Reinforcement of Rubber Magnetic Composites with Zinc Salts of Acrylic and Methacrylic Acids
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
2.2.1. Determinstion of Structural and Magnetic Characteristics of Ferrite
2.2.2. Preparation and Curing of Rubber Compounds
2.2.3. Determination of Cross-Link Density of Composites
- ν—Cross-link density (mol/cm3)
- Vr0—Volume fraction of rubber in equilibrium swelling sample of vulcanizate in absence of fillers
- Vr—Volume fraction of rubber in equilibrium swelling sample of filled vulcanizate
- VS—Molar volume of solvent (for acetone = 73.52 cm3/mol)
- χ—Huggins interaction parameter (for NBR-acetone χ = 0.3692)
2.2.4. Determination of Physical-Mechanical Properties
2.2.5. Evaluation of Dynamical-Mechanical Properties
2.2.6. Determination of Magnetic Characteristics
2.2.7. Microscopic Analysis of Composites
3. Results and Discussions
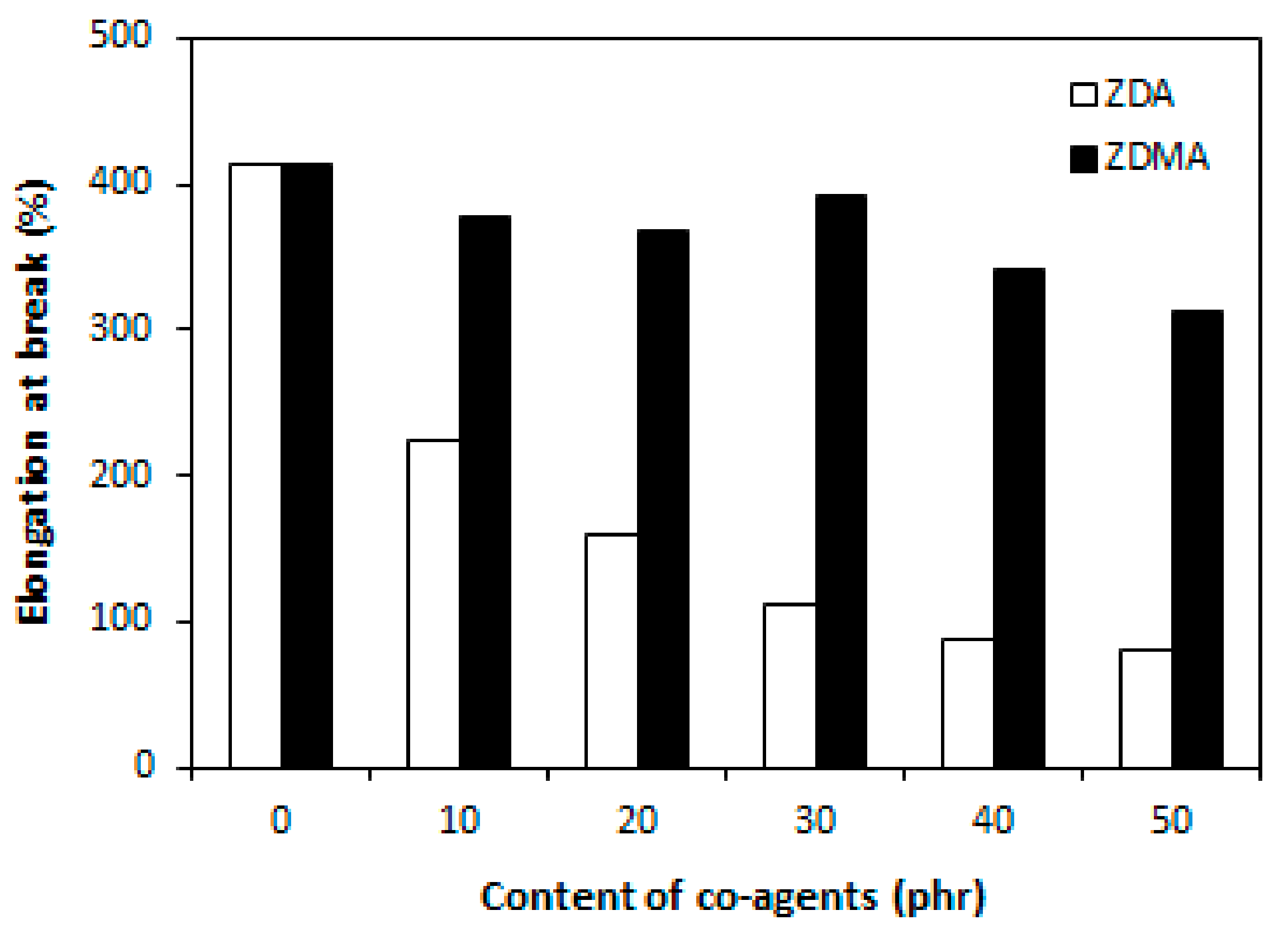
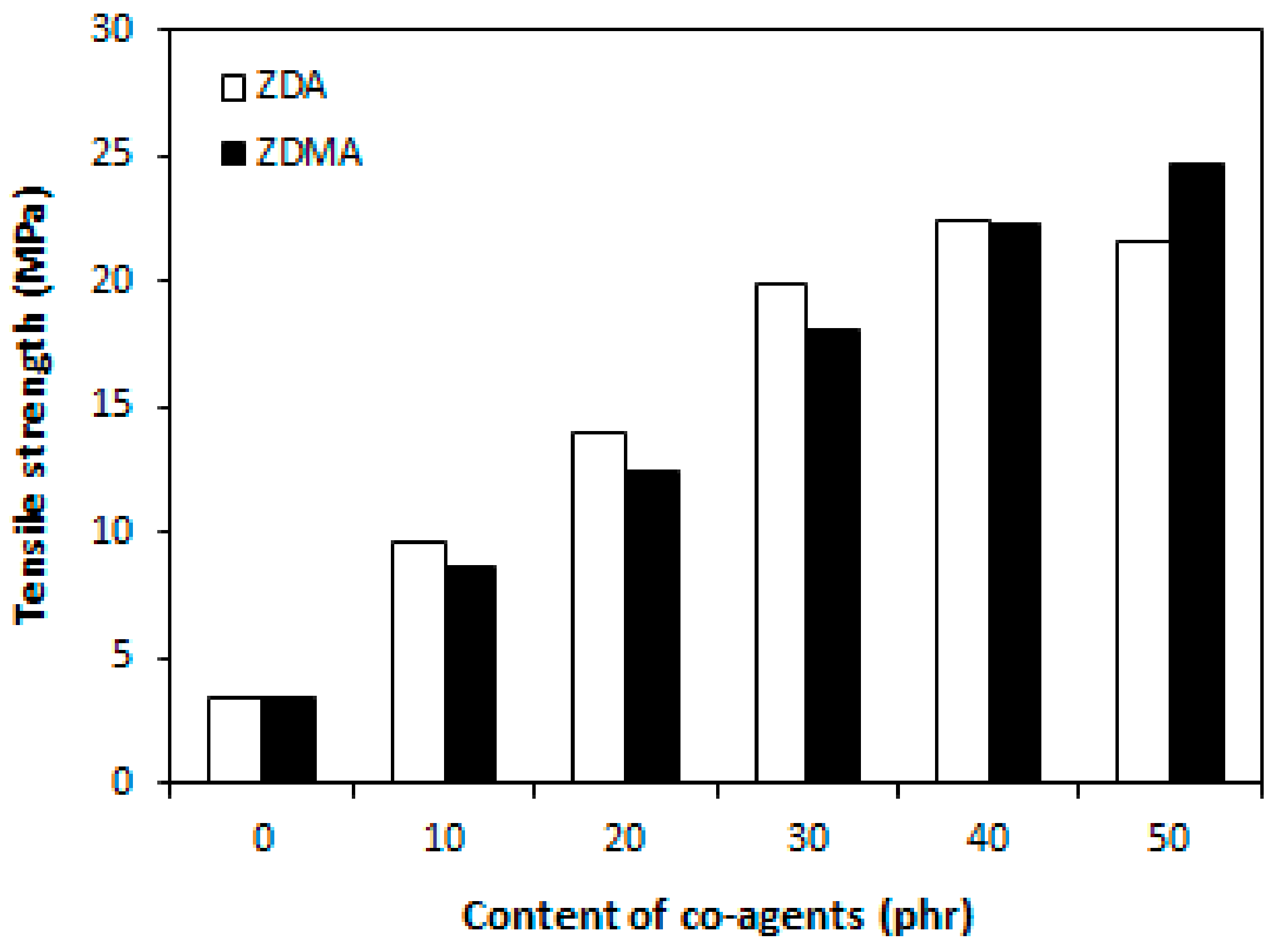
3.1. Influence of Co-Agents Content on Cross-Linking and Properties of Rubber Magnets Cured with Constant Amount of Dicumyl Peroxide
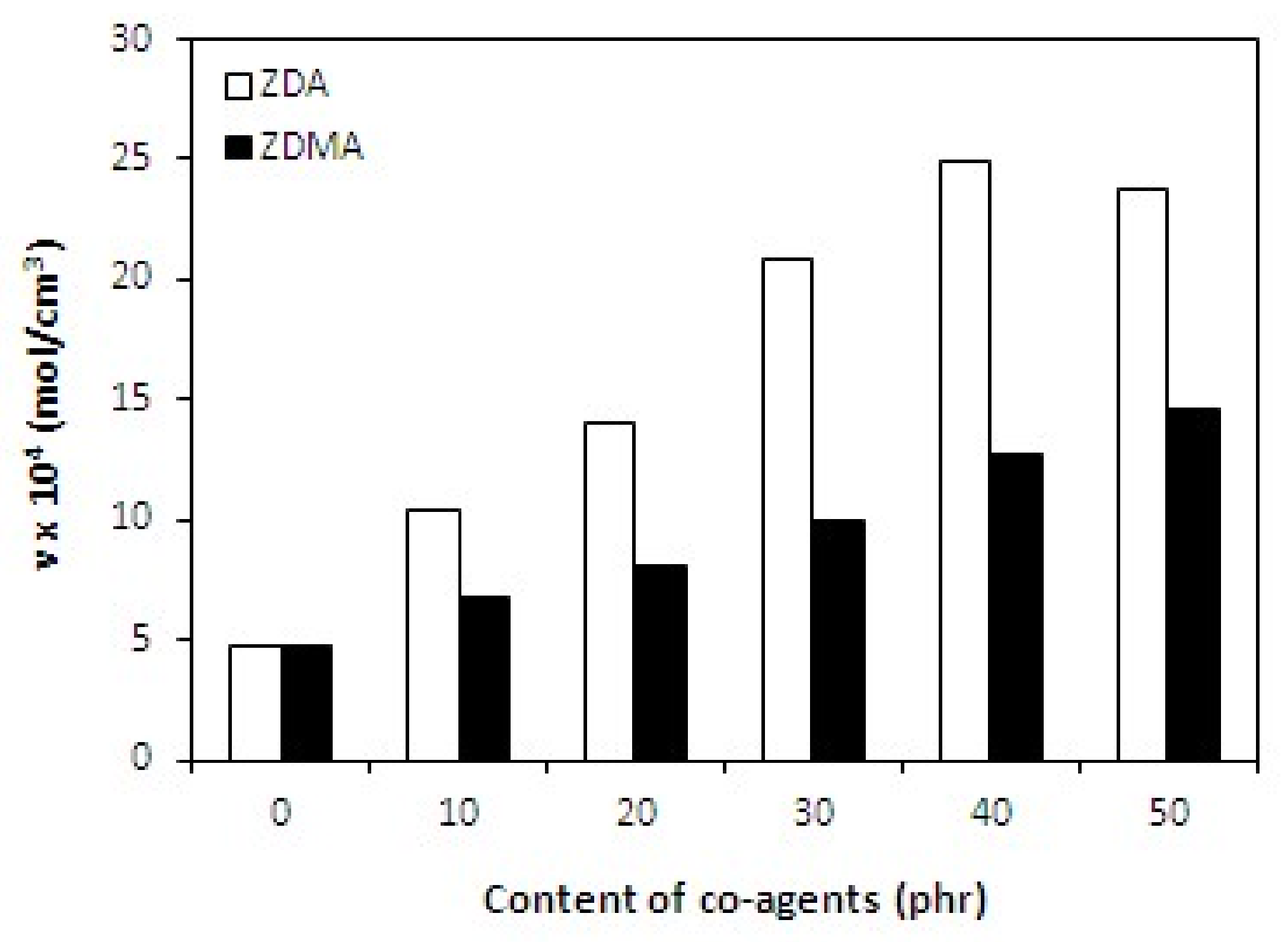
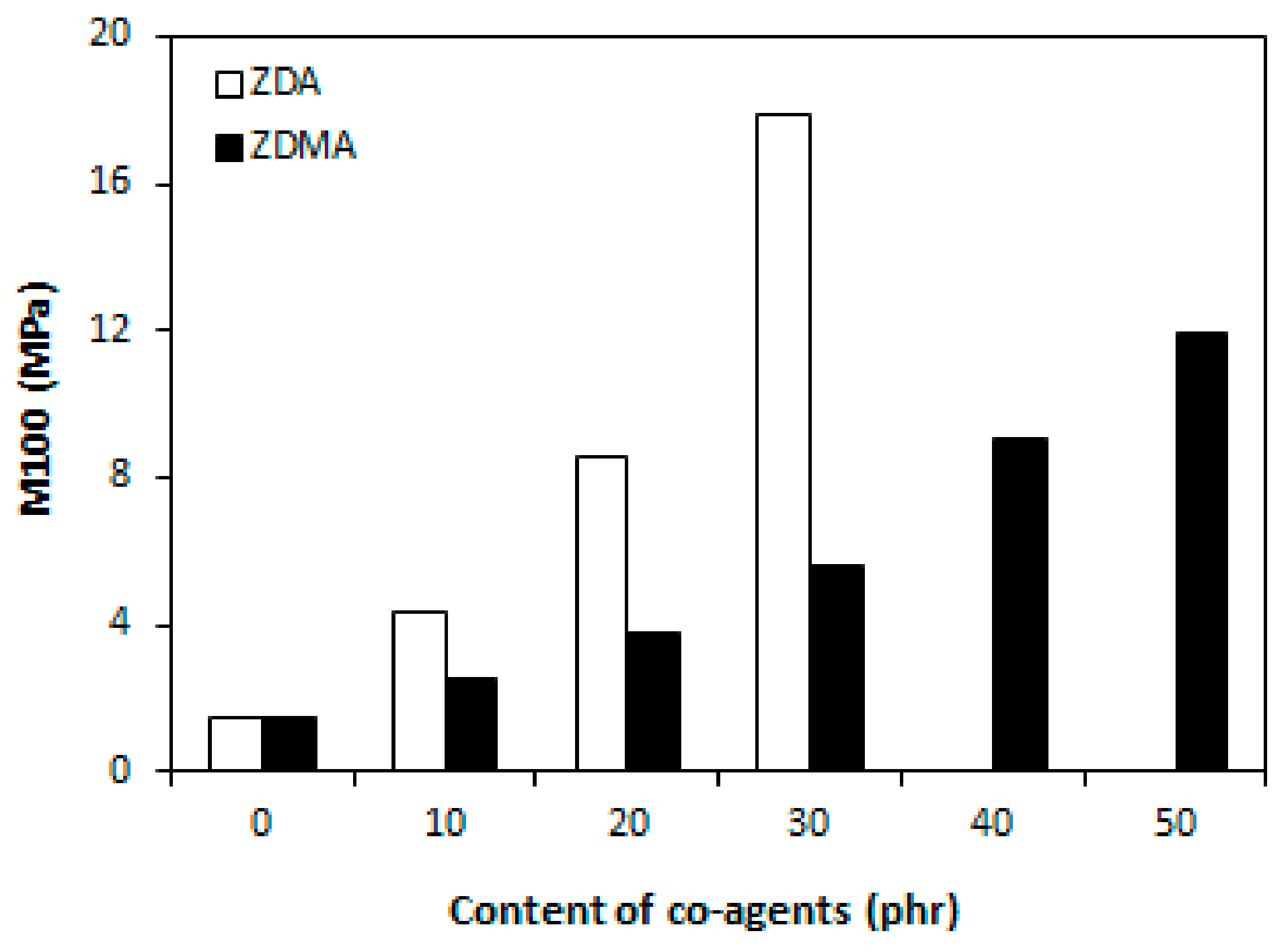
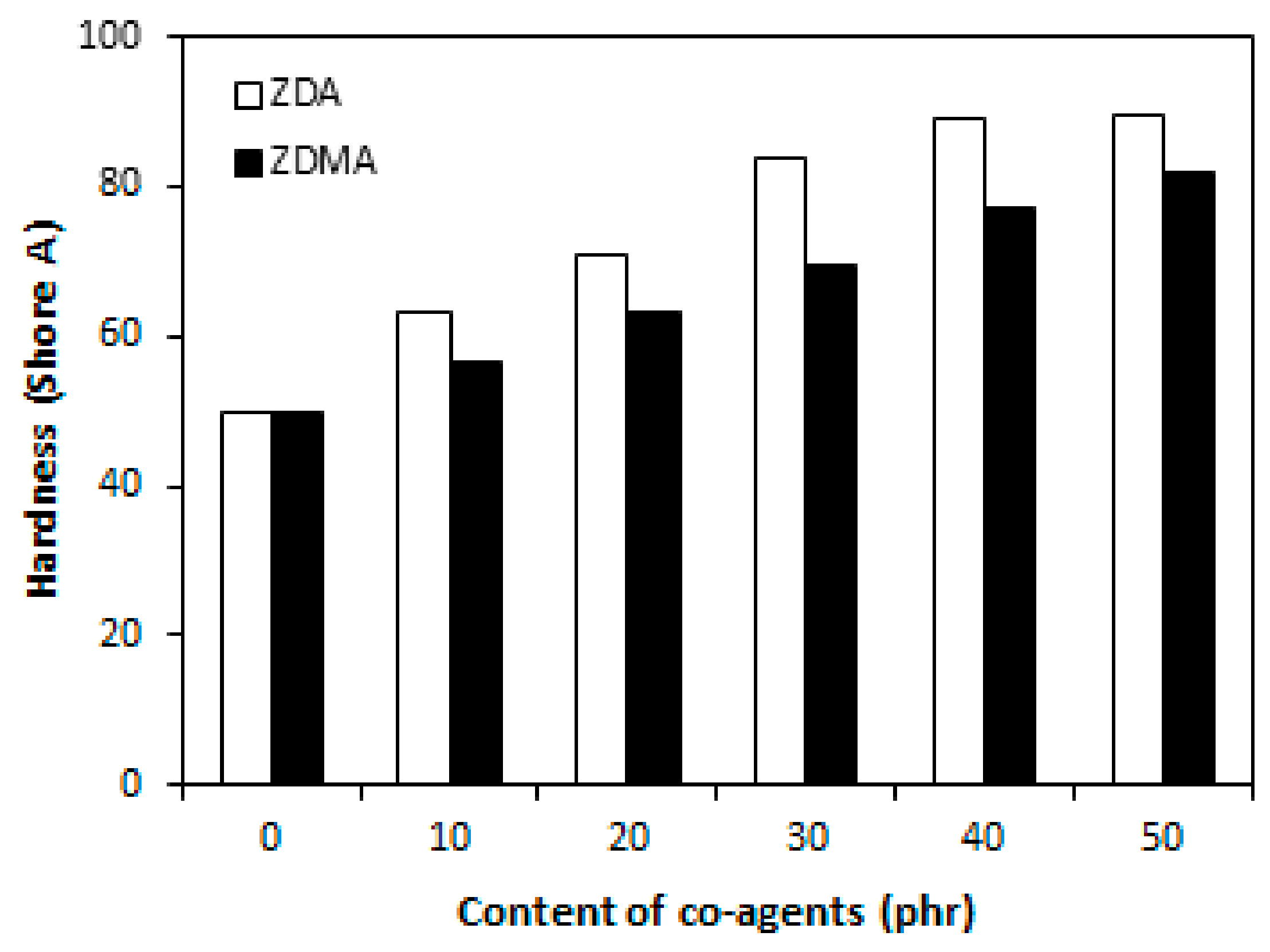
3.1.1. Cross-Link Density and Physical-Mechanical Properties
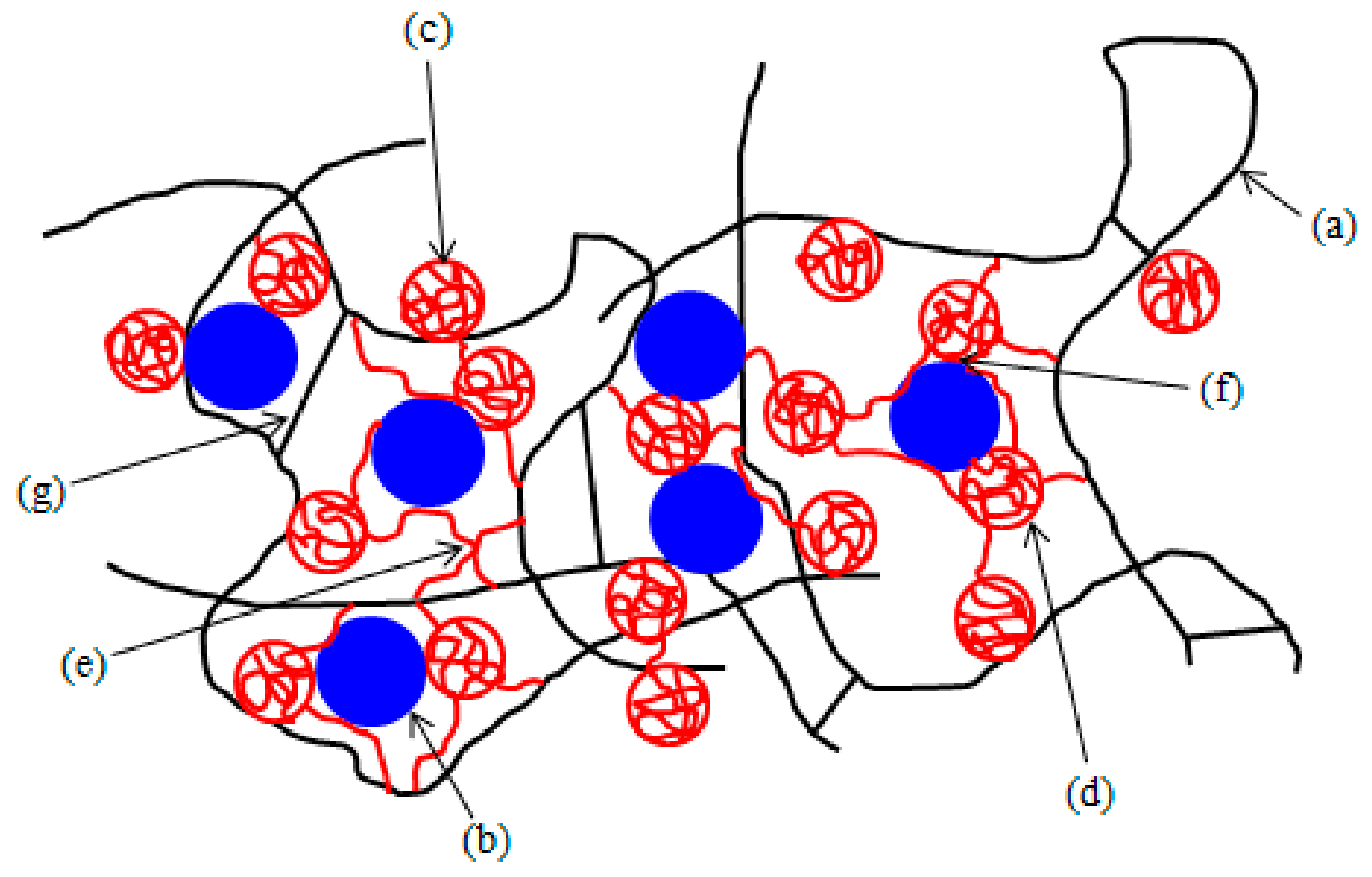
3.1.2. Microscopic Analysis
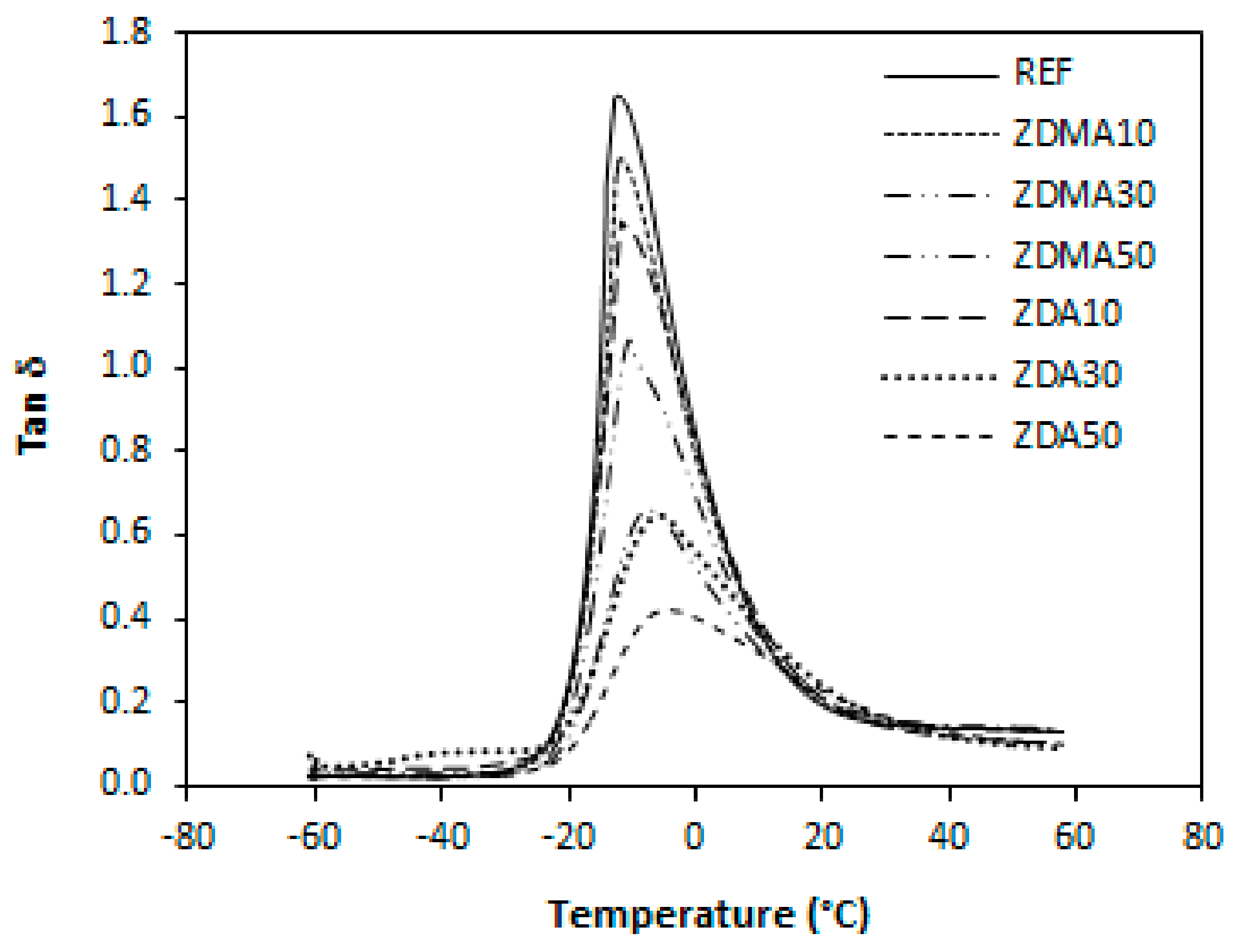
3.1.3. Dynamic-Mechanical Analysis
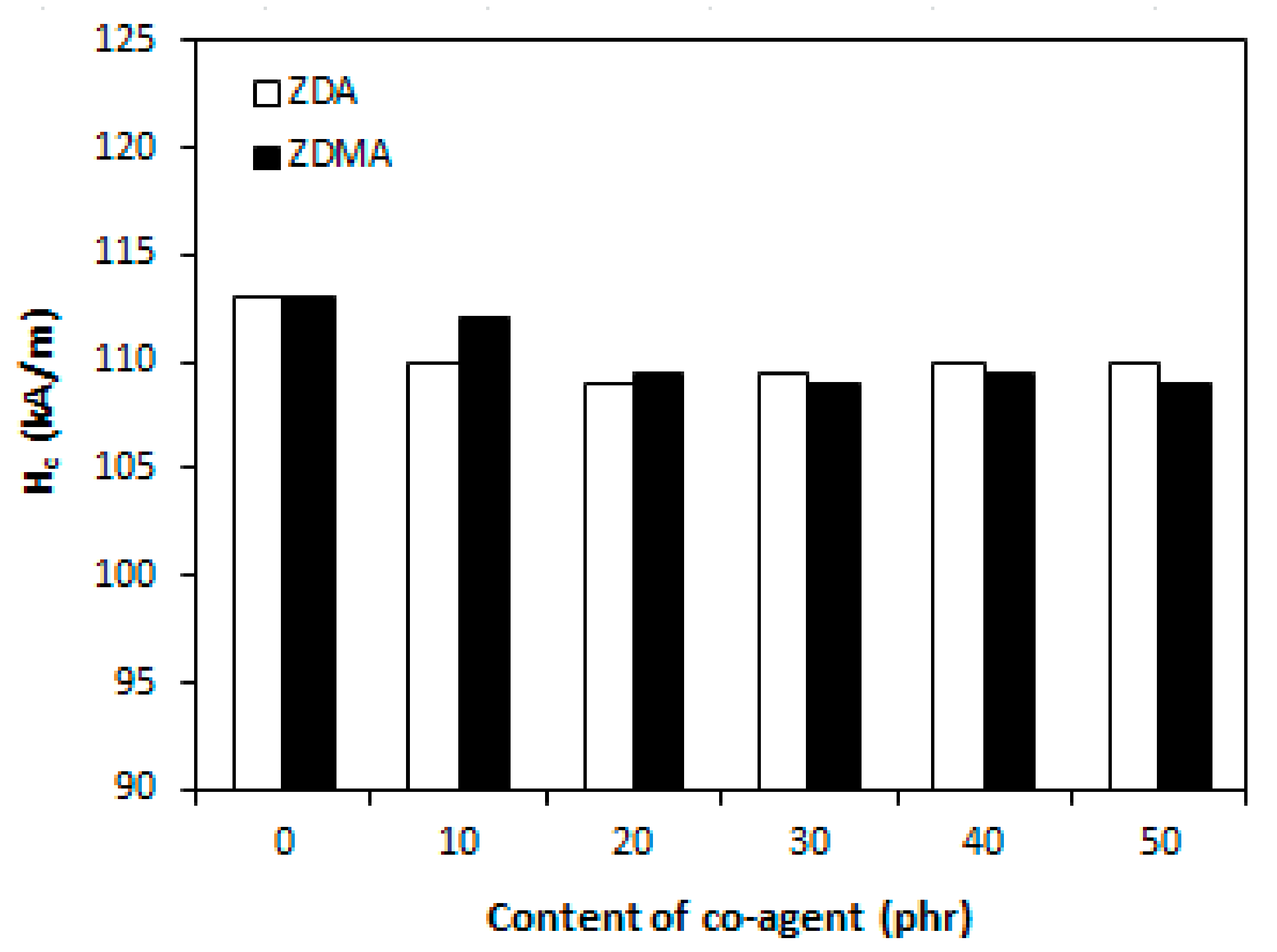
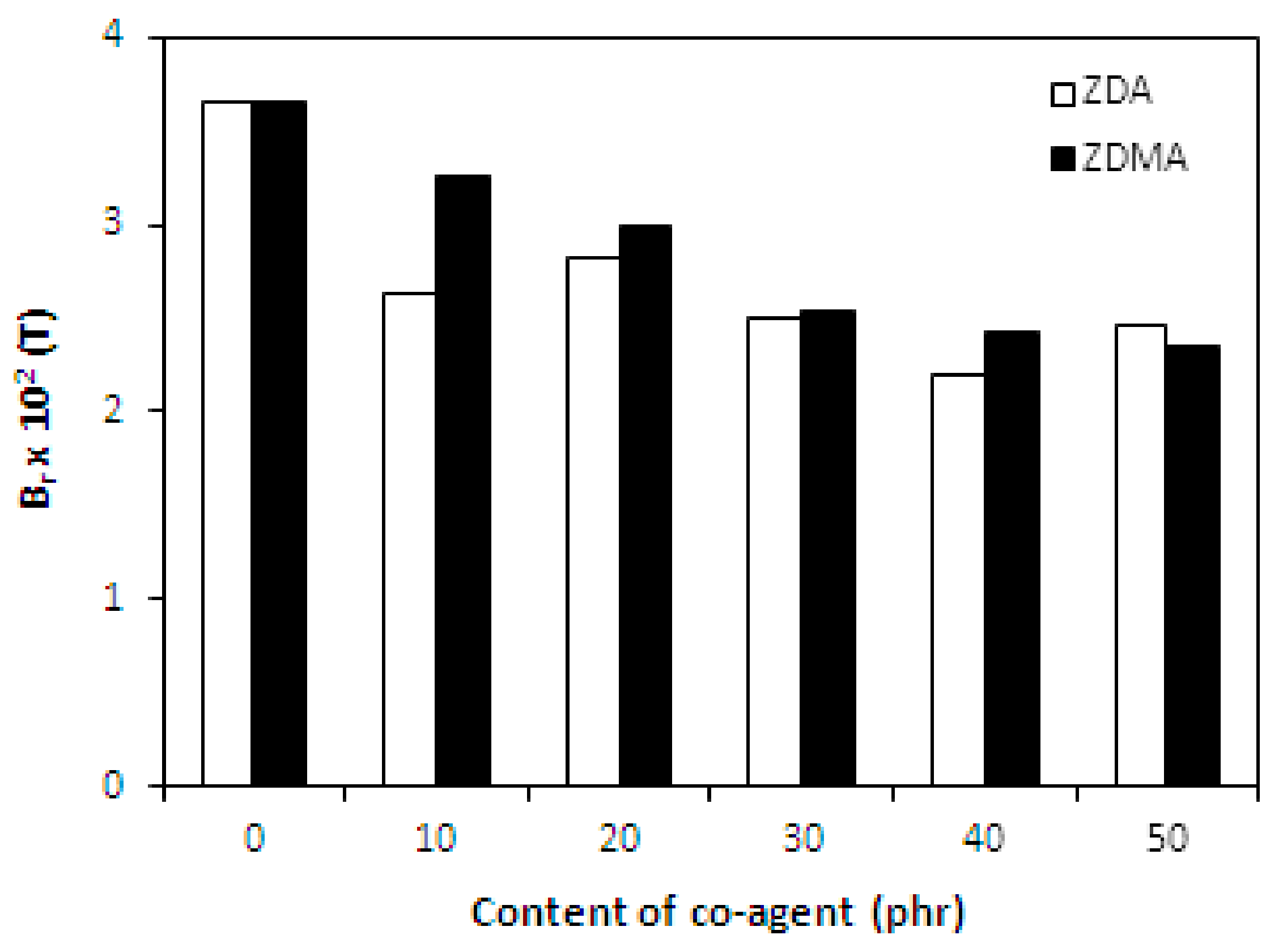
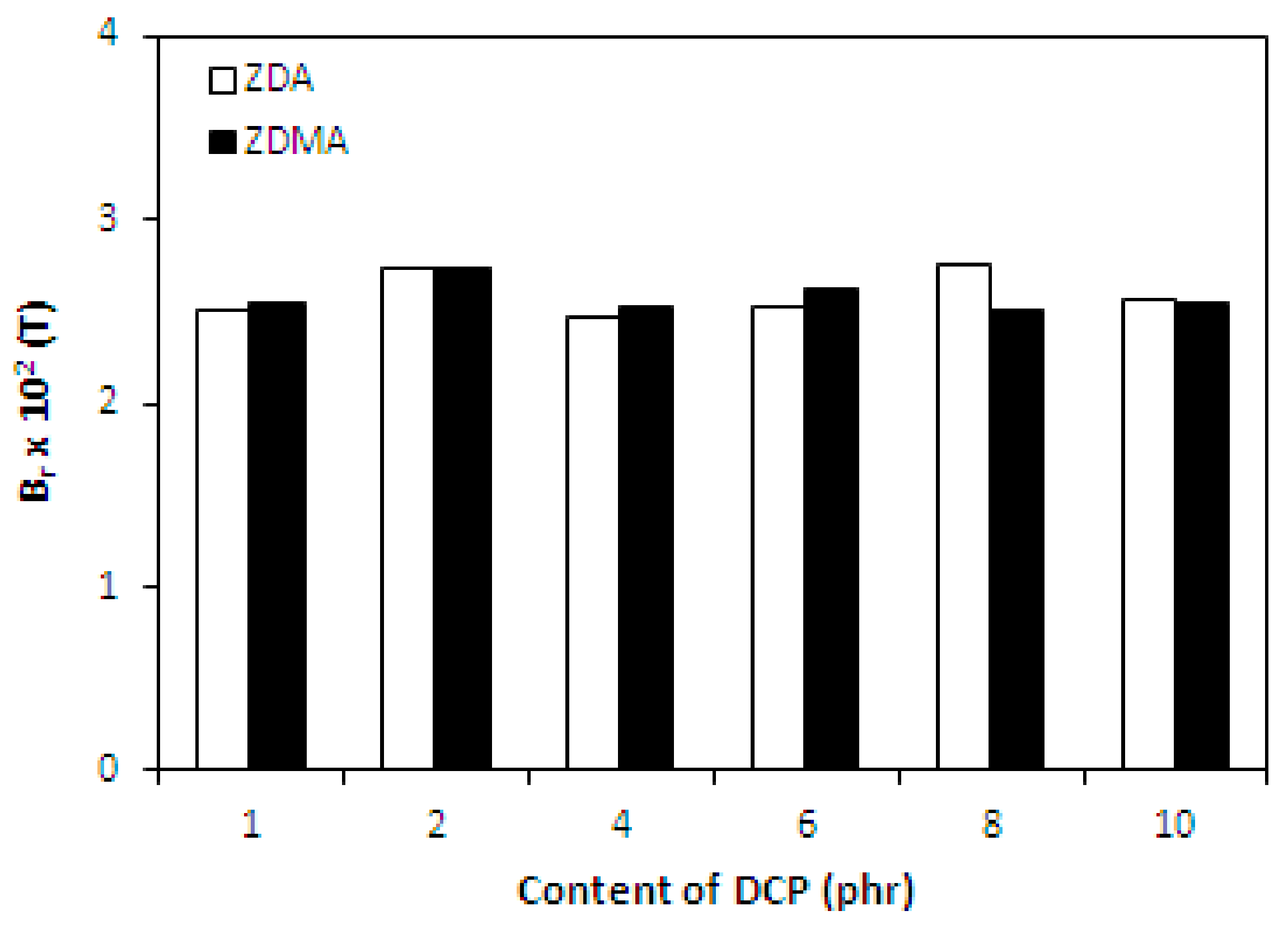
3.1.4. Magnetic Characteristics
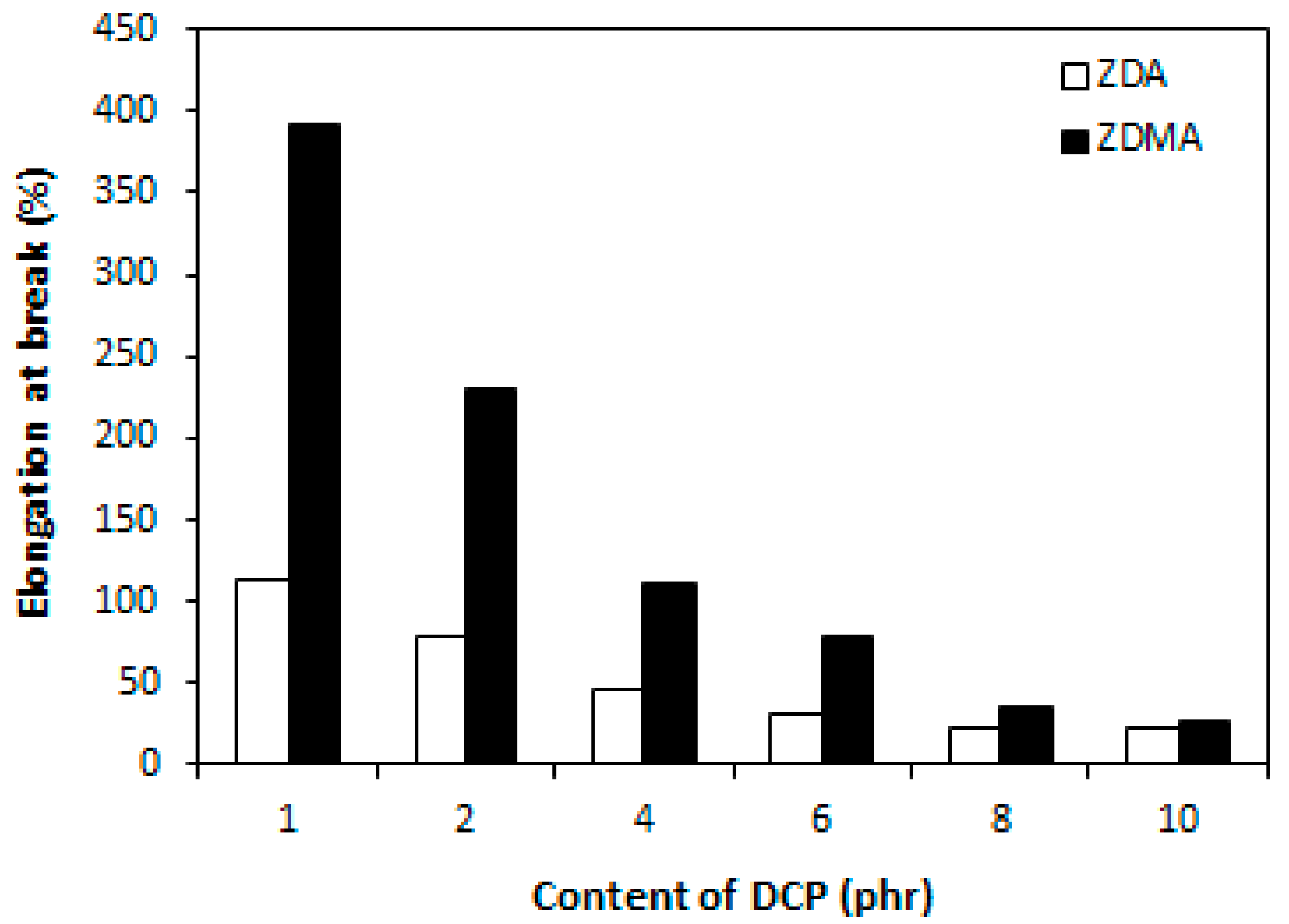
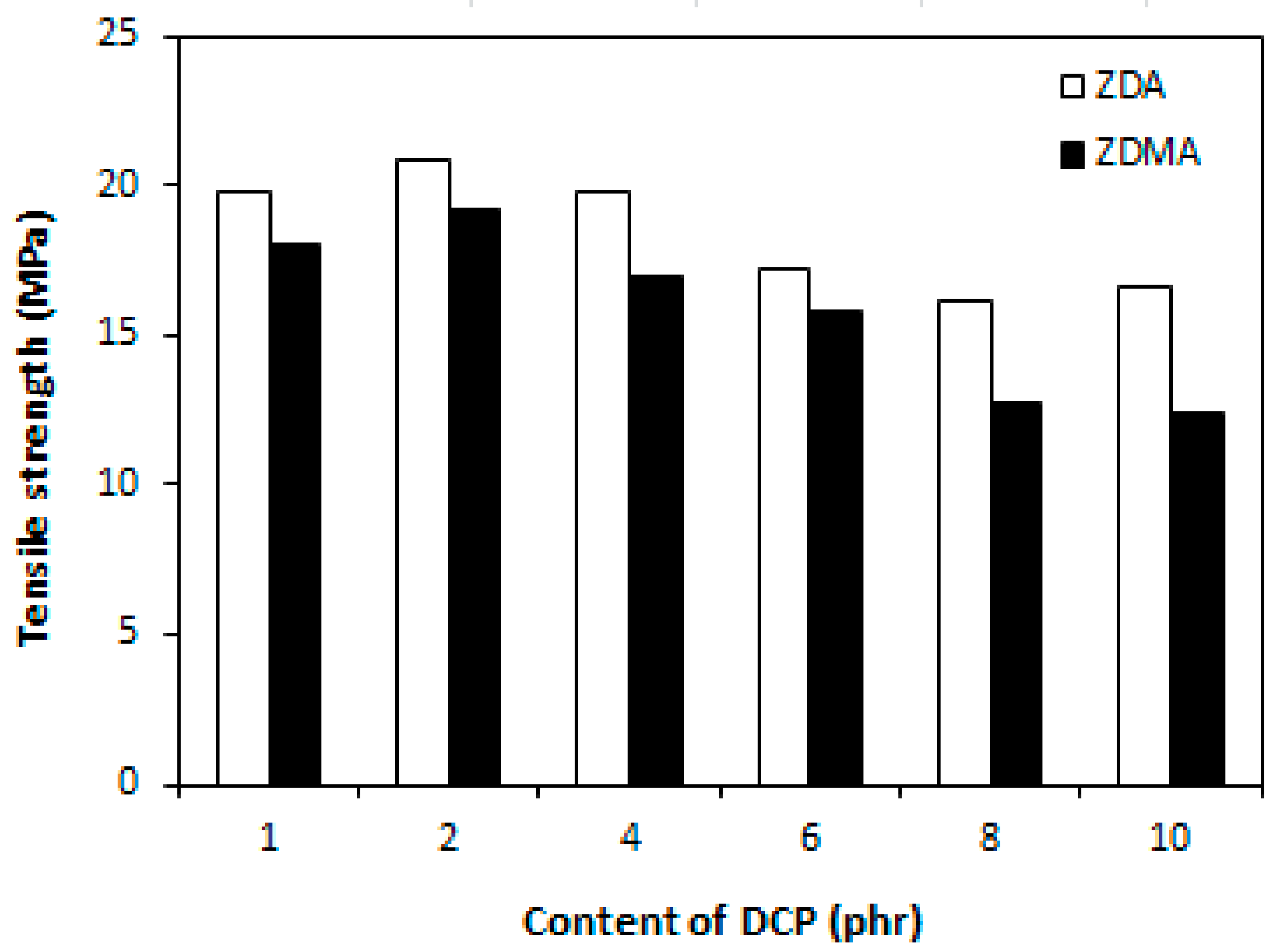
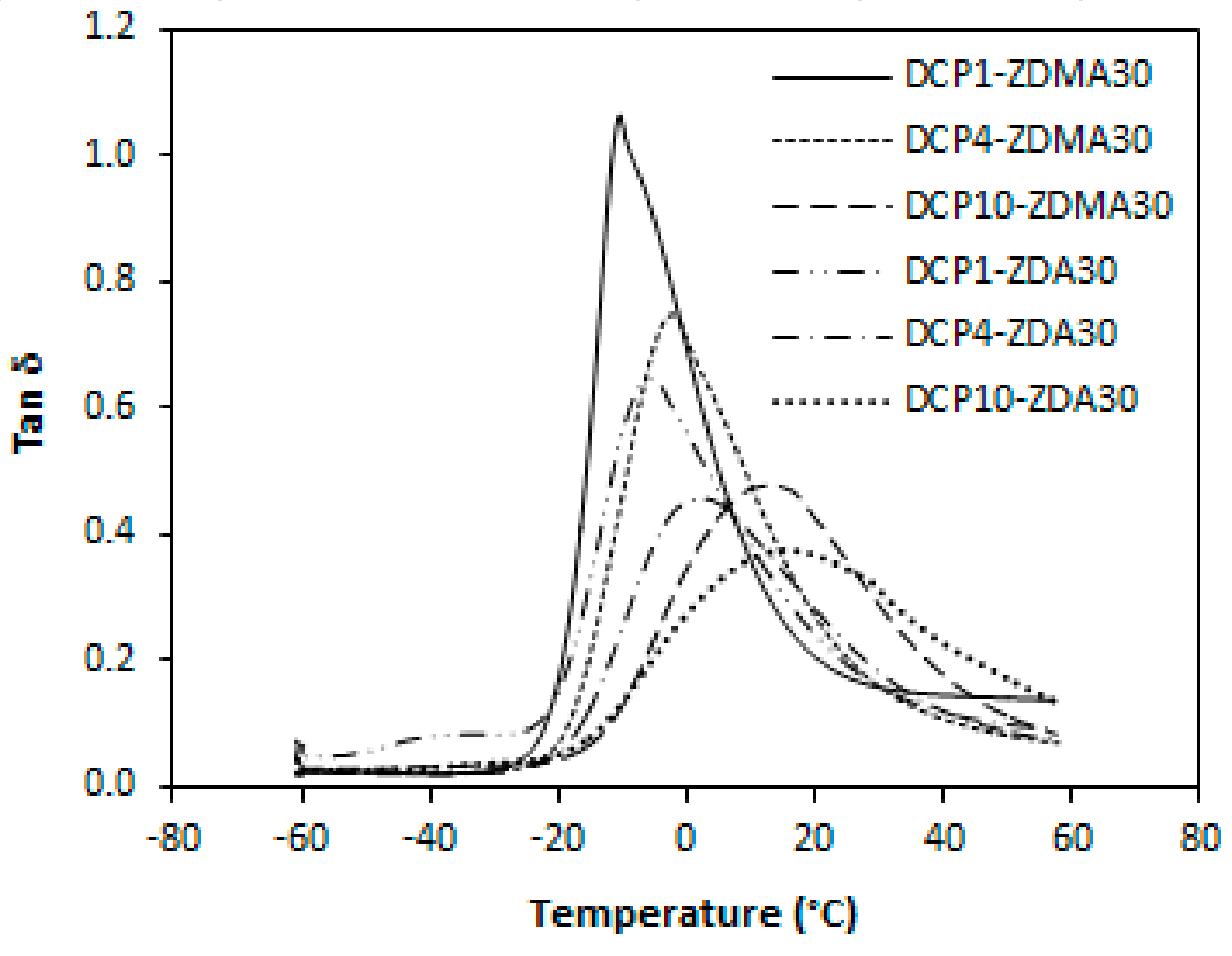
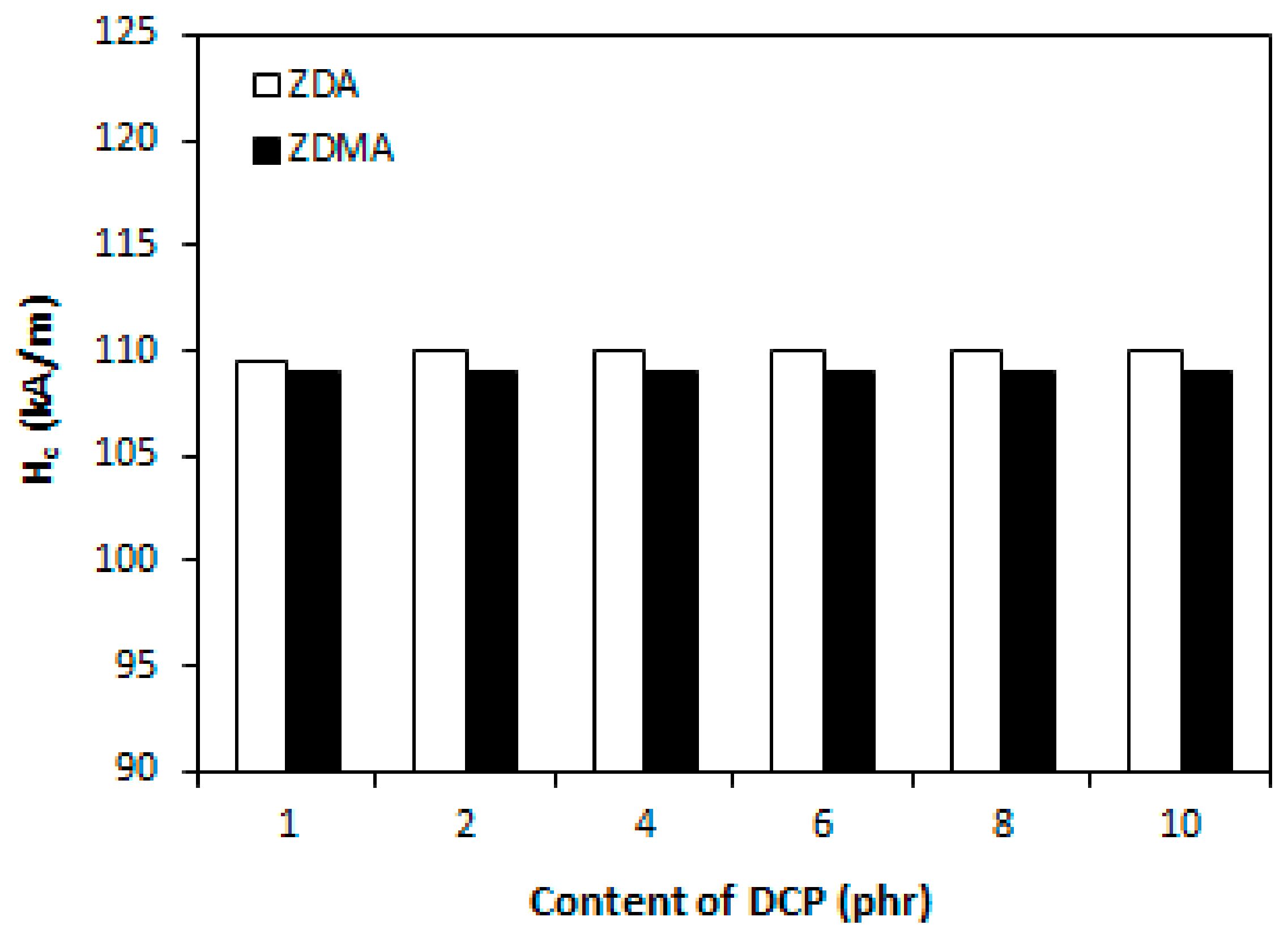
3.2. Influence of Peroxide Content on Cross-Link Density and Properties of Rubber Magnetic Composites Cured with Constant Amount of Co-Agents
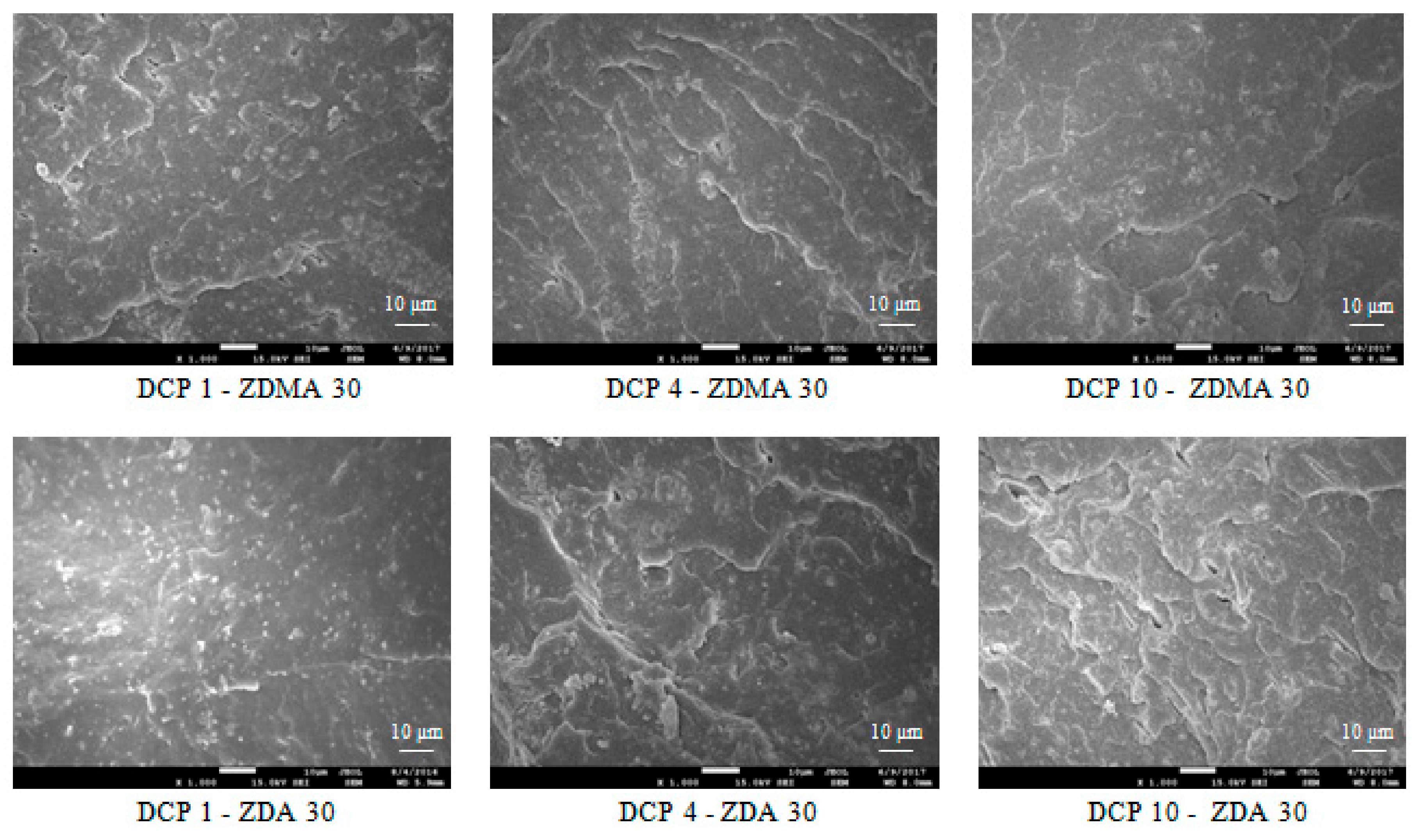
3.2.1. Microscopic Analysis
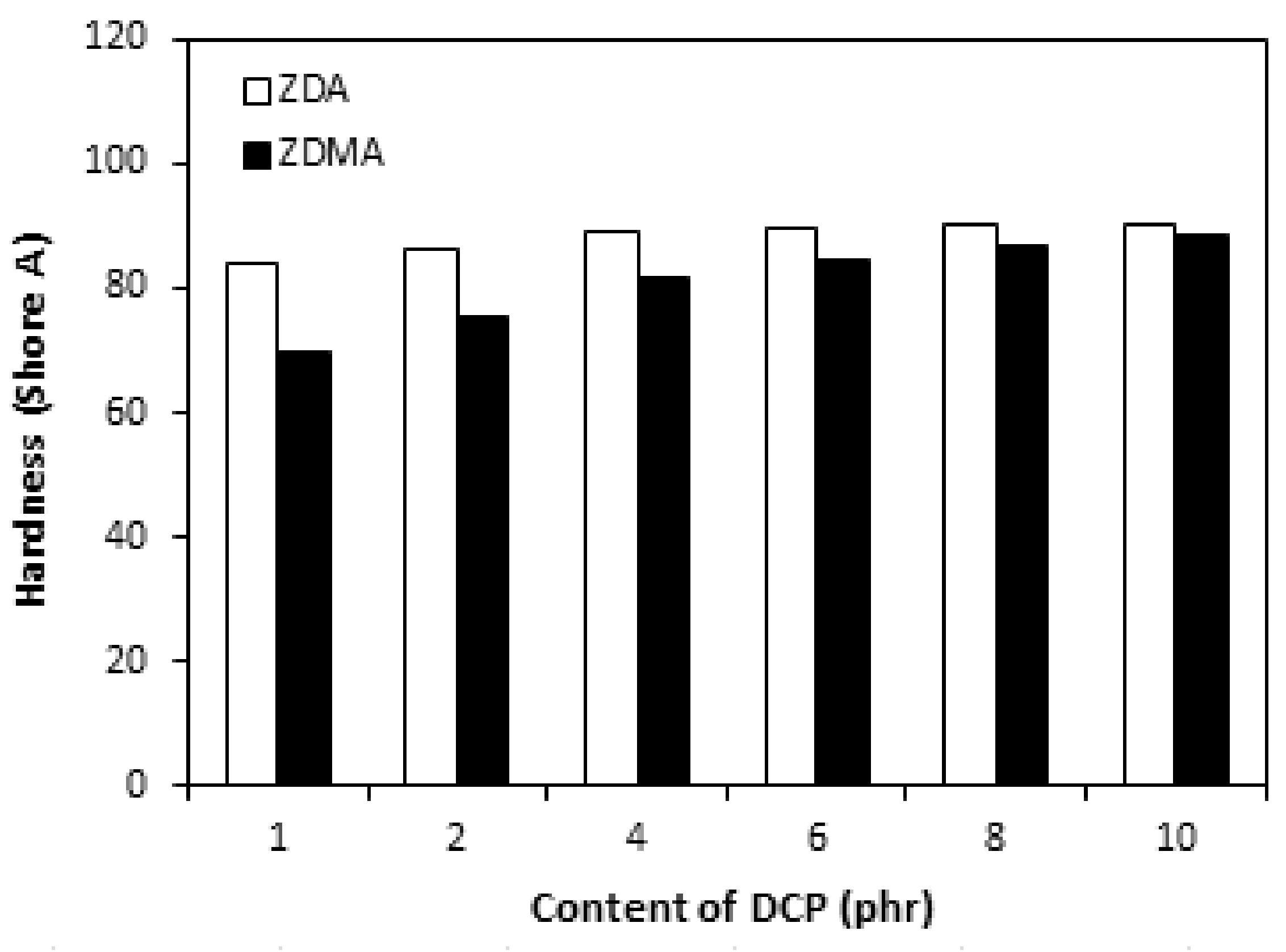
3.2.2. Cross-Link Density, Physical-Mechanical Properties, Dynamic and Magnetic Characteristics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hoang, N.; Zhang, N.; Du, H. An adaptive tunable vibration absorber using a new magnetorheological elastomer for vehicular powertrain transient vibration reduction. Smart Mater. Struct. 2011, 20, 015019. [Google Scholar] [CrossRef]
- Goshkoderia, A.; Rudykh, S. Stability of magnetoactive composites with periodic microstructures undergoing finite strains in the presence of a magnetic field. Compos. B Eng. 2017, 128, 19–29. [Google Scholar] [CrossRef]
- Das, S.; Nayak, G.C.; Sahu, S.K.; Routray, P.C.; Roy, A.K.; Baskey, H. Microwave absorption properties of double-layer composites using CoZn/NiZn/MnZn-ferrite and titanium dioxide. J. Magn. Magn. Mater. 2015, 377, 111–116. [Google Scholar] [CrossRef]
- Jung, H.S.; Kwon, S.H.; Choi, H.J.; Jung, J.H.; Kim, Y.G. Magnetic carbonyl iron/natural rubber composite elastomer and its magnetorheology. Compos. Struct. 2016, 136, 106–112. [Google Scholar] [CrossRef]
- Coran, A.Y. Chemistry of the vulcanization and protection of elastomers: A review of the achievements. J. Appl. Polym. Sci. 2003, 87, 24–30. [Google Scholar] [CrossRef]
- Choi, S.S.; Kim, E. A novel system for measurement of types and densities of sulfur crosslinks of a filled rubber vulcanizate. Polym. Test. 2015, 42, 62–68. [Google Scholar] [CrossRef]
- Dondi, D.; Buttafava, A.; Zeffiro, A.; Palamini, C.; Lostritto, A.; Giannini, L.; Faucitano, A. The mechanisms of the sulphur-only and catalytic vulcanization of polybutadiene: An EPR and DFT study. Eur. Polym. J. 2015, 62, 222–235. [Google Scholar] [CrossRef]
- Kruželák, J.; Sýkora, R.; Hudec, I. Sulphur and peroxide vulcanisation of rubber compounds—Overview. Chem. Pap. 2016, 70, 1533–1555. [Google Scholar] [CrossRef]
- Gupta, S.D.; Mukhopadhyay, R.; Baranwal, K.C.; Bhownick, A.K. Reverse Engineering of Rubber Products. Concepts, Tools and Techniques; CRC press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Visakh, P.M.; Thomas, S.; Chandra, A.K.; Mathew, A.P. Advances in Elastomers I: Blends and Interpenetrating Networks; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- González, L.; Rodríguez, A.; Marcos-Fernández, A.; Valentín, J.L.; Fernández-Torres, A. Effect of network heterogeneities on the physical properties of nitrile rubbers cured with dicumyl peroxide. J. Appl. Polym. Sci. 2007, 103, 3377–3382. [Google Scholar] [CrossRef]
- Kruželák, J.; Sýkora, R.; Hudec, I. Peroxide vulcanization of natural rubber. Part I: Effect of temperature and peroxide concentration. J. Polym. Eng. 2014, 34, 617–624. [Google Scholar] [CrossRef]
- Thitithammawong, A.; Nakason, C.; Sahakaro, K.; Noordermeer, J.W.M. Thermoplastic vulcanizates based on epoxidized natural rubber/polypropylene blends: Selection of optimal peroxide type and concentration in relation to mixing conditions. Eur. Polym. J. 2007, 43, 4008–4018. [Google Scholar] [CrossRef]
- Kyselá, G.; Hudec, I.; Alexy, P. Manufacturing and Processing of Rubber, 1st ed.; Slovak University of Technology Press: Bratislava, Slovakia, 2010. [Google Scholar]
- Sheng, C.; Hu, Z.; Martin, H.; Duan, Y.; Zhang, J. Effect of a small amount of sulfur on the physical and mechanical properties of peroxide-cured fully saturated HNBR compounds. J. Appl. Polym. Sci. 2015, 132, 41612. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, J.; Wang, C.; Gao, S.; Guo, S.; Zhang, Y. Influence of 1,2-polybutadiene on properties of dicumyl peroxide cured brominated butyl rubber. J. Appl. Polym. Sci. 2016, 2016. 133, 43280. [Google Scholar] [CrossRef]
- Rajan, R.; Varghese, S.; George, K.E. Role of coagents in peroxide vulcanization of natural rubber. Rubber Chem. Technol. 2013, 86, 488–502. [Google Scholar] [CrossRef]
- Wang, J.; Pan, S.; Zhang, Y.; Guo, S. Crosslink network evolution of BIIR/EPDM blends during peroxide vulcanization. Polym. Test. 2017, 59, 253–261. [Google Scholar] [CrossRef]
- Shanmugam, K.V.S.; Parent, J.S.; Whitney, R.A. C–H bond addition and copolymerization reactions of N-arylmaleimides: Fundamentals of coagent-assisted polymer cross-linking. Eur. Polym. J. 2012, 48, 841–849. [Google Scholar] [CrossRef]
- Wang, H.; Ding, Y.; Zhao, S. Effects of co-agents on the properties of peroxide-cured ethylene-propylene diene rubber (EPDM). J. Macromol. Sci. B 2016, 55, 433–444. [Google Scholar] [CrossRef]
- El-Nemr, K.F. Effect of different curing systems on the mechanical and physico-chemical properties of acrylonitrile butadiene rubber vulcanizates. Mater. Des. 2011, 32, 3361–3369. [Google Scholar] [CrossRef]
- Kruželák, J.; Sýkora, R.; Hudec, I. Peroxide vulcanization of natural rubber. Part II: Effect of peroxides and co-agents. J. Polym. Eng. 2015, 35, 21–29. [Google Scholar] [CrossRef]
- Vieira, E.R.; Mantovani, J.D.; de Camargo Forte, M.M. Comparison between peroxide/coagent cross-linking systems and sulfur for producing tire treads from elastomeric compounds. J. Elastom. Plast. 2015, 47, 347–359. [Google Scholar] [CrossRef]
- Bellucci, F.S.; de Almeida, F.C.L.; Nobre, M.A.L.; Rodríguez-Pérez, M.A.; Paschoalini, A.T.; Job, A.E. Magnetic properties of vulcanized natural rubber nanocomposites as a function of the concentration, size and shape of the magnetic fillers. Composites B 2016, 85, 196–206. [Google Scholar] [CrossRef]
- El-Nashar, D.E.; Ahmed, N.M.; Agami, W.R. The effect of new ferrite/kaolin pigment on the properties of acrylonitile-butadiene rubber composites. Mater. Des. 2013, 52, 108–117. [Google Scholar] [CrossRef]
- Makled, M.H.; Matsui, T. Magnetic and mechanical characterisation of natural rubber coprecipitated barium ferrite composites at high loading. Plast. Rubber Compos. 2009, 38, 297–301. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, F.; Yang, Q.; Chen, J.; Guan, H. Study on mechanical properties of nano-Fe3O4 reinforced nitrile butadiene rubber. Mater. Des. 2010, 31, 1023–1028. [Google Scholar] [CrossRef]
- Saramolee, P.; Lertsuriwat, P.; Hunyek, A.; Sirisathitkul, C. Cure and mechanical properties of recycled NdFeB–natural rubber composites. Bull. Mater. Sci. 2010, 33, 597–601. [Google Scholar] [CrossRef]
- Kraus, G. Swelling of filler-reinforced vulcanizates. J. Appl. Polym. Sci. 1963, 7, 861–871. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, L.; Tian, M.; Geng, H.; Zhang, L. Study on mechanical properties of elastomers reinforced by zinc dimethacrylate. Eur. Polym. J. 2005, 41, 589–598. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, L.; Shen, D.; Yang, C.; Zhang, L.Q. Infrared study on in situ polymerization of zinc dimethacrylate in poly (α-octylene-coethylene) elastomer. Polym. Int. 2004, 53, 802–808. [Google Scholar] [CrossRef]
- Oh, S.J.; Koenig, J.L. Studies of peroxide curing of polybutadiene/zinc diacrylate blends by fast FT-IR imaging. Rubber Chem. Technol. 2000, 73, 74–79. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, T.; Tang, Z.; Guo, B. Elastomeric composites based on zinc diacrylate-cured epoxidized natural rubber: mechanical properties and ageing resistance. KGK 2015, 7, 39–45. [Google Scholar]
- Chen, Y.; Xu, C. Crosslink network evolution of natural rubber/zinc dimethacrylate composite during peroxide vulcanization. Polym. Compos. 2011, 32, 1505–1514. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, L.; Yang, C.; Tian, M.; Zhang, L. The morphology of zinc dimethacrylate reinforced elastomers investigated by SEM and TEM. Eur. Polym. J. 2005, 41, 577–588. [Google Scholar] [CrossRef]
- Peng, Z.; Liang, X.; Zhang, Y.; Zhang, Y. Reinforcement of EPDM by in situ prepared zinc dimethacrylate. J. Appl. Polym. Sci. 2002, 84, 1339–1345. [Google Scholar] [CrossRef]
- Yuan, X.; Peng, Z.; Zhang, Y.; Zhang, Y. In situ preparation of zinc salts of unsaturated carboxylic acids to reinforce NBR. J. Appl. Polym. Sci. 2000, 77, 2740–2748. [Google Scholar] [CrossRef]
- Guo, W.; Shen, Z.; Guo, B.; Zhang, L.; Jia, D. Synthesis of bio-based copolyester and its reinforcement with zinc diacrylate for shape memory application. Polymer 2014, 55, 4324–4331. [Google Scholar] [CrossRef]
- Nie, T.; Huang, G.; Qu, L.; Zhang, P.; Weng, G.; Wu, J. Cure kinetics and morphology of natural rubber reinforced by the in situ polymerization of zinc dimethacrylate. J. Appl. Polym. Sci. 2010, 115, 99–106. [Google Scholar] [CrossRef]
- Nie, Y. Strain-induced crystallization of natural rubber/zinc dimethacrylate composites studied using synchrotron X-ray diffraction and molecular simulation. J. Polym. Res. 2015, 22, 1–10. [Google Scholar] [CrossRef]
- Cao, Y.; Gu, N.; Hu, S.; Jin, R.; Zhang, J. Preparation and properties of zinc-diacrylate-modified montmorillonite/rubber nanocomposite. Appl. Mech. Mater. 2012, 182, 47–51. [Google Scholar] [CrossRef]
- Henning, S.K.; Costin, R. Fundamentals of curing elastomers with peroxides and coagents. Rubber World 2006, 233, 28–35. [Google Scholar]
- Pullar, R.C. Hexagonal ferrites: A review of the synthesis, properties and applications of hexaferrite ceramics. Prog. Mater. Sci. 2012, 57, 1191–1334. [Google Scholar] [CrossRef]
- Xie, T.; Xu, L.; Liu, C.; Ding, S.; Yang, J.; Wu, W. Synthesis and adsorption properties of high specific area strontium ferrite from Industrial Strontium Residue. Vacuum 2013, 93, 71–78. [Google Scholar] [CrossRef]
- Kruželák, J.; Sýkora, R.; Hudec, I. Vulcanization of rubber compounds with peroxide curing systems. Rubber Chem. Technol. 2017, 90, 60–88. [Google Scholar] [CrossRef]
- Leblanc, J.L. Rubber-filler interactions and rheological properties in filed rubber compounds. Prog. Polym. Sci. 2002, 27, 627–687. [Google Scholar] [CrossRef]
- Fröhlich, J.; Niedermeier, W.; Luginsland, H.D. The effect of filler–filler and filler–elastomer interaction on rubber reinforcement. Composites A 2005, 36, 449–460. [Google Scholar] [CrossRef]
- Mokhtar, N.; Abdullah, M.H.J.; Ahmad, S.H.J. Structural and magnetic properties of type-M barium ferrite – thermoplastic natural rubber nanocomposites. Sans Malays. 2012, 41, 1125–1131. [Google Scholar]
- Gutiérrez, J.; Martins, P.; Gonçalves, R.; Sencadas, V.; Lasheras, A.; Lanceros-Mendez, S.; Barandiarán, J.M. Synthesis, physical and magnetic properties of BaFe12O19/P(VDF-TrFE) multifunctional composites. Eur. Polym. J. 2015, 69, 224–231. [Google Scholar] [CrossRef]
- Kruželák, J.; Ušáková, M.; Dosoudil, R.; Hudec, I.; Sýkora, R. Microstructure and performance of natural rubber based magnetic composites. Polym. Plast. Technol. Eng. 2014, 53, 1095–1104. [Google Scholar]
- Basfar, A.A.; Abdel-Aziz, M.M.; Mofti, S. Influence of different curing systems on the physico-mechanical properties and stability of SBR and NR rubbers. Radiat. Phys. Chem. 2002, 63, 81–87. [Google Scholar] [CrossRef]
- Kruželák, J.; Sýkora, R.; Dosoudil, R.; Hudec, I. Influence of peroxide curing systems on the performance of natural rubber-based magnetic composites. Compos. Interfaces 2015, 22, 473–488. [Google Scholar] [CrossRef]
- Kruželák, J.; Sýkora, R.; Dosoudil, R.; Hudec, I. Relationship between the cross-link structure and properties of peroxide and sulfur-cured magnetic composites based on NR and NBR. J. Elastom. Plast. 2017, 49, 459–480. [Google Scholar] [CrossRef]
- Kruželák, J.; Chodák, I.; Mošková, D.J.; Dosoudil, R.; Hudec, I. Cross-linking and properties of rubber magnetic composites cured with different curing systems. Polym. Adv. Technol. 2018, 29, 216–225. [Google Scholar] [CrossRef]
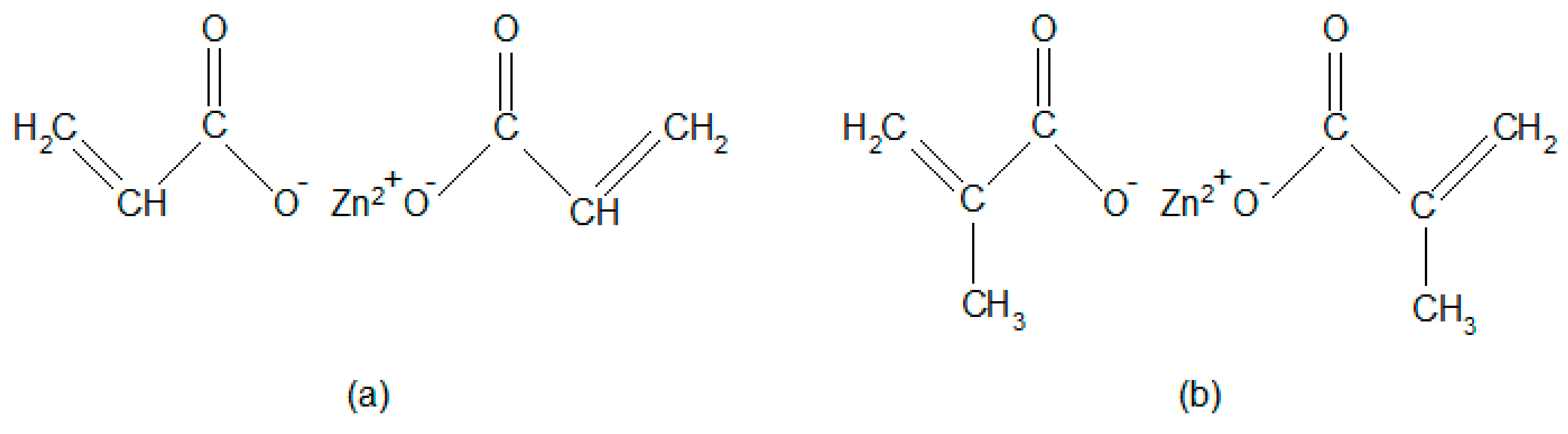


| Characteristics | Value |
|---|---|
| Total porosity (%) | 54.94 |
| Specific surface area (m2/g) | 3.30 |
| Total volume of pores (cm3/g) | 0.264 |
| Density (g/cm3) | 4.13 |
| Remanent magnetic induction (T) | 0.127 |
| Coercivity (kA/m) | 116 |
| Component | NBR | Ferrite | DCP | ZDA or ZDMA |
|---|---|---|---|---|
| Content (phr) | 100 | 50 | 1 | 0–50 |
| Component | NBR | Ferrite | DCP | ZDA or ZDMA |
|---|---|---|---|---|
| Content (phr) | 100 | 50 | 1–10 | 30 |
| Co-Agent Content (phr) | ν × 104 (mol/cm3) | Tg (°C) | ν × 104 (mol/cm3) | Tg (°C) |
|---|---|---|---|---|
| ZDMA | ZDA | |||
| 0 | 4.7 | −12.3 | 4.7 | −12.3 |
| 10 | 6.8 | −12.1 | 10.5 | −11.7 |
| 30 | 10.0 | −10.4 | 20.9 | −5.7 |
| 50 | 14.5 | −7.3 | 23.7 | −4.4 |
| Peroxide Content (phr) | ν × 104 (mol/cm3) | Tg (°C) | ν × 104 (mol/cm3) | Tg (°C) |
|---|---|---|---|---|
| ZDMA 30 phr | ZDA 30 phr | |||
| 1 | 10.0 | −10.4 | 20.9 | −5.7 |
| 4 | 22.3 | −2.2 | 37.1 | 2.1 |
| 10 | 51.3 | 13.1 | 68.3 | 15.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruželák, J.; Karlíková, V.; Dosoudil, R.; Tomanová, K.; Hudec, I. Reinforcement of Rubber Magnetic Composites with Zinc Salts of Acrylic and Methacrylic Acids. Materials 2018, 11, 2161. https://doi.org/10.3390/ma11112161
Kruželák J, Karlíková V, Dosoudil R, Tomanová K, Hudec I. Reinforcement of Rubber Magnetic Composites with Zinc Salts of Acrylic and Methacrylic Acids. Materials. 2018; 11(11):2161. https://doi.org/10.3390/ma11112161
Chicago/Turabian StyleKruželák, Ján, Viera Karlíková, Rastislav Dosoudil, Katarína Tomanová, and Ivan Hudec. 2018. "Reinforcement of Rubber Magnetic Composites with Zinc Salts of Acrylic and Methacrylic Acids" Materials 11, no. 11: 2161. https://doi.org/10.3390/ma11112161
APA StyleKruželák, J., Karlíková, V., Dosoudil, R., Tomanová, K., & Hudec, I. (2018). Reinforcement of Rubber Magnetic Composites with Zinc Salts of Acrylic and Methacrylic Acids. Materials, 11(11), 2161. https://doi.org/10.3390/ma11112161






