Effect of High Pressure on the Reducibility and Dispersion of the Active Phase of Fischer–Tropsch Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Catalysts
2.2. Characterization Techniques
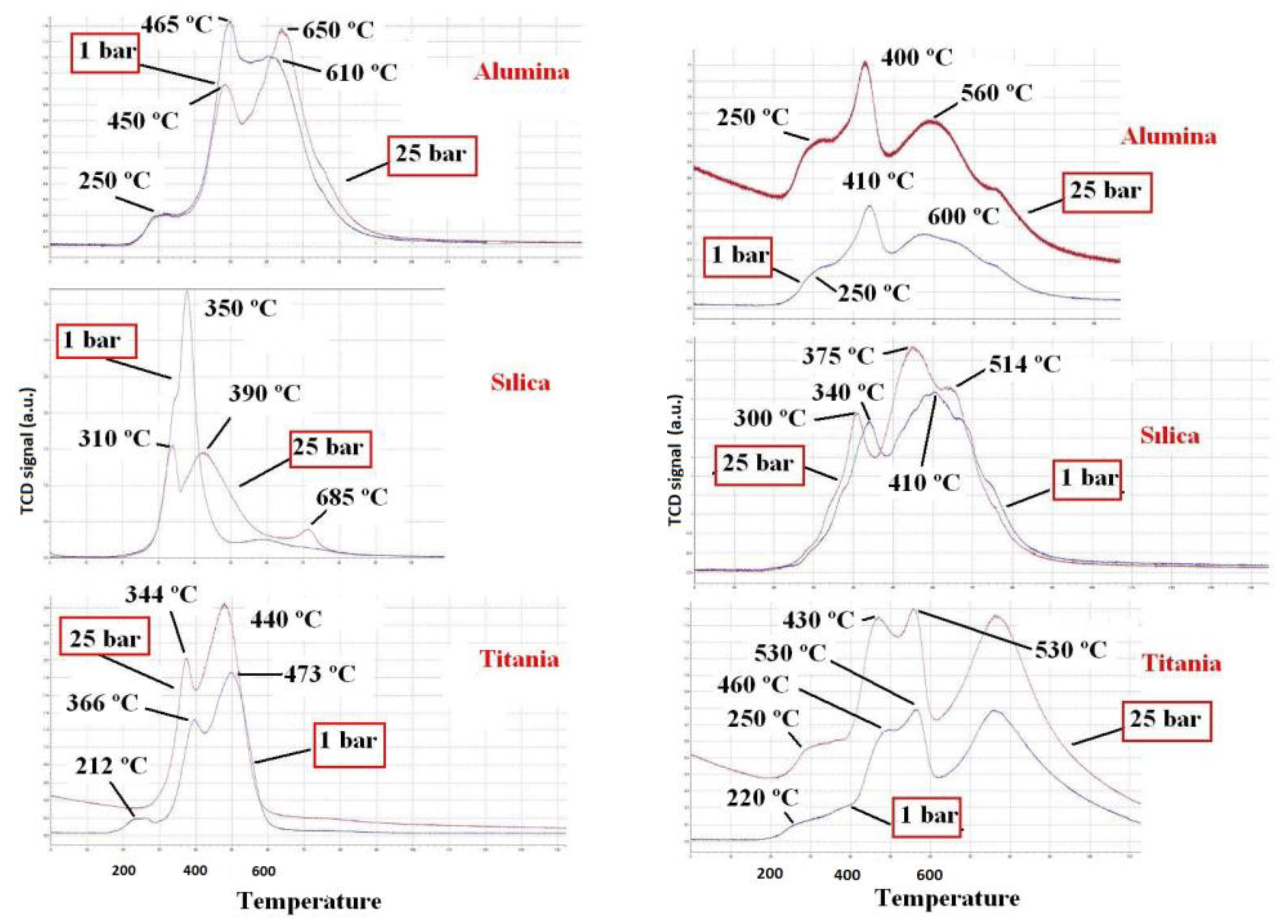
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Davis, B.H. Fischer-Tropsch synthesis: Comparison of performances of iron and cobalt catalysts. Ind. Eng. Chem. Res. 2007, 46, 8938–8945. [Google Scholar] [CrossRef]
- Iglesia, E. Design, synthesis, and use of cobalt-based Fischer-Tropsch synthesis catalysts. Appl. Catal. A 1997, 161, 59–78. [Google Scholar] [CrossRef]
- Jablonski, J.M.; Okal, J.; Potoczna-Petru, D.; Krajcyk, L. High temperature reduction with hydrogen, phase composition, and activity of cobalt/silica catalysts. J. Catal. 2003, 220, 146–160. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Ren, J.; Sun, Y. Chemical treatment of γ-Al2O3 and its influence on the properties of Co-based catalysts for Fischer–Tropsch synthesis. Appl. Catal. A 2003, 243, 121–133. [Google Scholar] [CrossRef]
- Chin, R.L.; Hercules, D.M. Surface spectroscopic characterization of cobalt-alumina catalysts. J. Phys. Chem. 1982, 86, 360–367. [Google Scholar] [CrossRef]
- Van Berge, P.J.; Van e Loosdrecht, J.; Barradas, S.; Van der Kraan, A.M. Oxidation of cobalt based Fischer–Tropsch catalysts as a deactivation mechanism. Catal. Today 2000, 58, 321–334. [Google Scholar] [CrossRef]
- Li, J.L.; Jacobs, G.; Das, T.; Zhang, Y.Q.; Davis, B. Fischer–Tropsch synthesis: Effect of water on the catalytic properties of a Co/SiO2 catalyst. Appl. Catal. A Gen. 2002, 236, 67–76. [Google Scholar] [CrossRef]
- Jacobs, G.; Patterson, P.M.; Zhang, Y.Q.; Das, T.; Li, J.L.; Davis, B.H. Fischer–Tropsch synthesis: Deactivation of noble metal-promoted Co/Al2O3 catalysts. Appl. Catal. A Gen. 2002, 233, 215–226. [Google Scholar] [CrossRef]
- Martens, J.H.A.; Van’t Blik, H.F.J.; Prins, R. Characterization of supported cobalt and cobalt-rhodium catalysts: II. Temperature-Programmed Reduction (TPR) and Oxidation (TPO) of CoTiO2 and CoRhTiO2. J. Catal. 1986, 97, 200–209. [Google Scholar] [CrossRef]
- Lapidus, A.; Krylova, A.; Kazanskii, V.; Borovkov, V.; Zaitsev, A.; Rathousky, J.; Zukal, A.; Jancalkova, M. Hydrocarbon synthesis from carbon monoxide and hydrogen on impregnated cobalt catalysts. Part I. Physico-chemical properties of 10% cobalt/alumina and 10% cobalt/silica. Appl. Catal. 1991, 73, 65–82. [Google Scholar] [CrossRef]
- Diehl, F.; Khodakov, A.Y. Promotion of cobalt Fischer-Tropsch catalysts with noble metals: A review. Oil Gas Sci. Technol. 2009, 64, 11–24. [Google Scholar] [CrossRef]
- Steynberg, A.; Dry, M. Fischer-Tropsch Technology; Studies in Surface Science and Catalysis; Elsevier B.V.: Amsterdam, The Netherlands, 2004; Volume 152, ISBN 0-444-51354-X. [Google Scholar]
- Den Breejen, J.P.; Radstake, P.B.; Bezemer, G.L.; Bitter, J.H.; Froseth, V.; Holmen, A.; De Jong, K.P. On the origin of the cobalt particle size effects in Fischer-Tropsch catalysis. J. Am. Chem. Soc. 2009, 131, 7197–7203. [Google Scholar] [CrossRef] [PubMed]
- Bezemer, G.L.; Bitter, J.H.; Kuipers, H.P.C.E.; Oosterbeek, H.; Holewijn, J.E.; Xu, X.; Kapteijn, F.; Van Dillen, A.J.; De Jong, K.P. Cobalt Particle Size Effects in the Fischer−Tropsch Reaction Studied with Carbon Nanofiber Supported Catalysts. J. Am. Chem. Soc. 2006, 128, 3956–3964. [Google Scholar] [CrossRef] [PubMed]
- Delannay, F. Characterization of Heterogeneous Catalysts; Marcel Dekker Inc.: New York, NY, USA, 1984; ISBN 978-0824771003. [Google Scholar]
- Haber, J.; Block, J.H.; Delmon, B. Manual of methods and procedures for catalyst characterization. Pure Appl. Chem. 1995, 67, 1257–1306. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K. Adsorption by Powders and Porous Solids—Principles, Methodology and Applications; Academic Press: Cambridge, MA, USA, 1998; ISBN 978-0-12-598920-6. [Google Scholar]
- Arnoldy, P.; Moulijn, J.A. Temperature-programmed reduction of CoOAl2O3 catalysts. J. Catal. 1985, 93, 38–54. [Google Scholar] [CrossRef]
- Jacobs, G.; Das, T.K.; Zhang, Y.; Li, J.; Racoillet, G.; Davis, B.H. Fischer-Tropsch synthesis: Support, loading, and promoter effects on the reducibility of cobalt catalysts. Appl. Catal. A Gen. 2002, 233, 263–281. [Google Scholar] [CrossRef]
- Borg, O.; Ronning, M.; Storster, S.; Van Beek, W.; Holmen, A. Identification of cobalt species during temperature programmed reduction of Fischer-Tropsch catalysts. Stud. Surf. Sci. Catal. 2007, 163, 255–272. [Google Scholar]
- De la Peña O’Shea, V.A.; Consuelo Álvarez Galván, M.; Platero Prats, A.E.; Campos-Martin, J.M.; Fierro, J.L.G. Direct evidence of the SMSI decoration effect: The case of Co/TiO2 catalyst. Chem. Commun. 2011, 47, 7131. [Google Scholar] [CrossRef] [PubMed]



| Catalyst | SBET (m2/g) | Vp (cm3/g) * | Metal Content (wt.%) |
|---|---|---|---|
| γ-Al2O3 | 183 | 0.410 | -- |
| SiO2 | 288 | 0.797 | -- |
| TiO2 | 51 | 0.147 | -- |
| Co/Al2O3 | 122 | 0.291 | 13.54 |
| Co/SiO2 | 225 | 0.615 | 13.79 |
| Co/TiO2 | 32 | 0.251 | 10.90 |
| Fe/Al2O3 | 132 | 0.290 | 9.11 |
| Fe/SiO2 | 220 | 0.562 | 13.30 |
| Fe/TiO2 | 38 | 0.235 | 9.62 |
| Catalyst | Q(H2) (cm3/goxide) | R (%) | D (%) |
|---|---|---|---|
| Co/TiO2 (25 bar) | 372 | 100 | 5 |
| Co/TiO2 (1 bar) | 315 | 85 | 1 |
| Co/SiO2 (25 bar) | 299 | 80 | 2 |
| Co/SiO2 (1 bar) | 296 | 80 | 1 |
| Co/Al2O3 (25 bar) | 296 | 80 | 6 |
| Co/Al2O3 (1 bar) | 271 | 73 | 3 |
| Fe/TiO2 (25 bar) | 421 | 100 | 7 |
| Fe/TiO2 (1 bar) | 306 | 73 | 1 |
| Fe/SiO2 (25 bar) | 346 | 82 | 3 |
| Fe/SiO2 (1 bar) | 295 | 70 | 1 |
| Fe/Al2O3 (25 bar) | 236 | 56 | 8 |
| Fe/Al2O3 (1 bar) | 175 | 42 | 3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yunes, S.; Vicente, M.Á.; Korili, S.A.; Gil, A. Effect of High Pressure on the Reducibility and Dispersion of the Active Phase of Fischer–Tropsch Catalysts. Materials 2019, 12, 1915. https://doi.org/10.3390/ma12121915
Yunes S, Vicente MÁ, Korili SA, Gil A. Effect of High Pressure on the Reducibility and Dispersion of the Active Phase of Fischer–Tropsch Catalysts. Materials. 2019; 12(12):1915. https://doi.org/10.3390/ma12121915
Chicago/Turabian StyleYunes, Simón, Miguel Ángel Vicente, Sophia A. Korili, and Antonio Gil. 2019. "Effect of High Pressure on the Reducibility and Dispersion of the Active Phase of Fischer–Tropsch Catalysts" Materials 12, no. 12: 1915. https://doi.org/10.3390/ma12121915
APA StyleYunes, S., Vicente, M. Á., Korili, S. A., & Gil, A. (2019). Effect of High Pressure on the Reducibility and Dispersion of the Active Phase of Fischer–Tropsch Catalysts. Materials, 12(12), 1915. https://doi.org/10.3390/ma12121915







