Enhancing Thermal Conductivity of SiC Matrix Pellets for Accident-Tolerant Fuel via Atomic Layer Deposition of Al2O3 Coating
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of SiC Pellets
2.3. Characterizations
3. Results
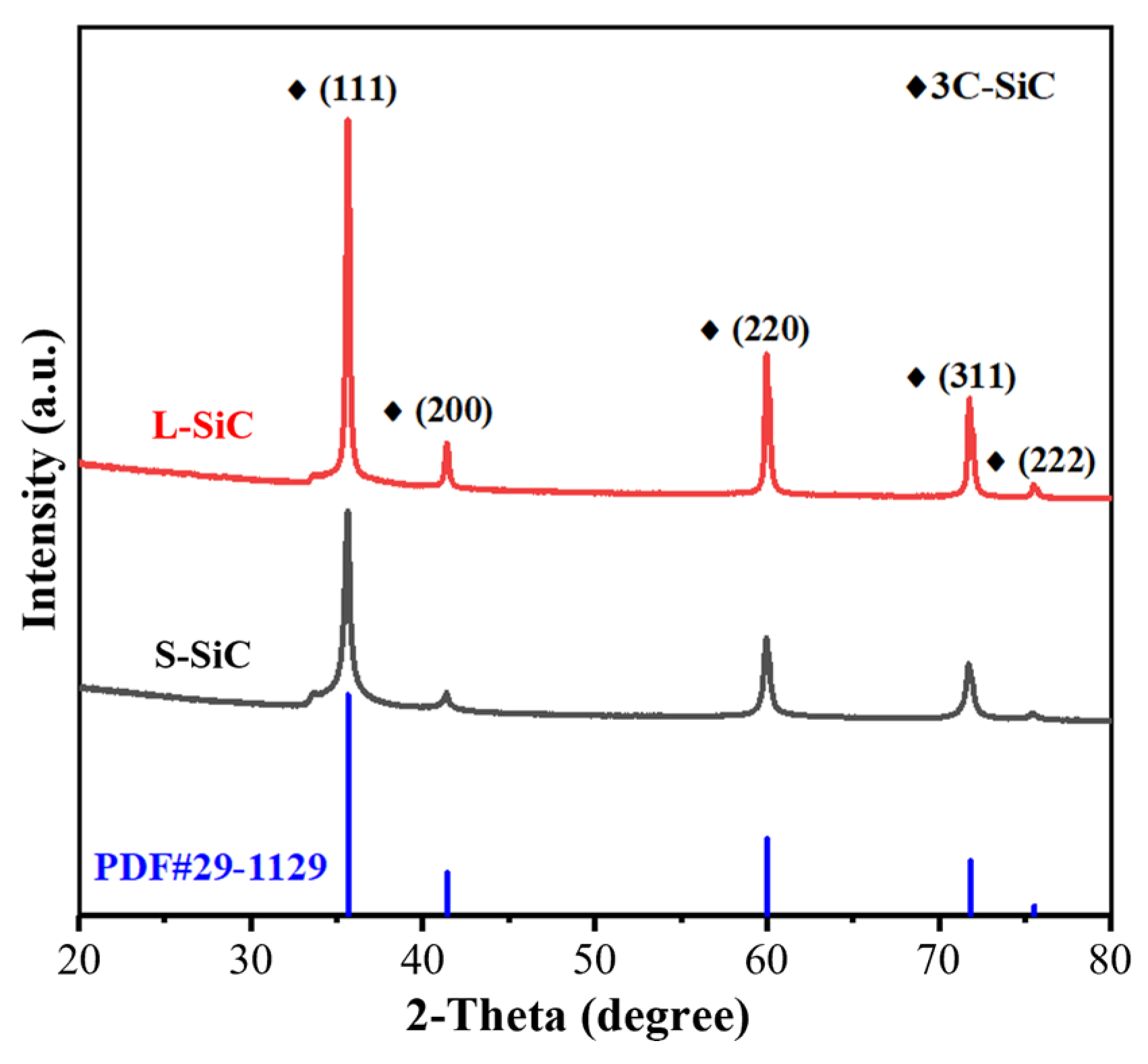
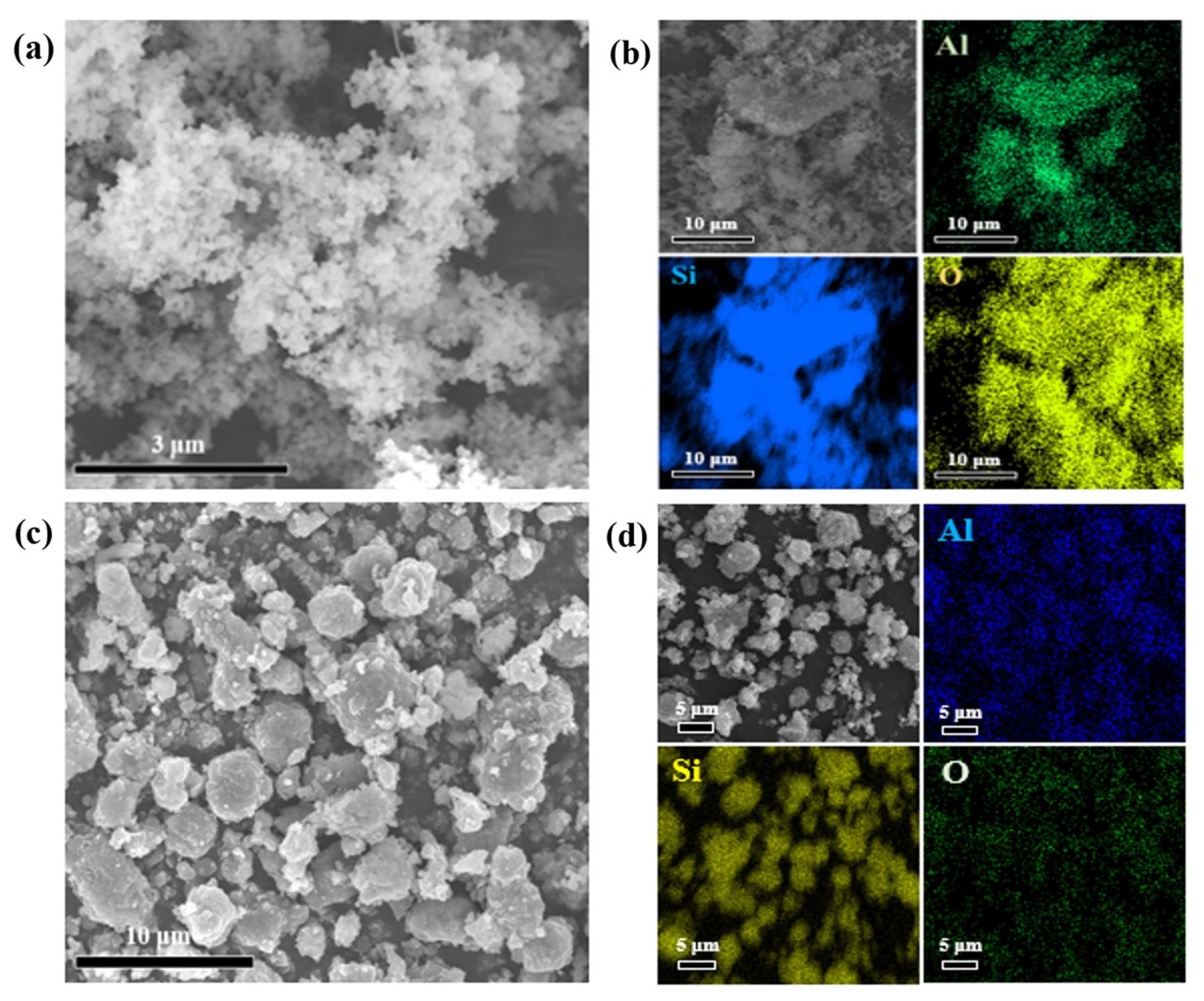
3.1. Features of the Raw SiC Powder
3.2. Optimization of the Atomic Layer Deposition Process
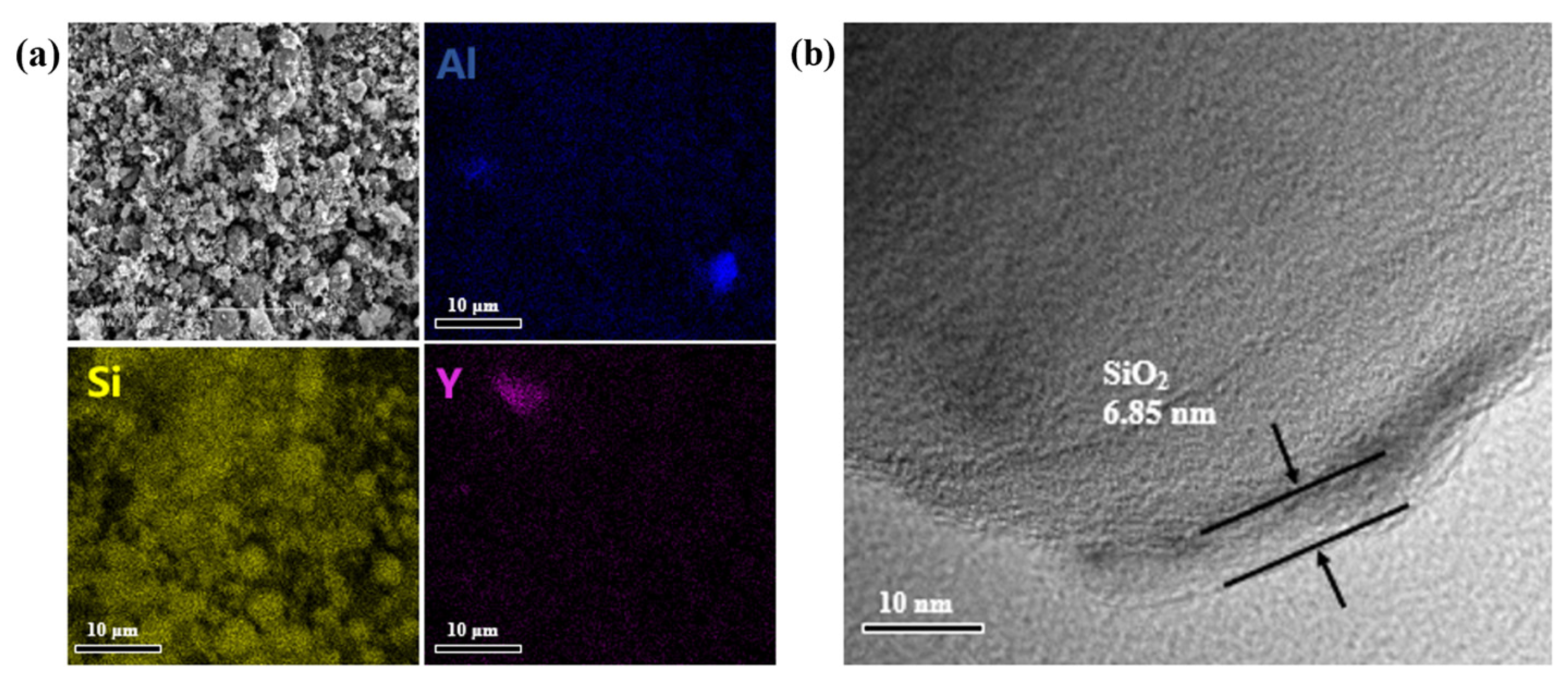
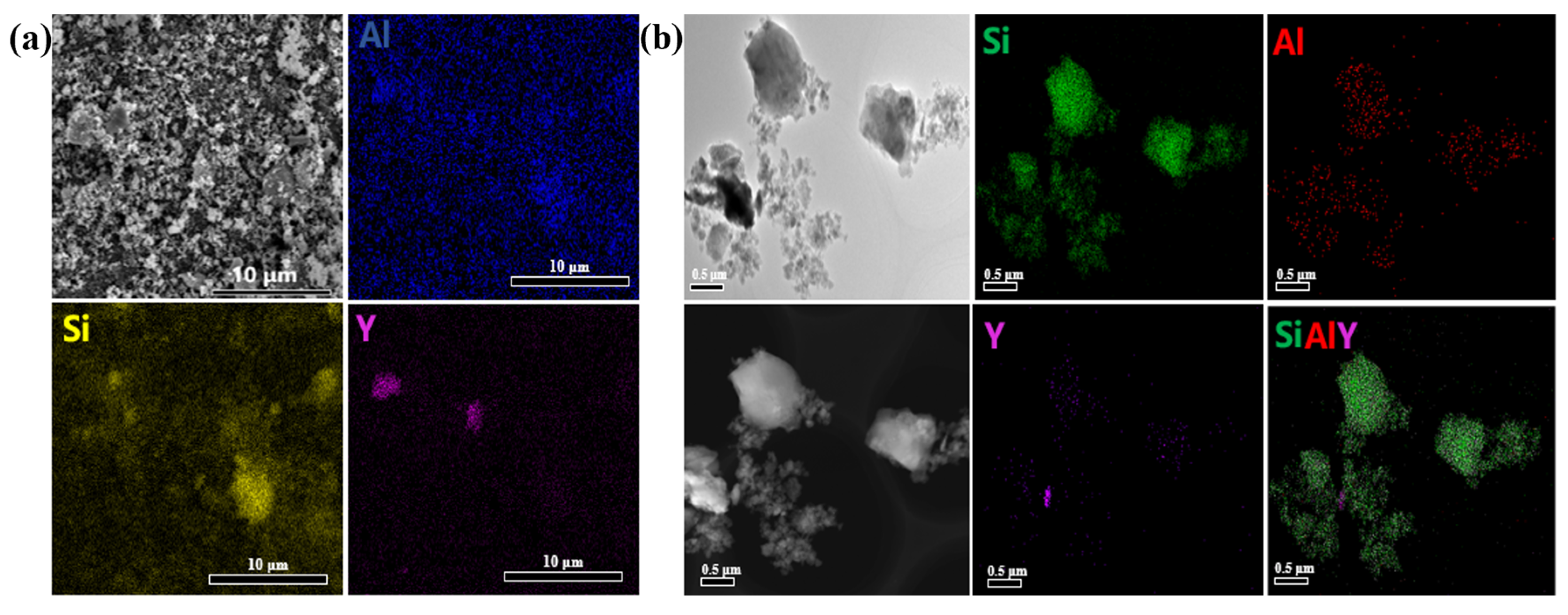
3.3. Morphological Characterization of SiC@Al2O3
3.4. Sintering and Performance Testing of SiC Pellets
4. Discussion
- Suppression of interfacial oxidation
- 2.
- Enhanced additive distribution and YAG phase formation
- 3.
- Thermal conductivity enhancement via interfacial engineering
- Interfacial characterization: employ advanced techniques such as atomic-resolution TEM or X-ray photoelectron spectroscopy to elucidate bonding mechanisms at the Al2O3/SiC interface.
- Al2O3-Y2O3 co-deposition via ALD: Explore the ALD process for the sequential or composite coating of Al2O3 and Y2O3 on SiC powders. Based on the research results presented in this work, this approach is expected to enhance the uniformity of the YAG phase during sintering while mitigating the influence of Al2O3 on the thermal conductivity of the SiC matrix.
- Scalability studies: transition from lab-scale ALD to pilot-scale systems to evaluate industrial feasibility and cost-performance trade-offs.
- Long-term stability testing: expose ALD-coated SiC pellets to simulated reactor conditions (high temperature, irradiation, mechanical stress, etc.) to assess durability over extended periods.
- Environmental impact mitigation: develop eco-friendly ALD precursors and recycling protocols to minimize chemical waste and align with sustainable manufacturing practices.
5. Conclusions
- The use of pressure-holding ALD protocols could effectively deposit Al2O3 on SiC, with a good deposition effect and a deposition rate of approximately 0.09 nm/cycle.
- ALD-Al2O3 + Y2O3 (5~7 wt.%) increased thermal conductivity by 14~18% compared to traditional mechanical mixing, despite slightly lower densities, highlighting the critical role of interfacial uniformity in reducing phonon scattering.
- A balance between sintering aid concentration and ALD-driven uniformity is essential, as excessive or insufficient Al2O3 content adversely affects densification.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Terrani, K.A. Accident Tolerant Fuel Cladding Development: Promise, Status, and Challenges. J. Nucl. Mater. 2018, 501, 13–30. [Google Scholar] [CrossRef]
- Ko, J.; Kim, J.W.; Min, H.W.; Kim, Y.; Yoon, Y.S. Review of Manufacturing Technologies for Coated Accident Tolerant Fuel Cladding. J. Nucl. Mater. 2022, 561, 153562. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, X.; Lu, Y.; Zhang, R. Preparation and research progress of accident tolerant fuel pellets. Powder Metall. Technol. 2022, 40, 334–339, 350. [Google Scholar] [CrossRef]
- Snead, L.L.; Venneri, F.; Kim, Y.; Terrani, K.A.; Tulenko, J.E.; Forsberg, C.W.; Peterson, P.F.; Lahoda, E.J. Fully Ceramic Microencapsulated Fuels: A Transformational Technology for Present and next Generation Reactors. Trans. Am. Nucl. Soc. 2011, 104, 4. [Google Scholar]
- Terrani, K.A.; Snead, L.L.; Gehin, J.C. Microencapsulated Fuel Technology for Commercial Light Water and Advanced Reactor Application. J. Nucl. Mater. 2012, 427, 209–224. [Google Scholar] [CrossRef]
- Terrani, K.A.; Kiggans, J.O.; Silva, C.M.; Shih, C.; Katoh, Y.; Snead, L.L. Progress on Matrix SiC Processing and Properties for Fully Ceramic Microencapsulated Fuel Form. J. Nucl. Mater. 2015, 457, 9–17. [Google Scholar] [CrossRef]
- Lee, Y.; Cho, B.; Cho, N.Z. Steady- and Transient-State Analyses of Fully Ceramic Microencapsulated Fuel with Randomly Dispersed Tristructural Isotropic Particles via Two-Temperature Homogenized Modeld I: Theory and Method. Nucl. Eng. Technol. 2016, 48, 650–659. [Google Scholar] [CrossRef]
- Olander, D. Nuclear Fuels-Present and Future. J. Nucl. Mater. 2009, 389, 1–22. [Google Scholar] [CrossRef]
- Cao, F.; Fan, X.; Liu, B.; Zhao, X.; Guo, F.; Xiao, P. Microstructure and Thermal Conductivity of Fully Ceramic Microencapsulated Fuel Fabricated by Spark Plasma Sintering. J. Am. Ceram. Soc. 2018, 101, 4224–4236. [Google Scholar] [CrossRef]
- Liu, W.; Shao, Z.; Liu, W.; Meng, Y.; Feng, S.; Cai, Z. Study on performance of coated particle dispersed fuel pellet prepared by spark plasma sintering. At. Energy Sci. Technol. 2023, 57, 1810–1816. [Google Scholar]
- Terrani, K.A.; Kiggans, J.O.; Katoh, Y.; Shimoda, K.; Montgomery, F.C.; Armstrong, B.L.; Parish, C.M.; Hinoki, T.; Hunn, J.D.; Snead, L.L. Fabrication and Characterization of Fully Ceramic Microencapsulated Fuels. J. Nucl. Mater. 2012, 426, 268–276. [Google Scholar] [CrossRef]
- Lee, J.K.; Kang, H.H.; Shim, D.J.; Lee, E.G.; Kim, H. Effects of YAG-Phase Amount on the Microstructure and Phase Transformation during the Liquid-Phase Sintering of β-SiC. Key Eng. Mater. 1999, 161–163, 263–266. [Google Scholar] [CrossRef]
- Gao, L.; Wang, H.; Kawaoka, H.; Sekino, T.; Niihara, K. Fabrication of YAG-SiC Nanocomposites by Spark Plasma Sintering. J. Eur. Ceram. Soc. 2002, 22, 785–789. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, H.; Xiaoyang, W.; Kan, H.; Long, H. Sintering Density of SiC-YAG Ceramic Composite Material. Bull. Chin. Ceram. Soc. 2015, 34, 2125–2129, 2138. [Google Scholar]
- Chai, Z.; Gao, Z.; Liu, H.; Zhang, X.; Glodan, G.; Xiao, P. Thermal Conductivity of Spark Plasma Sintered SiC Ceramics with Alumina and Yttria. J. Eur. Ceram. Soc. 2021, 41, 3264–3273. [Google Scholar] [CrossRef]
- Duan, C.-L.; Liu, X.; Shan, B.; Chen, R. Fluidized Bed Coupled Rotary Reactor for Nanoparticles Coating via Atomic Layer Deposition. Rev. Sci. Instrum. 2015, 86, 075101. [Google Scholar] [CrossRef]
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Cao, K.; Chen, R. Advances in Atomic Layer Deposition. Nanomanuf. Metrol. 2022, 5, 191–208. [Google Scholar] [CrossRef]
- Cui, H.; Gong, L.; Sun, Y.; Yang, G.Z.; Liang, C.L.; Chen, J.; Wang, C.X. Direct Synthesis of Novel SiC@Al2O3 Core-Shell Epitaxial Nanowires and Field Emission Characteristics. CrystEngComm 2011, 13, 1416–1421. [Google Scholar] [CrossRef]
- Shishkin, R.A. Oxide-Bonded Silicon Carbide and Alumina Ceramics Obtained from Template SCS Powders. Ceram. Int. 2025, 51, 10340–10350. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Liu, R.; Zhao, J.; Liu, M. Preparation of SiC@Al2O3–Y2O3 Core-Shell Nanoparticles by a Slow Precipitation Method for Dense SiC Sintering. Ceram. Int. 2020, 46, 24504–24511. [Google Scholar] [CrossRef]
- Chen, H.; Fraser Stoddart, J. From Molecular to Supramolecular Electronics. Nat. Rev. Mater. 2021, 6, 804–828. [Google Scholar] [CrossRef]
- Podder, S.; Basumatary, B.; Gogoi, D.; Bora, J.; Pal, A.R. Pyro-Phototronic Application in the Au/ZnO Interface for the Fabrication of a Highly Responsive Ultrafast UV Photodetector. Appl. Surf. Sci. 2021, 537, 147893. [Google Scholar] [CrossRef]
- Progelhof, R.C.; Throne, J.L.; Ruetsch, R.R. Methods for Predicting the Thermal Conductivity of Composite Systems: A Review. Polym. Eng. Sci. 1976, 16, 615–625. [Google Scholar] [CrossRef]







| No. | Composition of Sintering Aids | Total Amount of Sintering Aids (wt.%) | Relative Density (% T.D.) | Porosity (%) |
|---|---|---|---|---|
| 1 | 2.0% 1 Al2O3 + 2.0% Y2O3 | 4.0 | 80.61 | 13.71 |
| 2 | 3.0% Al2O3 + 3.0% Y2O3 | 6.0 | 90.09 | 6.89 |
| 3 | 4.0% Al2O3 + 4.0% Y2O3 | 8.0 | 95.79 | 1.10 |
| 4 | 2.0% ALD-Al2O3 2 + 2.0 Y2O3 | 4.0 | 82.24 | 12.95 |
| 5 | 2.5% ALD-Al2O3 + 2.5 Y2O3 | 5.0 | 92.93 | 3.95 |
| 6 | 3.5% ALD-Al2O3 + 3.5 Y2O3 | 7.0 | 92.57 | 4.21 |
| 7 | 3.0% ALD-Al2O3 + 1.0% Al2O3 + 4.0% Y2O3 | 8.0 | 95.18 | 1.11 |
| 8 | 2.0% ALD-Al2O3 + 2.0% Al2O3 + 4.0% Y2O3 | 8.0 | 94.87 | 1.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Wang, W.; Wang, J.; Liu, X.; Li, Y.; Li, Z.; Chen, R.; Liu, W. Enhancing Thermal Conductivity of SiC Matrix Pellets for Accident-Tolerant Fuel via Atomic Layer Deposition of Al2O3 Coating. Energies 2025, 18, 2130. https://doi.org/10.3390/en18082130
Zhao Y, Wang W, Wang J, Liu X, Li Y, Li Z, Chen R, Liu W. Enhancing Thermal Conductivity of SiC Matrix Pellets for Accident-Tolerant Fuel via Atomic Layer Deposition of Al2O3 Coating. Energies. 2025; 18(8):2130. https://doi.org/10.3390/en18082130
Chicago/Turabian StyleZhao, Yumeng, Wenqing Wang, Jiquan Wang, Xiao Liu, Yu Li, Zongshu Li, Rong Chen, and Wei Liu. 2025. "Enhancing Thermal Conductivity of SiC Matrix Pellets for Accident-Tolerant Fuel via Atomic Layer Deposition of Al2O3 Coating" Energies 18, no. 8: 2130. https://doi.org/10.3390/en18082130
APA StyleZhao, Y., Wang, W., Wang, J., Liu, X., Li, Y., Li, Z., Chen, R., & Liu, W. (2025). Enhancing Thermal Conductivity of SiC Matrix Pellets for Accident-Tolerant Fuel via Atomic Layer Deposition of Al2O3 Coating. Energies, 18(8), 2130. https://doi.org/10.3390/en18082130






