Overcoming the Difficulties of Thermophilic Co-Digestion of Sewage Sludge and Beverage Industry Wastes in the Presence of Zeolite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates and Inoculum
2.2. Experimental Design and Laboratory Installation
- T0—control series, mono-digestion of SS;
- T1—two-component AcD of OW and SS;
- T2—three-component AcD of OW, BSG, and SS;
- T3—three-component AcD of OW and SS with Z application;
- T4—four-component AcD of OW, BSG, and SS with Z application.
2.3. Analytical Methods
2.4. Kinetics of Methane Production and Energy Balance
- Modified Gompertz
- Logistic growth
- MP(t)—cumulative methane production (mL CH4/gVS);
- MP—methane production (mL CH4/gVS);
- Rm—maximum methane production rate (mL CH4/gVS d);
- e—constant (2.71828);
- λ—lag phase (d);
- DMP—daily methane production (m3 CH4/d);
- LVS—volatile solids load in the feedstock (kg VS/d);
- QTM—evaluated thermal energy obtained from the combustion of methane (MJ/d);
- QHF—thermal energy required for heating the feedstock (MJ/d);
- QHL—thermal energy required to cover the heat loss through the walls of the digester (MJ/d);
- CSS—specific heat of SS (4200 kJ/m3 K);
- D—diameter of the cylindrical part of the digester (m);
- A—surface of the digester walls (m2);
- QTD—total energy demand (MJ/d);
- HV—heating value of methane, (35.8 MJ/m3),
- CV—calorific value of methane, (10 kWh/m3);
- T—adopted temperature for AD (K);
- Tair—air temperature (K);
- TF—feedstock temperature (K);
- CHL—heat loss coefficient by permeation through the walls of the digester, (4.0 kJ m2/h K);
- qF—feedstock flow rate (m3/d);
- P—profit of thermal energy (%);
- α—margin factor, (1.1).
2.5. Adsorption Capacity of the Applied Z
- Ci—content of the selected parameter in an initial sample (mg/L);
- Ce—equilibrium content of the selected parameter (mg/L);
- V—volume of the solution (L);
- m—dose of applied the adsorbent (g).
2.6. Statistical Analysis
3. Results and Discussion
3.1. Removal of Organic Compounds and Process Stability
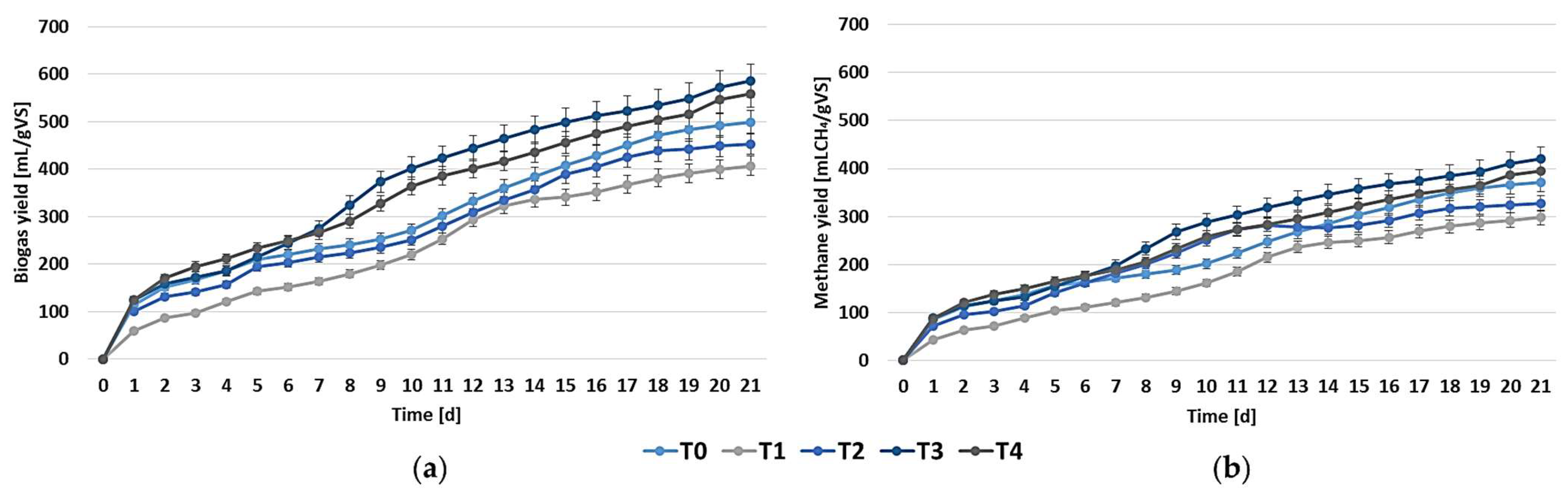
3.2. Methane Production and Its Kinetics
| Main Component | Co-Substrate | Mixing Ratio or Additive Mass | Method for Improving Methane Production % | VS Removal | Improvement of VS Removal (as Compared with Control) % | Methane Yield mLCH4/gVS | Improvement of Methane Yield (as Compared with Control) % | Reference |
| Thermophilic conditions | ||||||||
| WAS | OFMSW | 50:50 v/v | - | - | 330 | 233 | [64] | |
| SS | FOG | 52:48 on the VS basis | - | 51 | 66 | 670 | 169 | [65] |
| SS | GW | 73:27 on the COD basis | - | 45 | −10 | 230 | 6.5 | [66] |
| WAS | MA | 88:12 v/v | - | - | - | 388 | 7 | [67] |
| SS | FW + B | 50:50 v/v | Adsorption and immobilization using biochar | 432.2 | 46.2 | [61] | ||
| Cattle manure | Z | 98:2 v/v | Adsorption and immobilization additives using Z | - | - | 310 | 24 | [68] |
| Pig wastes | Z | 12 g | Adsorption and immobilization additives using Z | 75 | 198 | 278 | 66 | [29] |
| OW | - | - | Steam distillation | - | - | 332 | - | [54] |
| SS | - | - | Thermal pre-treatment | 39 | 7 | 480 | 47 | [69] |
| SS | - | - | Low-temperature pre-treatment (70 °C) | 36.55 | 10 | 180 | 20 | [70] |
| SS | OW + Z OW + BSG + Z | 97.9:2.1 VS + 6 g of Z 90.5:1.9:7.6 VS + 3 g of Z | - | 61% 62.7% | 3 6 | 420.0 395.0 | 14 6 | This study |
| Mesophilic conditions | ||||||||
| WAS | OFMSW | 40:60 | Alkaline | 67.5 | 35 | 337 | 28 | [71] |
| WAS | FW | 2:3 | Microwave | 56 | 56 | 367.6 | 92 | [72] |
| SS | GT | 90:10 | Enzyme | - | - | 434.3 | 78 | [73] |
| SS | OW + B | 1:1 VS + 30 g of B | Adsorption and immobilization using biochar | - | - | 704.10 | 136 | [57] |
| SS | OW | 97.9:2.1 VS | Ultrasound | 61.9 | 4 | 346 | 9 | [74] |
3.3. Energy Balance Evaluation
4. Conclusions
- The experiment should be continued in the continuous mode that involves the influence of operational parameters, i.e., hydraulic retention time and organic loading rate, on AD performance;
- The identification of the microbial community should be conducted; it would indicate the metabolic pathways with the anaerobic bioconversion of selected substrates and allow the full understanding of the impact of Z.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AcD | Anaerobic co-digestion |
| AD | Mono-digestion |
| BSG | Brewery spent grain |
| B | Biochar |
| COD | Chemical oxygen demand |
| GW | Grease waste |
| GT | Grease trap |
| FOG | Fat, oil and grease |
| FW | Food waste |
| MA | Microalgae |
| OW | Orange wastes |
| OFMSW | Organic fraction of municipal solid waste |
| sCOD | Soluble chemical oxygen demand |
| SS | Sewage sludge |
| TA | Total alkalinity |
| TAN | Ammonia nitrogen |
| TSs | Total solids |
| WWTP | Wastewater treatment plant |
| WAS | Waste activated sludge |
| VFAs | Volatile fatty acids |
| VSs | Volatile solids |
| Z | Natural zeolite |
References
- Li, P.; Zhao, H.; Cheng, C.; Hou, T.; Shen, D.; Jiao, Y. A review on anaerobic co-digestion of sewage sludge with other organic wastes for methane production: Mechanism, process, improvement and industrial application. Biomass Bioenergy 2024, 185, 107241. [Google Scholar] [CrossRef]
- Xie, S.; Wickham, R.; Nghiem, L.D. Synergistic effect from anaerobic co-digestion of sewage sludge and organic wastes. Int. Biodeterior. Biodegrad. 2017, 116, 191–197. [Google Scholar] [CrossRef]
- Jessy Hoon, Y.T.; Chan, Y.J.; Wan, Y.K.; Goh, Y.K.; Yazdi, S.K. Industrial-scale anaerobic Co-digestion (ACoD) of palm oil mill effluent (POME) and decanter cake (DC) for maximizing methane yield: An integrated machine learning and simulation-based economic analysis approach. Energy 2023, 289, 129939. [Google Scholar] [CrossRef]
- Paranjpe, A.; Saxena, S.; Jain, P. A Review on Performance Improvement of Anaerobic Digestion Using Co-Digestion of Food Waste and Sewage Sludge. J. Environ. Manag. 2023, 33, 117733. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Chandel, M.K. Anaerobic Co-digestion of sewage sludge and organic fraction of municipal solid waste: Focus on mix ratio optimization and synergistic effects. J. Environ. Manag. 2023, 345, 118821. [Google Scholar] [CrossRef] [PubMed]
- Alves, I.R.; Mahler, C.F.; Oliveira, L.B.; Reis, M.M.; Bassin, J.P. Assessing the use of crude glycerol from biodiesel production as an alternative to boost methane generation by anaerobic co-digestion of sewage sludge. Biomass Bioenergy 2020, 143, 105831. [Google Scholar] [CrossRef]
- Tandukar, M.; Pavlostathis, S.G. Anaerobic co-digestion of municipal sludge with fat-oil-grease (FOG) enhances the destruction of sludge solids. Chemosphere 2022, 292, 133530. [Google Scholar] [CrossRef]
- Chow, W.L.; Chong, S.; Lim, J.W.; Chan, Y.J.; Chong, M.F.; Tiong, T.J.; Chin, J.K.; Pan, G.-T. Anaerobic Co-Digestion of Wastewater Sludge: A Review of Potential Co-Substrates and Operating Factors for Improved Methane Yield. Processes 2020, 8, 39. [Google Scholar] [CrossRef]
- Rabii, A.; Aldin, S.; Dahman, Y.; Elbeshbishy, E. A Review on Anaerobic Co-Digestion with a Focus on the Microbial Populations and the Effect of Multi-Stage Digester Configuration. Energies 2019, 12, 1106. [Google Scholar] [CrossRef]
- Azevedo, A.L.; Lapa, N.; Moldão, M.; Duarte, E. Opportunities and Challenges in the Anaerobic co-Digestion of Municipal Sewage Sludge and Fruit and Vegetable Wastes: A review. Energy Nexus 2023, 10, 100202. [Google Scholar] [CrossRef]
- Sovacool, B.; Bazilian, M.D.; Griffiths, S.; Kim, J.; Foley, A.M.; Rooney, D.W. Decarbonizing the Food and Beverages Industry: A Critical and Systematic Review of Developments, Sociotechnical Systems and Policy Options. Renew. Sustain. Energy Rev. 2021, 143, 110856. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Lues, R. Anaerobic Digestion of Lignocellulosic Biomass: Substrate Characteristics (Challenge) and Innovation. Fermentation 2023, 9, 755. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, Y.; Li, L.; Zhou, H.; Wang, K.; Ding, J.; Jiang, J.; Wei, L.; Zhao, Q. The stress of bioactive compounds on microbes in anaerobic digestion of food waste and mitigation strategies: A critical review. Chem. Eng. J. 2024, 493, 152746. [Google Scholar] [CrossRef]
- Ruiz, B.; Flotats, X. Effect of limonene on batch anaerobic digestion of citrus peel waste. Biochem. Eng. J. 2016, 109, 9–18. [Google Scholar] [CrossRef]
- Szaja, A.; Golianek, P.; Kamiński, M. Process Performance of Thermophilic Anaerobic Co-Digestion of Municipal Sewage Sludge and Orange Peel. J. Ecol. Eng. 2022, 23, 66–76. [Google Scholar] [CrossRef]
- Singh, R.; Hans, M.; Kumar, S.; Yadav, Y.K. Thermophilic Anaerobic Digestion: An Advancement towards Enhanced Biogas Production from Lignocellulosic Biomass. Sustainability 2023, 15, 1859. [Google Scholar] [CrossRef]
- Labatut, R.A.; Angenent, L.T.; Scott, N.R. Conventional mesophilic vs. thermophilic anaerobic digestion: A trade-off between performance and stability? Water Res. 2014, 53, 249–258. [Google Scholar] [CrossRef]
- Liang, C.; Xie, B.; Su, Y.; Shi, J.; Liu, L.; Zhang, S. Mechanistic insights into the attenuation of antibiotic resistance genes in thermophilic anaerobic co-digestion of food waste: A comprehensive metagenomic and absolute quantification study. Chem. Eng. J. 2025, 505, 159794. [Google Scholar] [CrossRef]
- de Magalhães, L.F.; da Silva, G.R.; Peres, A.E.C.; Kooh, M.R.R. Zeolite Application in Wastewater Treatment. Adsorp. Sci. Technol. 2022, 2022, 4544104. [Google Scholar] [CrossRef]
- Gao, S.; Peng, H.; Song, B.; Zhang, J.; Wu, W.; Vaughan, J.; Zardo, P.; Vogrin, J.; Tulloch, S.; Zhu, Z.L. Synthesis of zeolite from low-cost feeds and its sustainable environmental applications. J. Environ. Chem. Eng. 2022, 11, 108995. [Google Scholar] [CrossRef]
- Rahman, R.O.A.; El-Kamash, A.M.; Hung, Y.-T. Applications of Nano-Zeolite in Wastewater Treatment: An Overview. Water 2022, 14, 137. [Google Scholar] [CrossRef]
- Tang, C.C.; Zhang, B.C.; Yao, X.Y.; Zhou, A.J.; Liu, W.; Ren, Y.X.; Li, Z.; Wang, A.; He, Z.W. Insights into response mechanism of anaerobic digestion of waste activated sludge to particle sizes of zeolite. Bioresour. Technol. 2023, 385, 129348. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dürr, V.; Guenne, A.; Mazéas, L.; Chapleur, O. Generic role of zeolite in enhancing anaerobic digestion and mitigating diverse inhibitions: Insights from degradation performance and microbial characteristics. J. Environ. Manag. 2024, 356, 120676. [Google Scholar] [CrossRef] [PubMed]
- Poirier, S.; D’ejean, S.; Chapleur, O. Support media can steer methanogenesis in the presence of phenol through biotic and abiotic effects. Water Res. 2018, 140, 24–33. [Google Scholar] [CrossRef]
- Palatsi, J.; Illa, J.; Prenafeta-Boldú, F.X.; Laureni, M.; Fernandez, B.; Angelidaki, I.; Flotats, X. Long-chain fatty acids inhibition and adaptation process in anaerobic thermophilic digestion: Batch tests, microbial community structure and mathematical modelling. Bioresour. Technol. 2010, 101, 2243–2251. [Google Scholar] [CrossRef]
- Oleszkiewicz, J.A.; Reimers, R.S.; Bartoszewski, K. Beztlenowa Stabilizacja Osadów, w Podstawy Oraz Praktyka Przeróbki i Zagospodaro Wania Osadów, Materiały Międzynarodowego Seminarium Szkoleniowego; LEM s.c.: Kraków, Poland, 1998; pp. 10-11–10-27. [Google Scholar]
- Woszuk, A.; Franus, W. Properties of the Warm Mix Asphalt involving clinoptilolite and Na-P1 zeolite additives. Constr. Build. Matter. 2016, 114, 556–563. [Google Scholar] [CrossRef]
- Szaja, A.; Montusiewicz, A.; Pasieczna-Patkowska, S.; Lebiocka, M. Technological and Energetic Aspects of Multi-Component Co-Digestion of the Beverage Industry Wastes and Municipal Sewage Sludge. Energies 2022, 15, 5395. [Google Scholar] [CrossRef]
- Kotsopoulos, T.A.; Karamanlis, X.; Dotas, D.; Martzopoulos, G. The impact of different natural zeolite concentrations on the methane production in thermophilic anaerobic digestion of pig waste. Biosyst. Eng. 2008, 99, 105–111. [Google Scholar] [CrossRef]
- Yi, J.; Dong, B.; Jin, J.; Dai, X. Effect of Increasing Total Solids Contents on Anaerobic Digestion of Food Waste under Mesophilic Conditions: Performance and Microbial Characteristics Analysis. PLoS ONE 2014, 9, 102548. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 22nd ed.; APHA: Washington, DC, USA, 2012. [Google Scholar]
- Szaja, A.; Montusiewicz, A. Enhancing the co-digestion efficiency of sewage sludge and cheese whey using brewery spent grain as an additional substrate. Bioresour. Technol. 2019, 291, 121863. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Wu, S.; Tan, Z.; Yang, C. Enhancing anaerobic digestion process with addition of conductive materials. Chemosphere 2021, 278, 130449. [Google Scholar] [CrossRef]
- Halim, A.A.; Aziz, H.A.; Johari, M.A.M.; Ariffin, K.S. Comparison study of ammonia and COD adsorption on zeolite, activated carbon and composite materials in landfill leachate treatment. Desalination 2010, 262, 31–35. [Google Scholar] [CrossRef]
- Montalvo, S.; Guerrero, L.; Borja, R.; Sánchez, E.G.; Milán, Z.; Cortés, I.; Rubia, M.A. Application of natural zeolites in anaerobic digestion processes: A review. Appl. Clay Sci. 2012, 58, 125–133. [Google Scholar] [CrossRef]
- Weiß, S.; Lebuhn, M.; Andrade, D.; Zankel, A.; Cardinale, M.; Birner-Gruenberger, R.; Somitsch, W.; Ueberbacher, B.J.; Guebitz, G.M. Activated zeolite—Suitable carriers for microorganisms in anaerobic digestion processes? Appl. Microbiol. Biotechnol. 2013, 97, 3225–3238. [Google Scholar] [CrossRef]
- Aworanti, O.A.; Ajani, A.O.; Agbede, O.O.; Agarry, S.E.; Ogunkunle, O.; Laseinde, O.T.; Kalam, M.A.; Fattah, I.M.R. Enhancing and upgrading biogas and biomethane production in anaerobic digestion: A comprehensive review. Front. Energy Res. 2023, 11, 1170133. [Google Scholar] [CrossRef]
- Palacios-Ruiz, B.; Méndez-Acosta, H.O.; Alcaraz-González, V.; González-Álvarez, V.; Pelayo-Ortiz, C. Regulation of Volatile Fatty Acids and Total Alkalinity in Anaerobic Digesters. IFAC Proc. Vol. 2008, 41, 13611–13616. [Google Scholar] [CrossRef]
- Lin, L.; Wan, C.; Liu, X.; Lei, Z.; Lee, D.J.; Zhang, Y.; Tay, J.H.; Zhang, Z. Anaerobic digestion of swine manure under natural zeolite addition: VFA evolution, cation variation, and related microbial diversity. Appl. Microbiol. Biotechnol. 2013, 97, 10575–10583. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Moscoviz, R.; Astals, S.; Robles, Á.; Steyer, J. Unraveling the literature chaos around free ammonia inhibition in anaerobic digestion. Renew. Sustain. Energy Rev. 2020, 117, 109487. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef]
- Cardona, L.; Mazéas, L.; Chapleur, O. Zeolite favours propionate syntrophic degradation during anaerobic digestion of food waste under low ammonia stress. Chemosphere 2021, 262, 127932. [Google Scholar] [CrossRef]
- Tzenos, C.A.; Kalamaras, S.D.; Economou, E.-A.; Romanos, G.E.; Veziri, C.M.; Mitsopoulos, A.; Menexes, G.C.; Sfetsas, T.; Kotsopoulos, T.A. The Multifunctional Effect of Porous Additives on the Alleviation of Ammonia and Sulfate Co-Inhibition in Anaerobic Digestion. Sustainability 2023, 15, 9994. [Google Scholar] [CrossRef]
- Wasielewski, S.; Rott, E.; Minke, R.; Steinmetz, H. Application of Natural Clinoptilolite for Ammonium Removal from Sludge Water. Molecules 2021, 26, 114. [Google Scholar] [CrossRef]
- He, J.; Luo, T.; Shi, Z.; Angelidaki, I.; Zhang, S.; Luo, G. Microbial shifts in anaerobic digestion towards phenol inhibition with and without hydrochar as revealed by metagenomic binning. J. Hazard. Mater. 2022, 440, 129718. [Google Scholar] [CrossRef]
- Yousef, R.I.; El-Eswed, B.; Al-Muhtaseb, A.H. Adsorption characteristics of natural zeolites as solid adsorbents for phenol removal from aqueous solutions: Kinetics, mechanism, and thermodynamics studies. Chem. Eng. J. 2011, 171, 1143–1149. [Google Scholar] [CrossRef]
- Ndao, A.; Adjallé, K. Overview of the Biotransformation of Limonene and α-Pinene from Wood and Citrus Residues by Microorganisms. Waste 2023, 1, 841–859. [Google Scholar] [CrossRef]
- Carvalho, A.; Fragoso, R.; Gominho, J.; Duarte, E. Effect of Minimizing D-Limonene Compound on Anaerobic Co-digestion Feeding Mixtures to Improve Methane Yield. Waste Biomass Valorization 2019, 10, 75–83. [Google Scholar] [CrossRef]
- Trujillo-Reyes, Á.; Pérez, A.G.; Cuéllar, S.G.; Serrano, A.; Cubero-Cardoso, J.; Jeison, D.; Fermoso, F.G. Evaluation of toxic effect of monoterpene compounds on anaerobic digestion. J. Environ. Chem. Eng. 2024, 12, 112035. [Google Scholar] [CrossRef]
- Feng, C.; Jiaqiang, E.; Han, W.; Deng, Y.; Zhang, B.; Zhao, X.; Han, D. Key technology and application analysis of zeolite adsorption for energy storage and heat-mass transfer process: A review. Renew. Sustain. Energy Rev. 2021, 144, 110954. [Google Scholar] [CrossRef]
- Bokarev, D.A.; Bragina, G.O.; Kolyadenkov, A.R.; Kazakov, A.V.; Stakheev, A.Y. Tuning the performance of Mn/Beta in ozone catalytic oxidation of VOCs by variation of the Mn content and its localization in the zeolite structure. Mendeleev Commun. 2024, 34, 837–839. [Google Scholar] [CrossRef]
- Liu, J.; Yang, L.; Dai, Z.; Jiang, W.; Ma, S.; Yao, L.; Chen, Y.; Zhou, Q.; Zheng, J. Zeolite-induced defect engineering for synthesis of CuBTC-derived novel carbon-zeolite bifunctional support catalyst for multicomponent VOCs catalytic oxidation removal. Chem. Eng. J. 2014, 500, 156830. [Google Scholar] [CrossRef]
- Wang, X.; Huang, S.; Wang, S.; Chen, S.; Dong, S.; Zhu, Y. Effect of D-limonene on volatile fatty acids production from anaerobic fermentation of waste activated sludge under pH regulation: Performance and mechanisms. J. Environ. Manag. 2024, 370, 122828. [Google Scholar] [CrossRef]
- Martín, M.A.; Siles, J.A.; Chica, A.F.; Martín, A. Biomethanization of orange peel waste. Bioresour. Technol. 2010, 101, 8993–8999. [Google Scholar] [CrossRef]
- Ruiz, B.; de Benito, A.; Rivera, J.D.; Flotats, X. Assessment of different pre-treatment methods for the removal of limonene in citrus waste and their effect on methane potential and methane production rate. Waste Manag. Res. 2016, 34, 1249–1257. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Fazzino, F.; Sidari, R.; Zema, D.A. Optimization of orange peel waste ensiling for sustainable anaerobic digestion. Renew. Energy 2020, 154, 849–862. [Google Scholar] [CrossRef]
- Martínez, E.J.; Rosas, J.G.; Sotres, A.; Moran, A.; Cara, J.; Sánchez, M.E.; Gómez, X. Codigestion of sludge and citrus peel wastes: Evaluating the effect of biochar addition on microbial communities. Biochem. Eng. J. 2018, 137, 314–325. [Google Scholar] [CrossRef]
- Chen, L.M.; Fang, W.; Liang, J.; Nabi, M.; Cai, Y.; Wang, Q.; Zhang, P.; Zhang, G. Biochar application in anaerobic digestion: Performances, mechanisms, environmental assessment and circular economy. Resour. Conserv. Recycl. 2023, 188, 106720. [Google Scholar] [CrossRef]
- Fagbohungbe, M.O.; Herbert, B.M.; Hurst, L.; Li, H.; Usmani, S.Q.; Semple, K.T. Impact of biochar on the anaerobic digestion of citrus peel waste. Bioresour. Technol. 2016, 216, 142–149. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Fazzino, F.; Folino, A.; Scibetta, S.; Sidari, R. Improvement of semi-continuous anaerobic digestion of pre-treated orange peel waste by the combined use of zero valent iron and granular activated carbon. Biomass Bioenergy 2019, 129, 105337. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Fang, Y.; Lai, W.; Xu, S.; Lichtfouse, E. Enhancing thermophilic anaerobic co-digestion of sewage sludge and food waste with biogas residue biochar. Renew. Energy 2022, 188, 465–475. [Google Scholar] [CrossRef]
- Fatima, B.; Liaquat, R.; Farooq, U.; Jamal, A.; Ali, M.I.; Liu, F.-J.; He, H.; Guo, H.; Urynowicz, M.; Huang, Z. Enhanced biogas production at mesophilic and thermophilic temperatures from a slaughterhouse waste with zeolite as ammonia adsorbent. Int. J. Environ. Sci. Technol. 2021, 18, 265–274. [Google Scholar] [CrossRef]
- Karthikeyan, P.K.; Bandulasena, H.C.; Radu, T. A comparative analysis of pre-treatment technologies for enhanced biogas production from anaerobic digestion of lignocellulosic waste. Ind. Crops Prod. 2024, 215, 118591. [Google Scholar] [CrossRef]
- Cavinato, C.; Bolzonella, D.; Pavan, P.; Fatone, F.; Cecchi, F. Mesophilic and thermophilic anaerobic co-digestion of waste activated sludge and source sorted biowaste in pilot-and full-scale reactors. Renew. Energy 2013, 55, 260–265. [Google Scholar] [CrossRef]
- Kabouris, J.C.; Tezel, U.; Pavlostathis, S.G.; Engelmann, M.; Dulaney, J.A.; Todd, A.C.; Gillette, R.A. Mesophilic and thermophilic anaerobic digestion of municipal sludge and fat, oil, and grease. Water Environ. Res. 2009, 81, 476–485. [Google Scholar] [CrossRef]
- Silvestre, G.; Illa, J.; Fernández, B.; Bonmatí, A. Thermophilic anaerobic co-digestion of sewage sludge with grease waste: Effect of long chain fatty acids in the methane yield and its dewatering properties. Appl. Energy 2014, 117, 87–94. [Google Scholar] [CrossRef]
- Olsson, J.; Feng, X.M.; Ascue, J.; Gentili, F.G.; Shabiimam, M.A.; Nehrenheim, E.; Thorin, E. Co-digestion of cultivated microalgae and sewage sludge from municipal waste water treatment. Bioresour. Technol. 2014, 171, 203–210. [Google Scholar] [CrossRef]
- Borja, R.; Sánchez, E.; Durán, M.M. Effect of the clay mineral zeolite on ammonia inhibition of anaerobic thermophilic reactors treating cattle manure. J. Environ. Sci. Health Part A Environ. Sci. Eng. Toxicol. 1996, 31, 479–500. [Google Scholar] [CrossRef]
- Gagliano, M.C.; Braguglia, C.M.; Gianico, A.; Mininni, G.; Nakamura, K.; Rossetti, S. Thermophilic anaerobic digestion of thermal pretreated sludge: Role of microbial community structure and correlation with process performances. Water Res. 2015, 68, 498–509. [Google Scholar] [CrossRef]
- Ferrer, I.; Ponsá, S.; Vázquez, F.; Font, X. Increasing biogas production by thermal (70 °C) sludge pre-treatment prior to thermophilic anaerobic digestion. Biochem. Eng. J. 2008, 42, 186–192. [Google Scholar] [CrossRef]
- Ahmadi-Pirlou, M.; Ebrahimi-Nik, M.; Khojastehpour, M.; Ebrahimi, S.H. Mesophilic co-digestion of municipal solid waste and sewage sludge: Effect of mixing ratio, total solids, and alkaline pretreatment. Int. Biodeterior. 2017, 125, 97–104. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, C.; Tong, J.; Liu, J.; Liu, J.; Yu, D.; Wang, Y.; Chen, M.; Wei, Y. Optimization and microbial community analysis of anaerobic co-digestion of food waste and sewage sludge based on microwave pretreatment. Bioresour. Technol. 2016, 200, 253–261. [Google Scholar] [CrossRef]
- Donoso-Bravo, A.; Fdz-Polanco, M. Anaerobic co-digestion of sewage sludge and grease trap: Assessment of enzyme addition. Process Biochem. 2013, 48, 936–940. [Google Scholar] [CrossRef]
- Szaja, A.; Bartkowska, I. Effect of microwave and ultrasonic pre-treatments on anaerobic co-digestion of orange wastes and municipal sewage sludge: A case study. Desalination Water Treat. 2024, 320, 100754. [Google Scholar] [CrossRef]
- Morais, N.W.; Coelho, M.M.; Silva, A.D.; Silva, F.S.; Ferreira, T.J.; Pereira, E.L.; dos Santos, A.B. Biochemical potential evaluation and kinetic modeling of methane production from six agro-industrial wastewaters in mixed culture. Environ. Pollut. 2021, 280, 116876. [Google Scholar] [CrossRef] [PubMed]
- López-Aguilar, H.A.; Morales-Durán, B.; Quiroz-Cardoza, D.; Pérez-Hernández, A. Lag Phase in the Anaerobic Co-Digestion of Sargassum spp. and Organic Domestic Waste. Energies 2023, 16, 5462. [Google Scholar] [CrossRef]
- Ore, O.T.; Akeremale, O.K.; Adeola, A.O.; Ichipi, E.; Olubodun, K.O. Production and Kinetic Studies of Biogas from Anaerobic Digestion of Banana and Cassava Wastes. Chem. Afr. 2023, 6, 477–484. [Google Scholar] [CrossRef]
- Martín, M.; Fernández, R.; Gutiérrez, M.D.; Siles, J.Á. Thermophilic anaerobic digestion of pre-treated orange peel: Modelling of methane production. Process. Saf. Environ. Prot. 2018, 117, 245–253. [Google Scholar] [CrossRef]
- Milán, Z.; Villa, P.; Sánchez, E.; Montalvo, S.; Borja, R.; Ilangovan, K.; Briones, R. Effect of natural and modified zeolite addition on anaerobic digestion of piggery waste. Water Sci. Technol. 2003, 48, 263–269. [Google Scholar] [CrossRef]



| Parameter | Unit | SS | OW | BSG | Inoculum |
|---|---|---|---|---|---|
| COD | g/L | 46.9 ± 3.2 | |||
| VFA | mg/L | 486 ± 17.4 | |||
| pH | 6.50 ± 0.02 | 7.94 ± 0.01 | |||
| Alkalinity | mg/L | 663 ± 13.3 | |||
| TS | g/kg | 43.89 ± 2.7 | 231.7 ± 9.2 | 961.5 ± 11.4 | 24.91 ± 0.7 |
| VS | g/kg | 35.32 ± 1.7 | 224.4 ± 17.6 | 889.5 ± 81.1 | 17.85 ± 0.55 |
| Phenols | mg/L | 2.95 ± 0.7 | 62.1 ± 3.7 | 49.8 ± 4.9 | |
| Limonene | ppm | nd. | 713 ± 37.1 | nd. | nd. |
| Series | pH | TA | VFA | TAN | Phenols | D-Limonene | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| - | mg/L | mg/L | mg/L | mg/L | ppb | |||||||
| F | D | F | D | F | D | F | D | F | D | F | D | |
| T0 | 6.50 ± 0.04 | 7.8 ± 0.1 | 663 ± 14.3 | 6463 ± 21.4 | 486 ± 3.4 | 1028 ± 12.4 | 59.4 ± 4.3 | 178 ± 11.2 | 2.95 ± 0.5 | 27.90 ± 2.3 | 18.27 ± 1.4 | 21.47 ± 2.1 |
| T1 | 5.59 ± 0.02 | 7.72 ± 0.06 | 650 ± 7.8 | 5950 ± 24.1 | 661 ± 4.7 | 1245 ± 11.4 | 65.4 ± 3.7 | 210 ± 7.8 | 5.03 ± 0.7 | 23.40 ± 3.1 | 2359.5 ± 14.3 | 839.67 ± 24.1 |
| T2 | 5.96 ± 0.07 | 7.68 ± 0.07 | 647 ± 9.8 | 5672 ± 17.6 | 645 ± 5.7 | 1487 ± 9.7 | 64.2 ± 4.9 | 194 ± 14.3 | 7.45 ± 0.7 | 33.80 ± 3.4 | 2332.1 ± 15.7 | 524.3 ± 14.2 |
| T3 | 6.43 ± 0.03 | 8.34 ± 0.1 | 690 ± 4.8 | 6399 ± 15.1 | 604 ± 4.8 | 788 ± 7.8 | 60.6 ± 5.1 | 179 ± 14.8 | 7.11 ± 0.6 | 15.33 ± 2.1 | 21,201.7 ± 31.3 | 451.4 ± 12.3 |
| T4 | 6.38 ± 0.06 | 8.23 ± 0.1 | 672 ± 7.4 | 6154 ± 11.1 | 638 ± 6.7 | 749 ± 8.9 | 62.3 ± 5.7 | 169 ± 11.7 | 8.23 ± 1.1 | 14.92 ± 2.0 | 21,660.4 ± 21.4 | 432.1 ± 11.7 |
| Series | Modified Gompertz | Logistic Growth | ||||||
| MP | Rm | λ | R2 | MP | Rm | λ | R2 | |
| mL CH4/gVS | mL CH4/gVS d | d | - | mL CH4/gVS | mL CH4/gVS d | d | - | |
| T0 | 380.1 | 17.0 | −2.98 | 0.984 | 343.1 | 17.7 | −2.30 | 0.983 |
| T1 | 335.8 | 16.8 | −0.53 | 0.993 | 317.9 | 17.2 | −0.23 | 0.993 |
| T2 | 362.3 | 23.7 | −1.06 | 0.991 | 320.7 | 23.5 | −0.85 | 0.989 |
| T3 | 446.9 | 25.6 | −1.29 | 0.993 | 415.5 | 25.9 | −0.93 | 0.991 |
| T4 | 443.3 | 20.2 | −2.69 | 0.988 | 406.7 | 20.2 | −2.49 | 0.984 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szaja, A.; Montusiewicz, A.; Lebiocka, M. Overcoming the Difficulties of Thermophilic Co-Digestion of Sewage Sludge and Beverage Industry Wastes in the Presence of Zeolite. Energies 2025, 18, 2085. https://doi.org/10.3390/en18082085
Szaja A, Montusiewicz A, Lebiocka M. Overcoming the Difficulties of Thermophilic Co-Digestion of Sewage Sludge and Beverage Industry Wastes in the Presence of Zeolite. Energies. 2025; 18(8):2085. https://doi.org/10.3390/en18082085
Chicago/Turabian StyleSzaja, Aleksandra, Agnieszka Montusiewicz, and Magdalena Lebiocka. 2025. "Overcoming the Difficulties of Thermophilic Co-Digestion of Sewage Sludge and Beverage Industry Wastes in the Presence of Zeolite" Energies 18, no. 8: 2085. https://doi.org/10.3390/en18082085
APA StyleSzaja, A., Montusiewicz, A., & Lebiocka, M. (2025). Overcoming the Difficulties of Thermophilic Co-Digestion of Sewage Sludge and Beverage Industry Wastes in the Presence of Zeolite. Energies, 18(8), 2085. https://doi.org/10.3390/en18082085





