Improved Optical and Morphological Properties of Vinyl-Substituted Hybrid Silica Materials Incorporating a Zn-Metalloporphyrin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Methods
2.1.1. Synthesis of Silica Xerogels
2.1.2. Porphyrin Derivative
2.1.3. Immobilization of Zn-TNMPyP in Silica Xerogels
2.2. Characterization of Samples
3. Results and Discussions
3.1. Gelation Time
3.2. Textural Properties
3.2.1. Nitrogen Adsorption
3.2.2. Small Angle Scattering
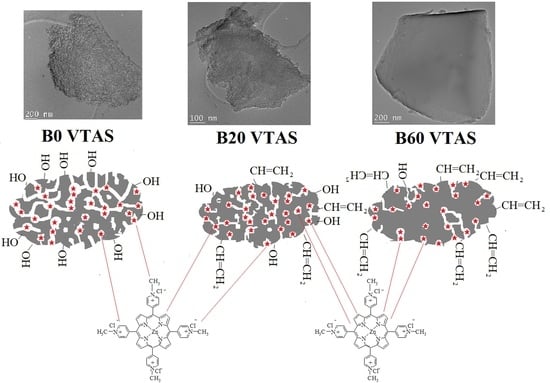
3.3. Morphologic Characterization
3.3.1. TEM
3.3.2. SEM
3.4. Chemical Composition
3.4.1. FT-IR Spectroscopy
3.4.2. Solid-State NMR
NMR Correlation Experiments
3.5. Contact Angle (CA) Measurements
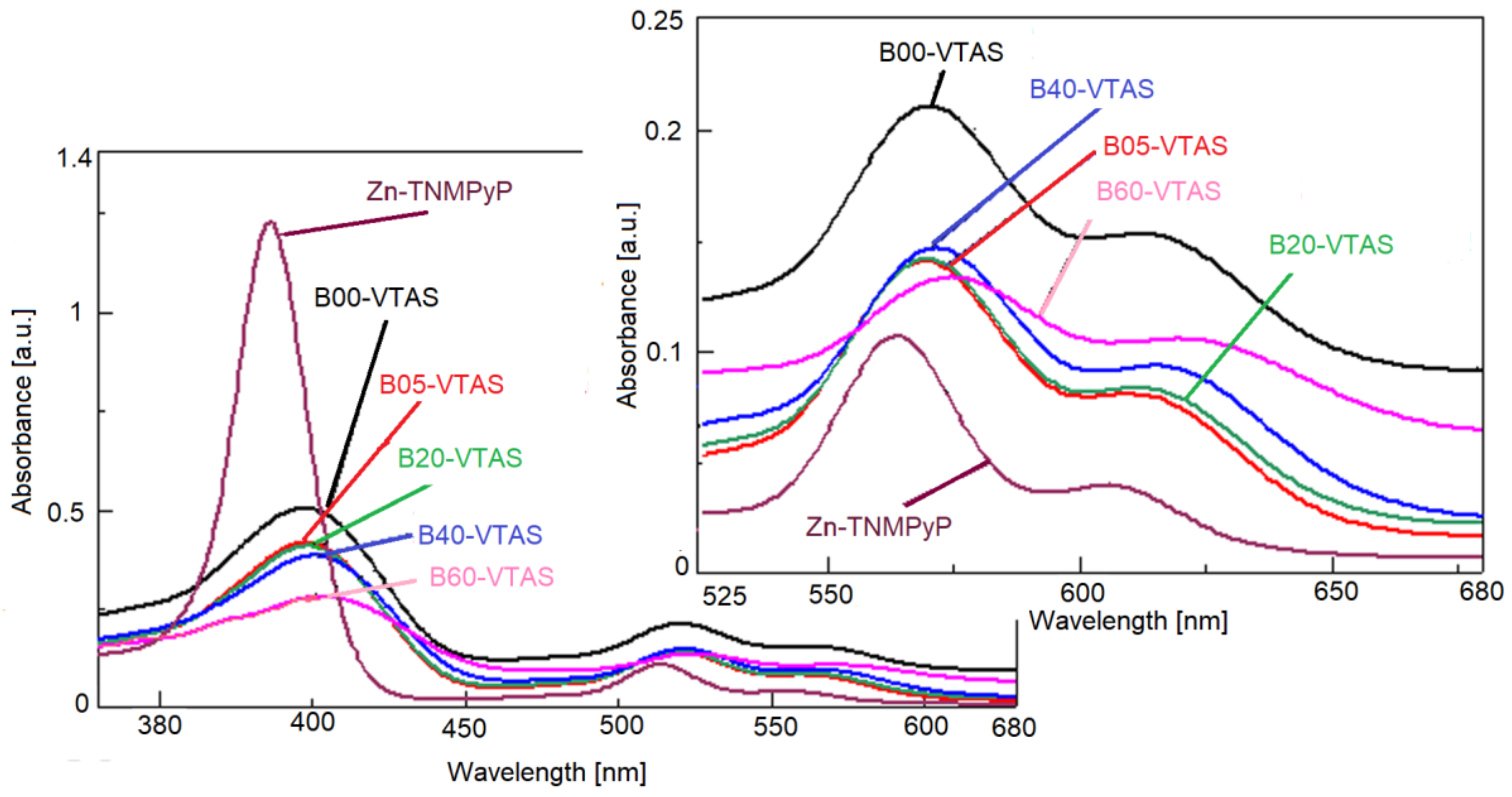
3.6. Fluorescence and UV-Vis Spectra
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Levy, D.; Zayat, M. The Sol-Gel Handbook: Synthesis, Characterization and Applications; Wiley-VCH: Weinheim, Germany, 2015; ISBN 9783527334865. [Google Scholar]
- Ciriminna, R.; Fidalgo, A.; Pandarus, V.; Béland, F.; Ilharco, L.M. The Sol-Gel Route to Advanced Silica-Based Materials and Recent Applications. Chem. Rev. 2013, 113, 6592–6620. [Google Scholar] [CrossRef] [PubMed]
- Zajickova, Z. Advances in the development and applications of organic–silica hybrid monoliths. J. Sep. Sci. 2017, 40, 25–48. [Google Scholar] [CrossRef] [PubMed]
- Membreno, D.; Smith, L.; Dunn, B. Silica sol-gel chemistry: Creating materials and architectures for energy generation and storage. J. Sol-Gel Sci. Technol. 2014, 70, 203–215. [Google Scholar] [CrossRef]
- Vidhya, S.; Murari, B.M. Sol-gel thin film based sensors and biosensors. Int. J. Pharm. Biol. Sci. 2016, 7, P297–P310. [Google Scholar]
- Gorni, G.; Velázquez, J.J.; Mosa, J.; Balda, R.; Fernández, J.; Durán, A.; Castro, Y. Transparent Glass-Ceramics Produced by Sol-Gel: A Suitable Alternative for Photonic Materials. Materials 2018, 11, 212. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Bierwagen, G.P. Sol-gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Sessler, J.L.; Seidel, D. Synthetic Expanded Porphyrin Chemistry. Angew. Chem. Int. Ed. 2003, 42, 5134–5175. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.; Selbo, P.K.; Weyergang, A.; Dietze, A.; Prasmickaite, L.; Bonsted, A.; Engesaeter, B.Ø.; Angell-Petersen, E.; Warloe, T.; Frandsen, N.; et al. Porphyrin-related photosensitizers for cancer imaging and therapeutic applications. J. Microsc. 2005, 218, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.F.; Konezny, S.J.; Song, H.; Milot, R.L.; Blakemore, J.D.; Lee, M.L.; Batista, V.S.; Schmuttenmaer, C.A.; Crabtree, R.H.; Brudvig, G.W. Bioinspired high-potential porphyrin photoanodes. J. Phys. Chem. C 2012, 116, 4892–4902. [Google Scholar] [CrossRef]
- Jiang, L.; Gan, C.R.R.; Gao, J.; Loh, X.J. A Perspective on the Trends and Challenges Facing Porphyrin-Based Anti-Microbial Materials. Small 2016, 12, 3609–3644. [Google Scholar] [CrossRef] [PubMed]
- Ormond, A.B.; Freeman, H.S. Dye Sensitizers for Photodynamic Therapy. Materials 2013, 6, 817–840. [Google Scholar] [CrossRef] [PubMed]
- Henriques, C.A.; Pinto, S.M.A.; Canotilho, J.; Ermelinda, M.; Eusébio, S. Synthesis of low melting point porphyrins: A quest for new materials. J. Porphyr. Phthalocyanines 2016, 20, 843–854. [Google Scholar] [CrossRef]
- Day, N.U.; Wamser, C.C.; Walter, M.G. Porphyrin polymers and organic frameworks. Polym. Int. 2015, 64, 833–857. [Google Scholar] [CrossRef]
- Pawlak, R.; Sadeghi, A.; Jöhr, R.; Hinaut, A.; Meier, T.; Kawai, S.; Zajac, L.; Olszowski, P.; Godlewski, S.; Such, B.; et al. Hydroxyl-Induced Partial Charge States of Single Porphyrins on Titania Rutile. J. Phys. Chem. C 2017, 121, 3607–3614. [Google Scholar] [CrossRef]
- Gonçalves, M.C.; Fortes, L.M.; Pimenta, A.R.; Pereira, J.C.G.; Almeida, R.M.; Carvalho, M.D.; Ferreira, L.P.; Cruz, M.M.; Godinho, M. Silica/Ormosil SPIONs for biomedical applications. Curr. Nanosci. 2013, 9, 599–608. [Google Scholar] [CrossRef]
- Ribeiro, T.; Baleizão, C.; Farinha, J.P.S. Functional Films from Silica/Polymer Nanoparticles. Materials 2014, 7, 3881–3900. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kathaperumal, M.; Chen, V.W.; Park, Y.; Fuentes-Hernandez, C.; Ming-Jen, P.; Kippelen, B.; Perry, J.W. Bilayer Structure with Ultrahigh Energy/Power Density Using Hybrid Sol-Gel Dielectric and Charge-Blocking Monolayer. Adv. Eng. Mater. 2015, 5. [Google Scholar] [CrossRef]
- Fard, A.K.; McKay, G.; Buekenhoudt, A.; Al Sulaiti, H.; Motmans, F.; Khraisheh, M.; Atieh, M. Inorganic Membranes: Preparation and Application for Water Treatment and Desalination. Materials 2018, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Li, G. Porphyrin-doped mesoporous silica films for rapid TNT detection. Colloid Polym. Sci. 2007, 285, 721–728. [Google Scholar] [CrossRef]
- Figueira, F.; Cavaleiro, J.A.S.; Tomé, J.P.C. Silica nanoparticles functionalized with porphyrins and analogs for biomedical studies. J. Porphyr. Phthalocyanines 2011, 15, 517–533. [Google Scholar] [CrossRef]
- Borzęcka, W.; Trindade, T.; Torres, T.; Tome, J. Targeting cancer cells with photoactive silica nanoparticles. Curr. Pharm. Des. 2016, 22, 6021–6038. [Google Scholar] [CrossRef] [PubMed]
- Nakagaki, S.; Ferreira, G.K.B.; Marçal, A.L.; Ciuffi, K.J. Metalloporphyrins immobilized on silica and modified silica as catalysts in heterogeneous processes. Curr. Org. Synth. 2014, 11, 67–88. [Google Scholar] [CrossRef]
- Elhamifar, D.; Badin, P.; Karimipoor, G. Alkyl-imidazolium based organosilica supported Fe/porphyrin complex: As novel, highly efficient and reusable catalyst for the unsymmetrical Hantzsch reaction. J. Colloid Interface Sci. 2017, 499, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, L.D.; Barbosa, I.A.; Zanardi, F.B.; De Sousa Filho, P.C.; Bolzon, L.B.; Ramos, A.P.; Serra, O.A.; Iamamoto, Y. Hydrocarbon oxidation by iron-porphyrin immobilized on SBA-15 as biomimetic catalyst: Role of silica surface. RSC Adv. 2016, 6, 104886–104896. [Google Scholar] [CrossRef]
- Gai, F.; Zhou, T.; Zhang, L.; Li, X.; Hou, W.; Yang, X.; Li, Y.; Zhao, X.; Xu, D.; Liu, Y.; et al. Silica cross-linked nanoparticles encapsulating fluorescent conjugated dyes for energy transfer-based white light emission and porphyrin sensing. Nanoscale 2012, 4, 6041–6049. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.F.; Santos, M.L.; Almeida, L.E.; Costa, N.B., Jr.; Gimenez, I.F.; Araki, K.; Mayer, I.; Engelmann, F.M.; Toma, H.E.; Barreto, L.S. Fluorescent tetraruthenated porphyrins embedded in monolithic SiO2 gels by the sol-gel process. J. Colloid Interface Sci. 2007, 305, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Spadavecchia, J.; Méthivier, C.; Landoulsi, J.; Pradier, C.-M. Interaction of ZnII porphyrin with TiO2 nanoparticles: From mechanism to synthesis of hybrid nanomaterials. ChemPhysChem 2013, 14, 2462–2469. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, G.; Zhang, L.; Liu, Z.; Cheng, Z.; Zhua, X. Photosensitizer cross-linked nano-micelle platform for multimodal imaging guided synergistic photothermal/photodynamic therapy. Nanoscale 2016, 8, 15323–15339. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yuan, J.; Liu, H.; Jiang, L.; Sun, S.; Cheng, S. Microgel-silica hybrid particles: Strategies for tunable nanostructure, composition, surface property and porphyrin functionalization. J. Colloid Interface Sci. 2010, 348, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.G.; Cattoën, X.; Durand, J.-O.; Man, M.W.C.; Khashab, N.M. Organosilica hybrid nanomaterials with a high organic content: Syntheses and applications of silsesquioxanes. Nanoscale 2016, 8, 19945–19972. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.Y.; Kim, J.S.; Kim, H.Y.; Ha, J.M.; Kim, Y.H.; Koo, S.M. One-pot synthesis and surface modifications of organically modified silica (ORMOSIL) particles having multiple functional groups. J. Colloid Interface Sci. 2012, 367, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.R.; Iacono, S.T. Progress in fluorinated organically modified silicas. Polym. Int. 2016, 65, 6–10. [Google Scholar] [CrossRef]
- Sándor, M.; Nistor, C.L.; Szalontai, G.; Stoica, R.; Nicolae, C.A.; Alexandrescu, E.; Fazakas, J.; Oancea, F.; Donescu, D. Aminopropyl-Silica Hybrid Particles as Supports for Humic Acids Immobilization. Materials 2016, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Vidinha, P.; Barreiros, S.; Cabral, J.M.S.; Nunes, T.G.; Fidalgo, A.; Ilharco, L.M. Enhanced Biocatalytic Activity of ORMOSIL-Encapsulated Cutinase: The Matrix Structural Perspective. J. Phys. Chem. C 2008, 112, 2008–2015. [Google Scholar] [CrossRef]
- Zheng, C.; Huang, L.; Li, W.; Chen, W. Encapsulation of cobalt porphyrins in organically modified silica gel glasses and their nonlinear optical properties. Appl. Phys. B 2017, 123, 27. [Google Scholar] [CrossRef]
- Hartono, S.B.; Qiao, S.Z.; Liu, J.; Jack, K.; Ladewig, B.P.; Hao, Z.; Lu, G.Q.M. Functionalized Mesoporous Silica with very Large Pores for Cellulase Immobilization. J. Phys. Chem. C 2010, 114, 8353–8362. [Google Scholar] [CrossRef] [Green Version]
- Harmoko, C.; Sucipto, K.I.; Retnoningtyas, E.S.; Hartono, S.B. Vinyl functionalized cubic mesoporous silica nanoparticles as supporting materials to enhance cellulase enzyme stability. ARPN J. Eng. Appl. Sci. 2016, 11, 2981–2992. [Google Scholar]
- Sangeetha, K.; Morris, K.B.V.; Abraham, B.T.E. Stability and catalytic properties of encapsulated subtilisin in xerogels of alkoxisilanes. Appl. Catal. A 2008, 341, 168–173. [Google Scholar] [CrossRef]
- Cimporescu, A.; Todea, A.; Badea, V.; Paul, C.; Peter, F. Efficient kinetic resolution of 1,5-dihydroxy-1,2,3,4-tetrahydronaphthalene catalyzed byimmobilized Burkholderia cepacia lipase in batch and continuous-flowsystem. Process Biochem. 2016, 51, 2076–2083. [Google Scholar] [CrossRef]
- Yang, J.; Liu, L.; Cao, X. Combination of bioimprinting and silane precursor alkyls improved the activity of sol-gel-encapsulated lipase. Enzyme Microb. Technol. 2010, 46, 257–261. [Google Scholar] [CrossRef]
- Todea, A.; Borza, P.; Cimporeanu, A.; Paul, C.; Peter, F. Continuous kinetic resolution of aliphatic and aromatic secondary alcohols by sol-gel entrapped lipases in packed bed bioreactors. Catal. Today 2018, 306, 223–232. [Google Scholar] [CrossRef]
- Zhang, L.; Chang, Z.X.; Li, D.L. The Surface Modification of Silica with Vinyltriethoxysilane. Adv. Mater. Res. 2012, 399–401, 1123–1130. [Google Scholar] [CrossRef]
- Li, Y.-S.; Wright, P.B.; Puritt, R.; Tran, T. Vibrational spectroscopic studies of vinyltriethoxysilane sol-gel and its coating. Spectrochim. Acta Part A 2004, 60, 2759–2766. [Google Scholar] [CrossRef] [PubMed]
- Donatti, D.A.; Ibañez Ruiz, A.; Vicelli, M.R.; Vollet, D.R. Small-angle X-ray scattering from wet gels prepared from co-hydrolysis of tetraethoxysilane and vinyltriethoxysilane. J. Sol-Gel Sci. Technol. 2009, 51, 222–227. [Google Scholar] [CrossRef]
- Chen, S.; Osaka, A.; Hayakawa, S.; Shirosakia, Y.; Tsurub, K. Morphology and structure of organosilica hybrid particles derived from tetramethoxysilane and vinyltrimethoxysilane via a catalyst-free sol-gel route. J. Mater. Chem. 2010, 20, 7337–7339. [Google Scholar] [CrossRef]
- Vollet, D.R.; Awano, C.M.; De Vicente, F.S.; Ruiz, A.I.; Donatti, D.A. Temperature effect on the structure and formation kinetics of vinyltriethoxysilane-derived organic/silica hybrids. Langmuir 2011, 27, 10986–10992. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.L.; Sun, Y.H.; Cao, D.X. Study on Properties of Organic Modified SiO2 Glass Prepared by Sol-Gel Method. Adv. Mater. Res. 2011, 306, 865–868. [Google Scholar] [CrossRef]
- Trabelsi, O.; Tighzert, L.; Jbara, O.; Hadjadj, A. Synthesis via sol-gel process and characterization of novel organic-inorganic coatings. J. Non-Cryst. Solids 2011, 357, 3910–3916. [Google Scholar] [CrossRef]
- Li, Y.S.; Ba, A.; Mahmood, M.S. An environmentally friendly coating for corrosion protection of aluminum and copper in sodium chloride solutions. Electrochim. Acta 2008, 53, 7859–7862. [Google Scholar] [CrossRef]
- Li, Y.S.; Ba, A.; Mahmood, M.S. Infrared and Raman spectra of triacetoxyvinylsilane, aqueous sol-gel and xerogel. Spectrochim. Acta Part A 2009, 72, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Grewe, D.D. Silane Bonded Medical Devices and Method of Making Same. U.S. Patent Application No. 9681939B2, 20 June 2017. [Google Scholar]
- Dudás, Z.; Vlad-Oros, B.; Preda, G.; Chiriac, A. Preliminary study for using vinyltriacetoxysilane as precursor in enzyme immobilization based on sol-gel method. Ann. West Univ. Timis. Ser. Chem. 2007, 16, 175–180. [Google Scholar]
- Vlad-Oros, B.; Dragomirescu, M.; Preda, G.; Savii, C.; Chiriac, A. Bioorganically doped sol-gel materials containing amyloglucosidase activity. Acta Period. Technol. 2006, 37, 179–186. [Google Scholar] [CrossRef]
- Qian, X.; Yan, R.; Shao, L.; Li, H.; Wang, X.; Hou, L. Triindole-modified push–pull type porphyrin dyes for dye-sensitized solar cells. Dyes Pigment. 2016, 134, 434–441. [Google Scholar] [CrossRef]
- Ladomenou, K.; Kitsopoulos, T.N.; Sharma, G.D.; Coutsolelos, A.G. The importance of various anchoring groups attached on porphyrins as potential dyes for DSSC applications. RSC Adv. 2014, 4, 21379–21404. [Google Scholar] [CrossRef]
- Brune, A.; Jeong, G.; Liddell, P.A.; Sotomura, T.; Moore, T.A.; Moore, A.L.; Gust, D. Porphyrin-sensitized nanoparticulate TiO2 as the photoanode of a hybrid photoelectrochemical biofuel cell. Langmuir 2004, 20, 8366–8371. [Google Scholar] [CrossRef] [PubMed]
- Angaridis, P.A.; Lazarides, T.; Coutsolelos, A.C. Functionalized porphyrin derivatives for solar energy conversion. Polyhedron 2014, 82, 19–32. [Google Scholar] [CrossRef]
- Mahendra, D.; Shirsat, M.D.; Sarkar, T.; Kakoullis, J., Jr.; Myung, N.V.; Konnanath, B.; Spanias, A.; Mulchandani, A. Porphyrin-Functionalized Single-Walled Carbon Nanotube Chemiresistive Sensor Arrays for VOCs. J. Phys. Chem. C 2012, 116, 3845–3850. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Deng, J.; Cao, D. Recent advances in Porphyrin-Derived sensors. Curr. Org. Chem. 2013, 17, 3078–3091. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Tarabukina, E.; Zakharova, N.; Birdeanu, M.; Taranu, B.; Palade, A.; Creanga, I.; Lascu, A.; Fagadar-Cosma, G. Hybrids formed between polyvinylpyrrolidone and an A3B porphyrin dye: Behavior in aqueous solutions and chemical response to CO2 presence. Polym. Int. 2016, 65, 200–209. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Sebarchievici, I.; Lascu, A.; Creanga, I.; Palade, A.; Birdeanu, M.; Taranu, B.; Fagadar-Cosma, G. Optical and electrochemical behavior of new nano-sized complexes based on gold-colloid and Co-porphyrin derivative in the presence of H2O2. J. Alloys Compd. 2016, 686, 896–904. [Google Scholar] [CrossRef]
- Mak, C.A.; Pericas, M.A.; Fagadar-Cosma, E. Functionalization of A3B-type porphyrin with Fe3O4 MNPs. Supramolecular assemblies, gas sensor and catalytic applications. Catal. Today 2017, in press. [Google Scholar] [CrossRef]
- Zoltan, T.; Rosales, M.C.; Yadarola, C. Reactive oxygen species quantification and their correlation with the photocatalytic activity of TiO2 (anatase and rutile) sensitized with asymmetric porphyrins. J. Environ. Chem. Eng. 2016, 4, 3967–3980. [Google Scholar] [CrossRef]
- Vasapollo, G.; Mele, G.; Del Sole, R.; Pio, I.; Li, J.; Mazzetto, S.E. Use of novel cardanol-porphyrin hybrids and their TiO2-based composites for the photodegradation of 4-Nitrophenol in water. Molecules 2011, 16, 5769–5784. [Google Scholar] [CrossRef] [PubMed]
- Zoladek, S.; Rutkowska, I.A.; Blicharska, M.; Skorupska, K.; Kulesza, P.J. Enhancement of oxygen reduction at Co-porphyrin catalyst by supporting onto hybrid multi-layered film of polypyrrole and polyoxometalate-modified gold nanoparticles. J. Solid State Electrochem. 2016, 20, 1199–1208. [Google Scholar] [CrossRef]
- Rogers, L.; Sergeeva, N.N.; Paszko, E.; Vaz, G.M.F.; Senge, M.O. Lead structures for applications in photodynamic therapy. 6. Temoporfin anti-inflammatory conjugates to target the tumor microenvironment for in vitro PDT. PLoS ONE 2015, 10, e0125372. [Google Scholar] [CrossRef] [PubMed]
- Sáfar, G.A.M.; Gontijo, R.N.; Fantini, C.; Martins, D.C.S.; Idemori, Y.M.; Pinheiro, M.V.B.; Krambrock, K. Enhanced Oxygen Singlet Production by Hybrid System of Porphyrin and Enriched (6,5) Single-Walled Carbon Nanotubes for Photodynamic Therapy. J. Phys. Chem. C 2015, 119, 4344–4350. [Google Scholar] [CrossRef]
- Carvalho, C.M.B.; Alves, E.; Costa, L.; Tomé, J.P.C; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Almeida, A.; Cunha, A.; et al. Functional Cationic Nanomagnet−Porphyrin Hybrids for the Photoinactivation of Microorganisms. ACS Nano 2010, 4, 7133–7140. [Google Scholar] [CrossRef] [PubMed]
- Fagadar-Cosma, E.; Creanga, I.; Maranescu, B.; Palade, A.; Lőrinczi, A.; Fagadar-Cosma, G.; Popescu, M. Dependence of optical response on pH of a water-soluble Zn(II)-metalloporphyrin. Dig. J. Nanomater. Biostruct. 2011, 6, 75–80. [Google Scholar]
- Ohyama, T.; Mita, H.; Yamamoto, Y. Binding of 5,10,15,20-tetrakis(N-methylpyridinium-4-yl)-21H,23H-porphyrin to an AT-rich region of a duplex DNA. Biophys. Chem. 2005, 113, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, F.M.; Losco, P.; Winnischofer, H.; Araki, K.; Toma, H.E. Synthesis, electrochemistry, spectroscopy and photophysical properties of a series of meso-phenylpyridylporphyrins with one to four pyridyl rings coordinated to [Ru(bipy)2Cl]+ groups. J. Porphyr. Phthalocyanines 2002, 6, 33–42. [Google Scholar] [CrossRef]
- Hambright, P.; Gore, T.; Burton, M. Synthesis and characterization of new isomeric water-soluble porphyrins. Tetra(2-N-methylpyridyl)porphine and tetra(3-N-methylpyridyl)porphine. Inorg. Chem. 1976, 15, 2314–2315. [Google Scholar] [CrossRef]
- Bailey, S.L.; Hambright, P. Kinetics of the reactions of divalent copper, zinc, cobalt, and nickel with a deformed water soluble centrally monoproticporphyrin. Inorg. Chim. Acta 2003, 344, 43–48. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Yamakawa, N.; Uno, T. Synthetic control of interchromophoric interaction in cationic bis-porphyrins toward efficient DNA photocleavage and singlet oxygen production in aqueous solution. Bioorg. Med. Chem. 2007, 15, 5230–5238. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Yang, S.J. Effect of two-step sol-gel reaction on the mesoporous silica structure. J. Colloid Interface Sci. 2003, 261, 127–132. [Google Scholar] [CrossRef]
- Takai, C.; Ishino, T.; Fuji, M.; Shirai, T. Rapid and high yield synthesis of hollow silica nanoparticles using an NH4F catalyst. Colloids Surf. A 2014, 446, 46–49. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1052–1069. [Google Scholar] [CrossRef]
- Bjork, E.M.; Soderlind, F.; Oden, M. Tuning the Shape of Mesoporous Silica Particles by Alterations in Parameter Space: From Rods to Platelets. Langmuir 2013, 29, 13551–13561. [Google Scholar] [CrossRef] [PubMed]
- Dudas, Z.; Enache, C.; Fagadar-Cosma, G.; Armeanu, I.; Fagadar-Cosma, E. Hybrid silica-porphyrin materials with tailored pore sizes. Mater. Res. Bull. 2010, 45, 1150–1156. [Google Scholar] [CrossRef]
- Becauge, G. Small-Angle Scattering from Polymeric Mass Fractals of Arbitrary Mass-Fractal Dimension. J. Appl. Cryst. 1996, 29, 134–146. [Google Scholar] [CrossRef]
- Al-Oweini, R.; El-Rassy, H. Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R′′Si(OR′)3 precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Malfait, W.J.; Zhao, S.; Verel, R.; Iswar, S.; Rentsch, D.; Fener, R.; Zhang, Y.; Milow, B.; Koebel, M.M. Surface Chemistry of Hydrophobic Silica Aerogels. Chem. Mater. 2015, 27, 6737–6745. [Google Scholar] [CrossRef]
- Románszki, L.; Datsenko, I.; May, Z.; Telegdi, J.; Nyikos, L.; Sand, W. Polystyrene films as barrier layers for corrosion protection of copper and copper alloys. Bioelectrochemistry 2014, 97, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Románszki, L.; Mohos, M.; Telegdi, J.; Keresztes, Z.; Nyikos, L. A comparison of contact angle measurement results obtained on bare, treated, and coated alloy samples by both dynamic sessile drop and Wilhelmy method. Period. Polytech. Chem. Eng. 2014, 58, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. J. Chem. Soc. Faraday Trans. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Kelm, A.; Waluk, J. Simulations of fluorescence enhancement and emission profile changes in porphyrin attached to gold-silica core-shell nanoparticles. Methods Appl. Fluoresc. 2016, 4, 014002. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Enache, C.; Armeanu, I.; Fagadar-Cosma, G. Comparative investigations of the absorption and fluorescence spectra of tetrapyrydilporphyrine and Zn(II)tetrapyridylporphyrine. Dig. J. Nanomater. Biostruct. 2007, 2, 175–183. [Google Scholar]
- Senge, M. Stirring the porphyrin alphabet soup—Functionalization reactions for porphyrins. Chem. Commun. 2011, 47, 1943–1960. [Google Scholar] [CrossRef] [PubMed]
- Fagadar-Cosma, E.; Cseh, L.; Badea, V.; Fagadar-Cosma, G.; Vlascici, D. Combinatorial Synthesis and Characterization of New Asymmetric Porphyrins as Potential Photosensitizers in Photodynamic Therapy. Comb. Chem. High Throughput Screen. 2007, 10, 466–472. [Google Scholar] [CrossRef] [PubMed]













| Silica Hybrid Sample (A Series) 1 | Silica Hybrid Sample (B Series) 2 | VTAS mol % in the Precursor Mixture (VTAS/TEOS) |
|---|---|---|
| A00-VTAS | B00-VTAS | 0 |
| A05-VTAS | B05-VTAS | 5 |
| A10-VTAS | B10-VTAS | 10 |
| A20-VTAS | B20-VTAS | 20 |
| A30-VTAS | B30-VTAS | 30 |
| A40-VTAS | B40-VTAS | 40 |
| A50-VTAS | B50-VTAS | 50 |
| A60-VTAS | B60-VTAS | 60 |
| Sample | Zn-TNMPyP | Isotherm and Hysteresys Type | Dp [ads] 1 (nm) | Dp [des] 2 (nm) | Dp [DFT] 3 (nm) | SBET (m2/g) | Vp (cm3/g) | Vpmicro (cm3/g) |
|---|---|---|---|---|---|---|---|---|
| B00-VTAS | No | Iva–H3 | 3.64 | 13.35 | 7.31 | 531.3 | 0.147 | 0.025 |
| Yes | IVa–H1 | 3.64 | 4.35 | 5.69 | 695.6 | 0.756 | 0.000 | |
| B05-VTAS | No | IVa–H4 | 3.63 | 3.66 | 4.57 | 900.4 | 0.807 | 0.056 |
| Yes | IVa–H4 | 3.64 | 3.42 | 4.57 | 532.4 | 0.388 | 0.021 | |
| B20-VTAS | No | IVa–H4 | 3.63 | 3.65 | 2.58 | 842.0 | 0.488 | 0.305 |
| Yes | Iva–H4 | 3.63 | 3.09 | 4.57 | 657.0 | 0.373 | 0.202 | |
| B40-VTAS | No | Ib | 3.66 | 3.62 | 2.54 | 771.6 | 0.453 | 0.276 |
| Yes | Ib | 3.66 | 3.42 | 2.51 | 672.9 | 0.385 | 0.245 | |
| B60-VTAS | No | Ib | 3.63 | 3.64 | 2.11 | 421.7 | 0.254 | 0.166 |
| Yes | Ib | 3.63 | 3.43 | 2.11 | 428.2 | 0.278 | 0.145 |
| Vinyl Content | A Series | B Series | B Series + Porphyrin D (nm) SANS | ||
|---|---|---|---|---|---|
| SANS | SAXS | SANS | SAXS | ||
| D (nm) | D (nm) | D (nm) | D (nm) | ||
| 0% | 28.25 ± 0.46 | 20.15 ± 0.01 | 25.31 ± 0.29 | 16.15 ± 0.03 | 5.51 ± 0.02 |
| 5% | 14.95 ± 0.05 | 7.97 ± 0.01 | 7.10 ± 0.06 | 7.10 ± 0.03 | 4.49 ± 0.01 |
| 10% | 7.87 ± 0.04 | 5.37 ± 0.003 | 5.11 ± 0.04 | 5.17 ± 0.005 | - |
| 20% | 6.50 ± 0.06 | 4.36 ± 0.02 | 4.04 ± 0.07 | 4.39 ± 0.01 | 4.20 ± 0.02 |
| 30% | 7.64 ± 0.19 | 14.26 ± 0.5 | 4.28 ± 0.09 | 3.91 ± 0.01 | - |
| 40% | 8.45 ± 0.36 | 13.85 ± 0.34 | 5.03 ± 0.11 | 4.57 ± 0.03 | 5.02 ± 0.04 |
| 50% | - | - | 3.97 ± 0.2 | - | |
| 60% | - | - | 6.99 ± 0.21 | 27.30 ± 0.65 | |
| Sample | Q4 (%) | Q3 (%) | Q2 (%) | T3 (%) | T2 (%) | Q4 + T3 (%) | Q4 + T3 + Q3 (%) | Q2 + T2 (%) | Q3 + T3 (%) |
|---|---|---|---|---|---|---|---|---|---|
| B00-VTAS | 61.7 | 35.0 | 3.2 | 0.0 | 0.0 | 61.7 | 96.8 | 3.2 | 35.0 |
| B05-VTAS | 52.9 | 38.0 | 3.5 | 4.7 | 0.8 | 57.7 | 95.7 | 4.3 | 42.7 |
| B20-VTAS | 42.4 | 32.6 | 3.8 | 15.6 | 5.6 | 58.1 | 90.6 | 9.4 | 48.2 |
| B40-VTAS | 38.0 | 20.0 | 1.8 | 32.1 | 8.1 | 70.1 | 90.1 | 9.9 | 52.1 |
| B60-VTAS | 27.3 | 11.6 | 0.8 | 49.5 | 10.8 | 76.8 | 88.4 | 11.6 | 61.1 |
| A05-VTAS | 62.6 | 29.7 | 1.7 | 4.3 | 1.7 | 66.9 | 96.6 | 3.4 | 34.0 |
| A60-VTAS | 26.4 | 9.7 | 0.5 | 52.0 | 11.3 | 78.5 | 88.2 | 11.8 | 61.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudás, Z.; Fagadar-Cosma, E.; Len, A.; Románszki, L.; Almásy, L.; Vlad-Oros, B.; Dascălu, D.; Krajnc, A.; Kriechbaum, M.; Kuncser, A. Improved Optical and Morphological Properties of Vinyl-Substituted Hybrid Silica Materials Incorporating a Zn-Metalloporphyrin. Materials 2018, 11, 565. https://doi.org/10.3390/ma11040565
Dudás Z, Fagadar-Cosma E, Len A, Románszki L, Almásy L, Vlad-Oros B, Dascălu D, Krajnc A, Kriechbaum M, Kuncser A. Improved Optical and Morphological Properties of Vinyl-Substituted Hybrid Silica Materials Incorporating a Zn-Metalloporphyrin. Materials. 2018; 11(4):565. https://doi.org/10.3390/ma11040565
Chicago/Turabian StyleDudás, Zoltán, Eugenia Fagadar-Cosma, Adél Len, Loránd Románszki, László Almásy, Beatrice Vlad-Oros, Daniela Dascălu, Andraž Krajnc, Manfred Kriechbaum, and Andrei Kuncser. 2018. "Improved Optical and Morphological Properties of Vinyl-Substituted Hybrid Silica Materials Incorporating a Zn-Metalloporphyrin" Materials 11, no. 4: 565. https://doi.org/10.3390/ma11040565
APA StyleDudás, Z., Fagadar-Cosma, E., Len, A., Románszki, L., Almásy, L., Vlad-Oros, B., Dascălu, D., Krajnc, A., Kriechbaum, M., & Kuncser, A. (2018). Improved Optical and Morphological Properties of Vinyl-Substituted Hybrid Silica Materials Incorporating a Zn-Metalloporphyrin. Materials, 11(4), 565. https://doi.org/10.3390/ma11040565