Advances in Thermal Management for Liquid Hydrogen Storage: The Lunar Perspective
Abstract
1. Introduction
2. Lunar In Situ Hydrogen Resources and Application Analysis
2.1. Availability of Lunar Hydrogen Resources
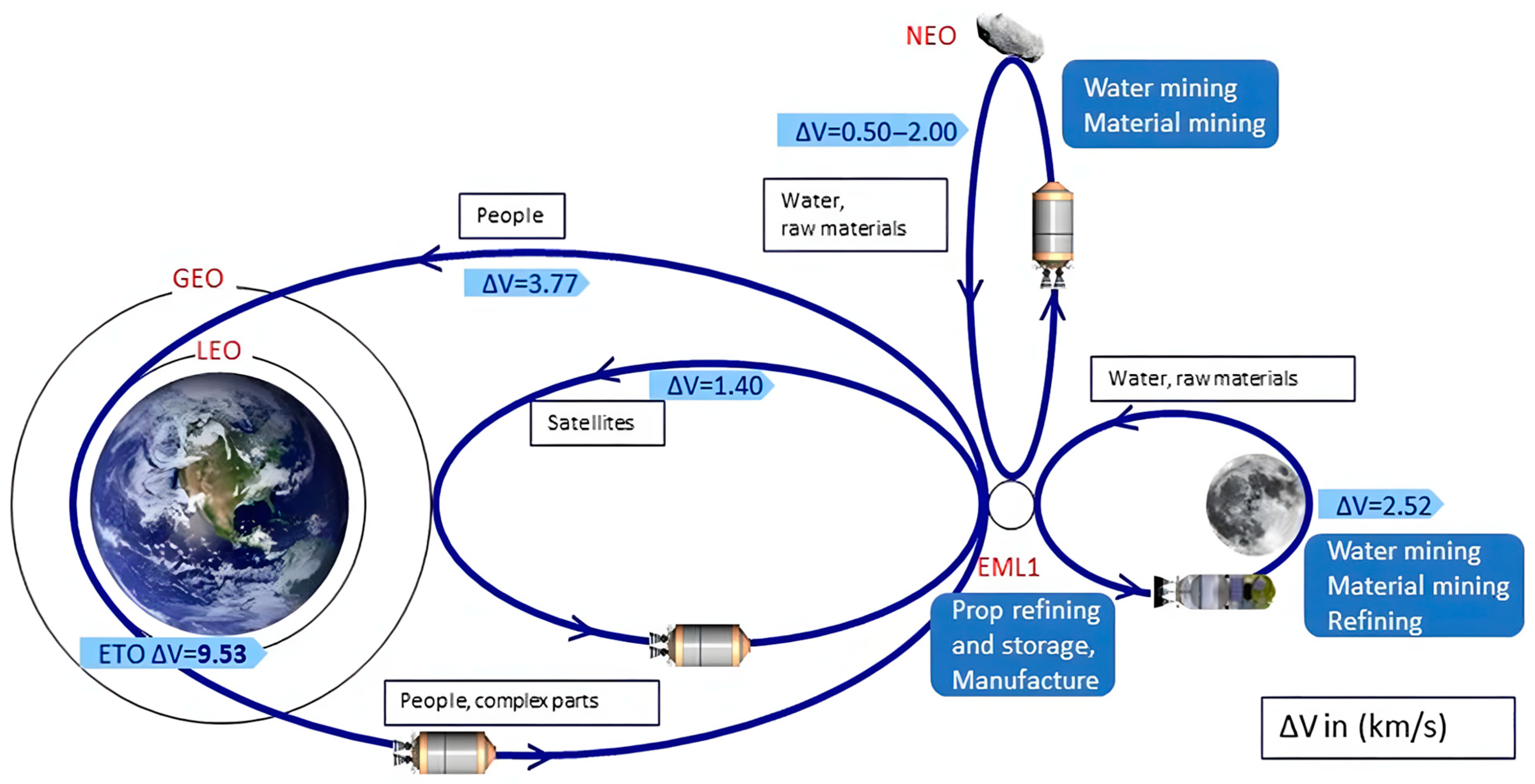
2.2. Economic Feasibility of In Situ-Derived LH2 on the Moon
2.3. Adaptability of LH2 Storage Technology to Lunar Environmental Conditions
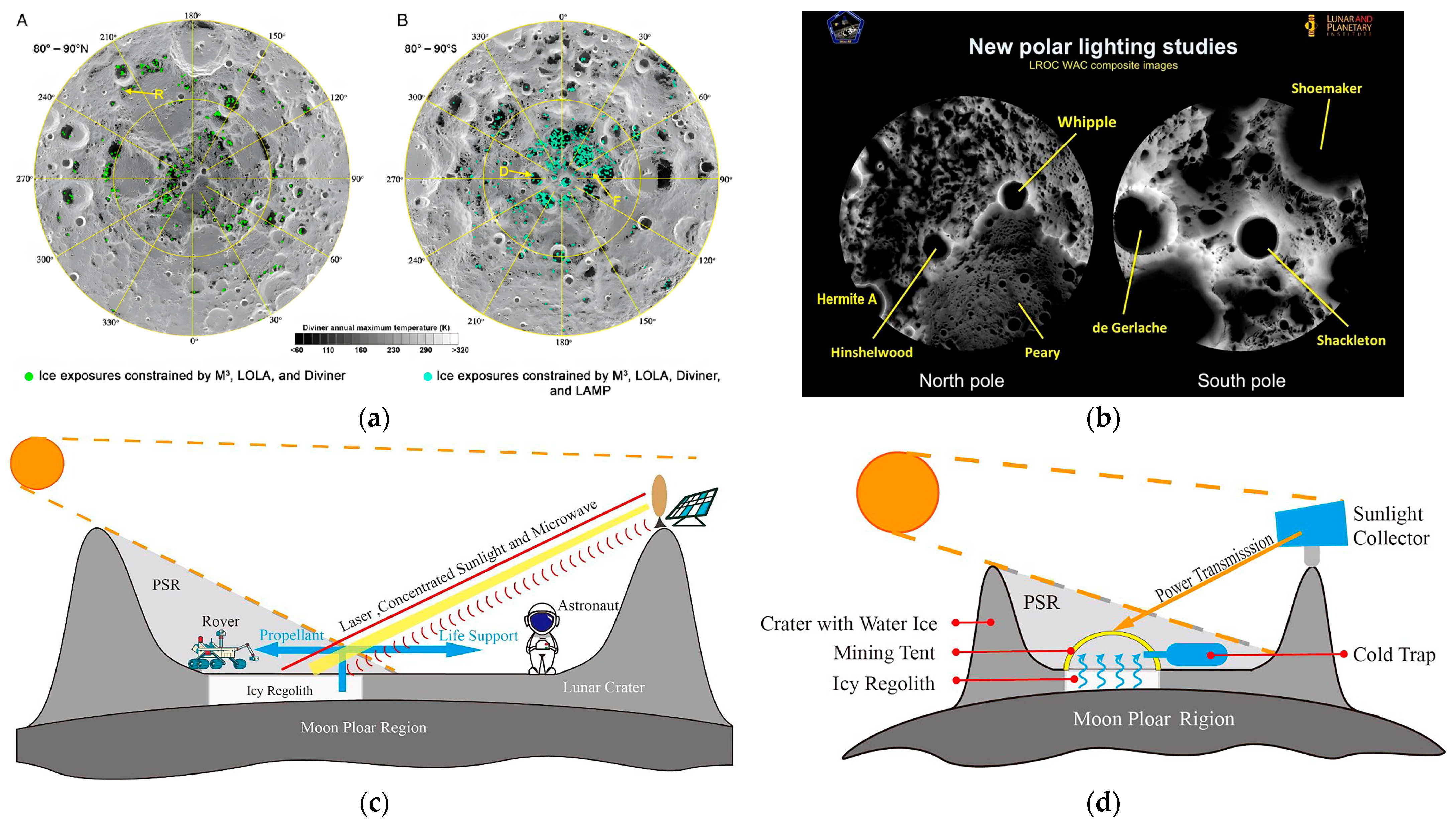
2.3.1. Vacuum and Radiation Conditions
2.3.2. Illumination and Thermal Environment
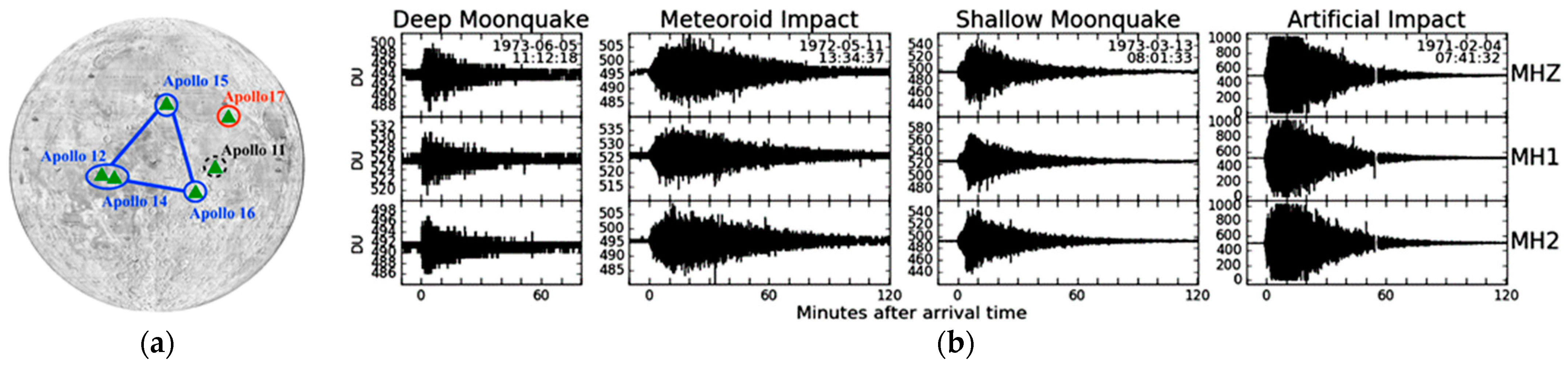
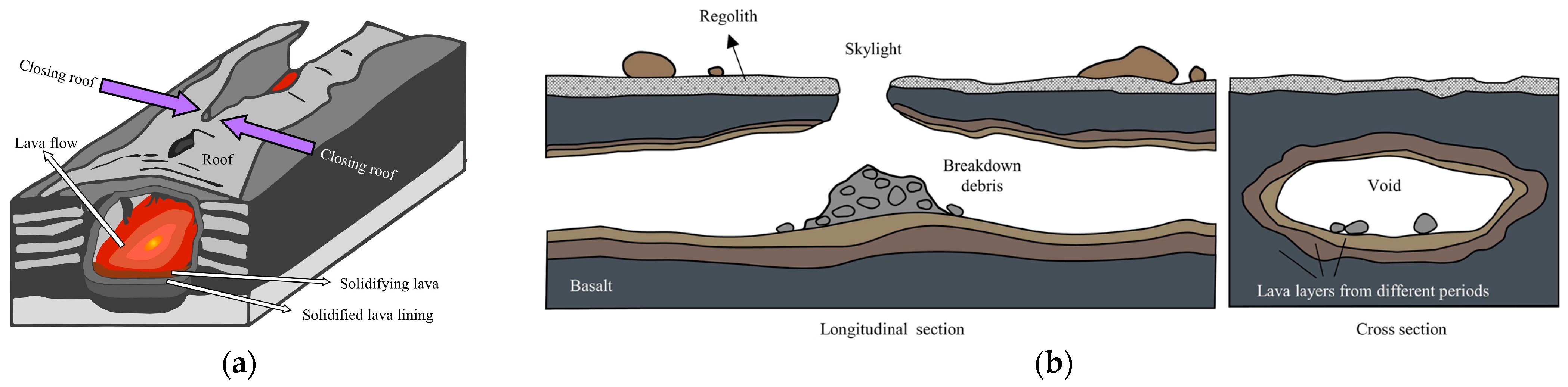
2.3.3. Dynamics and Geological Activity
2.3.4. Operating Environment and Orbital Characteristics
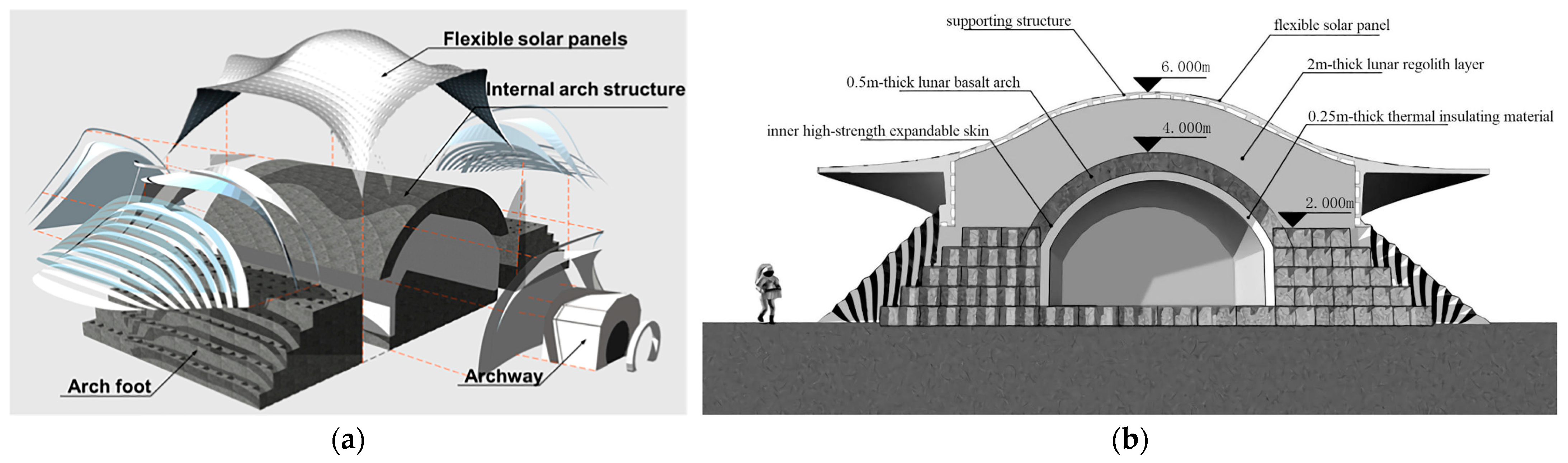
2.4. Deployment Strategies for Lunar LH2 Storage
3. Passive Thermal Protection Technologies for LH2 Storage Systems
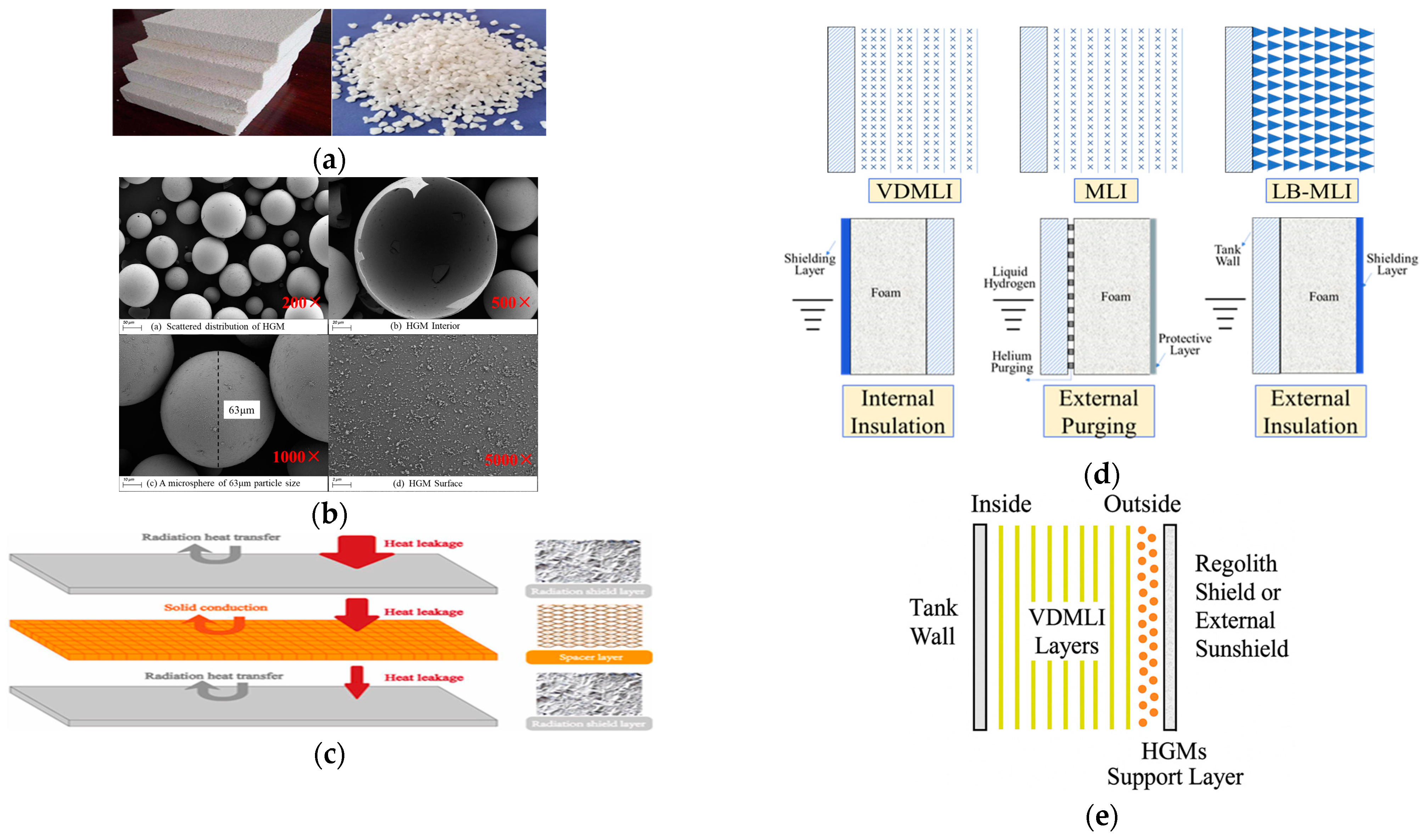
3.1. Advanced Insulation Material Technologies
3.2. Radiation Shielding
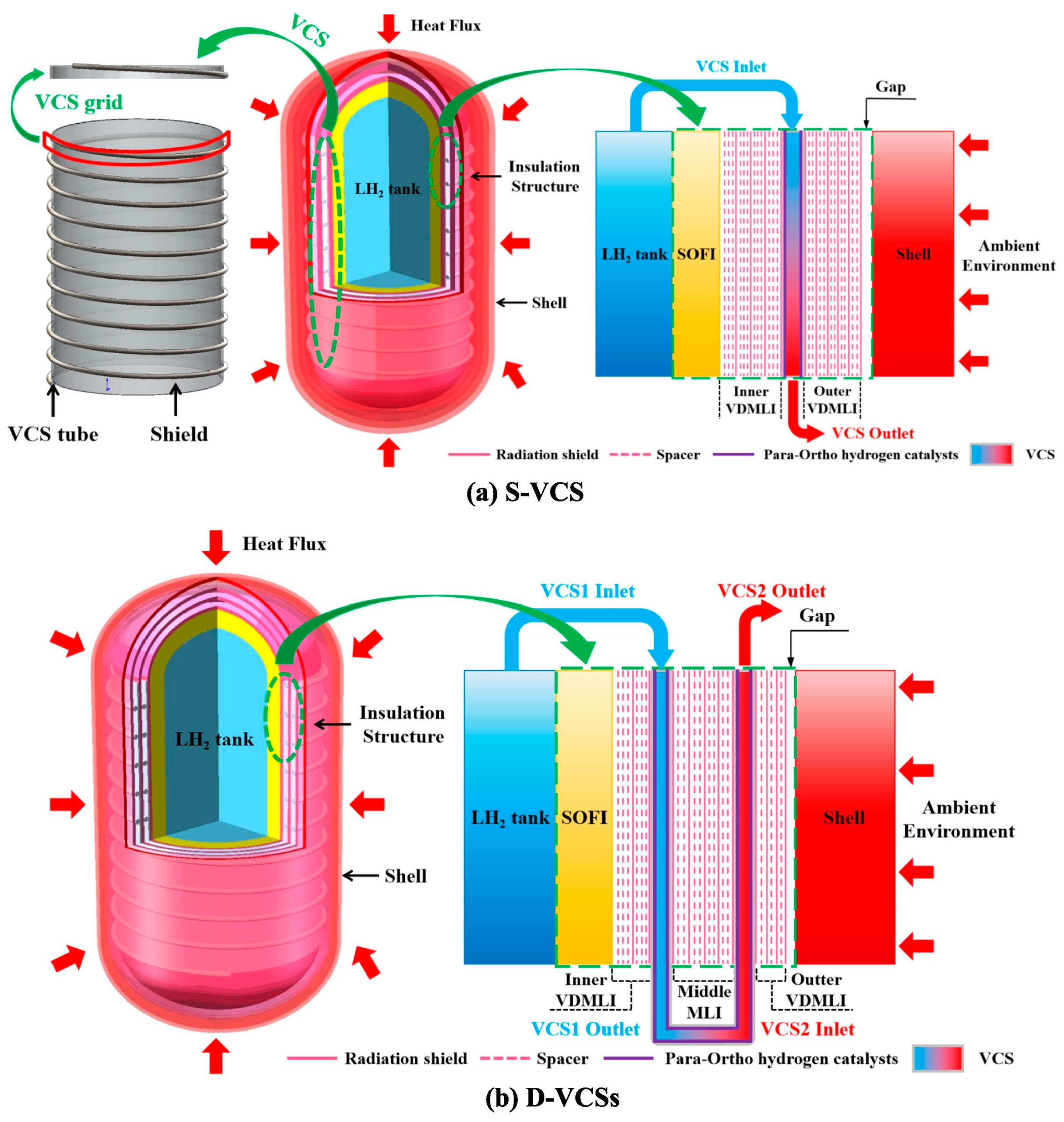
3.3. Vapor-Cooled Shield (VCS) Technology
3.4. Para-Hydrogen Refrigeration Technology
3.5. Passive Venting Technology
4. Active Cooling Technologies
4.1. Cryogenic Fluid Mixing Technology
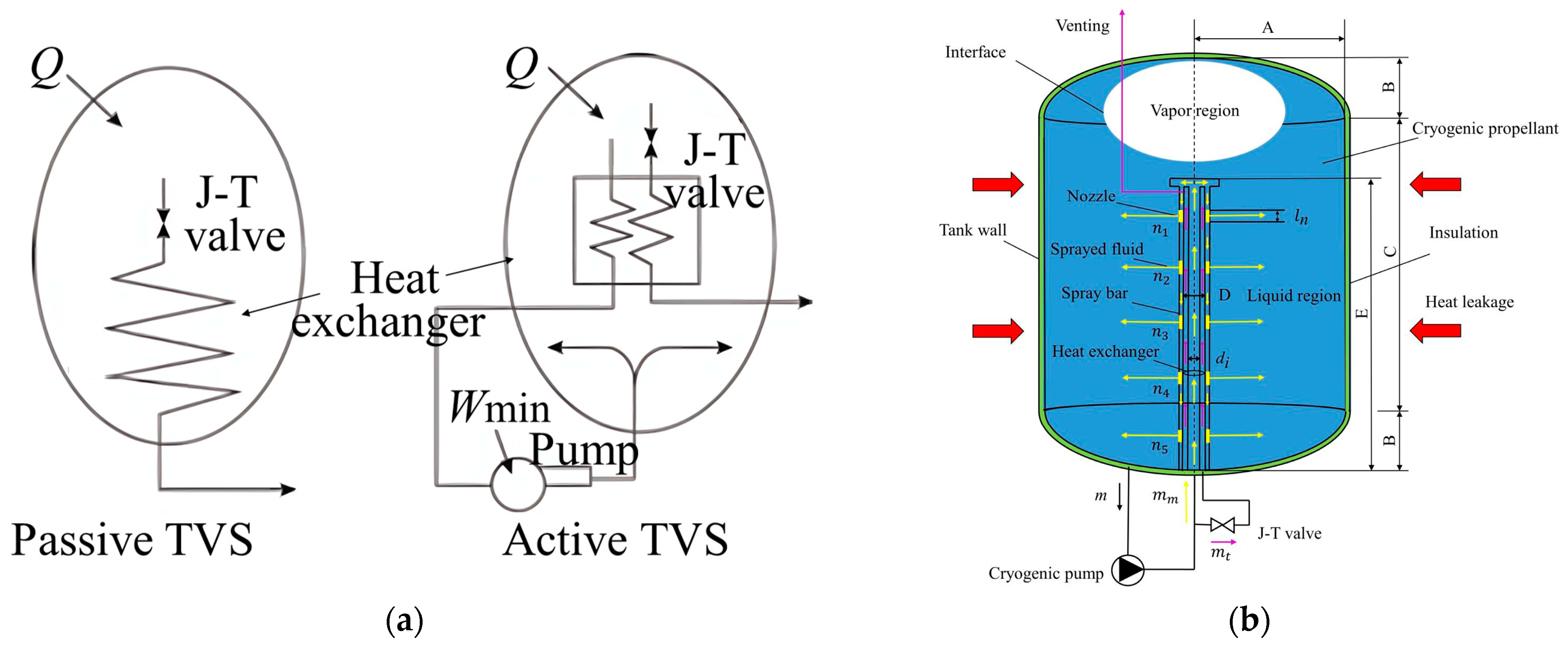
4.2. Thermodynamic Vent System (TVS)
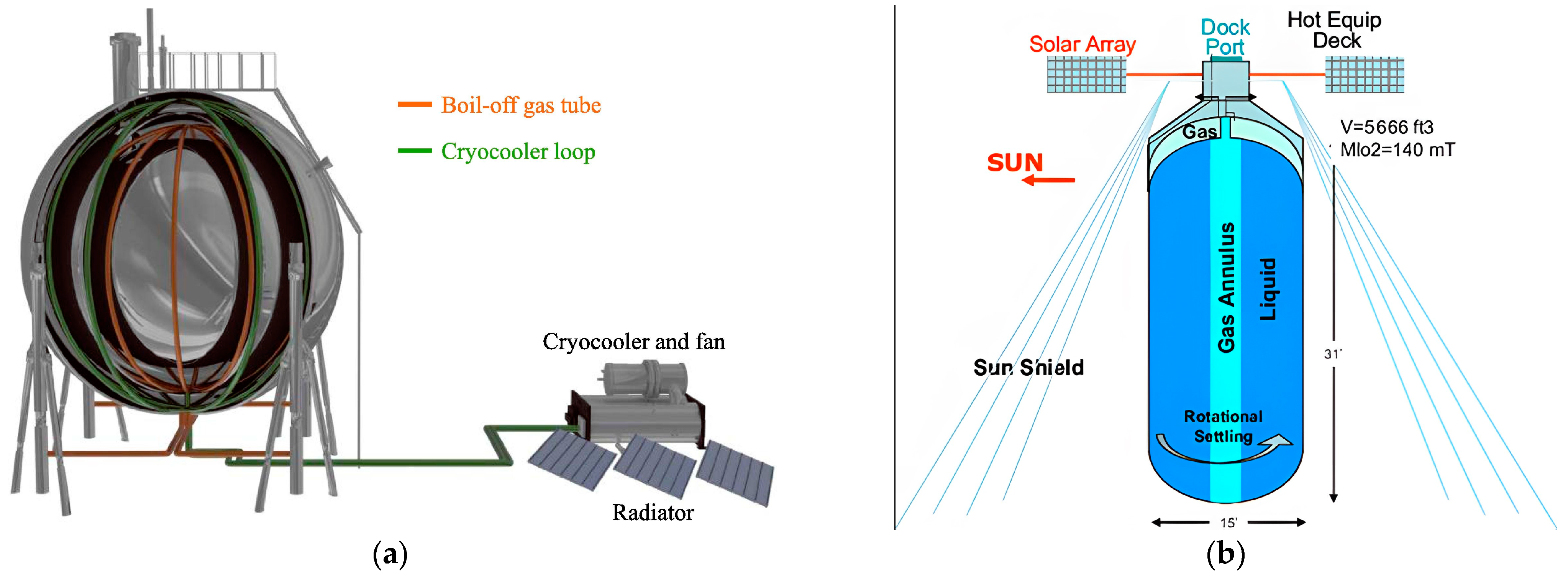
4.3. Cryocooler Technology
4.3.1. Typical Cryocooler Configurations
4.3.2. Large Area Cooling Technology
5. Advances in Boil-Off Hydrogen (BOH) Recovery Technologies
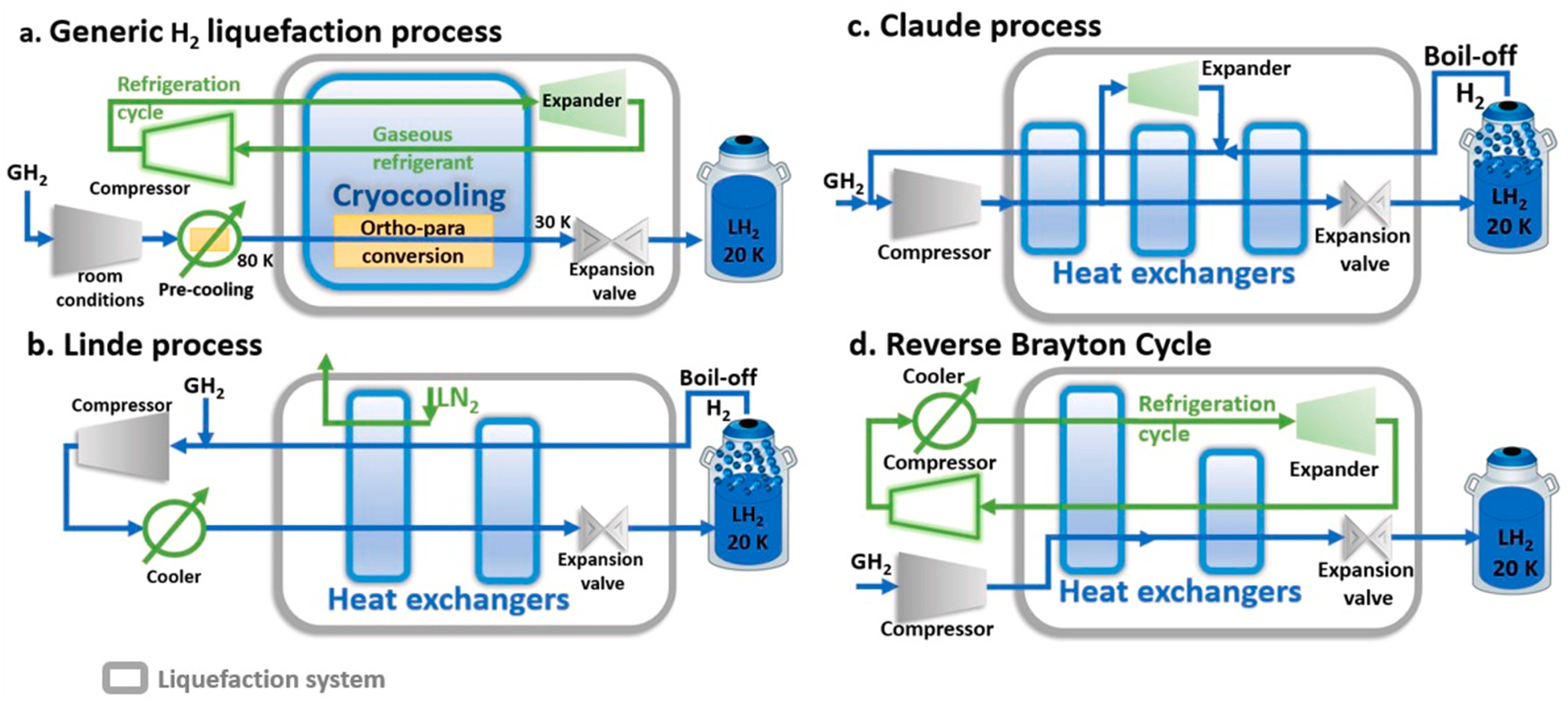
5.1. Hydrogen Reliquefaction
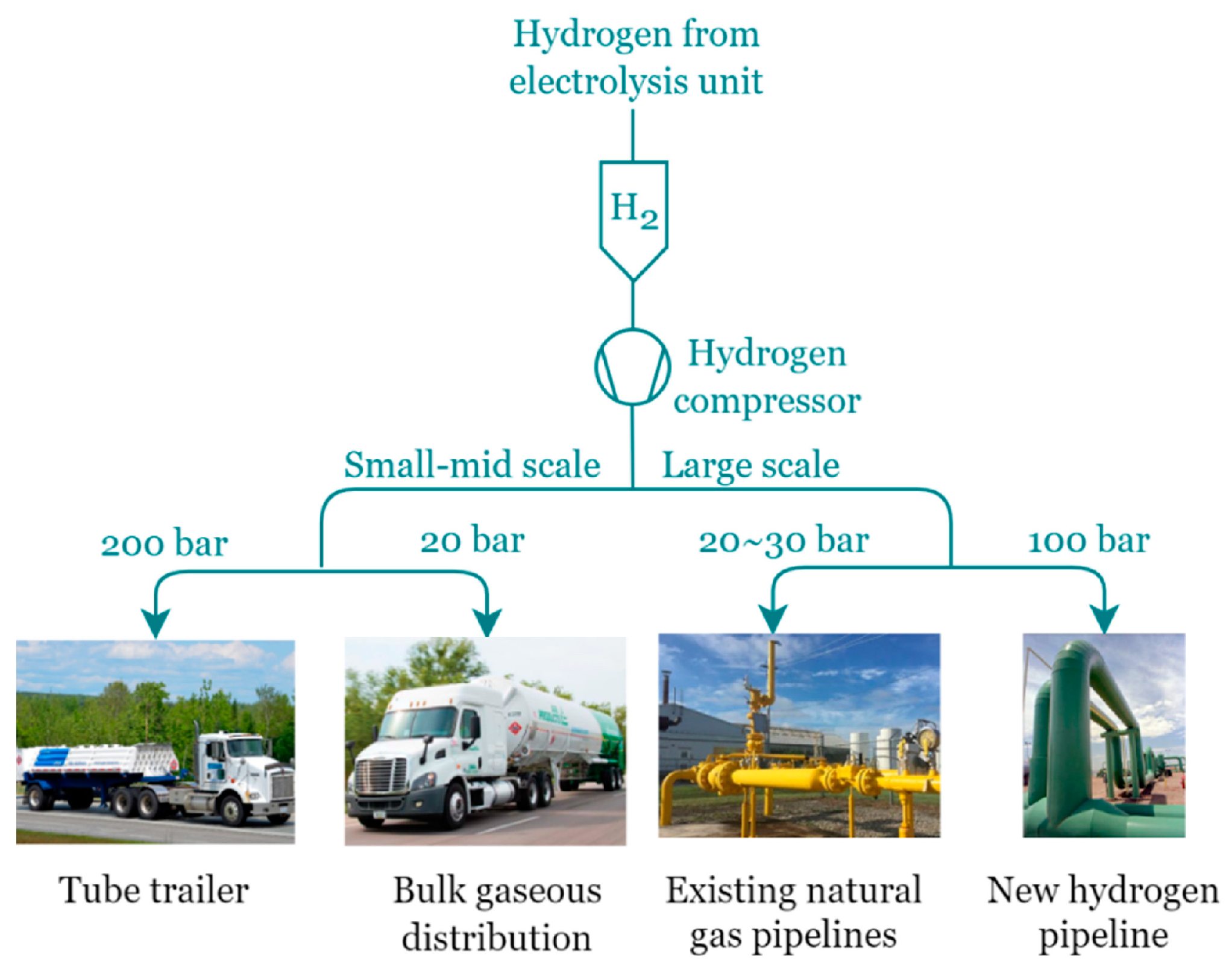
5.2. Hydrogen Recompression
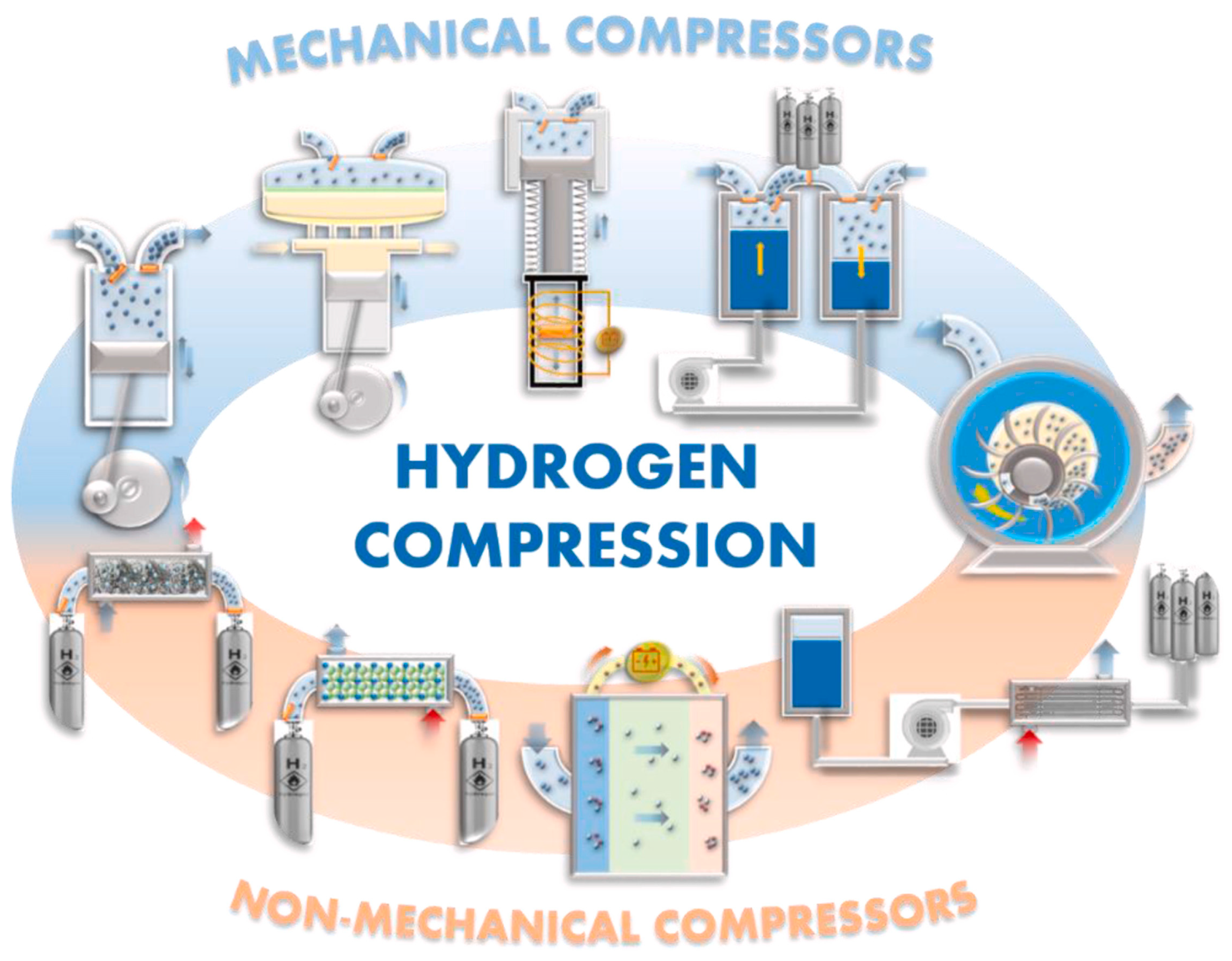
5.2.1. Mechanical Compression Technologies
5.2.2. Non-Mechanical Compression Technologies
6. Evaluation and Optimization of LH2 Storage Technologies
6.1. Technology Evaluation and Comparison
6.2. Feasible Scheme Design for Lunar LH2 Storage
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Saha, D.; Bazmohammadi, N.; Raya-Armenta, J.M.; Bintoudi, A.D.; Lashab, A.; Vasquez, J.C.; Guerrero, J.M. Space microgrids for future manned lunar bases: A review. IEEE Open Access J. Power Energy 2021, 8, 570–583. [Google Scholar] [CrossRef]
- Benaroya, H.; Bernold, L. Engineering of lunar bases. Acta Astronaut. 2008, 62, 277–299. [Google Scholar] [CrossRef]
- Wu, X. The International Lunar Research Station: China’s New Era of Space Cooperation and Its New Role in the Space Legal Order. Space Policy 2023, 65, 101537. [Google Scholar] [CrossRef]
- Smith, M.; Craig, D.; Herrmann, N.; Mahoney, E.; Krezel, J.; McIntyre, N.; Goodliff, K. The Artemis program: An overview of NASA’s activities to return humans to the moon. In Proceedings of the 2020 IEEE Aerospace Conference, Big Sky, MT, USA, 7–14 March 2020; pp. 1–10. [Google Scholar]
- Zuniga, A.; Modi, H.; Kaluthantrige, A.; Vertadier, H. Building an economical and sustainable lunar infrastructure to enable human missions. In Proceedings of the International Astronautical Congress, Washington, DC, USA, 21–25 October 2019. [Google Scholar]
- Vaniman, D.; Reedy, R.; Heiken, G.; Olhoeft, G.; Mendell, W. The lunar environment. Lunar Sourceb. 1991, 1, 27–60. [Google Scholar]
- Sherwood, B. Principles for a practical Moon base. Acta Astronaut. 2019, 160, 116–124. [Google Scholar] [CrossRef]
- Bennett, N.J.; Ellender, D.; Dempster, A.G. Commercial viability of lunar in-situ resource utilization (ISRU). Planet. Space Sci. 2020, 182, 104842. [Google Scholar] [CrossRef]
- Li, S.; Milliken, R.E. Water on the surface of the Moon as seen by the Moon Mineralogy Mapper: Distribution, abundance, and origins. Sci. Adv. 2017, 3, e1701471. [Google Scholar] [CrossRef]
- Guzik, M.C.; Jakupca, I.; Gilligan, R.; Bennett, W.R.; Smith, P.J.; Fincannon, J. Regenerative fuel cell power systems for lunar and Martian surface exploration. In Proceedings of the AIAA SPACE and Astronautics Forum and Exposition, Orlando, FL, USA, 12–14 September 2017; p. 5368. [Google Scholar]
- Doherty, M.; Gaby, J.; Salerno, L.; Sutherlin, S. Cryogenic fluid management technology for moon and mars missions. In Proceedings of the AIAA Space 2009 Conference & Exposition, Pasadena, CA, USA, 14–17 September 2009; p. 6532. [Google Scholar]
- Patterson, G.; Stickle, A.; Turner, F.; Jensen, J.; Bussey, D.; Spudis, P.; Espiritu, R.; Schulze, R.; Yocky, D.; Wahl, D. Bistatic radar observations of the Moon using Mini-RF on LRO and the Arecibo Observatory. Icarus 2017, 283, 2–19. [Google Scholar] [CrossRef]
- Honniball, C.; Lucey, P.; Li, S.; Shenoy, S.; Orlando, T.; Hibbitts, C.; Hurley, D.; Farrell, W. Molecular water detected on the sunlit Moon by SOFIA. Nat. Astron. 2021, 5, 121–127. [Google Scholar] [CrossRef]
- Luchsinger, K.M.; Chanover, N.J.; Strycker, P.D. Water within a permanently shadowed lunar crater: Further LCROSS modeling and analysis. Icarus 2021, 354, 114089. [Google Scholar] [CrossRef]
- Li, S.; Lucey, P.G.; Milliken, R.E.; Hayne, P.O.; Fisher, E.; Williams, J.-P.; Hurley, D.M.; Elphic, R.C. Direct evidence of surface exposed water ice in the lunar polar regions. Proc. Natl. Acad. Sci. USA 2018, 115, 8907–8912. [Google Scholar] [CrossRef] [PubMed]
- Rubanenko, L.; Venkatraman, J.; Paige, D.A. Thick ice deposits in shallow simple craters on the Moon and Mercury. Nat. Geosci. 2019, 12, 597–601. [Google Scholar] [CrossRef]
- Sanin, A.; Mitrofanov, I.; Litvak, M.; Bakhtin, B.; Bodnarik, J.; Boynton, W.V.; Chin, G.; Evans, L.; Harshman, K.; Fedosov, F. Hydrogen distribution in the lunar polar regions. Icarus 2017, 283, 20–30. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.-Z.; Zhang, J.-L. Review of energy supply techniques for ice in-situ thermal mining in lunar permanently shadowed regions. Adv. Space Res. 2024, 74, 784–799. [Google Scholar] [CrossRef]
- Kornuta, D.; Abbud-Madrid, A.; Atkinson, J.; Barr, J.; Barnhard, G.; Bienhoff, D.; Blair, B.; Clark, V.; Cyrus, J.; DeWitt, B. Commercial lunar propellant architecture: A collaborative study of lunar propellant production. Reach 2019, 13, 100026. [Google Scholar] [CrossRef]
- Sowers, G.F.; Dreyer, C.B. Ice mining in lunar permanently shadowed regions. New Space 2019, 7, 235–244. [Google Scholar] [CrossRef]
- LEAG Volatiles Specific Action Team: Final Report. Available online: https://lunarvolatiles.nasa.gov/wp-content/uploads/sites/46/2019/01/LEAG-VSAT-Report_123114.pdf (accessed on 23 April 2025).
- Bounova, G.; Ahn, J.; Hofstetter, W.; Wooster, P.; Hassan, R.; de Weck, O. Selection and technology evaluation of moon/mars transportation architectures. In Proceedings of the Space 2005, Pasadena, CA, USA, 18 January 2005; p. 6790. [Google Scholar]
- Sanders, G.B.; Kleinhenz, J.E.; Hilburger, M.W. Using ISRU and Surface Construction to Define Long-Term Lunar Infrastructure Needs. In Proceedings of the AIAA Aviation Forum and ASCEND, Las Vegas, NV, USA, 29 July–2 August 2024; p. 4840. [Google Scholar]
- Ellery, A. Sustainable in-situ resource utilization on the moon. Planet. Space Sci. 2020, 184, 104870. [Google Scholar] [CrossRef]
- Chin, G.; Brylow, S.; Foote, M.; Garvin, J.; Kasper, J.; Keller, J.; Litvak, M.; Mitrofanov, I.; Paige, D.; Raney, K. Lunar reconnaissance orbiter overview: Theáinstrument suite and mission. Space Sci. Rev. 2007, 129, 391–419. [Google Scholar] [CrossRef]
- Gibson Jr, E.K.; Bustin, R.; McKay, D.S. Lunar Hydrogen: A resource for future use at lunar bases and space activities. In Proceedings of the NASA, Lewis Research Center, Lunar Helium-3 and Fusion Power, Houston, TX, USA, 5–7 April 1988. [Google Scholar]
- Hampton, S.S. Conditions for Making Lunar In-Situ Resource Utilization (ISRU) Valuable for a Mars-Forward Future; University of Houston: Houston, TX, USA, 2020. [Google Scholar]
- Metzger, P.T.; Sapkota, D.; Fox, J.; Bennett, N. Aqua Factorem: Ultra Low Energy Lunar Water Extraction. Available online: https://www.nasa.gov/general/aqua-factorem-ultra-low-energy-lunar-water-extraction/ (accessed on 23 April 2025).
- Sowers, G.F. The business case for lunar ice mining. New Space 2021, 9, 77–94. [Google Scholar] [CrossRef]
- Carrato, P.; Demitz, J.; Mueller, R.; Gulen, J.; Benz, A. A Process Plant for Producing Rocket Fuel From Lunar Ice. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Salt Lake City, UT, USA, 11–14 November 2019; p. V006T06A108. [Google Scholar]
- Pelech, T.M.; Roesler, G.; Saydam, S. Technical evaluation of Off-Earth ice mining scenarios through an opportunity cost approach. Acta Astronaut. 2019, 162, 388–404. [Google Scholar] [CrossRef]
- Charania, A.; DePasquale, D. Economic analysis of a lunar in-situ resource utilization (ISRU) propellant services market. In Proceedings of the 58th International Astronautical Conference—IAC Paper, Hyderabad, India, 24–28 September 2007. Paper IAC-07-A5.1.03. [Google Scholar]
- Jones, C.A.; Klovstad, J.; Judd, E.; Komar, D. Cost breakeven analysis of cis-lunar ISRU for propellant. In Proceedings of the AIAA Scitech 2019 Forum, San Diego, CA, USA, 7–11 January 2019; p. 1372. [Google Scholar]
- Metzger, P.T. Economics of in-space industry and competitiveness of lunar-derived rocket propellant. Acta Astronaut. 2023, 207, 425–444. [Google Scholar] [CrossRef]
- Kutter, B.; Zegler, F.; Sowers, G.; Johnson, L. Cislunar-1000: Transportation Supporting a Self-Sustaining Space Economy. Available online: https://sciences.ucf.edu/class/wp-content/uploads/sites/23/2017/02/Cislunar-1000-Transporation-Space-2016.pdf (accessed on 23 April 2025).
- Lavoie, T.; Spudis, P.D. The purpose of human spaceflight and a lunar architecture to explore the potential of resource utilization. In Space 2016; AIAA: Reston, VA, USA, 2016; p. 5526. [Google Scholar]
- Wilcox, B. An architecture for sustainable human exploration of Mars enabled by water from the lunar poles. In Proceedings of the 2017 IEEE Aerospace Conference, Big Sky, MT, USA, 4–11 March 2017; pp. 1–8. [Google Scholar]
- Nunn, C.; Garcia, R.F.; Nakamura, Y.; Marusiak, A.G.; Kawamura, T.; Sun, D.; Margerin, L.; Weber, R.; Drilleau, M.; Wieczorek, M.A.; et al. Lunar seismology: A data and instrumentation review. Space Sci. Rev. 2020, 216, 89. [Google Scholar] [CrossRef]
- Chui, T.; Zhang, B.; Barmatz, M.; Hahn, I.; Penanen, K.; Hays, C.; Strayer, D.; Liu, Y.; Zhong, F.; Young, J.; et al. Cryogenics for lunar exploration. Cryogenics 2006, 46, 74–81. [Google Scholar] [CrossRef]
- Benaroya, H. Lunar habitats: A brief overview of issues and concepts. Reach 2017, 7, 14–33. [Google Scholar] [CrossRef]
- Kutter, B.; Zegler, F.; Bulk, T.; Pitchford, B.; Barr, J. Robust lunar exploration using an efficient lunar lander derived from existing upper stages. In Proceedings of the AIAA SPACE 2009 Conference & Exposition, Pasadena, CA, USA, 14–17 September 2009; p. 6566. [Google Scholar]
- Palos, M.F.; Serra, P.; Fereres, S.; Stephenson, K.; González-Cinca, R. Lunar ISRU energy storage and electricity generation. Acta Astronaut. 2020, 170, 412–420. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, R.; Xu, J.; Ding, L.; Luo, H.; Fan, J.; Chen, E.J.; Cai, L.; Tang, B. In-situ construction method for lunar habitation: Chinese Super Mason. Autom. Constr. 2019, 104, 66–79. [Google Scholar] [CrossRef]
- Zhou, C.; Tang, B.; Ding, L.; Sekula, P.; Zhou, Y.; Zhang, Z. Design and automated assembly of Planetary LEGO Brick for lunar in-situ construction. Autom. Constr. 2020, 118, 103282. [Google Scholar] [CrossRef]
- Haruyama, J.; Morota, T.; Kobayashi, S.; Sawai, S.; Lucey, P.G.; Shirao, M.; Nishino, M.N. Lunar holes and lava tubes as resources for lunar science and exploration. In Moon: Prospective Energy and Material Resources; Springer: Berlin/Heidelberg, Germany, 2012; pp. 139–163. [Google Scholar]
- Ding, J.; Xie, G.; Guo, L.; Xiong, X.; Han, Y.; Wang, X. Karst Cave as Terrestrial Simulation Platform to Test and Design Human Base in Lunar Lava Tube. Space Sci. Technol. 2022, 2022, 9875780. [Google Scholar] [CrossRef]
- Arya, A.; Rajasekhar, R.; Thangjam, G.; Ajai; Kumar, A.K. Detection of potential site for future human habitability on the Moon using Chandrayaan-1 data. Curr. Sci. 2011, 100, 524–529. [Google Scholar]
- Steigerwald, W. NASA’s LRO Finds Lunar Pits Harbor Comfortable Temperatures. National Aeronautics and Space Administration. Available online: https://www.nasa.gov/solar-system/nasas-lro-finds-lunar-pits-harbor-comfortable-temperatures/ (accessed on 23 April 2025).
- Kalita, H.; Quintero, A.; Wissing, A.; Haugh, B.; Angie, C.; Nail, G.; Wilson, J.; Richards, J.; Landin, J.; Kukkala, K. Evaluation of Lunar Pits and Lava Tubes for Use as Human Habitats. In Earth and Space 2021; ASCE: Reston, VA, USA, 2021; pp. 944–957. [Google Scholar]
- Xiao, L.; Huang, J.; Zhao, J.; Zhao, J. Significance and preliminary proposal for exploring the lunar lava tubes. Sci. Sin. Phys. Mech. Astron. 2018, 48, 119602. [Google Scholar] [CrossRef]
- Feng, Y.; Pan, P.-Z.; Tang, X.; Wang, Z.; Li, Y.; Hussain, A. A comprehensive review of lunar lava tube base construction and field research on a potential Earth test site. Int. J. Min. Sci. Technol. 2024, 34, 1201–1216. [Google Scholar] [CrossRef]
- Bennett, N.J.; Xie, R.; Dempster, A.G. The Moon and NEAs as sources of cislunar propellant; removing some constraints from a recent paper drives down lunar sourced propellant cost. Acta Astronaut. 2022, 190, 409–412. [Google Scholar] [CrossRef]
- Hurlbert, E.; Baine, M.; Grush, G. An open exploration architecture using an L-1 space propellant depot. In Proceedings of the SpaceOps 2010 Conference Delivering on the Dream Hosted by NASA Marshall Space Flight Center and Organized by AIAA, Huntsville, AL, USA, 25–30 April 2010; p. 2158. [Google Scholar]
- Goff, J.; Zegler, F.; Bienhoff, D.; Marchetta, J.; Kutter, B.; Chandler, F. Realistic near-term propellant depots: Implementation of a critical spacefaring capability. In Proceedings of the AIAA Space 2009 Conference & Exposition, Pasadena, CA, USA, 14–17 September 2009; p. 6756. [Google Scholar]
- Sommariva, A.; Gaudenzi, P.; Pianorsi, M.; Pasquali, M.; Vittori, E.; Eugeni, M.; Italiano, M.; Telli, C.; Di Nicola, M.; Gori, L. Preliminary analyses on technical and economic viability of moon-mined propellant for on-orbit refueling. Acta Astronaut. 2023, 204, 425–433. [Google Scholar] [CrossRef]
- Kinefuchi, K.; Miyakita, T.; Umemura, Y.; Nakajima, J.; Koga, M. Cooling system optimization of cryogenic propellant storage on lunar surface. Cryogenics 2022, 124, 103494. [Google Scholar] [CrossRef]
- Kutter, B.; Oneil, G.; Pitchford, B.; Zegler, F. A practical, affordable cryogenic propellant depot based on ULA’s flight experience. In Proceedings of the AIAA Space 2008 Conference & Exposition, San Diego, CA, USA, 9–11 September 2008; p. 7644. [Google Scholar]
- Bennett, N.J.; Dempster, A.G. Lowering the barriers to lunar sourced propellant via competitive parity pricing. Acta Astronaut. 2022, 191, 88–98. [Google Scholar] [CrossRef]
- Oeftering, R. A cis-lunar propellant infrastructure for flexible path exploration and space commerce. In Proceedings of the AIAA SPACE 2011 Conference & Exposition, Long Beach, CA, USA, 27–29 September 2011; p. 7113. [Google Scholar]
- Bennett, N.J.; Dempster, A.G. Geosynchronous transfer orbits as a market for impulse delivered by lunar sourced propellant. Planet. Space Sci. 2020, 182, 104843. [Google Scholar] [CrossRef]
- Black, J.; Slapakova, L.; Martin, K. Future Uses of Space out to 2050. RAND Corporation; RAND Corporation: Santa Monica, CA, USA; Cambridge, UK, 2022. [Google Scholar]
- Mueller, R.P. Lunar Base Construction Planning. In Earth and Space 2022; ASCE: Reston, VA, USA, 2022; pp. 858–870. [Google Scholar]
- Yin, L.; Yang, H.; Ju, Y. Review on the key technologies and future development of insulation structure for liquid hydrogen storage tanks. Int. J. Hydrogen Energy 2024, 57, 1302–1315. [Google Scholar] [CrossRef]
- Black, I. Basic Investigation of Multi-Layer Insulation Systems Final Report; NASA: Washington, DC, USA, 1964.
- Johnson, W.L. Thermal Performance of Cryogenic Multilayer Insulation at Various Layer Spacings; NASA Kennedy Space Center: Cape Canaveral, FL, USA, 2010. [Google Scholar]
- Hastings, L.J.; Martin, J.J. Experimental testing of a foam/multilayer insulation (FMLI) thermal control system (TCS) for use on a cryogenic upper stage. In Proceedings of the Space Technology and Applications International Forum—STAIF-98, Albuquerque, NM, USA, 15 January 1998; pp. 331–341. [Google Scholar]
- Johnson, W. Thermal analysis of low layer density multilayer insulation test results. In Proceedings of the 57th Cryogenic Engineering Conference (CEC), Spokane, WA, USA, 13–17 June 2011; Volume 1434, pp. 1519–1526. [Google Scholar]
- Hastings, L.; Hedayat, A.; Brown, T. Analytical Modeling and Test Correlation of Variable Density Multilayer Insulation for Cryogenic Storage; NASA Marshall Space Flight Center: Huntsville, AL, USA, 2004. [Google Scholar]
- Martin, J.; Hastings, L. Large-Scale Liquid Hydrogen Testing of Variable Density Multilayer Insulation with a Foam Substrate. 2001. Available online: https://ntrs.nasa.gov/api/citations/20010063699/downloads/20010063699.pdf (accessed on 23 April 2025).
- Wang, B.; Huang, Y.; Li, P.; Sun, P.; Chen, Z.; Wu, J. Optimization of variable density multilayer insulation for cryogenic application and experimental validation. Cryogenics 2016, 80, 154–163. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, L.; Cui, C.; Guo, J.; Zhu, W.; Zhou, Y.; Wang, J. Experimental study on composite insulation system of spray on foam insulation and variable density multilayer insulation. Appl. Therm. Eng. 2018, 130, 161–168. [Google Scholar] [CrossRef]
- Wang, P.; Ji, L.; Yuan, J.; An, Z.; Yan, K.; Zhang, J. Modeling and optimization of composite thermal insulation system with HGMs and VDMLI for liquid hydrogen on orbit storage. Int. J. Hydrogen Energy 2020, 45, 7088–7097. [Google Scholar] [CrossRef]
- Wang, P.; Ji, L.; Yuan, J.; An, Z.; Yan, K.; Zhang, J. The influence of inner material with different average thermal conductivity on the performance of whole insulation system for liquid hydrogen on orbit storage. Int. J. Hydrogen Energy 2021, 46, 10913–10923. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Dang, Z.; Jiao, J.; Pang, C. Research on effective thermal conductivity of hollow glass microspheres under vacuum at low temperature. Appl. Therm. Eng. 2024, 252, 123675. [Google Scholar] [CrossRef]
- Chen, L.; Lv, H.; Shang, Y.; Zhang, Z.; Chen, S.; Hou, Y. Strategies for improving the thermal insulation performance of liquid hydrogen tanks: A review of multilayer insulation structure. Int. J. Hydrogen Energy 2024, 94, 1419–1434. [Google Scholar] [CrossRef]
- Dew, M.; Lin, J.; Kutter, B.; Madlangbayan, A.; Willey, C.; Allwein, K.; Pitchford, B.; ONeil, G.; Ware, J. Design and development of an in-space deployable sun shield for Atlas Centaur. In Proceedings of the AIAA SPACE 2008 Conference & Exposition, San Diego, CA, USA, 9–11 September 2008; p. 7764. [Google Scholar]
- Plachta, D.; Christie, R.; Jurns, J.; Kittel, P. Passive ZBO storage of liquid hydrogen and liquid oxygen applied to space science mission concepts. Cryogenics 2006, 46, 89–97. [Google Scholar] [CrossRef]
- Chen, Q.; Gao, Y.; Ding, L.; Zhou, C.; Han, W.; Zhou, Y.; Shi, Y. Genetic Algorithm–Based Multiobjective Optimization for 3D Printable Design of a Double-Shell Lunar Habitat Structure. J. Aerosp. Eng. 2023, 36, 04023069. [Google Scholar] [CrossRef]
- Steiner, J.T.; Malla, R.B. A study of layered structural configurations as thermal and impact shielding of lunar habitats. In Earth and Space 2021; ASCE: Reston, VA, USA, 2021; pp. 1285–1296. [Google Scholar]
- Mottaghi, S.; Benaroya, H. Design of a lunar surface structure. I: Design configuration and thermal analysis. J. Aerosp. Eng. 2015, 28, 04014052. [Google Scholar] [CrossRef]
- Tripathi, S.; Steiner, J.T.; Malla, R.B. Three-dimensional temperature profile in a dome-shaped habitat structure on the moon. Acta Astronaut. 2023, 204, 263–280. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, Y.; Cheng, S.; Han, W.; Zhou, C.; Ding, L. Performance of lunar shell structure for moonbase subjected to low gravity coupled with changing temperature. Fundam. Res. 2024; in press. [Google Scholar]
- Zheng, J.; Chen, L.; Wang, J.; Xi, X.; Zhu, H.; Zhou, Y.; Wang, J. Thermodynamic analysis and comparison of four insulation schemes for liquid hydrogen storage tank. Energy Convers. Manag. 2019, 186, 526–534. [Google Scholar] [CrossRef]
- Xu, X.; Xu, H.; Yang, B.; Chen, L.; Wang, J. A Novel Composite Insulation System of Hollow Glass Microspheres and Multilayer Insulation with Self-Evaporating Vapor Cooled Shield for Liquid Hydrogen Storage. Energy Technol. 2020, 8, 2000591. [Google Scholar] [CrossRef]
- Jiang, W.; Yang, Y.; Hu, C.; Li, P.; Sun, P.; Huang, Y. Experimental study on composite insulation with foam, multilayer and vapor cooled shield for cryogen storage under different vacuum conditions. Cryogenics 2023, 129, 103604. [Google Scholar] [CrossRef]
- Jiang, W.; Sun, P.; Li, P.; Zuo, Z.; Huang, Y. Transient thermal behavior of multi-layer insulation coupled with vapor cooled shield used for liquid hydrogen storage tank. Energy 2021, 231, 120859. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, L.; Wang, J.; Zhou, Y.; Wang, J. Thermodynamic modelling and optimization of self-evaporation vapor cooled shield for liquid hydrogen storage tank. Energy Convers. Manag. 2019, 184, 74–82. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, L.; Wang, P.; Zhang, J.; Wang, J.; Zhou, Y. A novel cryogenic insulation system of hollow glass microspheres and self-evaporation vapor-cooled shield for liquid hydrogen storage. Front. Energy 2020, 14, 570–577. [Google Scholar] [CrossRef]
- Leng, Y.; Zhang, S.; Wang, X.; Pu, L.; Xu, P. Comparative study on thermodynamic performance of liquid hydrogen storage insulation system incorporating vapor-cooled shield with para–ortho hydrogen conversion by one-dimensional and quasi-two-dimensional model. Energy Convers. Manag. 2024, 321, 119068. [Google Scholar] [CrossRef]
- Meier, R.; Marple, J.; Lee, C. A Miniature Heat Exchanger for Simultaneous Para-Orthohydrogen Conversion and Heat Transfer. In Proceedings of the Advances in Cryogenic Engineering: Proceedings of the 1968 Cryogenic Engineering Conference Case Western Reserve University, Cleveland, OH, USA, 19–21 August 1968; pp. 185–193. [Google Scholar]
- WANG, L. Analysis on Cold Energy Release Schemes in Para Ortho Hydrogen Conversion and Its Utilization Potential in Space. J. Astronaut. 2019, 40, 109. [Google Scholar]
- Lv, H.; Zhang, Z.; Chen, L.; Zhang, Z.; Chen, S.; Hou, Y. Thermodynamic analysis of vapor-cooled shield with para-to-ortho hydrogen conversion in composite multilayer insulation structure for liquid hydrogen tank. Int. J. Hydrogen Energy 2024, 50, 1448–1462. [Google Scholar] [CrossRef]
- Shi, C.; Zhu, S.; Wan, C.; Bao, S.; Zhi, X.; Qiu, L.; Wang, K. Performance analysis of vapor-cooled shield insulation integrated with para-ortho hydrogen conversion for liquid hydrogen tanks. Int. J. Hydrogen Energy 2023, 48, 3078–3090. [Google Scholar] [CrossRef]
- Bliesner, R.M.; Leachman, J.W.; Adam, P.M. Parahydrogen–orthohydrogen conversion for enhanced vapor-cooled shielding of liquid oxygen tanks. J. Thermophys. Heat Transf. 2014, 28, 717–723. [Google Scholar] [CrossRef]
- Lin, C.S.; Van Dresar, N.T.; Hasan, M.M. Pressure control analysis of cryogenic storage systems. J. Propuls. Power 2004, 20, 480–485. [Google Scholar] [CrossRef]
- Bentz, M.D. Tank Pressure Control in Low Gravity by Jet Mixing. In NASA Contractor Report No. 191012; 1993; Available online: https://archive.org/details/NASA_NTRS_Archive_19950021580 (accessed on 23 April 2025).
- Lin, C.; Hasan, M.; Van Dresar, N. Experimental investigation of jet-induced mixing of a large liquid hydrogen storage tank. In Proceedings of the 6th Joint Thermophysics and Heat Transfer Conference, Colorado Springs, CO, USA, 20–23 June 1994; p. 2079. [Google Scholar]
- VanOverbeke, T. Modeling of pressure reduction due to axial-jet mixers in cryogenic tanks. In Proceedings of the 41st Aerospace Sciences Meeting and Exhibit, Reno, NV, USA, 6–9 January 2003; p. 998. [Google Scholar]
- Chang, H.-M.; Ryu, K.N.; Baik, J.H. Thermodynamic design of hydrogen liquefaction systems with helium or neon Brayton refrigerator. Cryogenics 2018, 91, 68–76. [Google Scholar] [CrossRef]
- Al Ghafri, S.Z.; Munro, S.; Cardella, U.; Funke, T.; Notardonato, W.; Trusler, J.M.; Leachman, J.; Span, R.; Kamiya, S.; Pearce, G. Hydrogen liquefaction: A review of the fundamental physics, engineering practice and future opportunities. Energy Environ. Sci. 2022, 15, 2690–2731. [Google Scholar] [CrossRef]
- Chang, H.-M.; Kim, B.H.; Choi, B. Hydrogen liquefaction process with Brayton refrigeration cycle to utilize the cold energy of LNG. Cryogenics 2020, 108, 103093. [Google Scholar] [CrossRef]
- Hastings, L.; Flachbart, R.; Martin, J.; Hedayat, A.; Fazah, M.; Lak, T.; Nguyen, H.; Bailey, J. Spray Bar Zero-Gravity Vent System for on-Orbit Liquid Hydrogen Storage; NASA Marshall Space Flight Center: Huntsville, AL, USA, 2003. [Google Scholar]
- Zheng, Y.; Yang, P.; Liu, Y.; Yang, Q.; Yan, C.; Wang, X. Parametric optimization and analysis of thermodynamic venting system in liquid hydrogen tank under microgravity. Int. J. Hydrogen Energy 2021, 46, 40041–40053. [Google Scholar] [CrossRef]
- Archipley, C.; Barclay, J.; Meinhardt, K.; Whyatt, G.; Thomsen, E.; Holladay, J.; Cui, J.; Anderson, I.; Wolf, S. Methane liquefaction with an active magnetic regenerative refrigerator. Cryogenics 2022, 128, 103588. [Google Scholar] [CrossRef]
- Gursu, S.; Sherif, S.; Veziroglu, T.; Sheffield, J. Analysis and optimization of thermal stratification and self-pressurization effects in liquid hydrogen storage systems—Part 1: Model development. Energy Resour. Technol. 1993, 115, 221–227. [Google Scholar] [CrossRef]
- Qiu, L.; He, Y.; Gan, Z.; Chen, G. A single-stage pulse tube cooler reached 12.6 K. Cryogenics 2005, 45, 641–643. [Google Scholar] [CrossRef]
- Hastings, L.J.; Plachta, D.; Salerno, L.; Kittel, P. An overview of NASA efforts on zero boiloff storage of cryogenic propellants. Cryogenics 2001, 41, 833–839. [Google Scholar] [CrossRef]
- Qiu, L.; He, Y.; Gan, Z.; Zhang, X.; Chen, G. Regenerator performance improvement of a single-stage pulse tube cooler reached 11.1 K. Cryogenics 2007, 47, 49–55. [Google Scholar] [CrossRef]
- Plachta, D.W. Hybrid Thermal Control Testing of a Cryogenic Propellant Tank; NASA Glenn Research Center: Cleveland, OH, USA, 1999.
- Nakano, A.; Maeda, T.; Ito, H.; Masuda, M.; Kawakami, Y.; Kato, A.; Tange, M.; Takahashi, T.; Matsuo, M. Small-scale hydrogen liquefaction with a two-stage Gifford–McMahon cycle refrigerator. Int. J. Hydrogen Energy 2010, 35, 9088–9094. [Google Scholar] [CrossRef]
- Hedayat, A.; Hastings, L.; Bryant, C.; Plachta, D. Large scale demonstration of liquid hydrogen storage with zero boiloff. In Proceedings of the AIP Conference Proceedings, Munich, Germany, 25–30 May 2002; pp. 1276–1283. [Google Scholar]
- Hastings, L.; Bryant, C.; Flachbart, R.; Holt, K.; Johnson, E.; Hedayat, A.; Hipp, B.; Plachta, D. Large-Scale Demonstration of Liquid Hydrogen Storage with Zero Boiloff for In-Space Applications; NASA: Washington, DC, USA, 2010.
- Plachta, D. Results of an Advanced Development Zero Boil-Off Cryogenic Propellant Storage Test. In Proceedings of the 40th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Fort Lauderdale, FL, USA, 11–14 July 2004; p. 3837. [Google Scholar]
- Plachta, D.W.; Christie, R.; Carlberg, E.; Feller, J. Cryogenic propellant boil-off reduction system. In Proceedings of the Advances in Cryogenic Engineering: Transactions of the Cryogenic Engineering Conference—CEC, Chattanooga, TN, USA, 16–20 July 2007; pp. 1457–1466. [Google Scholar]
- Plachta, D.W.; Guzik, M.C. Cryogenic boil-off reduction system. Cryogenics 2014, 60, 62–67. [Google Scholar] [CrossRef]
- Plachta, D.; Stephens, J.; Johnson, W.; Zagarola, M. NASA cryocooler technology developments and goals to achieve zero boil-off and to liquefy cryogenic propellants for space exploration. Cryogenics 2018, 94, 95–102. [Google Scholar] [CrossRef]
- Plachta, D.W.; Christie, R.J.; Feller, J.R.; Johnson, W.L. Cryogenic boil-off reduction system testing. In Proceedings of the 50th AIAA/ASME/SAE/ASEE Joint Propulsion Conference, Cleveland, OH, USA, 28–30 July 2014; p. 3579. [Google Scholar]
- Notardonato, W.; Swanger, A.; Fesmire, J.; Jumper, K.; Johnson, W.; Tomsik, T. Zero Boil-Off Methods for Large-Scale Liquid Hydrogen Tanks Using Integrated Refrigeration and Storage. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Madison, WI, USA, 9–13 July 2017; p. 012012. [Google Scholar]
- Notardonato, W.; Swanger, A.; Fesmire, J.; Jumper, K.; Johnson, W.; Tomsik, T. Final test results for the ground operations demonstration unit for liquid hydrogen. Cryogenics 2017, 88, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Petitpas, G. Boil-Off Losses Along LH2 Pathway; OSTI: Oak Ridge, TN, USA, 2018.
- Petitpas, G. Simulation of boil-off losses during transfer at a LH2 based hydrogen refueling station. Int. J. Hydrogen Energy 2018, 43, 21451–21463. [Google Scholar] [CrossRef]
- Yu, C.; Choi, M.; Chang, D. Economic analysis of cargo containment systems and boil-off hydrogen handling options for large-scale liquid hydrogen carriers considering insulation-layer thickness of liquid-hydrogen storage tank. Int. J. Hydrogen Energy 2025, 97, 1055–1067. [Google Scholar] [CrossRef]
- Choi, M.; Jung, W.; Lee, S.; Joung, T.; Chang, D. Thermal efficiency and economics of a boil-off hydrogen re-liquefaction system considering the energy efficiency design index for liquid hydrogen carriers. Energies 2021, 14, 4566. [Google Scholar] [CrossRef]
- Sadaghiani, M.S.; Mehrpooya, M. Introducing and energy analysis of a novel cryogenic hydrogen liquefaction process configuration. Int. J. Hydrogen Energy 2017, 42, 6033–6050. [Google Scholar] [CrossRef]
- Walnum, H.T.; Berstad, D.; Drescher, M.; Neksa, P.; Quack, H.; Haberstroh, C.; Essler, J. Principles for the liquefaction of hydrogen with emphasis on precooling processes. In Proceedings of the 12th Cryogenics, Dresden, Germany, 11–14 September 2012. [Google Scholar]
- Cao, H.; ter Brake, H. Progress and challenges in utilization of ejectors for cryogenic cooling. Appl. Therm. Eng. 2020, 167, 114783. [Google Scholar] [CrossRef]
- Morales-Ospino, R.; Celzard, A.; Fierro, V. Strategies to recover and minimize boil-off losses during liquid hydrogen storage. Renew. Sustain. Energy Rev. 2023, 182, 113360. [Google Scholar] [CrossRef]
- Stolzenburg, K.; Berstad, D.; Decker, L.; Elliott, A.; Haberstroh, C.; Hatto, C.; Klaus, M.; Mortimer, N.; Mubbala, R.; Mwabonje, O. Efficient liquefaction of hydrogen: Results of the IDEALHY project. In Proceedings of the XXth Energie—Symposium, Stralsund, Germany, 7–9 November 2013; pp. 1–8. [Google Scholar]
- Asadnia, M.; Mehrpooya, M. A novel hydrogen liquefaction process configuration with combined mixed refrigerant systems. Int. J. Hydrogen Energy 2017, 42, 15564–15585. [Google Scholar] [CrossRef]
- Krasae-In, S. Optimal operation of a large-scale liquid hydrogen plant utilizing mixed fluid refrigeration system. Int. J. Hydrogen Energy 2014, 39, 7015–7029. [Google Scholar] [CrossRef]
- Yin, L.; Ju, Y. Process optimization and analysis of a novel hydrogen liquefaction cycle. Int. J. Refrig. 2020, 110, 219–230. [Google Scholar] [CrossRef]
- Valenti, G.; Macchi, E. Proposal of an innovative, high-efficiency, large-scale hydrogen liquefier. Int. J. Hydrogen Energy 2008, 33, 3116–3121. [Google Scholar] [CrossRef]
- Lee, H.; Shao, Y.; Lee, S.; Roh, G.; Chun, K.; Kang, H. Analysis and assessment of partial re-liquefaction system for liquefied hydrogen tankers using liquefied natural gas (LNG) and H2 hybrid propulsion. Int. J. Hydrogen Energy 2019, 44, 15056–15071. [Google Scholar] [CrossRef]
- Elberry, A.M.; Thakur, J.; Santasalo-Aarnio, A.; Larmi, M. Large-scale compressed hydrogen storage as part of renewable electricity storage systems. Int. J. Hydrogen Energy 2021, 46, 15671–15690. [Google Scholar] [CrossRef]
- Peschel, A. Industrial perspective on hydrogen purification, compression, storage, and distribution. Fuel Cells 2020, 20, 385–393. [Google Scholar] [CrossRef]
- Tahan, M.-R. Recent advances in hydrogen compressors for use in large-scale renewable energy integration. Int. J. Hydrogen Energy 2022, 47, 35275–35292. [Google Scholar] [CrossRef]
- Sdanghi, G.; Maranzana, G.; Celzard, A.; Fierro, V. Review of the current technologies and performances of hydrogen compression for stationary and automotive applications. Renew. Sustain. Energy Rev. 2019, 102, 150–170. [Google Scholar] [CrossRef]
- Almasi, A. Latest practical notes and recent lessons learned on reciprocating compressors. Aust. J. Mech. Eng. 2016, 14, 138–150. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, B.; Yao, Y.; Jia, X.; Peng, X. Experimental study and sensitivity analysis of performance for a hydrogen diaphragm compressor. Renew. Energy 2024, 237, 121871. [Google Scholar] [CrossRef]
- Ren, S.; Jia, X.; Shi, L.; Li, K.; Peng, X. Theoretical and experimental study on improving diaphragm compressor design for hydrogen refueling stations through use of a free moving oil piston concept. J. Energy Storage 2023, 74, 109397. [Google Scholar] [CrossRef]
- Zhang, X.; Ziviani, D.; Braun, J.E.; Groll, E.A. Experimental validation and sensitivity analysis of a dynamic simulation model for linear compressors. Int. J. Refrig. 2020, 117, 369–380. [Google Scholar] [CrossRef]
- Zhang, X.; Ziviani, D.; Braun, J.E.; Groll, E.A. Theoretical analysis of dynamic characteristics in linear compressors. Int. J. Refrig. 2020, 109, 114–127. [Google Scholar] [CrossRef]
- Zhou, H.; Dong, P.; Zhu, S.; Li, S.; Zhao, S.; Wang, Y. Design and theoretical analysis of a liquid piston hydrogen compressor. J. Energy Storage 2021, 41, 102861. [Google Scholar] [CrossRef]
- Kermani, N.A.; Petrushina, I.; Rokni, M.M. Evaluation of ionic liquids as replacements for the solid piston in conventional hydrogen reciprocating compressors: A review. Int. J. Hydrogen Energy 2020, 45, 16337–16354. [Google Scholar] [CrossRef]
- Sdanghi, G.; Maranzana, G.; Celzard, A.; Fierro, V. Towards non-mechanical hybrid hydrogen compression for decentralized hydrogen facilities. Energies 2020, 13, 3145. [Google Scholar] [CrossRef]
- Orlova, S.; Mezeckis, N.; Vasudev, V. Compression of hydrogen gas for energy storage: A review. Latv. J. Phys. Tech. Sci. 2023, 60, 4–16. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Dong, X.; Yang, J.; Guo, H.; Gong, M. Thermodynamic analysis of low-temperature and high-pressure (cryo-compressed) hydrogen storage processes cooled by mixed-refrigerants. Int. J. Hydrogen Energy 2022, 47, 28932–28944. [Google Scholar] [CrossRef]
- Zohra, F.T.; Webb, C.J.; Lamb, K.E.; Gray, E.M. Degradation of metal hydrides in hydrogen-based thermodynamic machines: A review. Int. J. Hydrogen Energy 2024, 64, 417–438. [Google Scholar] [CrossRef]
- Malleswararao, K.; Dutta, P. Applications of metal hydride based thermal systems: A review. Appl. Therm. Eng. 2022, 215, 118816. [Google Scholar] [CrossRef]
- Pivac, I.; Pavasović, A.S.; Barbir, F. Recent advances and perspectives in diagnostics and degradation of electrochemical hydrogen compressors. Int. J. Hydrogen Energy 2024, 54, 387–396. [Google Scholar] [CrossRef]
- Gao, Z.; Fan, C.; Yin, Z.; Wang, S.; Zhang, L.; Xing, N.; Zhu, S.; Yao, Z.; Wu, H.; Jiang, Z. Electrochemical hydrogen Compression: Module design and membrane development. Chem. Eng. J. 2024, 488, 150733. [Google Scholar] [CrossRef]
- Myekhlai, M.; Park, S.; Webb, J.E.; Oh, H. Thermally-driven physisorption-based hydrogen compressors. Coord. Chem. Rev. 2024, 519, 216123. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Q.; Ding, Z.; Jiang, H.; Yang, H.; Du, W.; Zheng, Y.; Huo, K.; Shaw, L.L. MOFs-based materials for solid-state hydrogen storage: Strategies and perspectives. Chem. Eng. J. 2024, 485, 149665. [Google Scholar] [CrossRef]
- Yuvaraj, A.; Jayarama, A.; Sharma, D.; Nagarkar, S.S.; Duttagupta, S.P.; Pinto, R. Role of metal-organic framework in hydrogen gas storage: A critical review. Int. J. Hydrogen Energy 2024, 59, 1434–1458. [Google Scholar] [CrossRef]






| Non-Mechanical Compressor Type | Pin (MPa) | Pout (MPa) | Flowrate (kg/day) | Tin (K) | Supplier |
|---|---|---|---|---|---|
| Cryogenic | 0.1 | 69 | Up to 129 | 20 | Air Products |
| Metal hydride | 0.2–3 | 25 | 8.5–25.5 | 258–298 | Hystorsis |
| Electrochemical | 0.3–1.5 | Up to 90 | 2, 10, 120–200, 500–2000 | ≥298 | HyET Inc. |
| Adsorption | 0.25 | 35 | <155.52 | 80/114/172/298 | Toyota |
| Thermal Management Type | Technology | Efficiency | Energy Consumption | Complexity | Cost | Applicability |
|---|---|---|---|---|---|---|
| Passive cooling | Insulation material | 5 | 5 | 4 | 4 | 5 |
| Radiation shielding | 3 | 5 | 5 | 5 | 5 | |
| VCS | 4 | 4 | 3 | 4 | 4 | |
| Para-hydrogen | 4 | 4 | 3 | 4 | 5 | |
| Passive venting | 1 | 1 | 3 | 1 | 2 | |
| Active cooling | Fluid mixing | 2 | 3 | 4 | 4 | 2 |
| TVS | 4 | 3 | 3 | 3 | 4 | |
| Cryocooler | 3 | 2 | 2 | 2 | 3 | |
| BAC | 5 | 2 | 3 | 2 | 5 | |
| BOH recovery | Reliquefaction | 4 | 2 | 2 | 2 | 3 |
| Recompression | 3 | 3 | 3 | 3 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Fan, F.; Xu, J.; Li, H.; Mei, J.; Fei, T.; Sun, C.; Jiang, J.; Xue, R.; Yang, W.; et al. Advances in Thermal Management for Liquid Hydrogen Storage: The Lunar Perspective. Energies 2025, 18, 2220. https://doi.org/10.3390/en18092220
Li J, Fan F, Xu J, Li H, Mei J, Fei T, Sun C, Jiang J, Xue R, Yang W, et al. Advances in Thermal Management for Liquid Hydrogen Storage: The Lunar Perspective. Energies. 2025; 18(9):2220. https://doi.org/10.3390/en18092220
Chicago/Turabian StyleLi, Jing, Fulin Fan, Jingkai Xu, Heran Li, Jian Mei, Teng Fei, Chuanyu Sun, Jinhai Jiang, Rui Xue, Wenying Yang, and et al. 2025. "Advances in Thermal Management for Liquid Hydrogen Storage: The Lunar Perspective" Energies 18, no. 9: 2220. https://doi.org/10.3390/en18092220
APA StyleLi, J., Fan, F., Xu, J., Li, H., Mei, J., Fei, T., Sun, C., Jiang, J., Xue, R., Yang, W., & Song, K. (2025). Advances in Thermal Management for Liquid Hydrogen Storage: The Lunar Perspective. Energies, 18(9), 2220. https://doi.org/10.3390/en18092220










