike’s weight | 0.0549 | 0.1554 | 6.65634 × 10−10 | 1.33308 × 10−5 | |
| Logistic | Pmax | 334.65 | 341.81 | 409.4 | 386.44 |
| k | 0.2714 | 0.2611 | 0.1973 | 0.2289 | |
| t0 | 18.656 | 17.278 | 16.116 | 16.119 | |
| R2 | 0.9999 | 0.9999 | 0.9942 | 0.9955 | |
| RMSE | 1.9683 | 3.2779 | 13.342 | 11.117 | |
| NRMSE | 0.5857 | 0.9515 | 3.2039 | 2.8416 | |
| AIC | 62.881 | 105.73 | 223.64 | 208.32 | |
| Akaike’s weight | 0.3978 | 1.07736 × 10−6 | 1.27987 × 10−24 | 1.21099 × 10−22 | |
| Modified logistic | Pmax | 334.65 | 341.81 | 409.4 | 386.44 |
| Rmax | 22.709 | 22.312 | 20.196 | 22.113 | |
| λ | 11.288 | 9.6187 | 5.9804 | 7.3813 | |
| R2 | 0.9999 | 0.9999 | 0.9942 | 0.9955 | |
| RMSE | 1.9683 | 3.2779 | 13.342 | 11.117 | |
| NRMSE | 0.5857 | 0.9515 | 3.2039 | 2.8416 | |
| AIC | 62.881 | 105.73 | 223.64 | 208.32 | |
| Akaike’s weight | 0.3978 | 1.07736 × 10−6 | 1.27987 × 10−24 | 1.21099 × 10−22 | |
| Cone | Pmax | 349.55 | 359.909 | 473.5 | 422.93 |
| k | 0.0536 | 0.0578 | 0.0579 | 0.0608 | |
| n | 4.6672 | 4.0764 | 2.4466 | 3.0501 | |
| R2 | 0.9983 | 0.9987 | 0.9996 | 0.9997 | |
| RMSE | 6.3389 | 5.3697 | 3.9316 | 4.1621 | |
| NRMSE | 1.8862 | 1.5587 | 0.9441 | 1.0638 | |
| AIC | 161.12 | 147.18 | 120.99 | 125.79 | |
| Akaike’s weight | 1.8507 × 10−22 | 1.07549 × 10−15 | 0.0249 | 0.0001 | |
| Schnute | Pmax | 346.14 | 354.08 | 442.83 | 406.83 |
| r0 | 253.84 | 157.39 | 256.25 | 367.07 | |
| α | 0.1713 | 0.1619 | 0.1022 | 0.1302 | |
| β | 0.0612 | 0.0923 | 0.298 | 0.1902 | |
| R2 | 0.9977 | 0.9981 | 0.9997 | 0.9999 | |
| RMSE | 7.7492 | 6.4129 | 3.5178 | 3.2649 | |
| NRMSE | 2.3058 | 1.8615 | 0.8448 | 0.8345 | |
| AIC | 179.99 | 164.09 | 113.66 | 107.39 | |
| Akaike’s weight | 1.4783 × 10−26 | 2.28901 × 10−19 | 0.975035017 | 0.9996 | |
| Model | Parameter | Treatment | |||
|---|---|---|---|---|---|
| Control | 0.5% nZVI | 1.5% nZVI | 3% nZVI | ||
| Gompertz | Pmax | 221.58 | 222.49 | 272.4 | 264.03 |
| r0 | 5.2887 | 4.0257 | 0.8842 | 1.3165 | |
| α | 0.1918 | 0.1893 | 0.1341 | 0.1585 | |
| R2 | 0.9999 | 0.9999 | 0.9997 | 0.9998 | |
| RMSE | 2.8299 | 2.2328 | 3.2303 | 2.5219 | |
| NRMSE | 1.3029 | 1.0197 | 1.2236 | 0.9747 | |
| AIC | 93.382 | 73.474 | 104.49 | 83.701 | |
| Akaike’s weight | 4.51867 × 10−14 | 5.90669 × 10−6 | 1.16604 × 10−5 | 0.0035 | |
| Modified Gompertz | Pmax | 221.58 | 222.49 | 272.39 | 264.03 |
| Rmax | 15.64 | 15.495 | 13.439 | 15.401 | |
| λ | 12.077 | 10.867 | 6.6088 | 7.0439 | |
| R2 | 0.9999 | 0.9999 | 0.9997 | 0.9998 | |
| RMSE | 2.8299 | 2.2328 | 3.2303 | 2.5219 | |
| NRMSE | 1.3029 | 1.0197 | 1.2236 | 0.9747 | |
| AIC | 93.382 | 73.474 | 104.49 | 83.701 | |
| Akaike’s weight | 4.51867 × 10−14 | 5.90669 × 10−6 | 1.16604 × 10−5 | 0.0035 | |
| Richards | Pmax | 217.64 | 219.88 | 272.85 | 264.1 |
| Rmax | 11.831 | 9.1118 | 0.3392 | 0.0488 | |
| v | 0.5096 | 0.3312 | 0.0094 | 0.0012 | |
| λ | 12.305 | 11.01 | 6.5779 | 7.0355 | |
| R2 | 0.9999 | 0.9999 | 0.9996 | 0.9998 | |
| RMSE | 1.3621 | 1.6781 | 3.3011 | 2.5263 | |
| NRMSE | 0.6271 | 0.7664 | 1.2504 | 0.9764 | |
| AIC | 33.959 | 51.484 | 108.32 | 85.848 | |
| Akaike’s weight | 0.3619 | 0.3519 | 1.71807 × 10−6 | 0.0012 | |
| Modified Richards | Pmax | 217.41 | 220.13 | 272.29 | 263.92 |
| P0 | 11.548 | 7.5148 | 0.0835 | 0.0394 | |
| Rmax | 0.5388 | 0.2914 | 0.0008 | 0.0004 | |
| ν | 2.9558 | 3.3062 | 0.0889 | 0.0841 | |
| λ | 12.344 | 10.975 | 6.6143 | 7.0233 | |
| R2 | 0.9999 | 0.9999 | 0.9997 | 0.9998 | |
| RMSE | 1.3539 | 1.6665 | 3.2348 | 2.5262 | |
| NRMSE | 0.6234 | 0.7611 | 1.2253 | 0.9764 | |
| AIC | 35.451 | 52.9 | 108.61 | 87.843 | |
| Akaike’s weight | 0.1716 | 0.1734 | 1.48617 × 10−6 | 0.0004 | |
| Logistic | Pmax | 215.01 | 216.19 | 260.15 | 255.48 |
| k | 0.2979 | 0.2918 | 0.2137 | 0.2473 | |
| t0 | 19.364 | 18.255 | 16.799 | 15.835 | |
| R2 | 0.9999 | 0.9999 | 0.9979 | 0.9982 | |
| RMSE | 2.2344 | 3.4573 | 7.9469 | 6.9838 | |
| NRMSE | 1.0288 | 1.5789 | 3.0103 | 2.6992 | |
| AIC | 73.534 | 110.2 | 180.11 | 169.26 | |
| Akaike’s weight | 9.22464 × 10−10 | 6.25741 × 10−14 | 4.4263 × 10−22 | 9.10726 × 10−22 | |
| Modified logistic | Pmax | 215.01 | 216.19 | 260.15 | 255.48 |
| Rmax | 16.015 | 15.771 | 13.897 | 15.793 | |
| λ | 12.651 | 11.401 | 7.4396 | 7.7469 | |
| R2 | 0.9999 | 0.9999 | 0.9979 | 0.9982 | |
| RMSE | 2.2344 | 3.4573 | 7.9469 | 6.9838 | |
| NRMSE | 1.0288 | 1.5789 | 3.0103 | 2.6992 | |
| AIC | 73.534 | 110.2 | 180.11 | 169.26 | |
| Akaike’s weight | 9.22464 × 10−10 | 6.25741 × 10−14 | 4.4263 × 10−22 | 9.10726 × 10−22 | |
| Cone | Pmax | 222.87 | 224.7 | 290.63 | 274.33 |
| k | 0.0517 | 0.0549 | 0.0574 | 0.0627 | |
| n | 5.3314 | 4.896 | 2.888 | 3.334 | |
| R2 | 0.9999 | 0.9999 | 0.9998 | 0.9999 | |
| RMSE | 2.1576 | 1.9209 | 2.9226 | 2.8108 | |
| NRMSE | 0.9934 | 0.8772 | 1.1071 | 1.0864 | |
| AIC | 70.597 | 60.833 | 96.087 | 92.81 | |
| Akaike’s weight | 4.006 × 10−9 | 0.0032 | 0.0008 | 3.63324 × 10−5 | |
| Schnute | Pmax | 221.79 | 222.88 | 277.34 | 266.27 |
| r0 | 359.42 | 282.69 | 288.49 | 367.28 | |
| α | 0.1889 | 0.1849 | 0.1176 | 0.1456 | |
| β | 0.0384 | 0.0513 | 0.2121 | 0.1552 | |
| R2 | 0.9999 | 0.9999 | 0.9999 | 0.9999 | |
| RMSE | 2.9999 | 2.4217 | 2.4067 | 2.1522 | |
| NRMSE | 1.3813 | 1.1059 | 0.9117 | 0.8318 | |
| AIC | 100.28 | 82.294 | 81.773 | 72.384 | |
| Akaike’s weight | 1.43592 × 10−15 | 7.17968 × 10−8 | 0.9992 | 0.9902 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usevičiūtė, L.; Januševičius, T.; Danila, V.; Mažeikienė, A.; Zagorskis, A.; Pranskevičius, M.; Marčiulaitienė, E. Performance and Kinetics of Anaerobic Digestion of Sewage Sludge Amended with Zero-Valent Iron Nanoparticles, Analyzed Using Sigmoidal Models. Energies 2025, 18, 1425. https://doi.org/10.3390/en18061425
Usevičiūtė L, Januševičius T, Danila V, Mažeikienė A, Zagorskis A, Pranskevičius M, Marčiulaitienė E. Performance and Kinetics of Anaerobic Digestion of Sewage Sludge Amended with Zero-Valent Iron Nanoparticles, Analyzed Using Sigmoidal Models. Energies. 2025; 18(6):1425. https://doi.org/10.3390/en18061425
Chicago/Turabian StyleUsevičiūtė, Luiza, Tomas Januševičius, Vaidotas Danila, Aušra Mažeikienė, Alvydas Zagorskis, Mantas Pranskevičius, and Eglė Marčiulaitienė. 2025. "Performance and Kinetics of Anaerobic Digestion of Sewage Sludge Amended with Zero-Valent Iron Nanoparticles, Analyzed Using Sigmoidal Models" Energies 18, no. 6: 1425. https://doi.org/10.3390/en18061425
APA StyleUsevičiūtė, L., Januševičius, T., Danila, V., Mažeikienė, A., Zagorskis, A., Pranskevičius, M., & Marčiulaitienė, E. (2025). Performance and Kinetics of Anaerobic Digestion of Sewage Sludge Amended with Zero-Valent Iron Nanoparticles, Analyzed Using Sigmoidal Models. Energies, 18(6), 1425. https://doi.org/10.3390/en18061425






