Oxytree Pruned Biomass Torrefaction: Process Kinetics
Abstract
1. Introduction
2. Materials and Methods
2.1. Oxytree Biomass Samples
- S(G−)(I−),
- S(G+)(I−),
- S(G−)(I+),
- S(G+)(I+),
- C(G+)(I−),
- C(G−)(I−),
- C(G+)(I+),
- C(G−)(I+),
2.2. Ultimate and Proximate Analysis of Samples
- Moisture content determined in accordance with [28], using a laboratory dryer (WAMED, model KBC-65W, Warsaw, Poland),
- Organic matter determined in accordance with [29], using a muffle furnace (SNOL, 8.1/1100, Utena, Lithuania).
- Combustibles and ash content determined in accordance with [30], using a muffle furnace (SNOL, 8.1/1100, Utena, Lithuania),
- Higher heating value (HHV) and low heating value (LHV) determined in accordance with [31] using calorimeters (IKA POL, model C 200, Warsaw, Poland).
2.3. Thermogravimetric Analysis—Experimental Design and Procedure
2.4. Data Analysis
- ms—mass after torrefaction time t, g;
- mo—initial mass, g;
- k—a constant rate of the reaction, s−1;
- t—time, s.
- Ea—activation energy, J∙mol−1;
- a—the slope coefficient of the linear Equation (2), k;
- R—gas constant, J∙mol−1∙K−1.
2.5. Statistical Analysis
- Δm and k—the variable grouping by the cultivation type;
- Δm and k—the variable grouping by torrefaction temperatures;
- Δm—the variable grouping by the torrefaction time.
3. Results
3.1. Oxytree Biomass Characterization
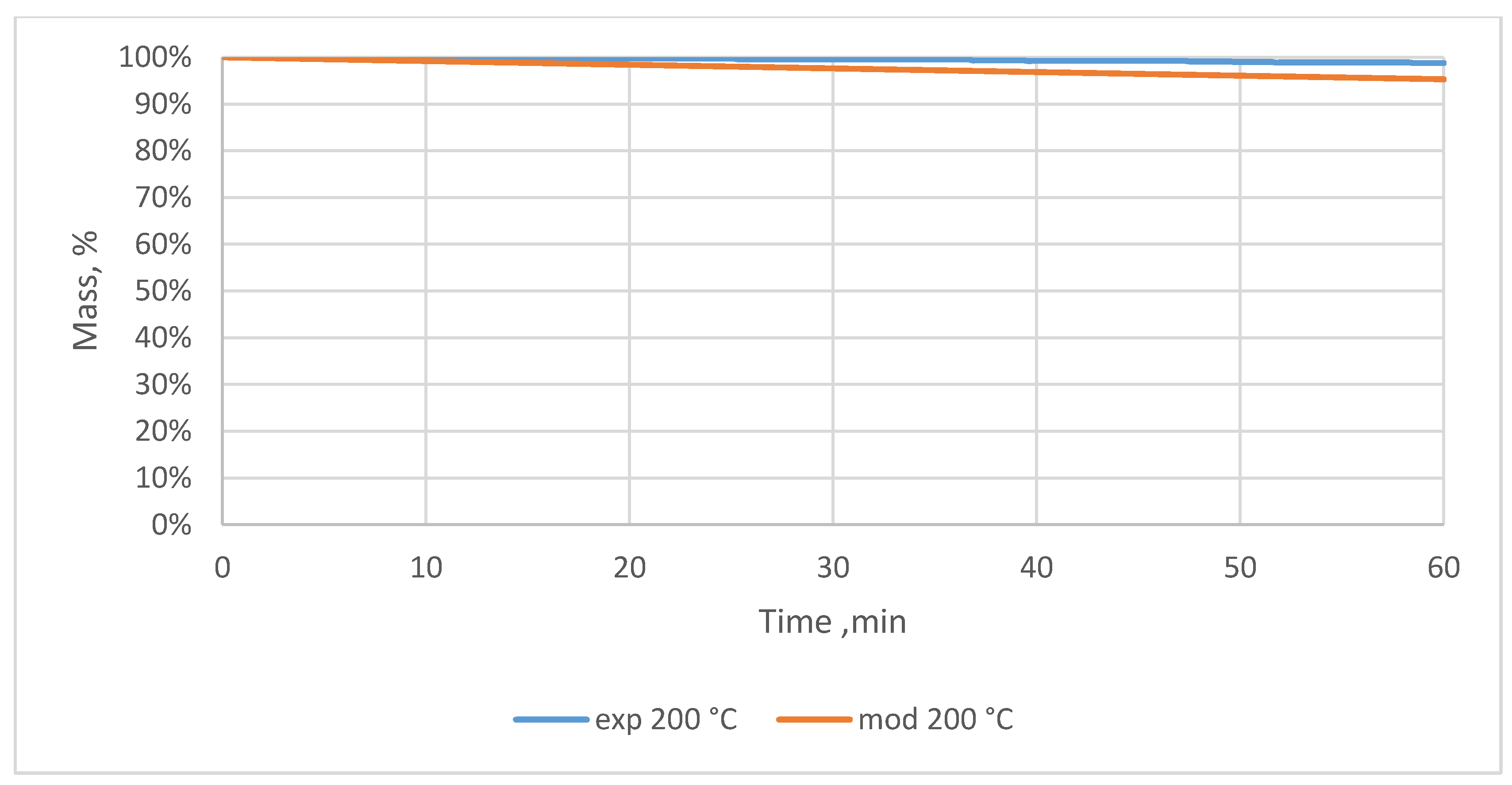
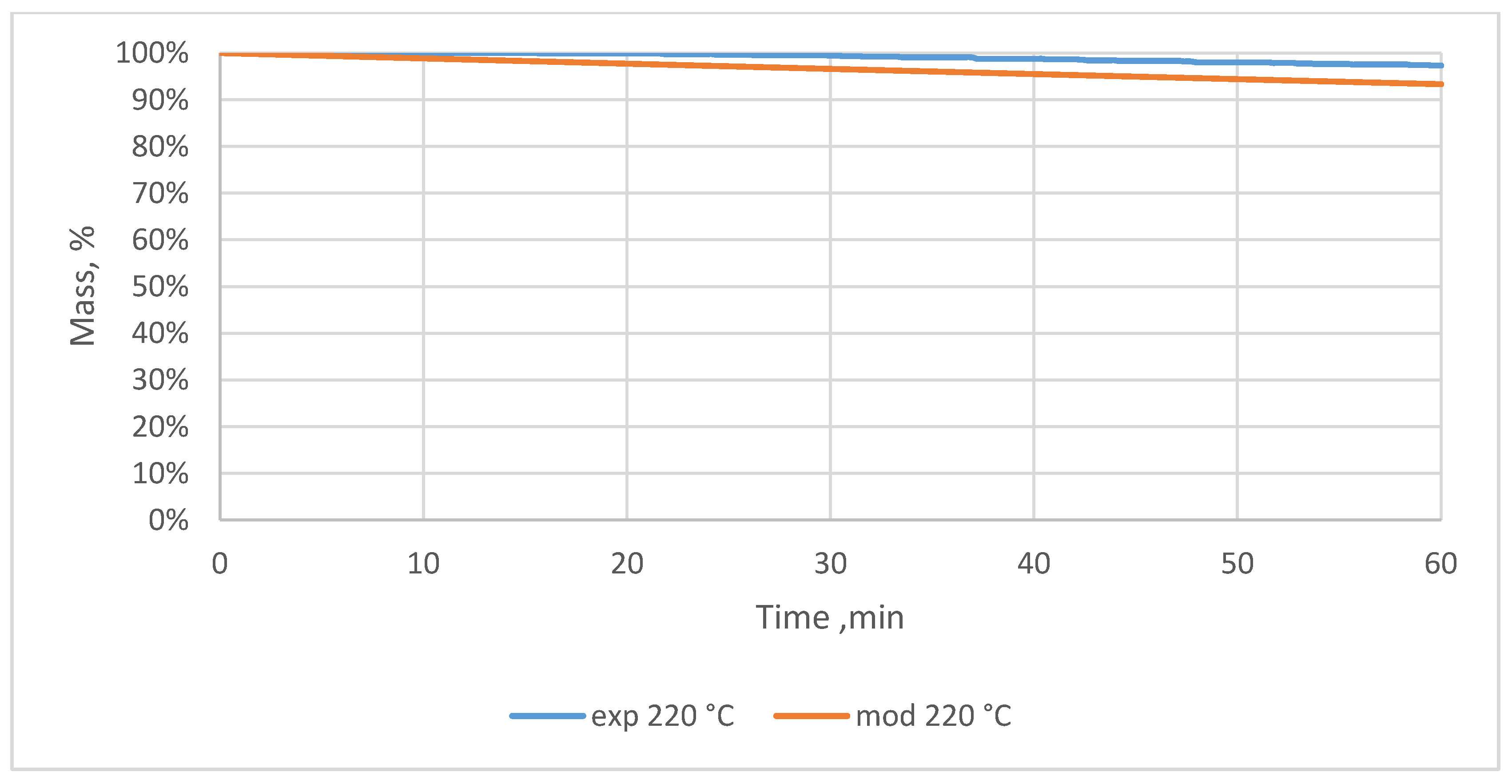
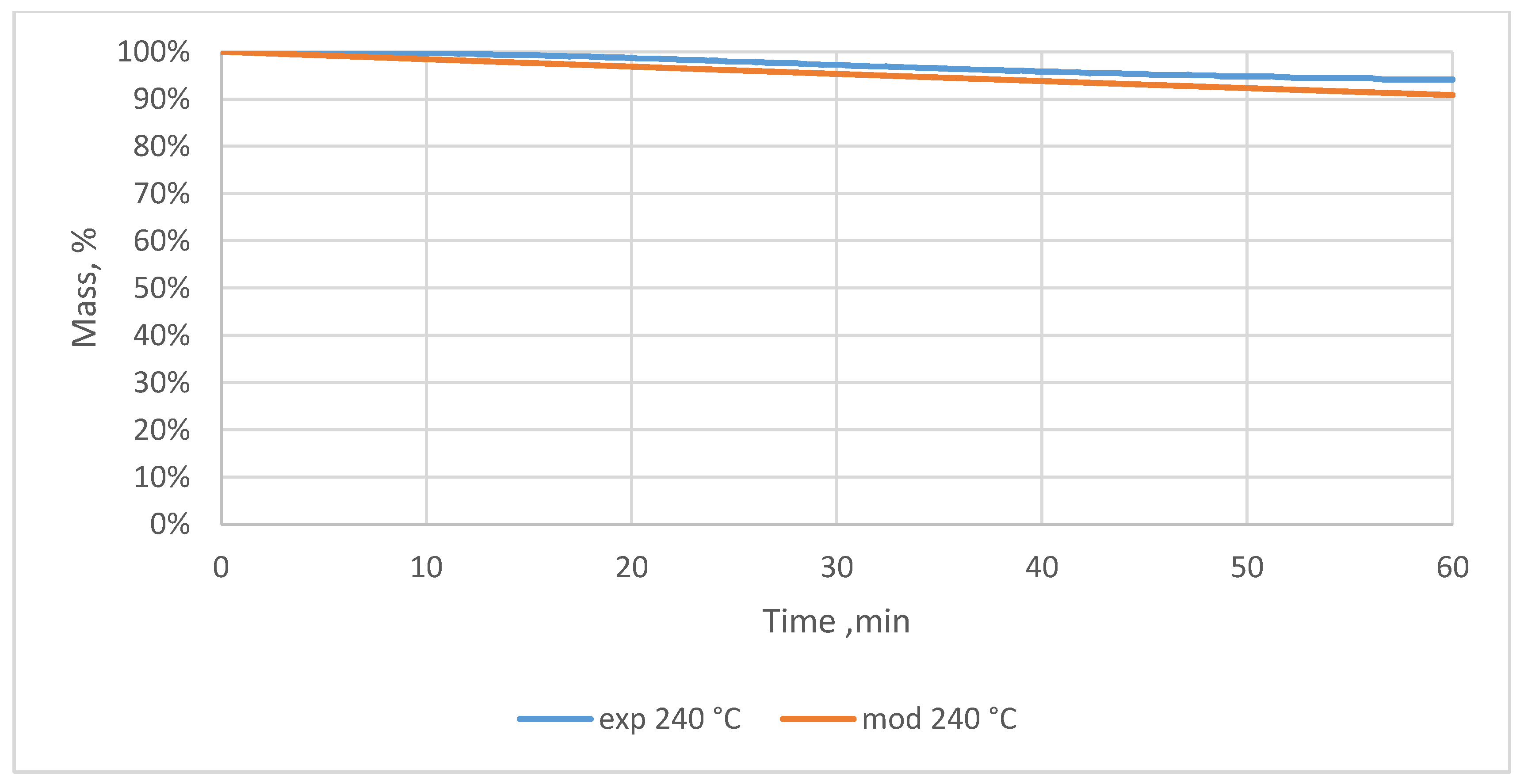
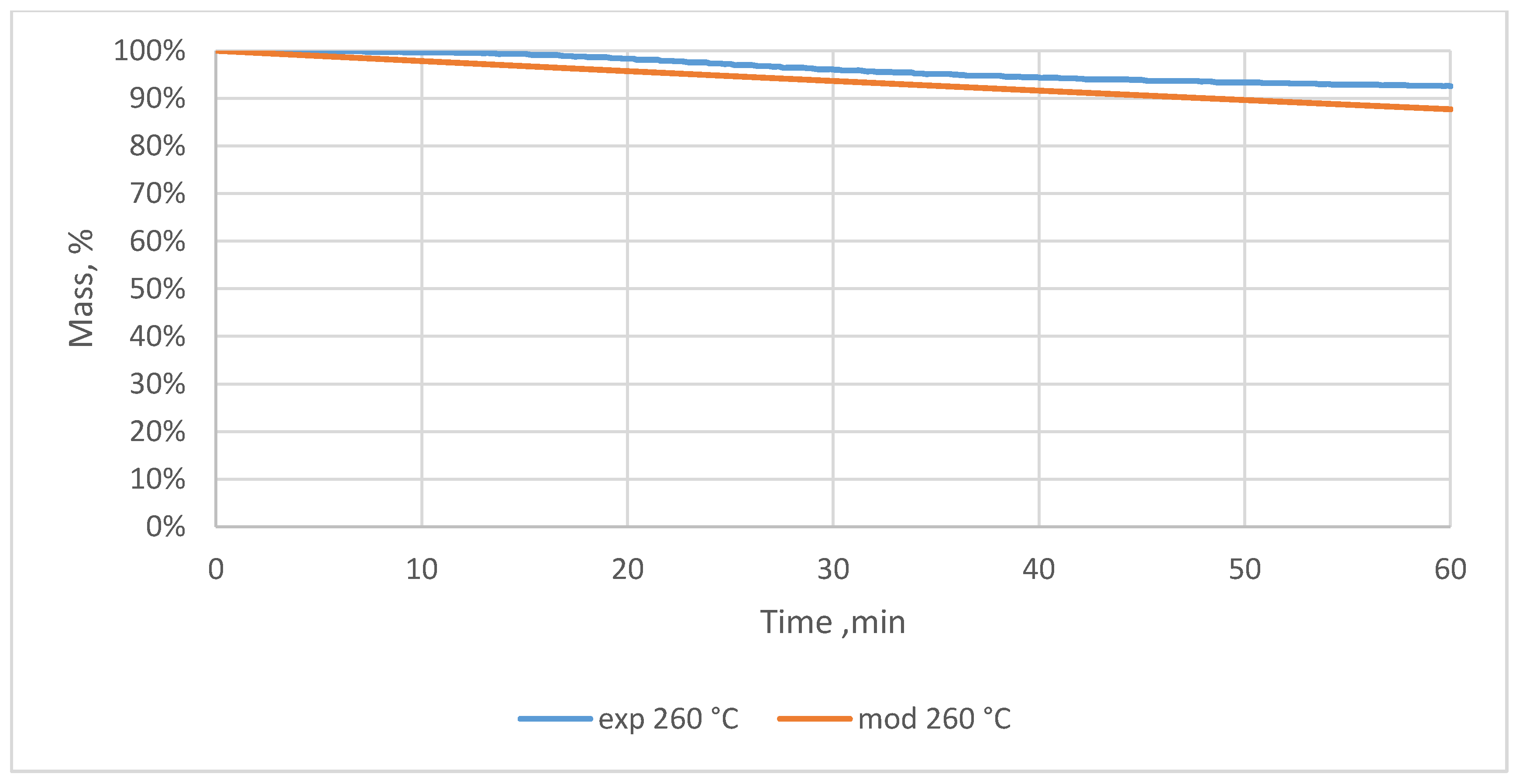
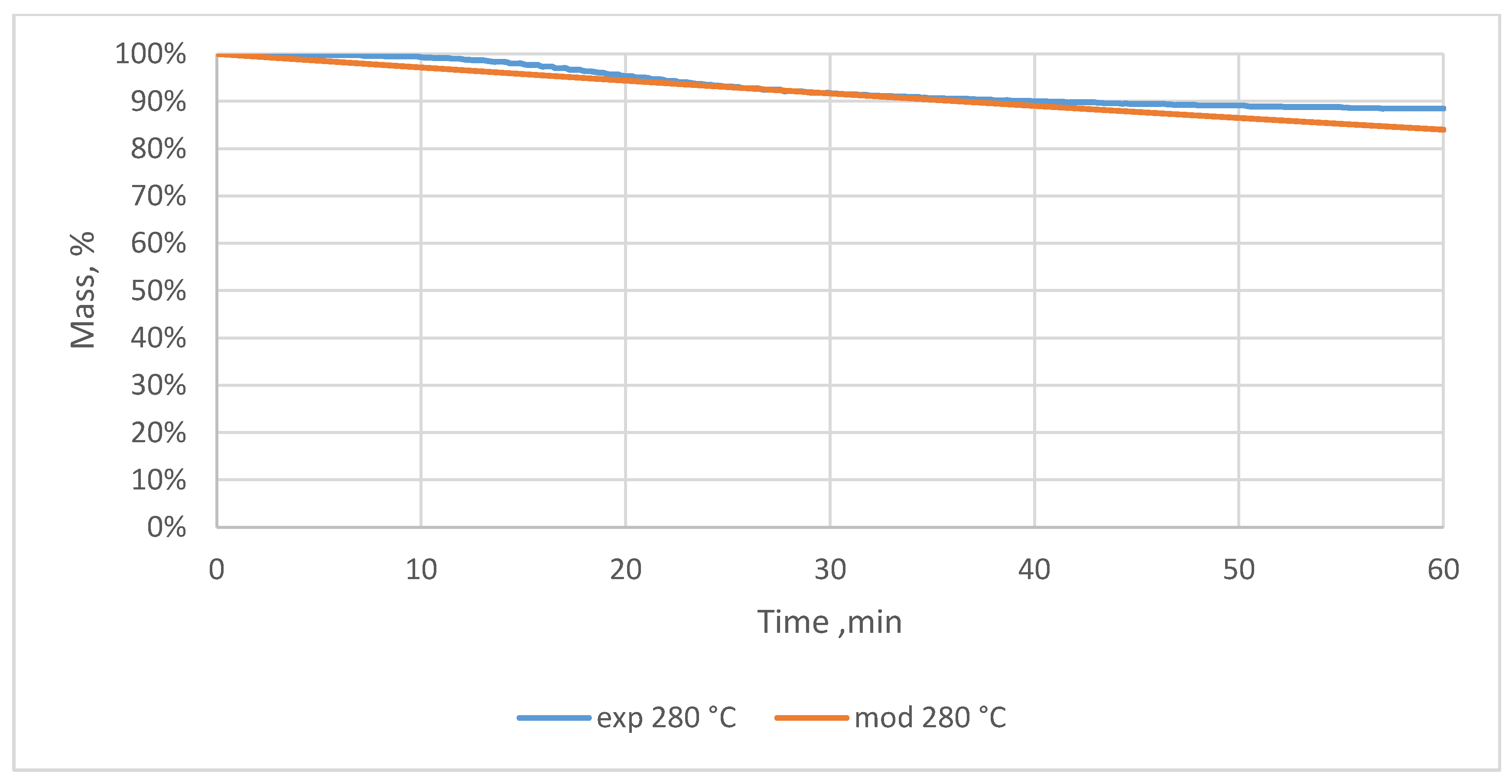
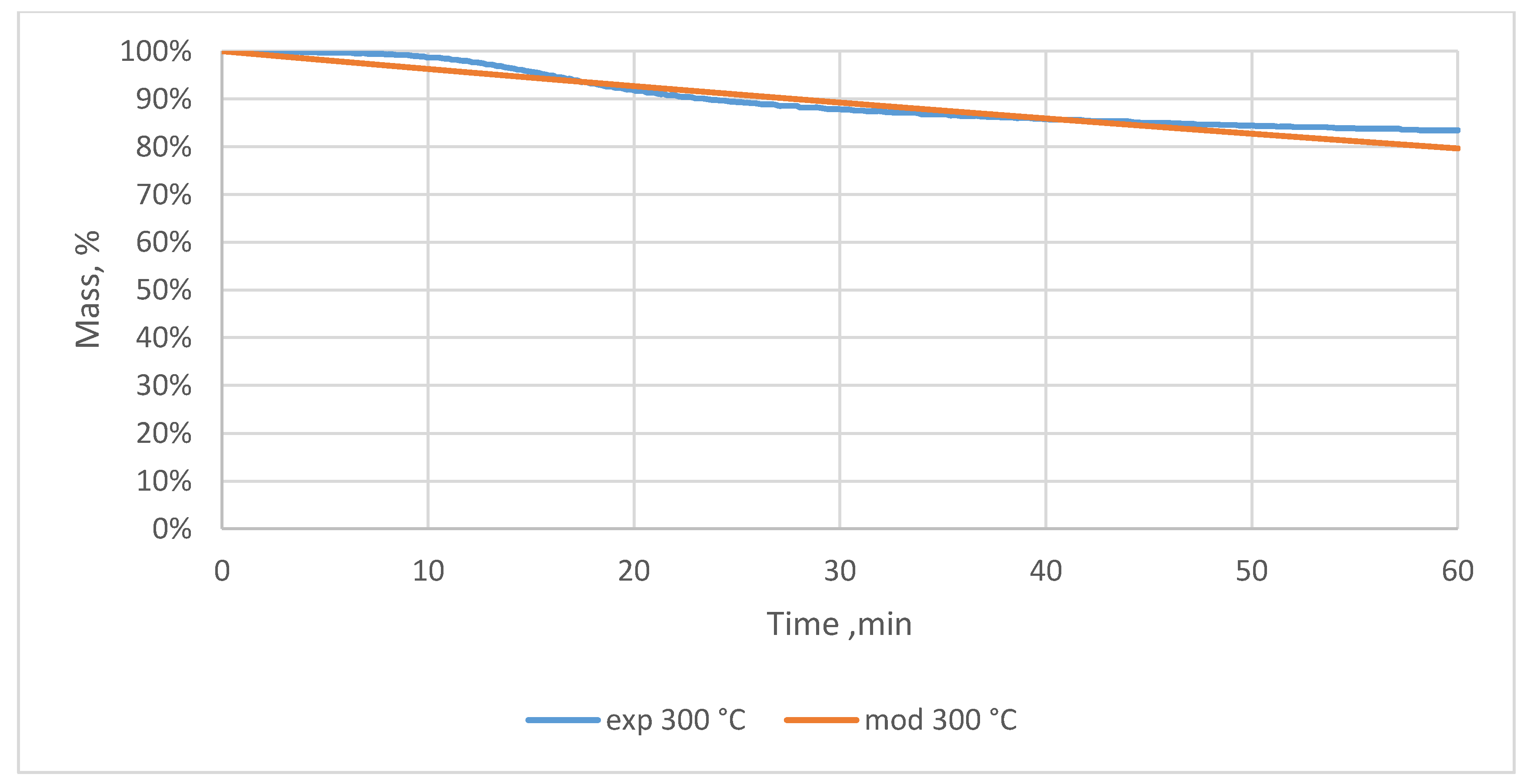
3.2. Relative Mass Loss During Torrefaction of Oxytree
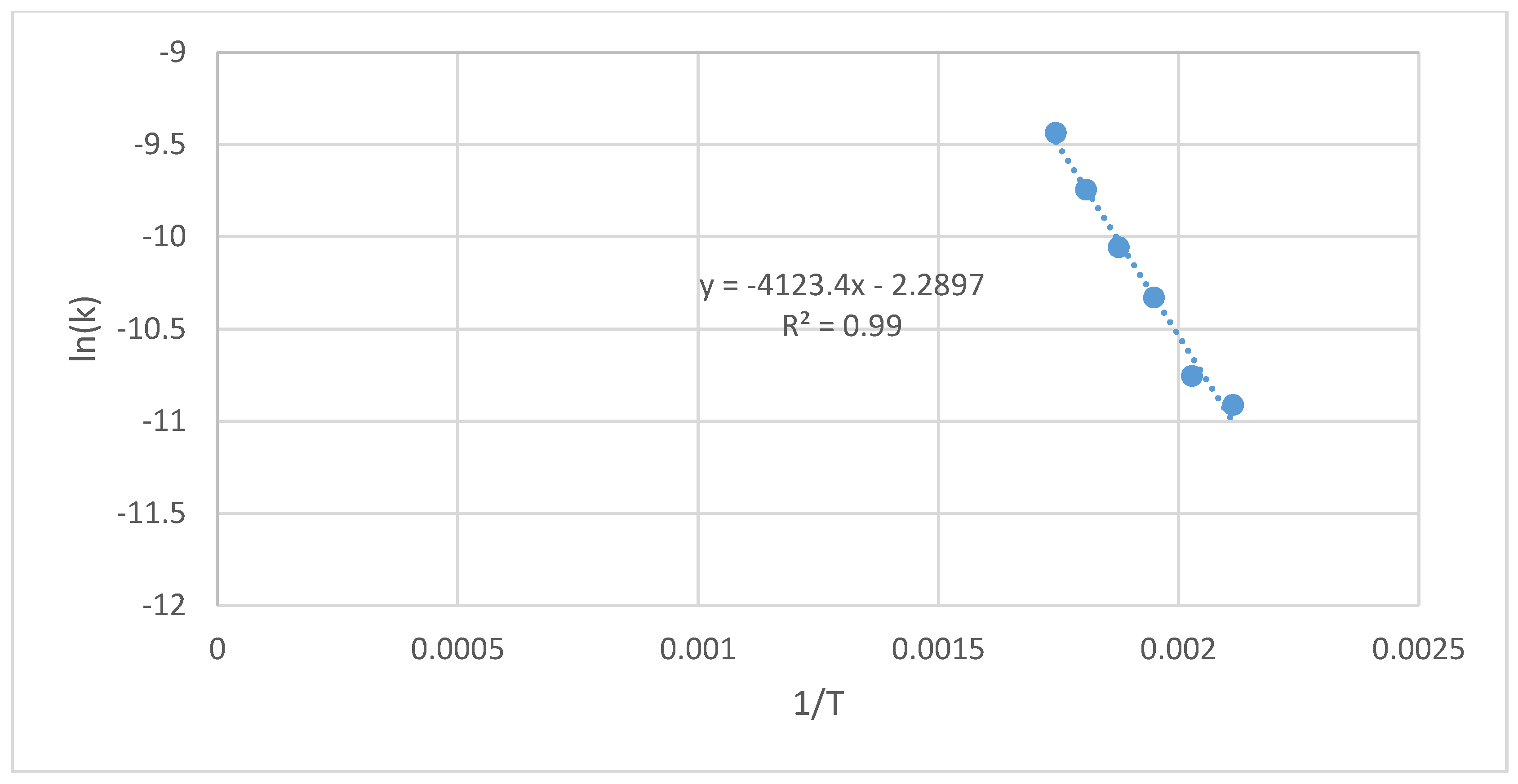
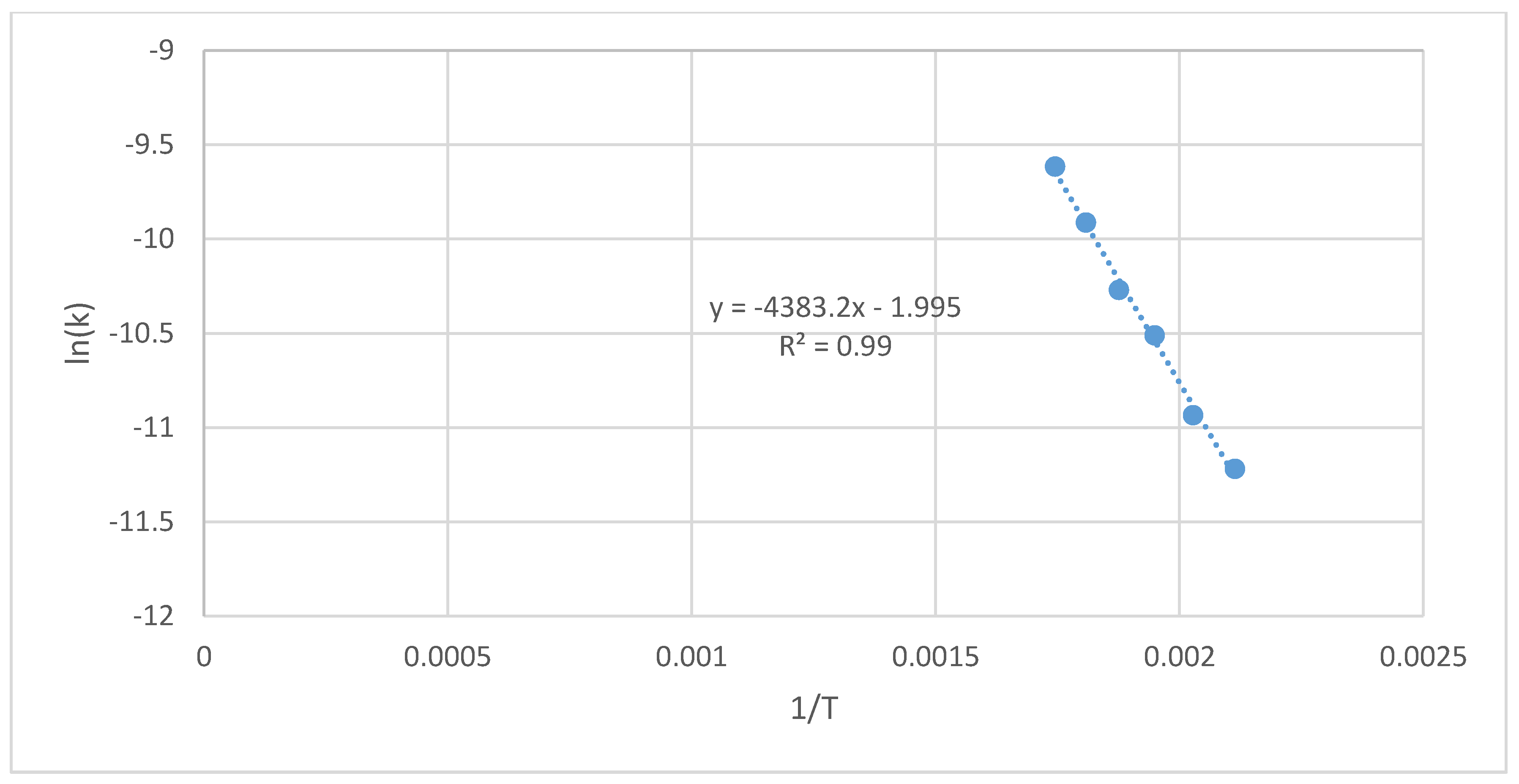
3.3. Oxytree Torrefaction Kinetics
4. Discussion
4.1. Oxytree Biomass Sample Characterization
4.2. Mass Loss During Torrefaction of Oxytree
4.3. Oxytree Torrefaction Kinetics
5. Conclusions
- the cultivation method and soil type did not have an effect on the relative mass loss, k value, and energy activation (p < 0.05);
- the relative mass loss increased with the torrefaction temperature increase (as it is commonly reported for other types of biowaste torrefaction). The smallest relative mass loss was recorded at 200 °C, 10 min and the highest at 300 °C, 60 min, respectively 0.1% and 19.6% (p < 0.05);
- the constant reaction rate (k value) significantly increased with the torrefaction temperature increase. The smallest k value was 1.26 × 10−5 s−1, while the largest was 7.96 × 105 s−1 at 200 °C and 300 °C, respectively;
- the average energy activation of the torrefaction in 200–300 °C was 36.5 kJ·mol−1.
Author Contributions
Funding
Conflicts of Interest
Appendix A
Appendix A.1. Statistical Evaluation of the k Value
Appendix A.1.1. Results of Distribution Normality Evaluation
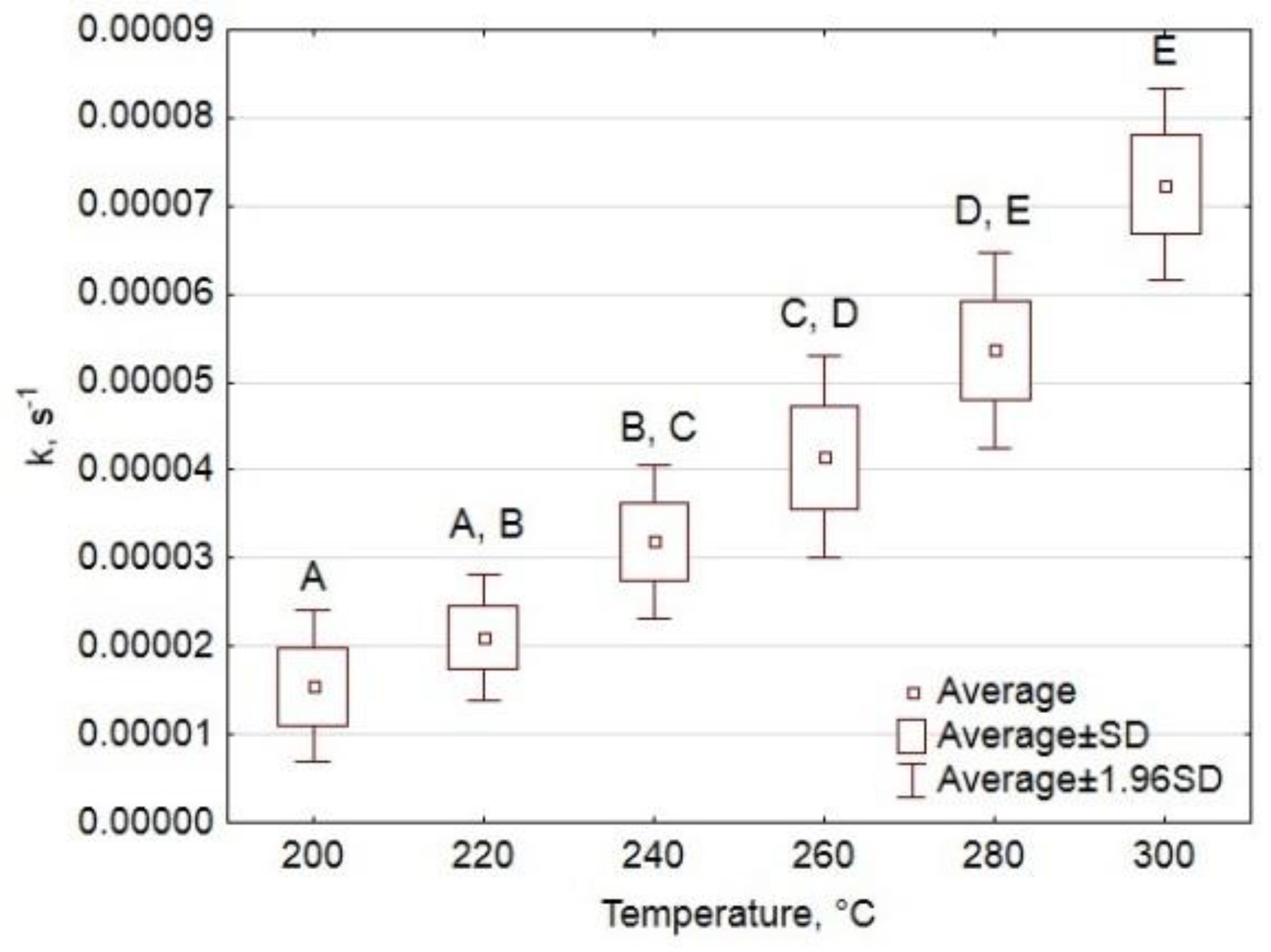
Appendix A.1.2. Analysis of Variance
- k relative to the variable grouping the cultivation type;
- k relative to the variable that groups the process temperature.
Appendix A.1.3. Results and Interpretation
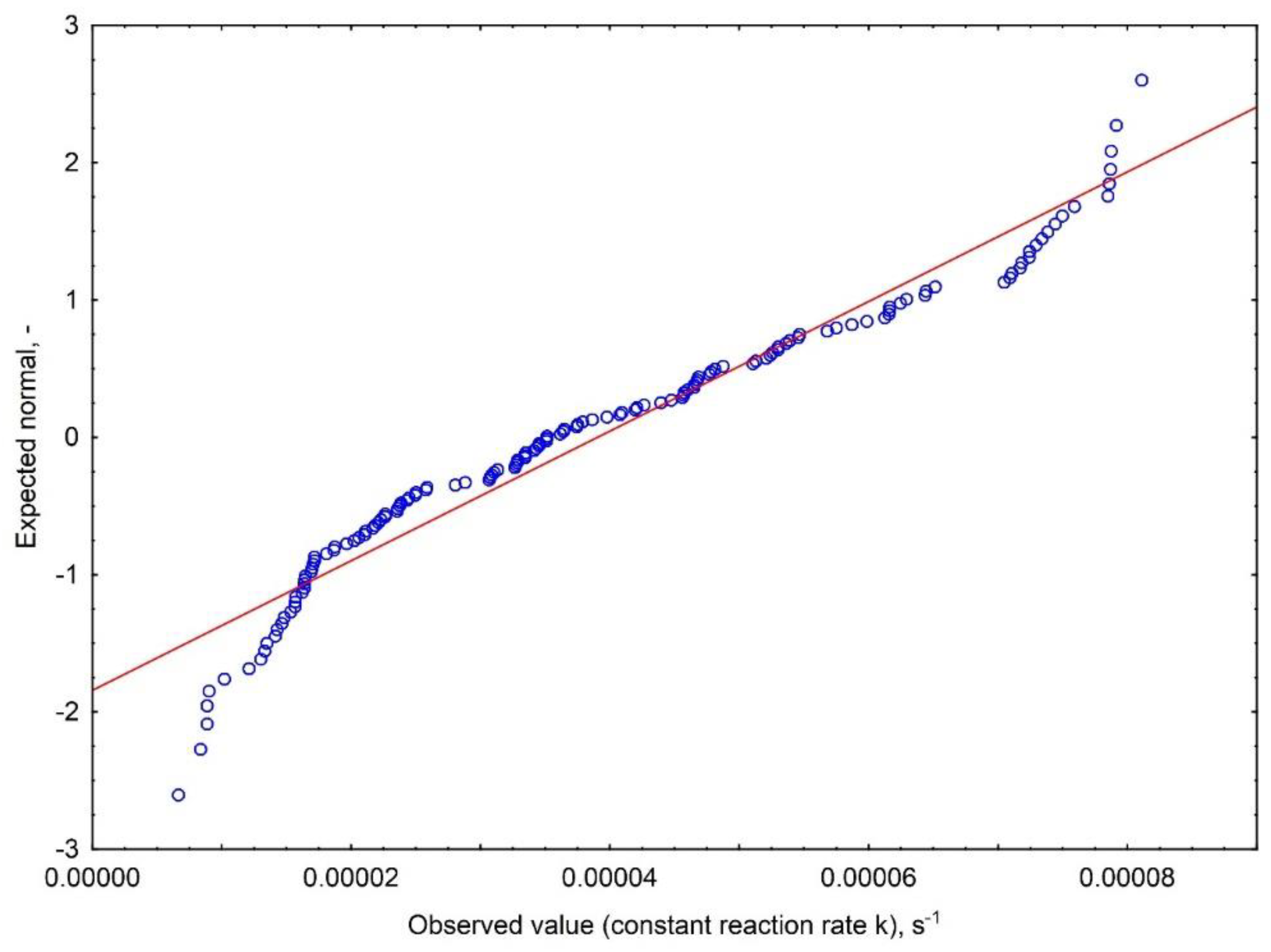
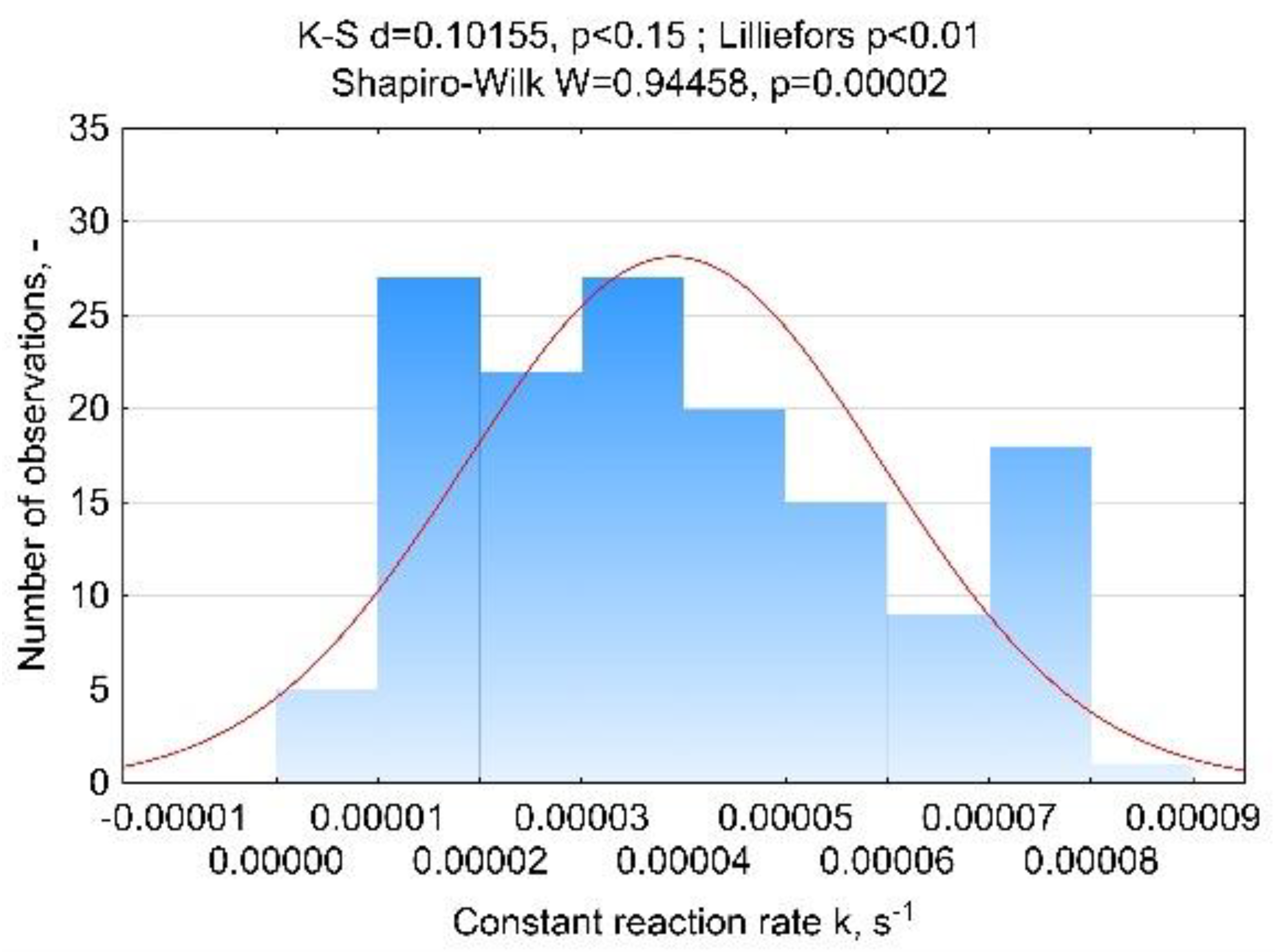

Appendix A.2. Relative Mass Loss Δm, %
Appendix A.2.1. Results of the Distribution Normality Evaluation
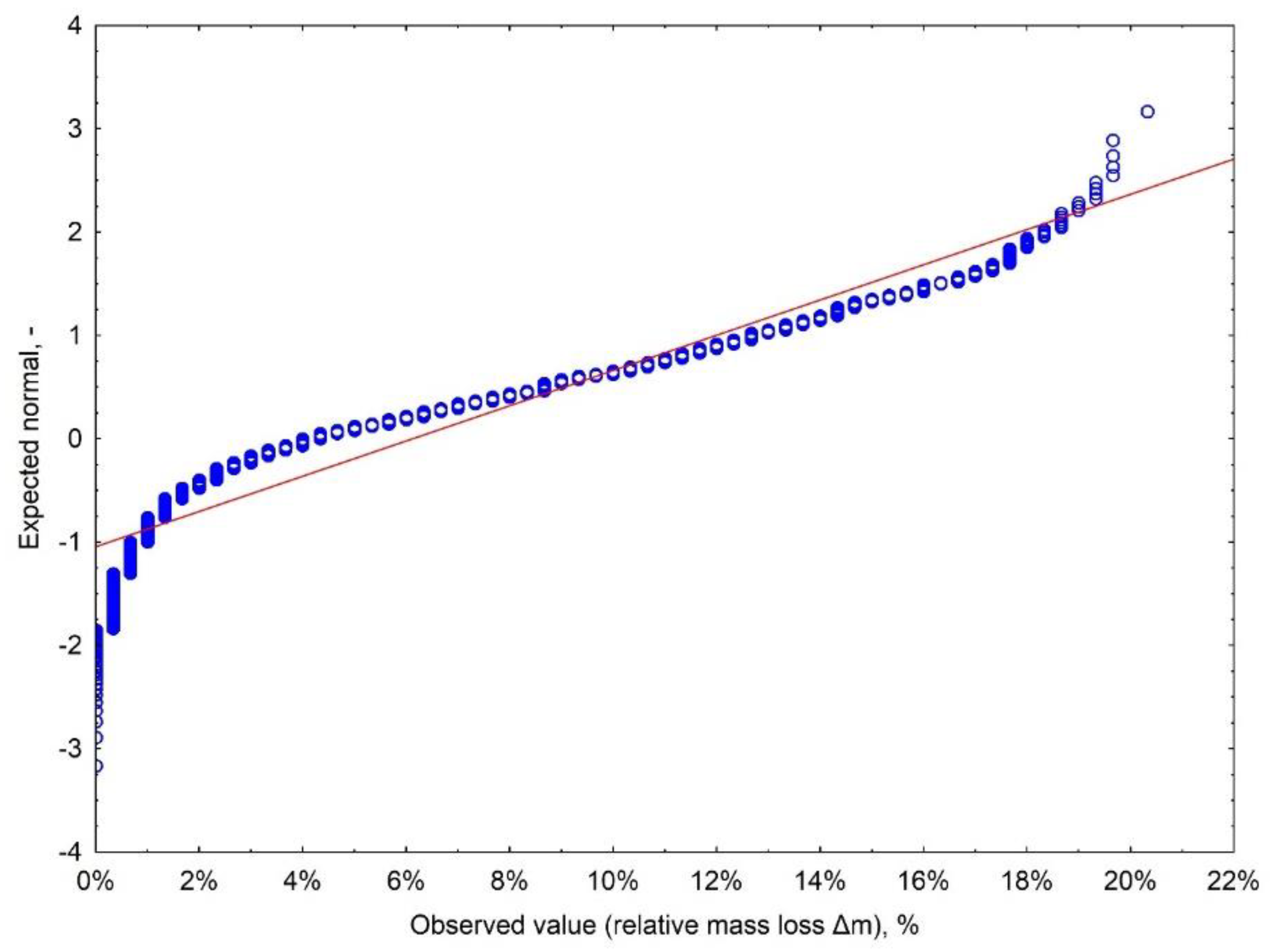
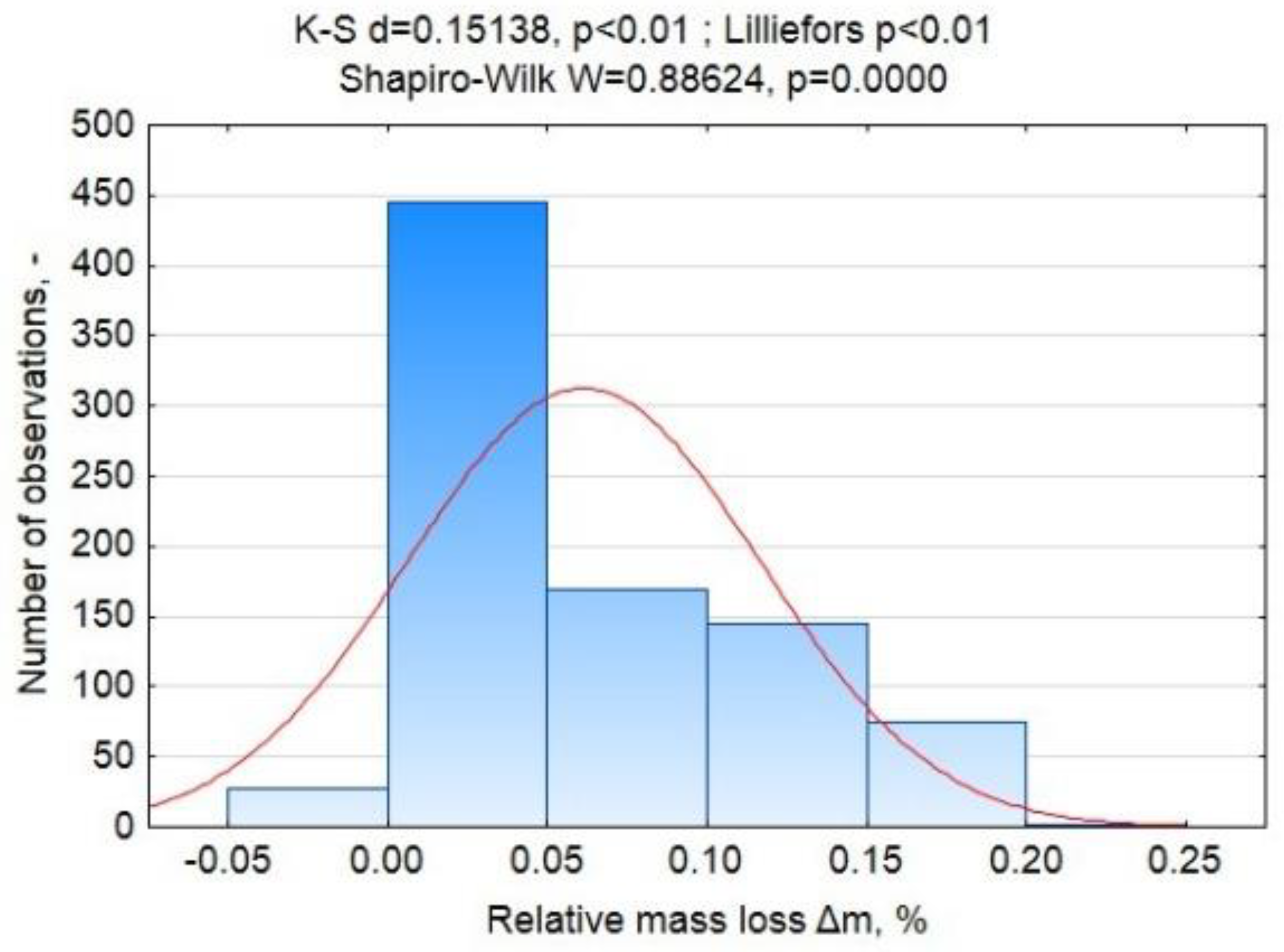
Appendix A.2.2. Analysis of Variance
- Δm relative to the variable grouping the cultivation type;
- Δm relative to the variable grouping process temperatures;
- Δm relative to the variable that groups the process time.
Appendix A.2.3. Results and Interpretation

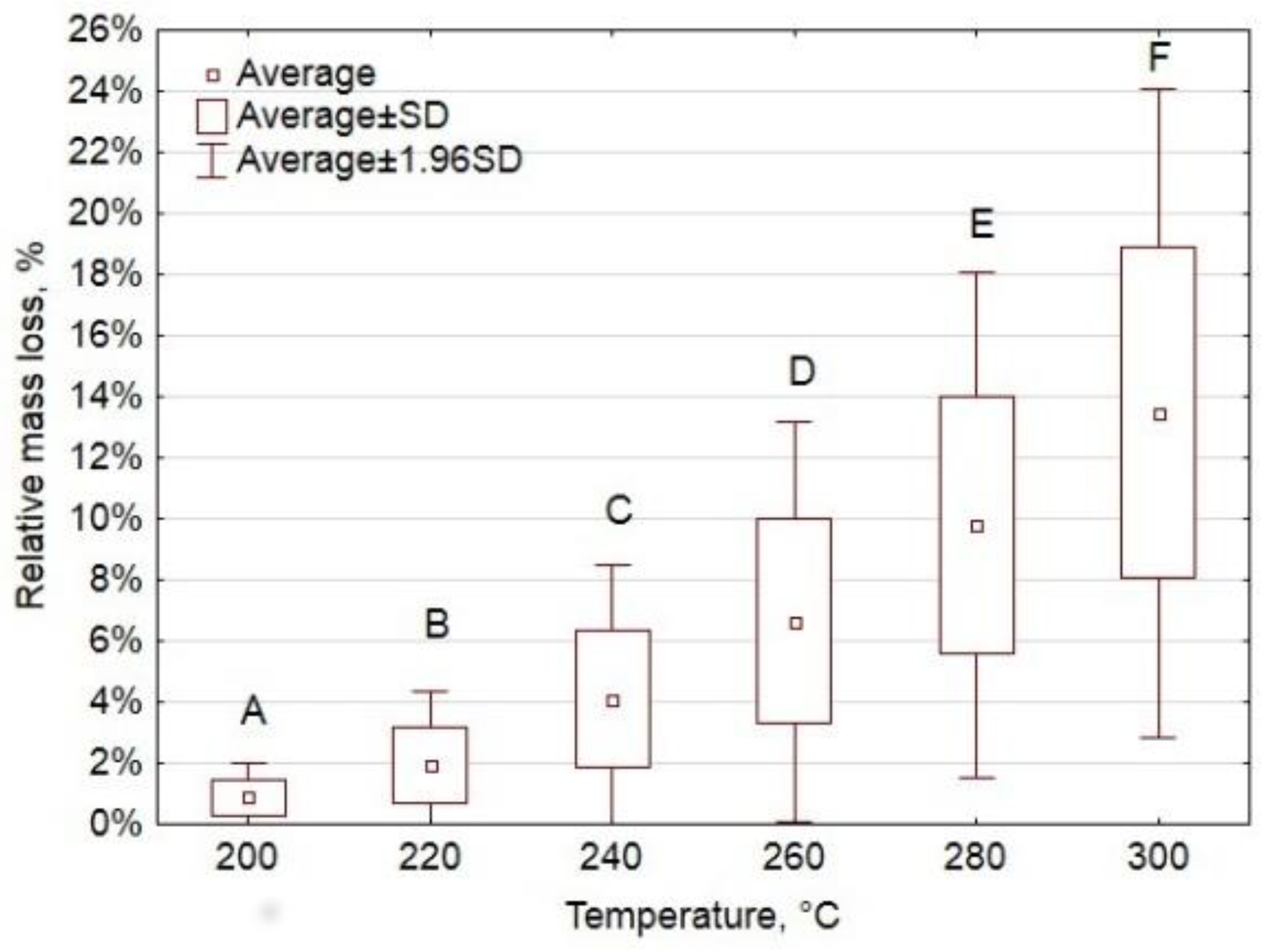
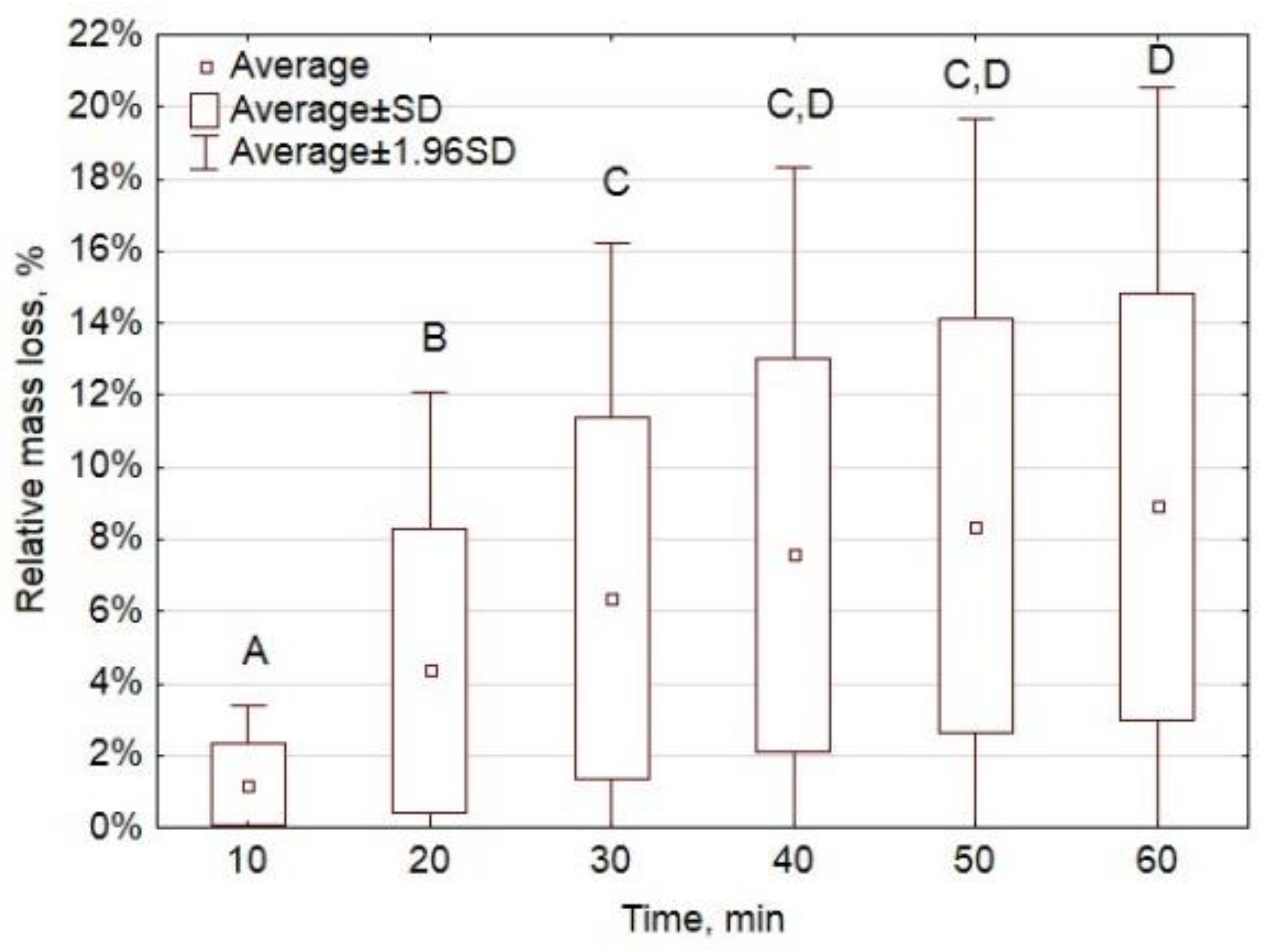
Appendix A.3. Oxytree Biomass Characterization
Appendix A.3.1. Results of the Distribution Normality Evaluation
Appendix A.3.2. Analysis of Variance
- Organic matter content relative to the variable grouping of the cultivation type;
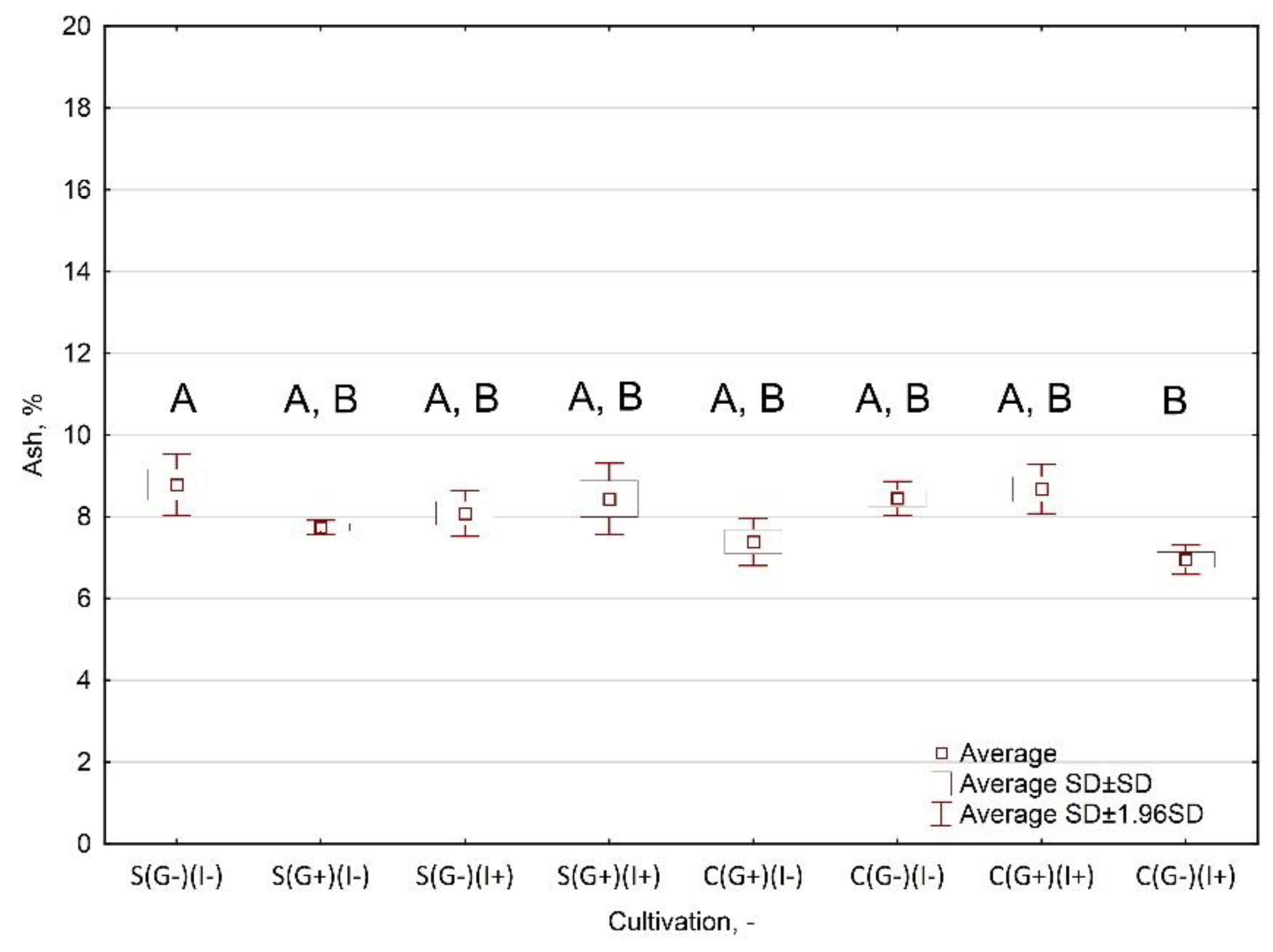
- Ash relative to the variable grouping of the cultivation type;
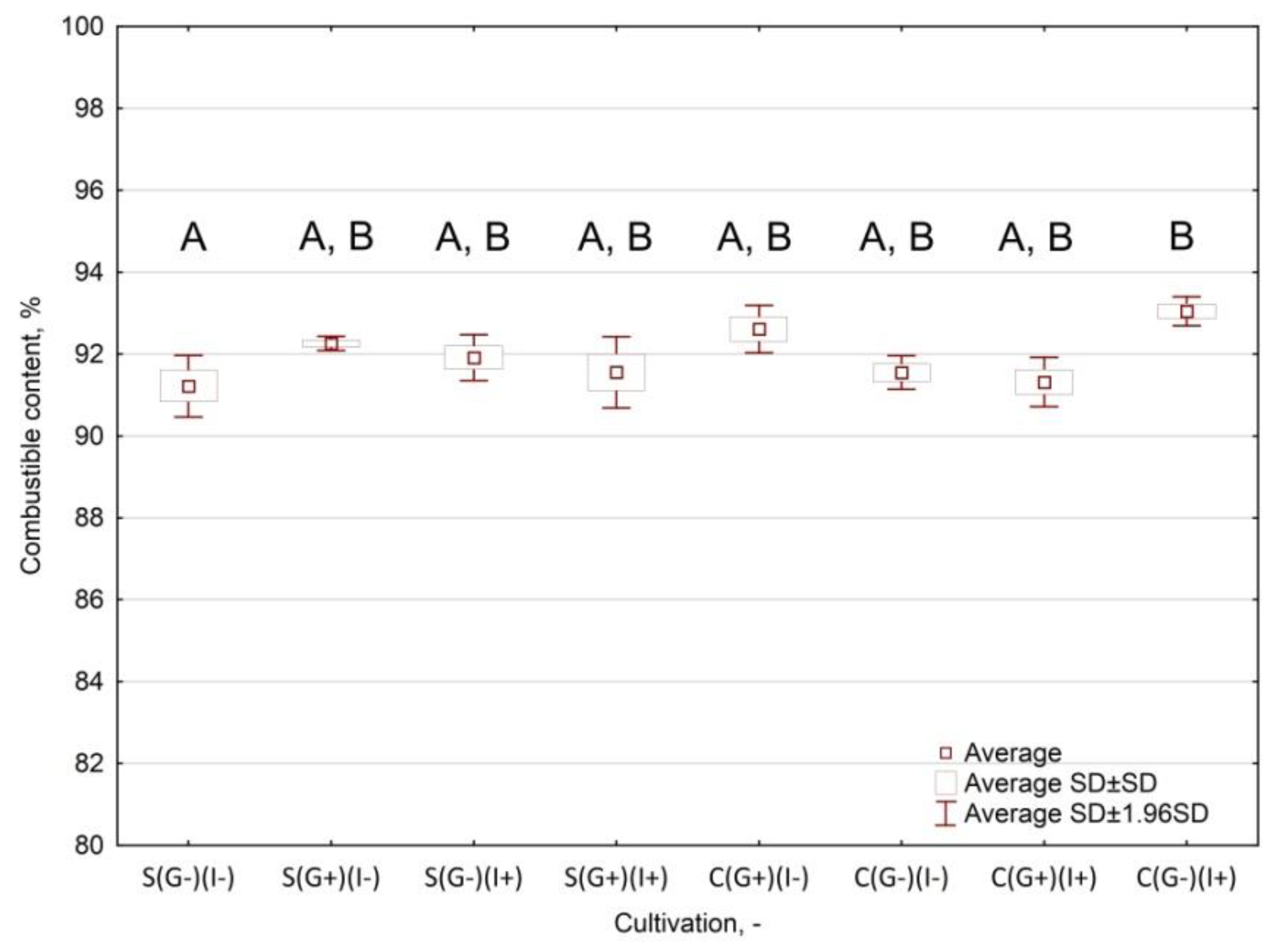
- Combustible content relative to the variable grouping of the cultivation type;
- High heating value relative to the variable grouping of the cultivation type; and
- Low heating value relative to the variable grouping of the cultivation type.
- Hypotheses 0 showed that the grouping variable does not affect the Δm; and
- Hypothesis 1 tests the influence of the grouping variable on the Δm value.
Appendix A.3.3. Results and Interpretation
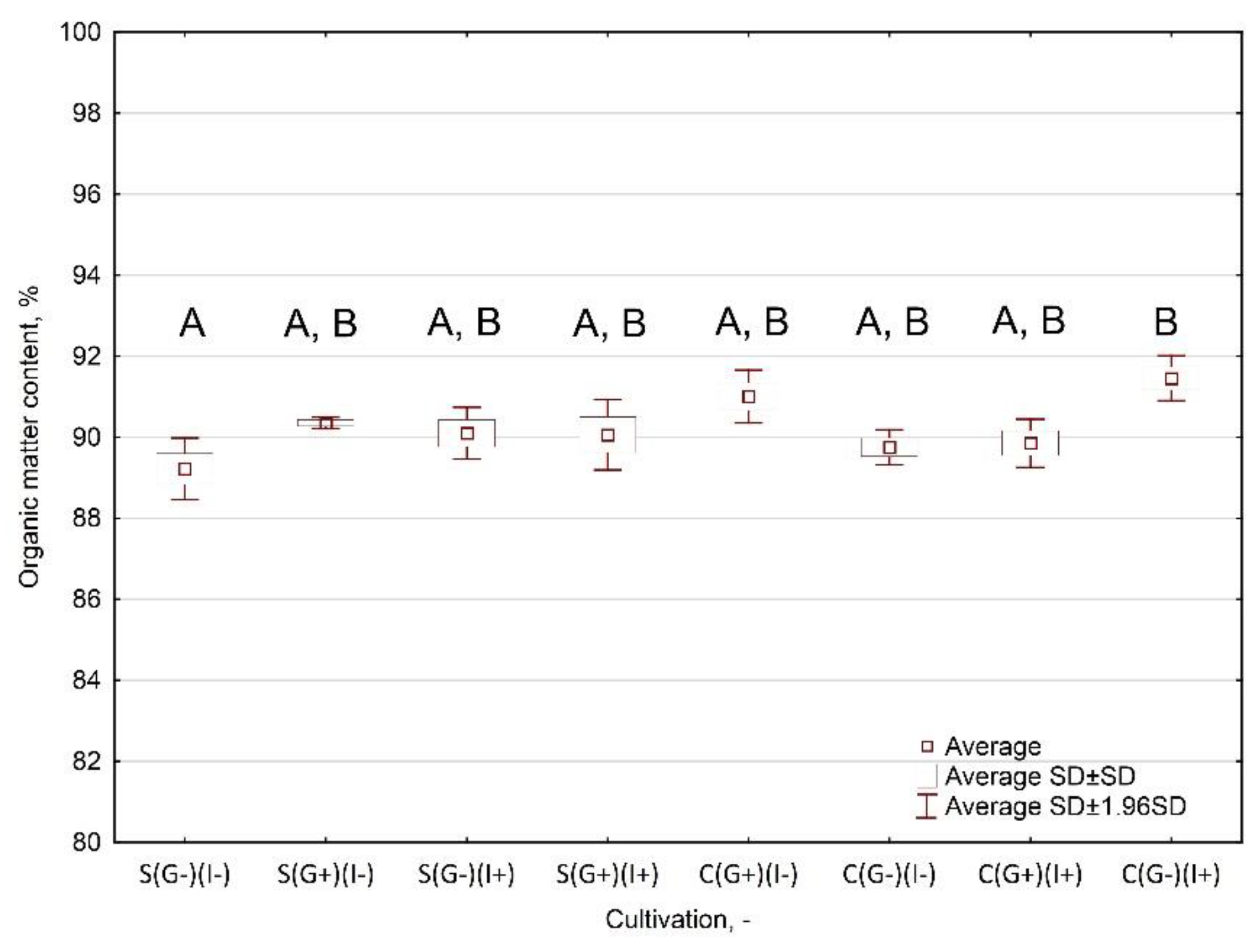


Appendix B




References
- Bentsen, N.S.; Felby, C. Biomass for energy in the European Union - a review of bioenergy resource assessments. Biotechnol. Biofuels 2012, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Scarlat, N.; Dallemand, J.-F.; Monforti-Ferrario, F.; Banja, M.; Motola, V. Renewable energy policy framework and bioenergy contribution in the European Union—An overview from National Renewable Energy Action Plans and Progress Reports. Renew. Sustain. Energy Rev. 2015, 51, 969–985. [Google Scholar] [CrossRef]
- Di Fulvio, F.; Forsell, N.; Korosuo, A.; Obersteiner, M.; Hellweg, S. Spatially explicit LCA analysis of biodiversity losses due to different bioenergy policies in the European Union. Sci. Total Environ. 2019, 651, 1505–1516. [Google Scholar] [CrossRef] [PubMed]
- Tuskan, G. Short-rotation woody crop supply systems in the United States: What do we know and what do we need to know? Biomass Bioenergy 1998, 14, 307–315. [Google Scholar] [CrossRef]
- Tullus, A.; Rytter, L.; Tullus, T.; Weih, M.; Tullus, H. Short-rotation forestry with hybrid aspen (Populus tremulaL.×P. tremuloidesMichx.) in Northern Europe. Scand. J. For. Res. 2012, 27, 10–29. [Google Scholar] [CrossRef]
- Sage, R.F.; Sultmanis, S. Why are there no C 4 forests? J. Plant Physiol. 2016, 203, 55–68. [Google Scholar] [CrossRef]
- Wang, D.; Jaiswal, D.; Lebauer, D.S.; Wertin, T.M.; Bollero, G.A.; Leakey, A.D.B.; Long, S.P. A physiological and biophysical model of coppice willow (Salix spp.) production yields for the contiguous USA in current and future climate scenarios. Plant Cell Environ. 2015, 38, 1850–1865. [Google Scholar] [CrossRef]
- Covshoff, S.; Hibberd, J.M. Integrating C4 photosynthesis into C3 crops to increase yield potential. Curr. Opin. Biotechnol. 2012, 23, 209–214. [Google Scholar] [CrossRef]
- Wang, P.; Vlad, D.; Langdale, J.A. Finding the genes to build C4 rice. Curr. Opin. Plant Boil. 2016, 31, 44–50. [Google Scholar] [CrossRef]
- Icka, P.; Damo, R.; Icka, E. Paulownia Tomentosa, a Fast Growing Timber. Ann. Valahia Univ. Targoviste Agric. 2016, 10, 14–19. [Google Scholar] [CrossRef]
- Moreno, J.L.; Bastida, F.; Ondoño, S.; García, C.; Andrés-Abellán, M.; López-Serrano, F.R. Agro-forestry management of Paulownia plantations and their impact on soil biological quality: The effects of fertilization and irrigation treatments. Appl. Soil Ecol. 2017, 117, 46–56. [Google Scholar] [CrossRef]
- Woods, V.B. Paulownia as a Novel Biomass Crop for Northern Ireland? In A Review of Current Knowledge, 7th ed.; Agri-Food and Biosciences Institute: Belfast, Northern Ireland, 2008; Available online: https://www.doc-developpement-durable.org/file/Arbres-Bois-de-Rapport-Reforestation/FICHES_ARBRES/Paulownia/Paulownia%20as%20a%20novel%20biomass%20crop_Ireland.pdf (accessed on 22 July 2019).
- Huseinovic, S. Paulownia elongata sy hu in function of improving the quality of the environment. Period. Eng. Nat. Sci. (PEN) 2017, 5, 117–123. [Google Scholar] [CrossRef]
- Bortniak, M.; Sekutowski, T.R.; Zajączkowska, O.; Kucharski, M. Influence of the soil from Oxytree [Paulownia elongata S. Y. Hu × Paulownia fortunei (Seem.) Hemsl.] plantation on germination and initial growth of winter wheat and winter rape. Prog. Plant Prot. 2018, 58, 247–250. [Google Scholar] [CrossRef]
- Lisiecka, B.; Bokůvka, O.; Tańśki, T.; Krzemiński, Ł.; Jambor, M. Obtaining of biomorphic composites based on carbon materials. Prod. Eng. Arch. 2018, 19, 22–25. [Google Scholar] [CrossRef]
- Paulownia112.com. Available online: https://www.paulownia112.com/wp-content/uploads/2015/10/informe-de-resultados-ms344-analisis-biomasa-clon-in-vitro-112r.pdf (accessed on 15 December 2018).
- Pradhan, P.; Mahajani, S.M.; Arora, A. Production and utilization of fuel pellets from biomass: A review. Fuel Process. Technol. 2018, 181, 215–232. [Google Scholar] [CrossRef]
- Kihedu, J. Torrefaction and Combustion of Ligno-Cellulosic Biomass. Energy Procedia 2015, 75, 162–167. [Google Scholar] [CrossRef]
- Poudel, J.; Oh, S.C. Effect of Torrefaction on the Properties of Corn Stalk to Enhance Solid Fuel Qualities. Energies 2014, 7, 5586–5600. [Google Scholar] [CrossRef]
- Phanphanich, M.; Mani, S. Impact of torrefaction on the grindability and fuel characteristics of forest biomass. Bioresour. Technol. 2011, 102, 1246–1253. [Google Scholar] [CrossRef]
- Bates, R.B.; Ghoniem, A.F. Bioresource Technology Biomass torrefaction: Modeling of volatile and solid product evolution kinetics. Bioresour. Technol. 2012, 124, 460–469. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. Kinetic Analysis of Biomass Pyrolysis; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 39–83. [Google Scholar]
- Świechowski, K.; Liszewski, M.; Bąbelewski, P.; Koziel, J.A.; Białowiec, A. Oxytree Pruned Biomass Torrefaction: Mathematical Models of the Influence of Temperature and Residence Time on Fuel Properties Improvement. Materials 2019, 12, 2228. [Google Scholar] [CrossRef]
- Rodrigues, A.; Vanbeveren, S.P.; Costa, M.; Ceulemans, R. Relationship between soil chemical composition and potential fuel quality of biomass from poplar short rotation coppices in Portugal and Belgium. Biomass Bioenergy 2017, 105, 66–72. [Google Scholar] [CrossRef]
- Achinelli, F.G.; Doffo, G.; Barotto, A.J.; Luquez, V.; Monteoliva, S. Effects of irrigation, plantation density and clonal composition on woody biomass quality for bioenergy in a short rotation culture system with willows (Salix spp.). Revista Árvore 2018, 42, 42. [Google Scholar] [CrossRef]
- Świechowski, K.; Liszewski, M.; Bąbelewski, P.; Koziel, J.A.; Białowiec, A. Fuel Properties of Torrefied Biomass from Pruning of Oxytree. Data 2019, 4, 55. [Google Scholar] [CrossRef]
- FAO World Reference Base for Soil Resources 2014 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. 2015. Available online: http://www.fao.org/3/i3794en/I3794en.pdf (accessed on 22 July 2019).
- Polish Committee for Standardization. PN-EN 14346:2011 Standard. Waste characteristics. Calculation of Dry Mass on the Basis of Dry Residue or Water Content. Available online: https://infostore.saiglobal.com/enau/Standards/pn-en-14346-2011-932471_saig_pkn_pkn_2197939/ (accessed on 22 July 2019).
- Polish Committee for Standardization. PN-EN 15169:2011 Standard. Waste characteristics. Determination of Organic Matter Content for Waste, Slurry and Sludge. Available online: http://sklep.pkn.pl/pn-en-15169-2011p.html (accessed on 22 July 2019).
- Polish Committee for Standardization. PN-Z-15008-04:1993 Standard. Municipal Solid Waste. Analysis of Combustible and Non-Combustible Content. Available online: http://sklep.pkn.pl/pn-z-15008-04-1993p.html (accessed on 22 July 2019).
- Polish Committee for Standardization. PN-G-04513:1981 Standard. Solid Fuels. Determination of the Higher Heating Value and the Lower Heating Value. Available online: http://sklep.pkn.pl/pn-g-04513-1981p.html (accessed on 22 July 2019).
- Stępień, P.; Pulka, J.; Serowik, M.; Białowiec, A. Thermogravimetric and Calorimetric Characteristics of Alternative Fuel in Terms of Its Use in Low-Temperature Pyrolysis. Waste Biomass Valorization 2018, 10, 1669–1677. [Google Scholar] [CrossRef]
- Stępień, P.; Białowiec, A. Kinetic parameters of torrefaction process of alternative fuel produced from municipal solid waste and characteristic of carbonized refuse derived fuel. Detritus 2018, 3, 75–83. [Google Scholar] [CrossRef]
- Peleg, M.; Normand, M.D.; Corradini, M.G. The Arrhenius Equation Revisited. Crit. Rev. Food Sci. Nutr. 2012, 52, 830–851. [Google Scholar] [CrossRef]
- Liaqat, F. Effects of Storage and Geographical Location on Fuel Quality of Norway Spruce Forest Residues, Swedish University of Agricultural Sciences Examensarbete. 2011. Available online: https://stud.epsilon.slu.se/3174/4/liaqat_f_110826.pdf (accessed on 22 July 2019).
- Chen, W.-H.; Peng, J.; Bi, X.T. A state-of-the-art review of biomass torrefaction, densification and applications. Renew. Sustain. Energy Rev. 2015, 44, 847–866. [Google Scholar] [CrossRef]
- Klasnja, B.; Kopitovic, S.; Orlovic, S. Wood and bark of some poplar and willow clones as fuelwood. Biomass Bioenergy 2002, 23, 427–432. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, C.; Hidayat, W.; Jang, J.-H.; Kim, N.-H. Solid Bioenergy Properties of Paulownia tomentosa Grown in Korea. J. Korean Wood Sci. Technol. 2016, 44, 890–896. [Google Scholar] [CrossRef][Green Version]
- Vusić, D.; Migalić, M.; Željko, Z.; Trkmić, M.; Bešlić, A.; Drvodelić, D. Fuel properties of paulownia biomass. Nat. Resour. Green Technol. Sustain. Dev. 2018, 126–130. Available online: https://pdfs.semanticscholar.org/3275/d126918947d36a46da5cd3337f1a3cd1b121.pdf (accessed on 22 July 2019).
- Krzyżaniak, M.; Stolarski, M.J.; Waliszewska, B.; Szczukowski, S.; Tworkowski, J.; Załuski, D.; Śnieg, M. Willow biomass as feedstock for an integrated multi-product biorefinery. Ind. Crop. Prod. 2014, 58, 230–237. [Google Scholar] [CrossRef]
- Fang, S.; Zhai, X.; Wan, J.; Tang, L. Clonal variation in growth, chemistry and calorific value of new poplar hybrids at nursery stage. Biomass Bioenergy 2013, 54, 303–311. [Google Scholar] [CrossRef]
- Yorgun, S.; Yıldız, D. Preparation and characterization of activated carbons from Paulownia wood by chemical activation with H3PO4. J. Taiwan Inst. Chem. Eng. 2015, 53, 122–131. [Google Scholar] [CrossRef]
- Campbell, W.A.; Evitts, R.W. Determining the Severity of Torrefaction for Multiple Biomass Types Using Carbon Content. Energy Fuels 2018, 32, 9448–9458. [Google Scholar] [CrossRef]
- Ribeiro, J.M.C.; Godina, R.; Matias, J.C.D.O.; Nunes, L.J.R. Future Perspectives of Biomass Torrefaction: Review of the Current State-Of-The-Art and Research Development. Sustainability 2018, 10, 2323. [Google Scholar] [CrossRef]
- Ren, S.; Lei, H.; Wang, L.; Bu, Q.; Chen, S.; Wu, J. Thermal behaviour and kinetic study for woody biomass torrefaction and torrefied biomass pyrolysis by TGA. Biosyst. Eng. 2013, 116, 420–426. [Google Scholar] [CrossRef]
- Becker, A.; Scherer, V. A comparison of the torrefaction behavior of wood, miscanthus and palm kernel shells: Measurements on single particles with geometries of technical relevance. Fuel 2018, 224, 507–520. [Google Scholar] [CrossRef]
- Wang, L.; Barta-Rajnai, E.; Skreiberg, Ø.; Khalil, R.; Czégény, Z.; Jakab, E.; Barta, Z.; Grønli, M. Effect of torrefaction on physiochemical characteristics and grindability of stem wood, stump and bark. Appl. Energy 2018, 227, 137–148. [Google Scholar] [CrossRef]
- Gucho, E.M.; Shahzad, K.; Bramer, E.A.; Akhtar, N.A.; Brem, G. Experimental Study on Dry Torrefaction of Beech Wood and Miscanthus. Energies 2015, 8, 3903–3923. [Google Scholar] [CrossRef]
- Basu, P.; Rao, S.; Dhungana, A. An investigation into the effect of biomass particle size on its torrefaction. Can. J. Chem. Eng. 2013, 91, 466–474. [Google Scholar] [CrossRef]
- Bridgeman, T.; Jones, J.; Shield, I.; Williams, P.; Jones, J. Torrefaction of reed canary grass, wheat straw and willow to enhance solid fuel qualities and combustion properties. Fuel 2008, 87, 844–856. [Google Scholar] [CrossRef]
- Dhanavath, K.N.; Bankupalli, S.; Bhargava, S.K.; Parthasarathy, R. An experimental study to investigate the effect of torrefaction temperature on the kinetics of gas generation. J. Environ. Chem. Eng. 2018, 6, 3332–3341. [Google Scholar] [CrossRef]
- Carrasco, J.C.; Oporto, G.S.; Zondlo, J.; Jingxin, W. Torrefaction Kinetics of Red Oak (Quercus rubra) in a Fluidized Reactor. BioResources 2013, 8, 5067–5082. [Google Scholar] [CrossRef]
- Walkowiak, M.; Bartkowiak, M. The kinetics of the thermal decomposition of the willow wood (Salix viminalis L.) exposed to the torrefaction process. Drewno 2012, 55, 37–49. Available online: http://drewno-wood.pl/pobierz-55 (accessed on 22 July 2019).
- Saddawi, A.; Jones, J.M.; Williams, A.; Wójtowicz, M.A. Kinetics of the Thermal Decomposition of Biomass. Energy Fuels 2010, 24, 1274–1282. [Google Scholar] [CrossRef]
- Diaz, I.; Rodriguez, M.; Arnaiz, C.; Miguel, G.S.; Dominguez, M. Biomass pyrolysis kinetics through thermogravimetric analysis. Comput. Aided Chem. Eng. 2013, 32, 1–6. [Google Scholar]
- Roesler, J.F.; Sanz, E.; Nastoll, W.; Lu, P. Exothermicity in Wood Torrefaction and its Impact on Product Mass Yields: From Micro to Pilot Scale. Can. J. Chem. Eng. 2015, 93, 331–339. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Torrefaction of wood: Part 1. Weight loss kinetic. J. Anal. Appl. Pyrolysis 2006, 77, 28–34. [Google Scholar] [CrossRef]
- Peng, J.H. A Study of Softwood Torrefaction and Densification for the Production of High Quality Wood Pellets, The University of British Columbia. 2012. Available online: https://www.collectionscanada.gc.ca/obj/thesescanada/vol2/BVAU/TC-BVAU-42654.pdf (accessed on 22 July 2019).
- Bach, Q.; Khalil, R.A.; Tran, K.; Skreiberg, Ø. Torrefaction Kinetics of Norwegian Biomass Fuels. Chem. Eng. Trans. 2014, 37, 49–54. [Google Scholar] [CrossRef]
- Chen, L.; Wang, S.; Meng, H.; Wu, Z.; Zhao, J. Synergistic effect on thermal behavior and char morphology analysis during co-pyrolysis of paulownia wood blended with different plastics waste. Appl. Therm. Eng. 2017, 111, 834–846. [Google Scholar] [CrossRef]
- Pulka, J.; Manczarski, P.; Koziel, J.A.; Białowiec, A. Torrefaction of Sewage Sludge: Kinetics and Fuel Properties of Biochars. Energies 2019, 12, 565. [Google Scholar] [CrossRef]
- Syguła, E.; Koziel, J.A.; Białowiec, A. Proof-of-Concept of Spent Mushrooms Compost Torrefaction—Studying the Process Kinetics and the Influence of Temperature and Duration on the Calorific Value of the Produced Biocoal. Energies 2019, 12, 3060. [Google Scholar] [CrossRef]

| Cultivation Type Symbol, - | Moisture Content, % | Organic Matter Content in d.m., % | Combustible Content in d.m., % | Ash in d.m., % | HHV, J·g d.m.−1 | LHV, J·g d.m.−1 |
|---|---|---|---|---|---|---|
| S(G−)(I−) | 4.64 ± 0.09 | 89.22 ± 0.39 | 91.22 ± 0.38 | 8.78 ± 0.38 | 18,251 ± 90 | 16,666 ± 90 |
| S(G+)(I−) | 5.84 ± 0.04 | 90.36 ± 0.07 | 92.26 ± 0.09 | 7.74 ± 0.09 | 18,577 ± 195 | 17,135 ± 195 |
| S(G−)(I+) | 5.96 ± 0.02 | 90.10 ± 0.33 | 91.91 ± 0.29 | 8.09 ± 0.29 | 18,407 ± 199 | 16,762 ± 199 |
| S(G+)(I+) | 5.77 ± 0.05 | 90.06 ± 0.44 | 91.56 ± 0.44 | 8.44 ± 0.44 | 18,499 ± 69 | 16,858 ± 69 |
| C(G+)(I−) | 5.22 ± 0.01 | 91.00 ± 0.33 | 92.61 ± 0.29 | 7.39 ± 0.29 | 18,453 ± 135 | 16,920 ± 135 |
| C(G−)(I−) | 5.04 ± 0.01 | 89.75 ± 0.22 | 91.55 ± 0.21 | 8.45 ± 0.21 | 18,099 ± 441 | 16,463 ± 441 |
| C(G+)(I+) | 5.62 ± 0.04 | 89.86 ± 0.30 | 91.32 ± 0.31 | 8.68 ± 0.31 | 17,910 ± 407 | 16,430 ± 407 |
| C(G−)(I+) | 5.57 ± 0.01 | 91.46 ± 0.28 | 93.05 ± 0.18 | 6.95 ± 0.18 | 18,167 ± 377 | 16,450 ± 377 |
| Mean | 5.46 ± 0.43 | 90.23 ± 0.73 | 91.93 ± 0.67 | 8.07 ± 0.67 | 18,295 ± 317 | 16,711 ± 336 |
| Cultivation Type Symbol, - | C, % | H, % | N, % | S, % | O, % |
|---|---|---|---|---|---|
| S(G−)(I−) | 44.20 | 6.74 | 2.05 | 0.17 | 36.06 |
| S(G+)(I−) | 44.10 | 5.95 | 1.95 | 0.20 | 38.16 |
| S(G−)(I+) | 44.90 | 6.87 | 2.22 | 0.21 | 35.90 |
| S(G+)(I+) | 45.10 | 6.87 | 2.83 | 0.20 | 35.06 |
| C(G+)(I−) | 44.10 | 6.44 | 2.72 | 0.21 | 37.53 |
| C(G−)(I−) | 40.20 | 6.93 | 2.46 | 0.22 | 39.95 |
| C(G+)(I+) | 41.80 | 6.15 | 2.06 | 0.19 | 39.66 |
| C(G−)(I+) | 45.70 | 7.24 | 2.09 | 0.19 | 36.23 |
| Mean | 43.76 ± 1.84 | 6.65 ± 0.43 | 2.30 ± 0.33 | 0.20 ± 0.02 | 37.32 ± 1.81 |
| Temperature, °C | Time, min | Relative Mass Loss, % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| S(G−)(I−) | S(G+)(I−) | S(G−)(I+) | S(G+)(I+) | C(G+)(I−) | C(G−)(I−) | C(G+)(I+) | C(G−)(I+) | ||
| 200 | 10 | 0.1 ± 0.2 | 0.2 ± 0.2 | 0.2 ± 0.2 | 0.4 ± 0.2 | 0.1 ± 0.2 | 0.1 ± 0.2 | 0.2 ± 0.2 | 0.1 ± 0.2 |
| 20 | 0.2 ± 0.4 | 0.3 ± 0.0 | 0.4 ± 0.2 | 0.9 ± 0.2 | 0.4 ± 0.2 | 0.4 ± 0.2 | 0.6 ± 0.2 | 0.4 ± 0.2 | |
| 30 | 0.4 ± 0.5 | 0.7 ± 0.0 | 0.7 ± 0.3 | 1.3 ± 0.3 | 0.6 ± 0.2 | 0.8 ± 0.2 | 0.9 ± 0.2 | 0.8 ± 0.2 | |
| 40 | 0.8 ± 0.5 | 0.9 ± 0.2 | 1.0 ± 0.3 | 1.8 ± 0.2 | 0.8 ± 0.2 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | |
| 50 | 1.0 ± 0.7 | 1.0 ± 0.0 | 1.2 ± 0.5 | 2.1 ± 0.5 | 1.0 ± 0.0 | 1.2 ± 0.2 | 1.3 ± 0.2 | 1.2 ± 0.2 | |
| 60 | 1.2 ± 0.5 | 1.3 ± 0.0 | 1.4 ± 0.7 | 2.4 ± 0.5 | 1.2 ± 0.2 | 1.3 ± 0.0 | 1.6 ± 0.0 | 1.4 ± 0.2 | |
| 220 | 10 | 0.0 ± 0.0 | 0.3 ± 0.3 | 0.2 ± 0.2 | 0.3 ± 0.2 | 0.4 ± 0.2 | 0.2 ± 0.2 | 0.4 ± 0.2 | 0.3 ± 0.0 |
| 20 | 0.1 ± 0.2 | 0.8 ± 0.7 | 0.7 ± 0.3 | 1.2 ± 0.2 | 1.2 ± 0.2 | 0.8 ± 0.7 | 1.1 ± 0.2 | 1.2 ± 0.2 | |
| 30 | 0.6 ± 0.7 | 1.6 ± 0.8 | 1.3 ± 0.3 | 2.1 ± 0.2 | 1.9 ± 0.2 | 1.8 ± 0.7 | 2.0 ± 0.3 | 2.0 ± 0.3 | |
| 40 | 1.3 ± 0.9 | 2.2 ± 0.8 | 1.9 ± 0.5 | 2.8 ± 0.2 | 2.3 ± 0.0 | 2.6 ± 0.8 | 2.8 ± 0.2 | 3.0 ± 0.3 | |
| 50 | 2.0 ± 0.9 | 2.9 ± 0.8 | 2.3 ± 0.3 | 3.4 ± 0.2 | 2.9 ± 0.2 | 3.2 ± 0.8 | 3.2 ± 0.2 | 3.6 ± 0.4 | |
| 60 | 2.7 ± 1.2 | 3.3 ± 0.7 | 2.7 ± 0.3 | 4.0 ± 0.0 | 3.2 ± 0.2 | 3.7 ± 0.6 | 3.7 ± 0.3 | 4.1 ± 0.5 | |
| 240 | 10 | 0.3 ± 0.0 | 0.8 ± 0.2 | 0.4 ± 0.0 | 0.8 ± 0.2 | 0.6 ± 0.4 | 0.9 ± 0.2 | 0.7 ± 0.0 | 0.4 ± 0.4 |
| 20 | 1.4 ± 0.2 | 2.2 ± 0.2 | 1.8 ± 0.2 | 2.7 ± 0.2 | 1.9 ± 1.0 | 2.6 ± 0.5 | 2.2 ± 0.2 | 2.0 ± 0.9 | |
| 30 | 2.9 ± 0.2 | 4.0 ± 0.3 | 3.3 ± 0.2 | 4.7 ± 0.3 | 3.7 ± 1.5 | 4.3 ± 0.7 | 4.2 ± 0.2 | 3.9 ± 0.8 | |
| 40 | 4.2 ± 0.2 | 5.3 ± 0.3 | 4.8 ± 0.2 | 6.0 ± 0.3 | 4.9 ± 1.3 | 5.7 ± 0.7 | 5.3 ± 0.3 | 5.3 ± 0.3 | |
| 50 | 5.2 ± 0.2 | 6.2 ± 0.2 | 5.9 ± 0.2 | 7.0 ± 0.2 | 5.8 ± 1.1 | 6.3 ± 0.7 | 6.0 ± 0.3 | 6.4 ± 0.2 | |
| 60 | 5.9 ± 0.2 | 6.8 ± 0.2 | 6.6 ± 0.2 | 7.4 ± 0.2 | 6.3 ± 0.8 | 6.8 ± 0.8 | 6.4 ± 0.4 | 7.0 ± 0.3 | |
| 260 | 10 | 0.3 ± 0.3 | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.0 ± 0.7 | 1.1 ± 0.4 | 1.8 ± 0.2 | 1.1 ± 0.4 | 1.2 ± 0.2 |
| 20 | 1.7 ± 0.9 | 4.2 ± 0.2 | 4.3 ± 0.3 | 3.9 ± 1.4 | 4.1 ± 0.4 | 6.0 ± 0.3 | 4.4 ± 0.4 | 5.1 ± 0.2 | |
| 30 | 4.1 ± 1.5 | 7.2 ± 0.2 | 7.4 ± 0.4 | 6.9 ± 1.3 | 6.7 ± 0.3 | 8.8 ± 0.2 | 6.8 ± 0.4 | 7.8 ± 0.2 | |
| 40 | 5.7 ± 1.5 | 8.7 ± 0.0 | 9.0 ± 0.3 | 8.3 ± 1.2 | 8.0 ± 0.0 | 10.3 ± 0.3 | 8.0 ± 0.3 | 9.1 ± 0.2 | |
| 50 | 6.7 ± 1.5 | 9.6 ± 0.2 | 9.9 ± 0.5 | 9.4 ± 1.0 | 8.7 ± 0.0 | 11.3 ± 0.3 | 8.7 ± 0.3 | 9.9 ± 0.4 | |
| 60 | 7.4 ± 1.6 | 10.0 ± 0.0 | 10.3 ± 0.3 | 10.1 ± 0.7 | 9.2 ± 0.2 | 12.2 ± 0.4 | 9.1 ± 0.2 | 10.4 ± 0.2 | |
| 280 | 10 | 0.8 ± 0.2 | 1.3 ± 0.9 | 2.1 ± 0.4 | 2.2 ± 0.2 | 2.1 ± 0.2 | 2.4 ± 0.2 | 1.9 ± 0.8 | 2.1 ± 0.4 |
| 20 | 4.7 ± 0.0 | 6.7 ± 2.0 | 8.2 ± 0.5 | 8.2 ± 0.5 | 7.7 ± 0.3 | 8.7 ± 0.0 | 7.6 ± 1.3 | 8.3 ± 0.3 | |
| 30 | 8.4 ± 0.4 | 10.0 ± 1.2 | 11.3 ± 0.3 | 11.3 ± 0.3 | 10.4 ± 0.2 | 11.3 ± 0.0 | 10.1 ± 1.3 | 11.2 ± 0.4 | |
| 40 | 10.0 ± 0.3 | 11.7 ± 0.9 | 12.9 ± 0.2 | 12.8 ± 0.5 | 12.0 ± 0.3 | 12.6 ± 0.2 | 11.7 ± 1.2 | 12.8 ± 0.2 | |
| 50 | 11.1 ± 0.4 | 12.7 ± 0.7 | 14.0 ± 0.3 | 13.8 ± 0.5 | 13.0 ± 0.3 | 13.6 ± 0.2 | 12.6 ± 1.1 | 13.7 ± 0.3 | |
| 60 | 11.6 ± 0.5 | 13.3 ± 0.7 | 14.7 ± 0.3 | 14.3 ± 0.3 | 13.7 ± 0.3 | 14.2 ± 0.2 | 13.3 ± 0.9 | 14.3 ± 0.3 | |
| 300 | 10 | 1.3 ± 0.9 | 2.9 ± 0.2 | 3.4 ± 0.2 | 3.6 ± 0.7 | 3.1 ± 1.3 | 3.4 ± 0.7 | 3.3 ± 0.0 | 2.9 ± 1.6 |
| 20 | 8.3 ± 1.2 | 11.3 ± 0.3 | 12.0 ± 0.3 | 12.1 ± 0.5 | 10.7 ± 1.5 | 11.4 ± 0.7 | 11.1 ± 0.2 | 11.2 ± 1.9 | |
| 30 | 12.2 ± 1.1 | 14.7 ± 0.3 | 15.7 ± 0.3 | 15.7 ± 0.3 | 14.0 ± 1.2 | 14.9 ± 0.5 | 14.4 ± 0.2 | 14.7 ± 1.7 | |
| 40 | 14.3 ± 1.2 | 16.4 ± 0.4 | 17.7 ± 0.3 | 17.4 ± 0.4 | 15.8 ± 1.8 | 16.6 ± 0.5 | 16.1 ± 0.2 | 16.6 ± 1.6 | |
| 50 | 15.7 ± 1.2 | 17.7 ± 0.3 | 19.0 ± 0.3 | 18.8 ± 0.4 | 16.9 ± 0.8 | 17.7 ± 0.3 | 17.3 ± 0.3 | 17.9 ± 1.3 | |
| 60 | 16.6 ± 1.2 | 18.3 ± 0.3 | 19.8 ± 0.5 | 19.6 ± 0.2 | 17.7 ± 0.7 | 18.4 ± 0.4 | 18.0 ± 0.3 | 18.8 ± 1.3 | |
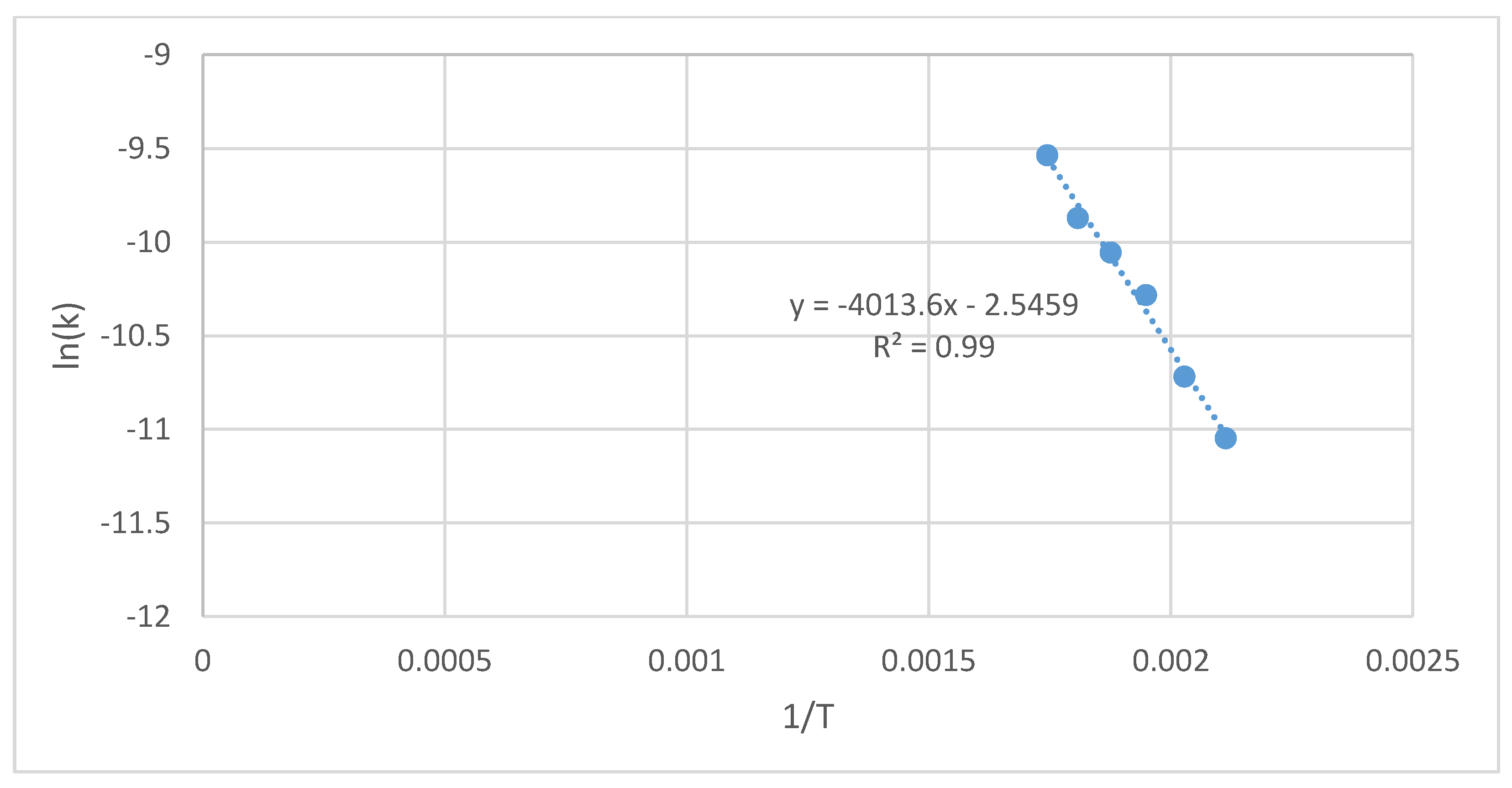
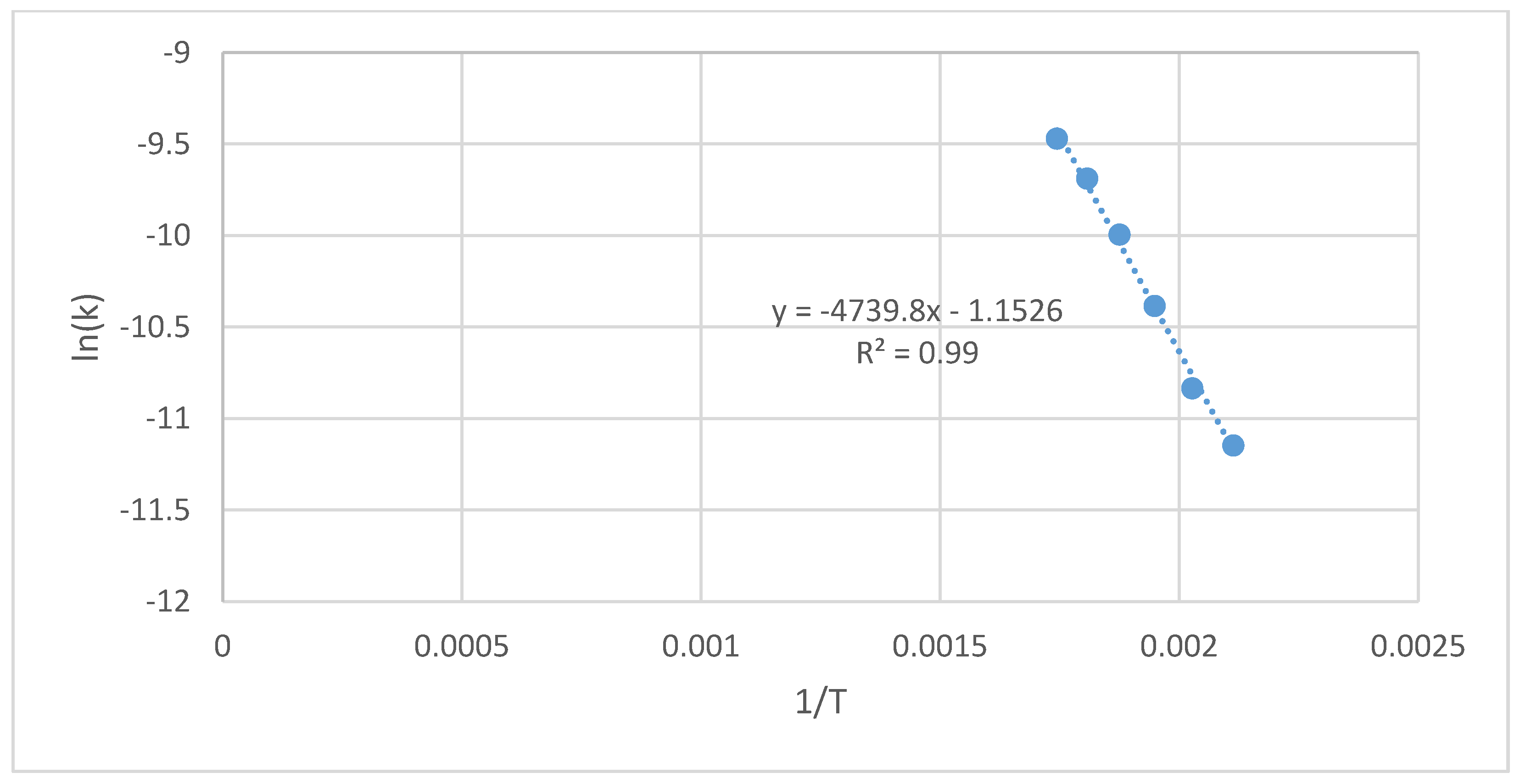
| Cultivation Type Symbol | Process Temperature | Constant Rate of the Reaction | Arrhenius Plot Parameters | Activation Energy | Determination Coefficient | ||
|---|---|---|---|---|---|---|---|
| - | T, °C | T, K | k, s−1 | 1/T, K−1 | ln(k), s−1 | Ea, J·mol−1 | R2, - |
| S(G−)(I−) | 200 | 473 | 1.30 × 10−5 | 2.11 × 10−3 | −11.3 | 35,028 | 0.98 |
| 220 | 493 | 1.95 × 10−5 | 2.03 × 10−3 | −10.9 | |||
| 240 | 513 | 2.99 × 10−5 | 1.95 × 10−3 | −10.4 | |||
| 260 | 533 | 3.24 × 10−5 | 1.88 × 10−3 | −10.3 | |||
| 280 | 553 | 4.69 × 10−5 | 1.81 × 10−3 | −9.97 | |||
| 300 | 573 | 6.64 × 10−5 | 1.75 × 10−3 | −9.62 | |||
| S(G+)(I−) | 200 | 473 | 1.59 × 10−5 | 2.11 × 10−3 | −11.1 | 33,369 | 0.99 |
| 220 | 493 | 2.21 × 10−5 | 2.03 × 10−3 | −10.7 | |||
| 240 | 513 | 3.42 × 10−5 | 1.95 × 10−3 | −10.3 | |||
| 260 | 533 | 4.29 × 10−5 | 1.88 × 10−3 | −10.1 | |||
| 280 | 553 | 5.16 × 10−5 | 1.81 × 10−3 | −9.87 | |||
| 300 | 573 | 7.21 × 10−5 | 1.75 × 10−3 | −9.54 | |||
| S(G−)(I+) | 200 | 473 | 1.44 × 10−5 | 2.11 × 10−3 | −11.2 | 39,407 | 0.99 |
| 220 | 493 | 1.97 × 10−5 | 2.03 × 10−3 | −10.8 | |||
| 240 | 513 | 3.09 × 10−5 | 1.95 × 10−3 | −10.4 | |||
| 260 | 533 | 4.56 × 10−5 | 1.88 × 10−3 | −10.0 | |||
| 280 | 553 | 6.19 × 10−5 | 1.81 × 10−3 | −9.69 | |||
| 300 | 573 | 7.70 × 10−5 | 1.75 × 10−3 | −9.47 | |||
| S(G+)(I+) | 200 | 473 | 1.82 × 10−5 | 2.11 × 10−3 | −10.9 | 34,282 | 0.99 |
| 220 | 493 | 2.13 × 10−5 | 2.03 × 10−3 | −10.8 | |||
| 240 | 513 | 3.26 × 10−5 | 1.95 × 10−3 | −10.3 | |||
| 260 | 533 | 4.28 × 10−5 | 1.88 × 10−3 | −10.1 | |||
| 280 | 553 | 5.85 × 10−5 | 1.81 × 10−3 | −9.75 | |||
| 300 | 573 | 7.96 × 10−5 | 1.75 × 10−3 | −9.44 | |||
| C(G+)(I−) | 200 | 473 | 1.26 × 10−5 | 2.11 × 10−3 | −11.3 | 38,436 | 0.98 |
| 220 | 493 | 1.81 × 10−5 | 2.03 × 10−3 | −10.9 | |||
| 240 | 513 | 3.22 × 10−5 | 1.95 × 10−3 | −10.3 | |||
| 260 | 533 | 4.15 × 10−5 | 1.88 × 10−3 | −10.1 | |||
| 280 | 553 | 5.15 × 10−5 | 1.81 × 10−3 | −9.87 | |||
| 300 | 573 | 6.86 × 10−5 | 1.75 × 10−3 | −9.59 | |||
| C(G−)(I−) | 200 | 473 | 1.39 × 10−5 | 2.11 × 10−3 | −11.2 | 36,210 | 0.98 |
| 220 | 493 | 2.31 × 10−5 | 2.03 × 10−3 | −10.7 | |||
| 240 | 513 | 3.44 × 10−5 | 1.95 × 10−3 | −10.3 | |||
| 260 | 533 | 4.63 × 10−5 | 1.88 × 10−3 | −9.98 | |||
| 280 | 553 | 5.37 × 10−5 | 1.81 × 10−3 | −9.83 | |||
| 300 | 573 | 7.36 × 10−5 | 1.75 × 10−3 | −9.52 | |||
| C(G+)(I+) | 200 | 473 | 1.34 × 10−5 | 2.11 × 10−3 | −11.2 | 36,442 | 0.99 |
| 220 | 493 | 1.78 × 10−5 | 2.03 × 10−3 | −10.9 | |||
| 240 | 513 | 2.72 × 10−5 | 1.95 × 10−3 | −10.5 | |||
| 260 | 533 | 3.46 × 10−5 | 1.88 × 10−3 | −10.3 | |||
| 280 | 553 | 4.95 × 10−5 | 1.81 × 10−3 | −9.91 | |||
| 300 | 573 | 6.66 × 10−5 | 1.75 × 10−3 | −9.62 | |||
| C(G−)(I+) | 200 | 473 | 1.29 × 10−5 | 2.11 × 10−3 | −11.3 | 38,907 | 0.99 |
| 220 | 493 | 2.17 × 10−5 | 2.03 × 10−3 | −10.7 | |||
| 240 | 513 | 3.39 × 10−5 | 1.95 × 10−3 | −10.3 | |||
| 260 | 533 | 4.57 × 10−5 | 1.88 × 10−3 | −9.99 | |||
| 280 | 553 | 5.61 × 10−5 | 1.81 × 10−3 | −9.79 | |||
| 300 | 573 | 7.57 × 10−5 | 1.75 × 10−3 | −9.49 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świechowski, K.; Stegenta-Dąbrowska, S.; Liszewski, M.; Bąbelewski, P.; Koziel, J.A.; Białowiec, A. Oxytree Pruned Biomass Torrefaction: Process Kinetics. Materials 2019, 12, 3334. https://doi.org/10.3390/ma12203334
Świechowski K, Stegenta-Dąbrowska S, Liszewski M, Bąbelewski P, Koziel JA, Białowiec A. Oxytree Pruned Biomass Torrefaction: Process Kinetics. Materials. 2019; 12(20):3334. https://doi.org/10.3390/ma12203334
Chicago/Turabian StyleŚwiechowski, Kacper, Sylwia Stegenta-Dąbrowska, Marek Liszewski, Przemysław Bąbelewski, Jacek A. Koziel, and Andrzej Białowiec. 2019. "Oxytree Pruned Biomass Torrefaction: Process Kinetics" Materials 12, no. 20: 3334. https://doi.org/10.3390/ma12203334
APA StyleŚwiechowski, K., Stegenta-Dąbrowska, S., Liszewski, M., Bąbelewski, P., Koziel, J. A., & Białowiec, A. (2019). Oxytree Pruned Biomass Torrefaction: Process Kinetics. Materials, 12(20), 3334. https://doi.org/10.3390/ma12203334









