Advanced Electrochemical Performance of NiWO4/Graphene Oxide as Cathode Material for Zinc Ion Battery
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of NiWO4
2.2. Preparation of NiWO4/Graphene Oxide
2.3. Preparation of Zn//NiWO4/Graphene Oxide Button Batteries
2.4. Electrochemical Experiments
3. Results and Discussions
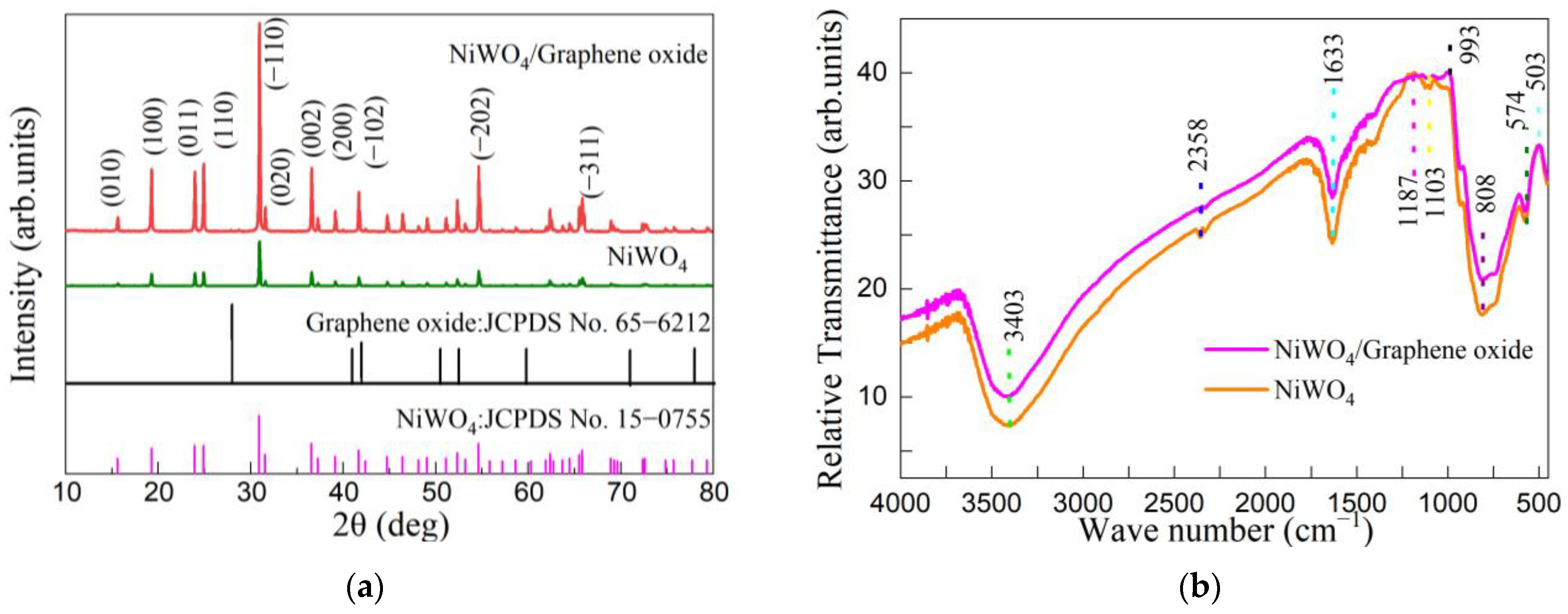
3.1. Phase Structure and FTIR Analysis
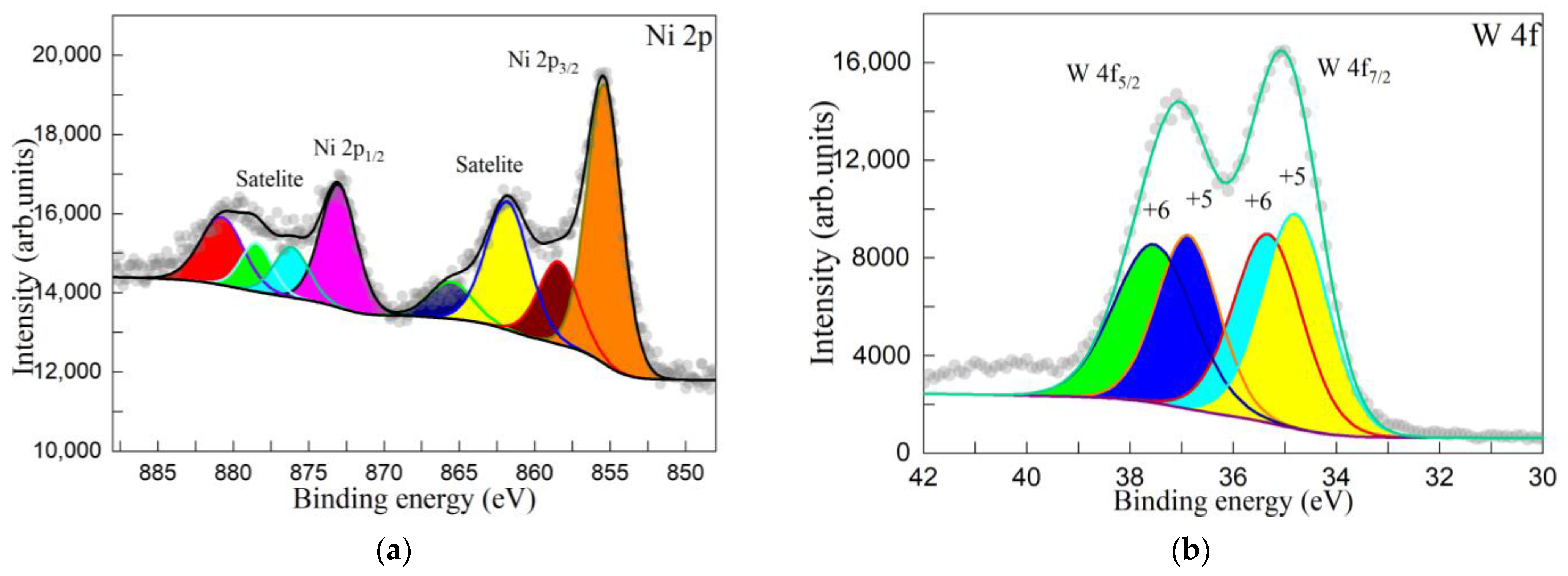
3.2. Microstructural Analysis
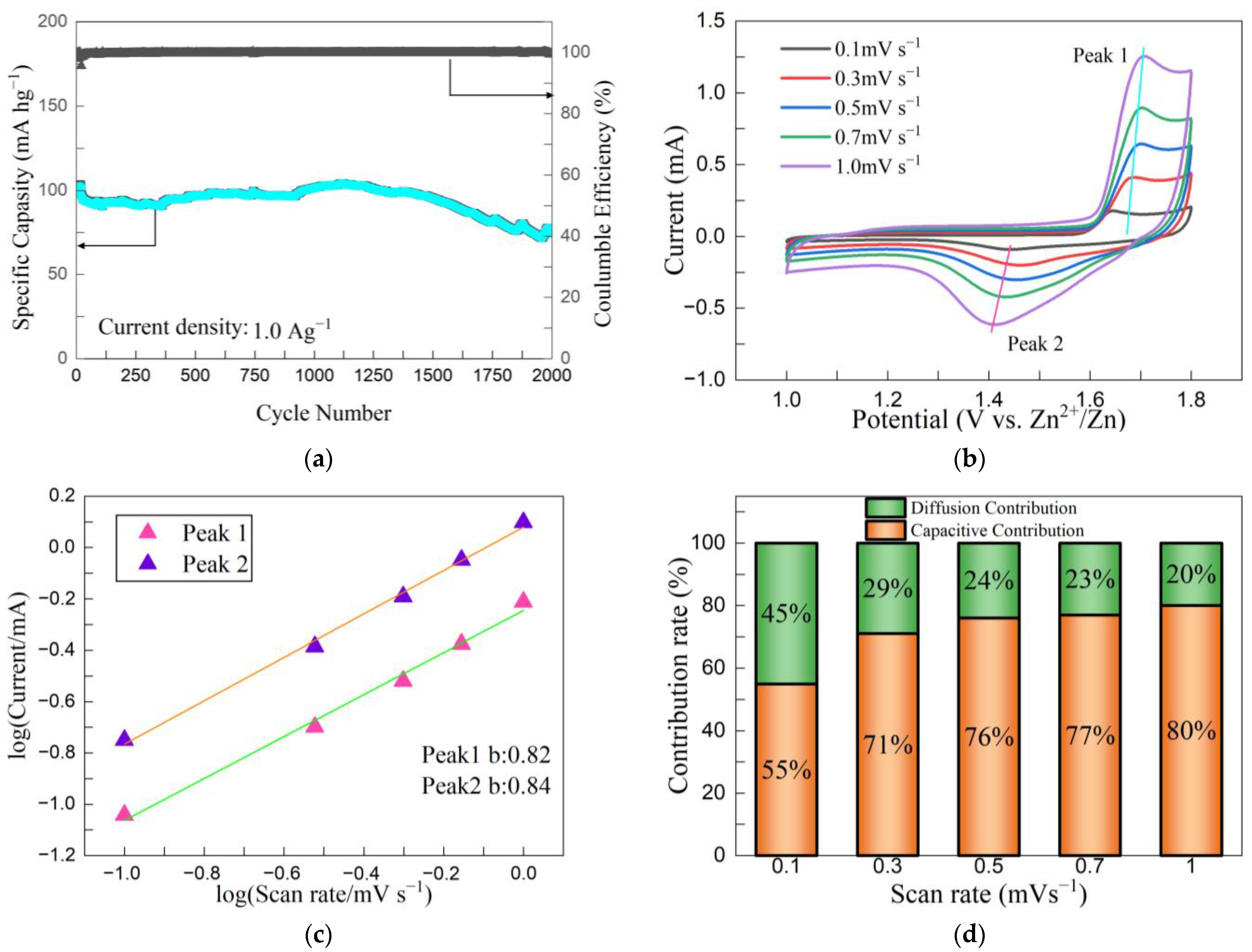
3.3. Electrochemical Testing
3.4. Battery Performance
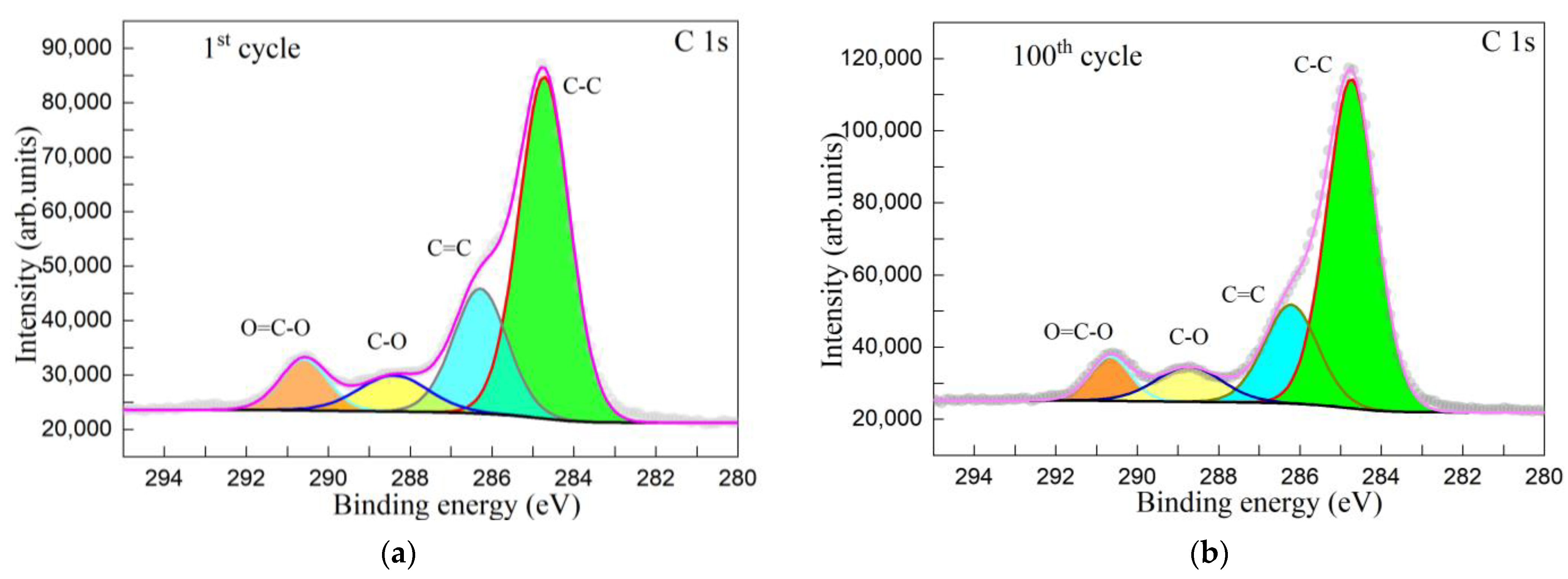
3.5. Battery Cycle Stability
4. Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Toman, M.A.; Jemelkova, B. Energy and economic development: An assessment of the state of knowledge. Energy J. 2003, 24, 93–112. [Google Scholar] [CrossRef]
- Dincer, I. Renewable energy and sustainable development: A crucial review. Renew. Sustain. Energy Rev. 2000, 4, 157–175. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, X.; Yu, M.; Niu, Z.; Cheng, F.; Chen, J. Materials chemistry for rechargeable zinc-ion batteries. Chem. Soc. Rev. 2020, 49, 4203–4219. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Hong, E.; Yan, T.; Fang, X. Effective Dual Cation Release in Quasi-2D Perovskites for Ultrafast UV Light-Powered Imaging. Adv. Mater. 2025, 37, 2412014. [Google Scholar] [CrossRef] [PubMed]
- Blomgren, G.E. The development and future of lithium ion batteries. J. Electrochem. Soc. 2016, 164, A5019. [Google Scholar] [CrossRef]
- Xie, J.; Lu, Y.C. A retrospective on lithium-ion batteries. Nat. Commun. 2020, 11, 2499. [Google Scholar] [CrossRef]
- Tang, B.; Shan, L.; Liang, S.; Zhou, J. Issues and opportunities facing aqueous zinc-ion batteries. Energy Environ. Sci. 2019, 12, 3288–3304. [Google Scholar] [CrossRef]
- Wang, F.; Borodin, O.; Gao, T.; Fan, X.; Sun, W.; Han, F.; Faraone, A.; Dura, J.A.; Xu, K.; Wang, C. Highly reversible zinc metal anode for aqueous batteries. Nat. Mater. 2018, 17, 543–549. [Google Scholar] [CrossRef]
- Ruan, P.; Liang, S.; Lu, B.; Fan, H.J.; Zhou, J. Design strategies for high-energy-density aqueous zinc batteries. Angew. Chem. 2022, 134, e202200598. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, Y.; Shi, L.; Wang, K.; Wang, B.; Li, L.; Ma, Y.; Li, Y.; Sun, Z.; Ali, W.; et al. An overview and future perspectives of rechargeable zinc batteries. Small 2020, 16, 2000730. [Google Scholar] [CrossRef]
- Xu, X.; Pei, L.; Yang, Y.; Shen, J.; Ye, M. Facile synthesis of NiWO4/reduced graphene oxide nanocomposite with excellent capacitive performance for supercapacitors. J. Alloys Compd. 2016, 654, 23–31. [Google Scholar] [CrossRef]
- Kumar, R.; Gupta, P.K.; Agrawal, A.; Nagarale, R.K.; Sharma, A. Hydrothermally synthesized reduced graphene oxide-NiWO4 nanocomposite for lithium-ion battery anode. J. Electrochem. Soc. 2017, 164, A785. [Google Scholar] [CrossRef]
- Xing, X.; Wang, J. Reduced graphene oxide incorporated NiWO4 for high-performance energy storage. J. Mater. Sci. Mater. Electron. 2016, 27, 11613–11622. [Google Scholar] [CrossRef]
- Hussain, D.; Khan, S.A.; Khan, T.A. Fabrication and characterization of mesoporous guar gum/NiWO4 nanocomposite for efficient adsorption of phloxine B and crystal violet from aqueous solution and evaluation of its antioxidant activity. Colloid Interface Sci. Commun. 2021, 44, 100488. [Google Scholar] [CrossRef]
- Wu, F.; Gao, X.; Xu, X.; Jiang, Y.; Gao, X.; Yin, R.; Cao, X. MnO2 nanosheet-assembled hollow polyhedron grown on carbon cloth for flexible aqueous zinc-ion batteries. ChemSusChem 2020, 13, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Yang, L.; Ren, X.; Cui, G.; Xiong, X.; Sun, X. Full water splitting electrocatalyzed by NiWO4 nanowire array. ACS Sustain. Chem. Eng. 2018, 6, 9555–9559. [Google Scholar] [CrossRef]
- Ming, F.; Liang, H.; Lei, Y.; Kandambeth, S.; Eddaoudi, M.; Alshareef, H.N. Layered MgxV2O5· n H2O as cathode material for high-performance aqueous zinc ion batteries. ACS Energy Lett. 2018, 3, 2602–2609. [Google Scholar] [CrossRef]
- Wang, Z.; Mao, Z.; Lai, L.; Okubo, M.; Song, Y.; Zhou, Y.; Liu, X.; Huang, W. Sub-micron silicon/pyrolyzed carbon@ natural graphite self-assembly composite anode material for lithium-ion batteries. Chem. Eng. J. 2017, 313, 187–196. [Google Scholar] [CrossRef]
- Botsa, S.M.; Babu, M.J.; Suresh, P.; Kalyani, P.; Venkateswararao, B.; Muralikrishna, R. Spherical NiWO4-reduced graphene oxide nanocomposite for effective visible light driven photocatalytic activity for the decolourisation of organic pollutants. Arab. J. Chem. 2020, 13, 8489–8497. [Google Scholar] [CrossRef]
- Yu, X.; Wang, S.; Zhang, Y.; Yu, X.; Gao, H.; Yang, H.; Fang, L.; Zhang, H.; Syed, A. Utilization of stable and efficient high-entropy (Ni0.2Zn0.2Mg0.2Cu0.2Co0.2)Al2O4 catalyst with polyvalent transition metals to boost peroxymonosulfate activation toward pollutant degradation. Small 2025, 21, 2410819. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Han, Y.; Yu, X.; Deng, L.; Hu, L.; Gao, H.; Jagadeesha, A.V.; Shaikh, S.F.; Ubaidullah, M. Double internal magnetic fields significantly improve photocatalytic activity over Ba0.5Sr0.5TiO3/BaFe12O19/SrFe12O19 photocatalysts. Colloids Surf. A Physicochem. Eng. Asp. 2025, 705, 135608. [Google Scholar] [CrossRef]
- Yu, X.; Wang, S.; Zhang, Y.; Gao, H.; Zhou, X.; Li, D.; Yang, H.; Fang, L.; Zhang, H.; Syed, A. Novel high entropy alloy/NiAl2O4 photocatalysts for the degradation of tetracycline hydrochloride: Heterojunction construction, performance evaluation and mechanistic insights. Ceram. Int. 2024, 50, 29528–29546. [Google Scholar] [CrossRef]
- Wang, S.; Li, M.; Gao, H.; Yin, Z.; Chen, C.; Yang, H.; Fang, L.; Angadi, V.J.; Yi, Z.; Li, D. Construction of CeO2/YMnO3 and CeO2/MgAl2O4/YMnO3 photocatalysts and adsorption of dyes and photocatalytic oxidation of antibiotics: Performance prediction, degradation pathway and mechanism insight. Appl. Surf. Sci. 2023, 608, 154977. [Google Scholar] [CrossRef]
- Pourmasoud, S.; Sobhani-Nasab, A.; Behpour, M.; Rahimi-Nasrabadi, M.; Ahmadi, F. Investigation of optical properties and the photocatalytic activity of synthesized YbYO4 nanoparticles and YbVO4/NiWO4 nanocomposites by polymeric capping agents. J. Mol. Struct. 2018, 1157, 607–615. [Google Scholar] [CrossRef]
- AlShehri, S.M.; Ahmed, J.; Alzahrani, A.M.; Ahamad, T. Synthesis, characterization, and enhanced photocatalytic properties of NiWO4 nanobricks. New J. Chem. 2017, 41, 8178–8186. [Google Scholar] [CrossRef]
- Punetha, D.; Pandey, S.K. Enhancement and optimization in sensing characteristics of ammonia gas sensor based on light assisted nanostructured WO3 thin film. IEEE Sens. J. 2020, 20, 14617–14623. [Google Scholar] [CrossRef]
- Chavan, S.; Vitillo, J.G.; Gianolio, D.; Zavorotynska, O.; Civalleri, B.; Jakobsen, S.; Nilsen, M.H.; Valenzano, L.; Lamberti, C.; Lillerud, K.P. H2 storage in isostructural UiO-67 and UiO-66 MOFs. Phys. Chem. Chem. Phys. 2012, 14, 1614–1626. [Google Scholar] [CrossRef]
- Wang, X.; Lin, S.; Cui, N.; Qi, K.; Liu, S.-y.; Khan, I. Synthesis of ZnWO4/NiWO4 photocatalysts and their application in tetracycline hydrochloride degradation and antibacterial activities. J. Taiwan Inst. Chem. Eng. 2024, 157, 105408. [Google Scholar] [CrossRef]
- Zhu, J.; Li, W.; Li, J.; Li, Y.; Hu, H.; Yang, Y. Photoelectrochemical activity of NiWO4/WO3 heterojunction photoanode under visible light irradiation. Electrochim. Acta 2013, 112, 191–198. [Google Scholar] [CrossRef]
- Sadiq, M.M.; Bhat, D.K. Novel NiWO4-ZnO-NRGO Ternary Nanocomposites with Enhanced Photocatalytic Activity. Mater. Today Proceeding 2018, 5, 22412–22420. [Google Scholar] [CrossRef]
- Yadav, N.; Khamsanga, S.; Kheawhom, S.; Qin, J.; Pattananuwat, P. Mnco2o4 spinel microsphere assembled with flake structure as a cathode for high-performance zinc ion battery. J. Energy Storage 2023, 64, 107148. [Google Scholar] [CrossRef]
- Zhang, B.; Shang, X.; Jiang, Z.; Song, C.; Maiyalagan, T.; Jiang, Z.-J. Atmospheric-pressure plasma jet-induced ultrafast construction of an ultrathin nonstoichiometric nickel oxide layer with mixed Ni3+/Ni2+ ions and rich oxygen defects as an efficient electrocatalyst for oxygen evolution reaction. ACS Appl. Energy Mater. 2021, 4, 5059–5069. [Google Scholar] [CrossRef]
- Liu, Z.; Xi, M.; Li, G.; Huang, Y.; Mao, L.; Xu, J.; Wang, W.; Qi, Z.; Ding, J.; Zhang, S. Reviving Zn Dendrites to Electroactive Zn2+ by Ion Sieve Interface. Adv. Mater. 2025, 37, 2413677. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wang, L.; Gao, J.; Yan, T.; Li, H.; Mao, J.; Song, F.; Fedotov, S.; Chang, L.Y.; Li, N. Facile Zn2+ desolvation enabled by local coordination engineering for long-cycling aqueous zinc-ion batteries. Adv. Funct. Mater. 2023, 33, 2301648. [Google Scholar] [CrossRef]
- Kumar, S.; Goud, P.; Ghanem, M.A.; Gutturu, R.R.; Joo, S.W. Constructing graphene oxide-wrapped wolframite-type 3D cube-like CuWO4 composite as a promising redox-active electrode for battery-type supercapacitors. Ceram. Int. 2024, 50, 34270–34282. [Google Scholar]
- Murugesan, M.; Nagavenkatesh, K.; Devendran, P.; Nallamuthu, N.; Uthayakumar, T. Facile synthesis of Zn2V2O7/RGO nanocomposite for enhancing the electrochemical performance and evaluating the supercapacitor device. Inorg. Chem. Commun. 2024, 165, 112544. [Google Scholar] [CrossRef]
- Naskar, I.; Ghosal, P.; Deepa, M. Zinc-ion hybrid supercapacitor-batteries with a leaf-like ZIF-L/MgNiO2 micro-sphere composite and a Zn2+/sulfonated poly (ether ether ketone) gel. Sustain. Energy Fuels 2023, 7, 2627–2644. [Google Scholar] [CrossRef]
- Sun, Q.; Chang, L.; Liu, Y.; Nie, W.; Duan, T.; Xu, Q.; Cheng, H.; Lu, X. Chrysanthemum-like polyaniline-anchored PANI0. 22· V2O5· 0.88 H2O-hybridized cathode for high-stable aqueous zinc-ion batteries. ACS Appl. Energy Mater. 2023, 6, 3102–3112. [Google Scholar] [CrossRef]
- Xing, F.; Shen, X.; Chen, Y.; Liu, X.; Chen, T.; Xu, Q. A carbon-coated spinel zinc cobaltate doped with manganese and nickel as a cathode material for aqueous zinc-ion batteries. Dalton Trans. 2021, 50, 5795–5806. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Z.; Qin, H.; Zeng, X.; Meng, J. Advanced electrochemical performance of ZnMn2O4/N-doped graphene hybrid as cathode material for zinc ion battery. J. Power Sources 2019, 425, 162–169. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Mathew, V.; Gim, J.; Kim, S.; Song, J.; Baboo, J.P.; Choi, S.H.; Kim, J. Electrochemically induced structural transformation in a γ-MnO2 cathode of a high capacity zinc-ion battery system. Chem. Mater. 2015, 27, 3609–3620. [Google Scholar] [CrossRef]
- Ji, J.; Wan, H.; Zhang, B.; Wang, C.; Gan, Y.; Tan, Q.; Wang, N.; Yao, J.; Zheng, Z.; Liang, P.; et al. Co2+/3+/4+-Regulated electron state of Mn-O for superb aqueous zinc-manganese oxide batteries. Adv. Energy Mater. 2021, 11, 2003203. [Google Scholar] [CrossRef]
- Liu, Z.; Pulletikurthi, G.; Endres, F.A. prussian blue/zinc secondary battery with a bio-ionic liquid–water mixture as electrolyte. ACS Appl. Mater. Interfaces 2016, 8, 12158–12164. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Yan, M.; Zhu, T.; Wang, X.; Wei, X.; Li, J.; Zhou, L.; Li, Z.; Chen, L.; Mai, L. Zn/V2O5 aqueous hybrid-ion battery with high voltage platform and long cycle life. ACS Appl. Mater. Interfaces 2017, 9, 42717–42722. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Mou, J.; Zhang, J.; Dong, L.; Liu, W.; Xu, C.; Kang, F. Electrochemically induced spinel-layered phase transition of Mn3O4 in high performance neutral aqueous rechargeable zinc battery. Electrochim. Acta 2018, 259, 170–178. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Islam, S.; Putro, D.Y.; Mathew, V.; Kim, S.; Jo, J.; Kim, S.; Sun, Y.; Kim, K.; Kim, J. Structural transformation and electrochemical study of layered MnO2 in rechargeable aqueous zinc-ion battery. Electrochim. Acta 2018, 276, 1–11. [Google Scholar] [CrossRef]
- Lee, B.; Lee, H.R.; Kim, H.; Chung, K.Y.; Cho, B.W.; Oh, S.H. Elucidating the intercalation mechanism of zinc ions into α-MnO2 for rechargeable zinc batteries. Chem. Commun. 2015, 51, 9265–9268. [Google Scholar] [CrossRef]
- Bai, J.; Hu, S.; Feng, L.; Jin, X.; Wang, D.; Zhang, K.; Guo, X. Manganese vanadium oxide composite as a cathode for high-performance aqueous zinc-ion batteries. Chin. Chem. Lett. 2024, 35, 109326. [Google Scholar] [CrossRef]
- Tang, C.; Wang, X.; Ma, M.; Wang, Z.; Li, Y.; Li, H.; Li, B.; Zhang, Y.; Zhu, X. Optimizing the electrons/ions diffusion kinetics in δ-MnO2 for realizing an ultra-high rate-capability supercapacitor. Chem. Eng. J. 2023, 471, 144784. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, D.; Zhang, X.; Sawangphruk, M.; Qin, J.; Liu, R. A universal and facile approach to suppress dendrite formation for a Zn and Li metal anode. J. Mater. Chem. A 2020, 8, 9331–9344. [Google Scholar] [CrossRef]
- Yang, X.; Qiao, Z.; Liu, F.; Yang, S.; Zhang, L.; Cao, B. In-depth study of electrochemical capacitor performance of MnO2 during phase transition from Ramsdellite-MnO2 to Birnessite-MnO2. Electrochim. Acta 2018, 280, 77–85. [Google Scholar] [CrossRef]
- Yang, J.; Ju, Z.; Jiang, Y.; Xing, Z.; Xi, B.; Feng, J.; Xiong, S. Enhanced capacity and rate capability of nitrogen/oxygen dual-doped hard carbon in capacitive potassium-ion storage. Adv. Mater. 2018, 30, 1700104. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Huang, J.; Yi, J.; Liu, X.; Liu, Y.; Wang, Y.; Zhang, J.; Xia, Y. Recent advances in polymer electrolytes for zinc ion batteries: Mechanisms, properties, and perspectives. Adv. Energy Mater. 2020, 10, 1903977. [Google Scholar] [CrossRef]
- Xu, C.; Li, X.; Zhang, Y.; Yang, T.; Mao, H.; Zhang, Q.; Zhou, X.; Zhang, S.; Fang, Y.; Peng, F. Augmenting energy storage in photorechargeable supercapacitors via Ni2+/Ni3+ interconversion mechanism within TiO2/CdS/Ni2(OH)2CO3 composite photoelectrode. J. Power Sources 2024, 609, 234627. [Google Scholar] [CrossRef]
- Kosova, N.; Devyatkina, E.; Kaichev, V. Optimization of Ni2+/Ni3+ ratio in layered Li(Ni, Mn, Co)O2 cathodes for better electrochemistry. J. Power Sources 2007, 174, 965–969. [Google Scholar] [CrossRef]
- Madhu, G.; Biju, V. Effect of Ni2+ and O2− vacancies on the electrical and optical properties of nanostructured nickel oxide synthesized through a facile chemical route. Phys. E Low-Dimens. Syst. Nanostructures 2014, 60, 200–205. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, L.; Ma, J.; Wang, H.; Zhang, C.; Deng, H.; He, H. Investigation into the enhanced catalytic oxidation of o-xylene over MOF-derived Co3O4 with different shapes: The role of surface twofold-coordinate lattice oxygen (O2f). ACS Catal. 2021, 11, 6614–6625. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]







| Electrode | Electrolyte | Current (mA g−1) | Cycle Number | Capacity (mA h g−1) | Capacity Retention | Voltage (V) | Ref. |
|---|---|---|---|---|---|---|---|
| γ-MnO2 | 1 M ZnSO4 + 0.1 M MnSO4 | - | 40 | 158.0 | 63.0% | 1.0–1.8 | [41] |
| Co-Mn3O4 | 2 M ZnSO4 + 0.2 M MnSO4 | 2000 | 1100 | 81.1 | 72.5% | 0.2–2.2 | [42] |
| FeFe(CN)6 | 3 M ZnSO4 + 0.2 M MnSO4 | 42 | 10 | 112.0 | 70.0% | 0.8–1.8 | [43] |
| V2O5 | 3 M Zn(CF3SO3)2 | 100 | 90 | 292.0 | 25.0% | 0.2–1.6 | [44] |
| MnCo2O4 | 2 M ZnSO4 + 0.5 M MnSO4 | 100 | 100 | 405.0 | 11.0% | 0.8–1.8 | [31] |
| MnO2/Carbon cloth | 1 M ZnSO4 + 0.1 M MnSO4 | 100 | 30 | 91.7 | 82.4% | 0.8–1.8 | [15] |
| Mn3O4 | 2 M ZnSO4 + 0.1 M MnSO4 | 500 | 300 | 106.1 | - | 1.0–1.8 | [45] |
| ZnMn2O4 | 3 M Zn(CF3SO3)2 | 500 | 500 | 150.0 | 98.0% | 1.0–2.0 | [46] |
| α-MnO2 | 1 M ZnSO4 | 42 | 30 | 140 | 70.0% | 0.8–1.9 | [47] |
| NiWO4/graphene oxide | 2 M ZnSO4 | 100 | 100 | 287.0 | 41.4% | 1.0–1.8 | This work |
| 1000 | 2000 | 113.4 | 75.2% |
| Sample | Zn | |
|---|---|---|
| 2p1/2 | 2p3/2 | |
| 1st Cycle NiWO4/graphene oxide | 1045.55 | 1022.41 |
| 100th cycle NiWO4/graphene oxide | 1045.16 | 1022.06 |
| Sample | Ni | Satellite | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2p3/2 | 2p1/2 | |||||||||
| 1st cycle NiWO4/graphene oxide | 856.23 | 873.05 | 851.59 | 854.02 | 859.04 | 862.01 | 874.43 | 876.46 | 878.29 | 880.89 |
| 100th cycle NiWO4/graphene oxide | 856.20 | 873.65 | 852.04 | 854.22 | 858.26 | 862.22 | 857.08 | 876.46 | 878.83 | 881.49 |
| Sample | W | |||
|---|---|---|---|---|
| 4f7/2 | 4f5/2 | |||
| +5 | +6 | +5 | +6 | |
| 1st cycle NiWO4/graphene oxide | 35.82 | 35.48 | 38.00 | 37.57 |
| 100th cycle NiWO4/graphene oxide | 35.95 | 35.39 | 38.05 | 37.52 |
| Sample | Adsorbed Oxygen | Defective Oxygen | Lattice Oxygen |
|---|---|---|---|
| 1st cycle NiWO4/graphene oxide | 533.76 | 532.01 | 529.98 |
| 100th cycle NiWO4/graphene oxide | 533.99 | 532.01 | 530.17 |
| Sample | C-C | C=C | C-O | O=C-O |
|---|---|---|---|---|
| 1st cycle NiWO4/graphene oxide | 284.69 | 286.27 | 288.33 | 290.62 |
| 100th cycle NiWO4/graphene oxide | 284.74 | 286.25 | 288.72 | 290.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, L.; Wang, S. Advanced Electrochemical Performance of NiWO4/Graphene Oxide as Cathode Material for Zinc Ion Battery. Energies 2025, 18, 2023. https://doi.org/10.3390/en18082023
Deng L, Wang S. Advanced Electrochemical Performance of NiWO4/Graphene Oxide as Cathode Material for Zinc Ion Battery. Energies. 2025; 18(8):2023. https://doi.org/10.3390/en18082023
Chicago/Turabian StyleDeng, Likai, and Shifa Wang. 2025. "Advanced Electrochemical Performance of NiWO4/Graphene Oxide as Cathode Material for Zinc Ion Battery" Energies 18, no. 8: 2023. https://doi.org/10.3390/en18082023
APA StyleDeng, L., & Wang, S. (2025). Advanced Electrochemical Performance of NiWO4/Graphene Oxide as Cathode Material for Zinc Ion Battery. Energies, 18(8), 2023. https://doi.org/10.3390/en18082023





