Reactivity and Microstructure of Metakaolin Based Geopolymers: Effect of Fly Ash and Liquid/Solid Contents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Methods for Powder Precursors
2.3. Manufacture of Geopolymers
2.4. Characterization Methods for Fresh Geopolymers
2.5. Characterization Methods for Hardened Geopolymers
3. Results
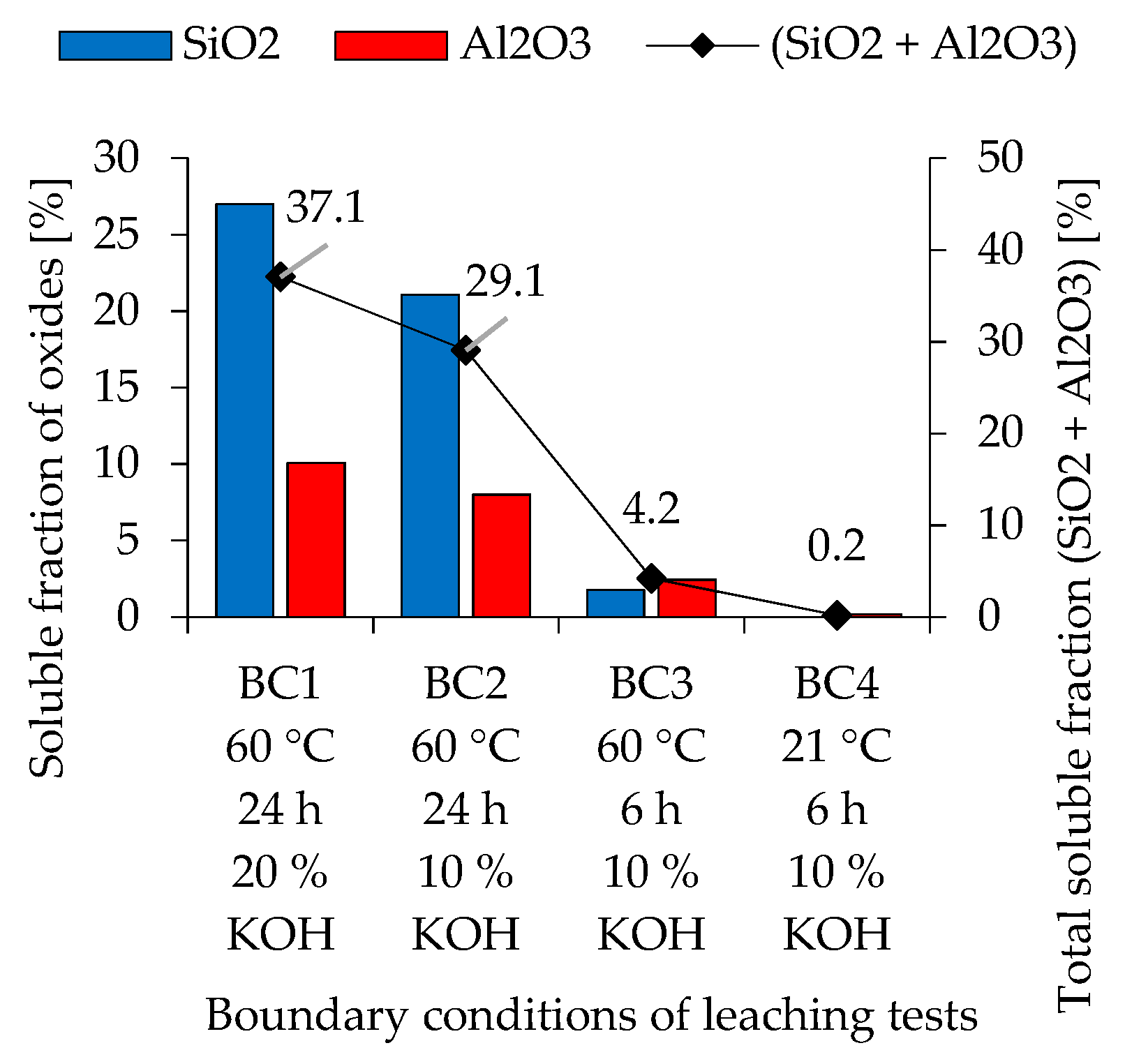
3.1. Reactivity of Metakaolin and Fly Ash
3.2. Workability of Fresh Paste
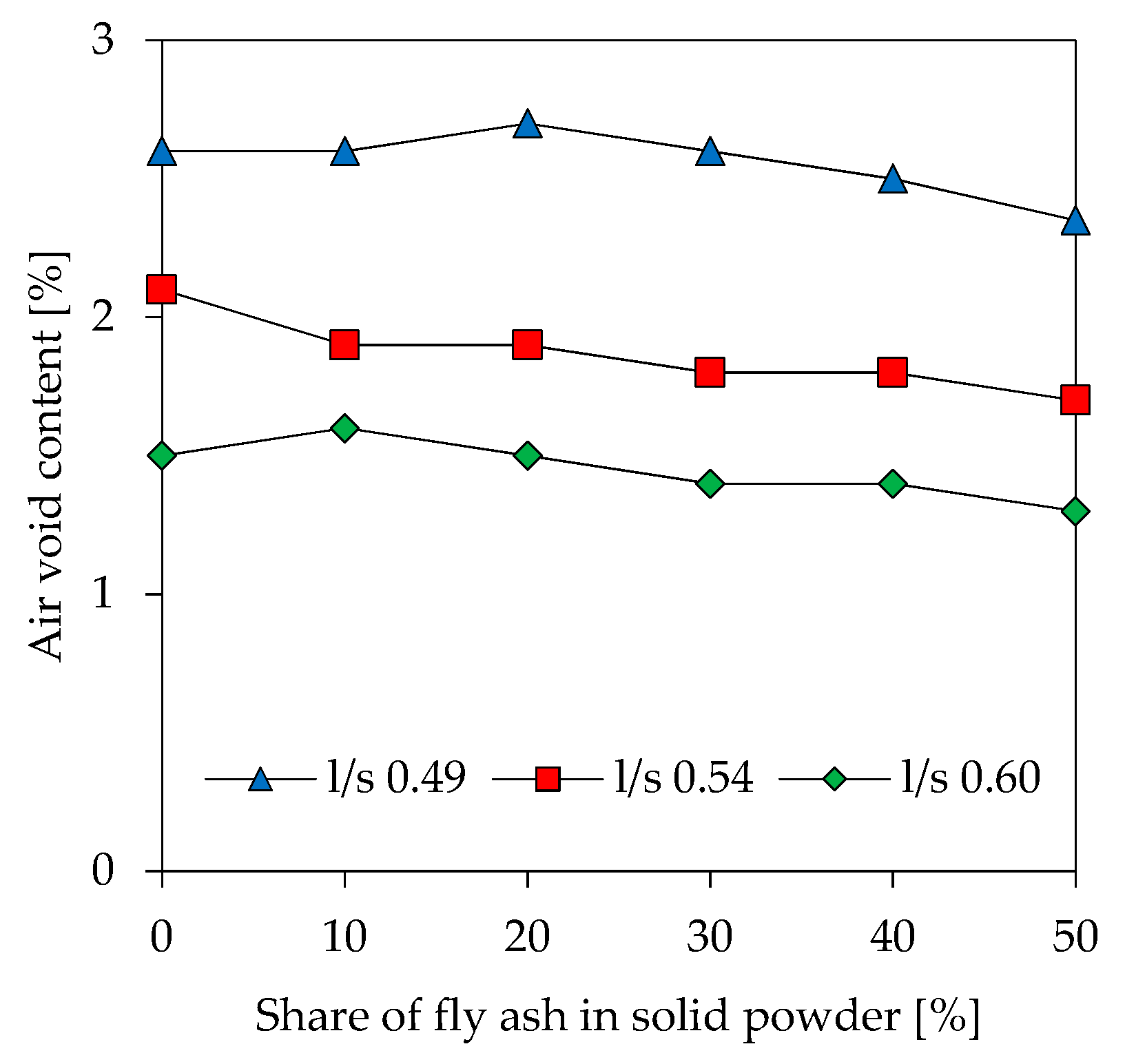
3.3. Air Void Content of Fresh Paste
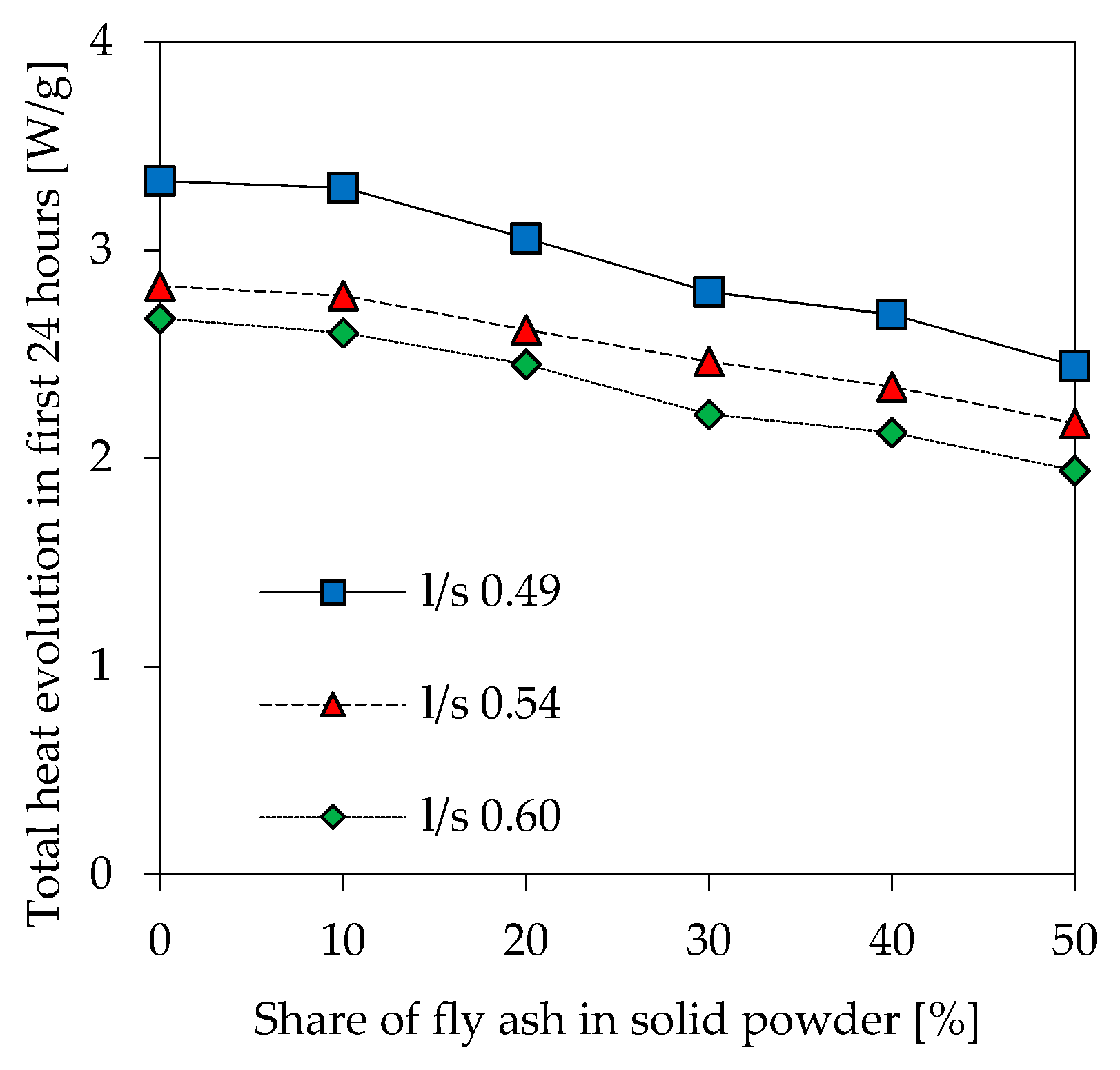
3.4. Isothermal Calorimetry
3.5. Setting Time
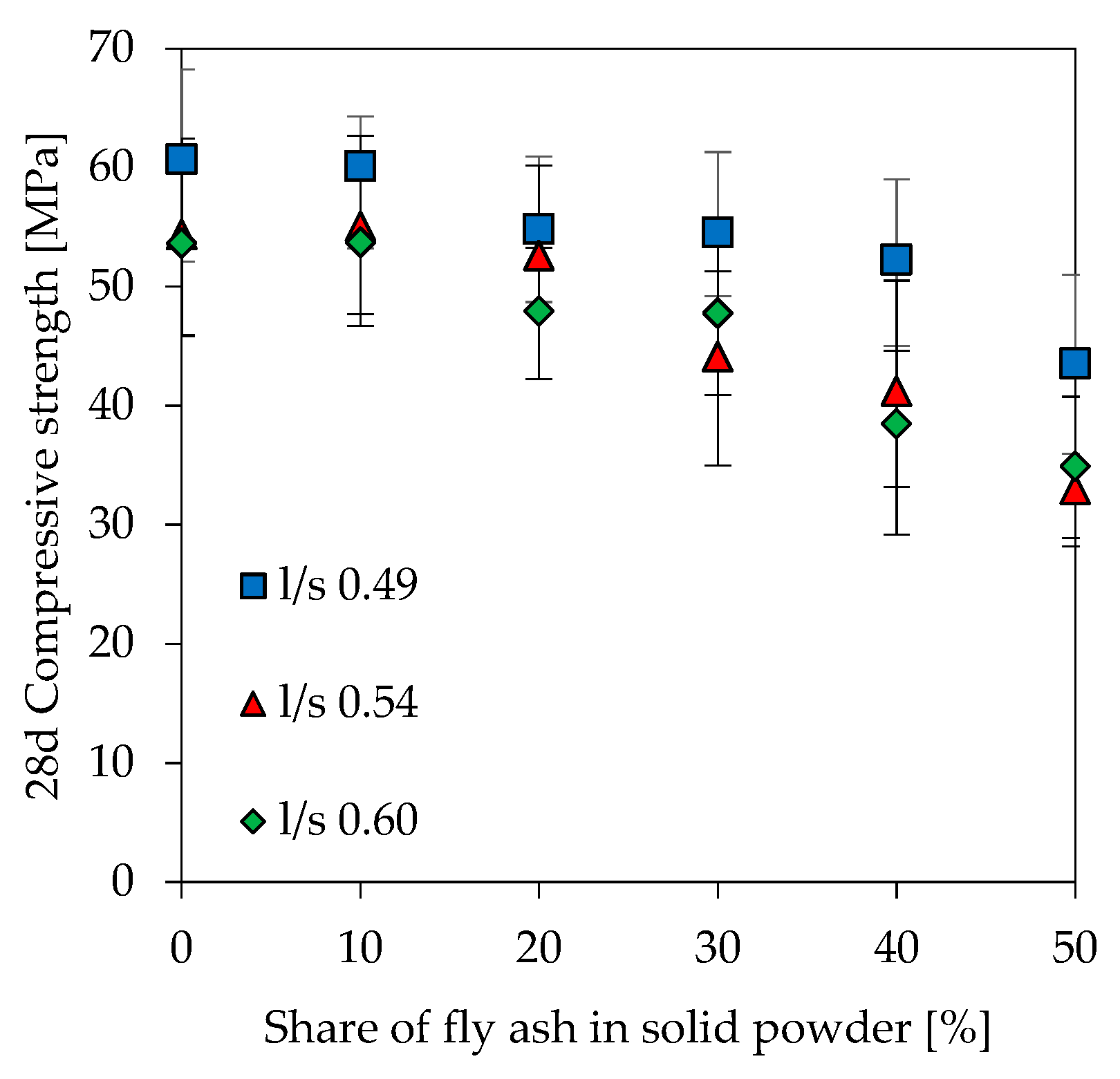
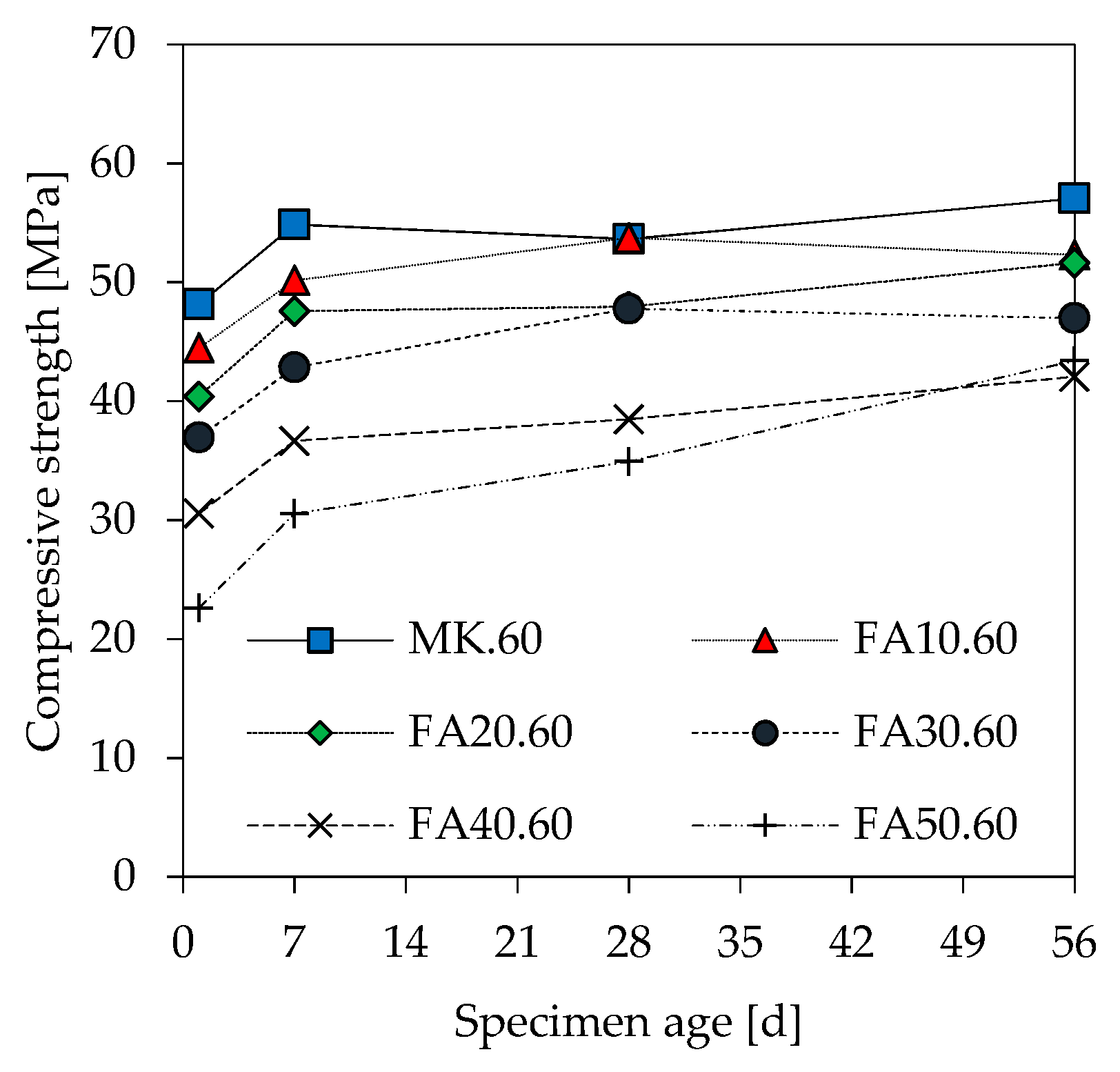
3.6. Compressive Strength
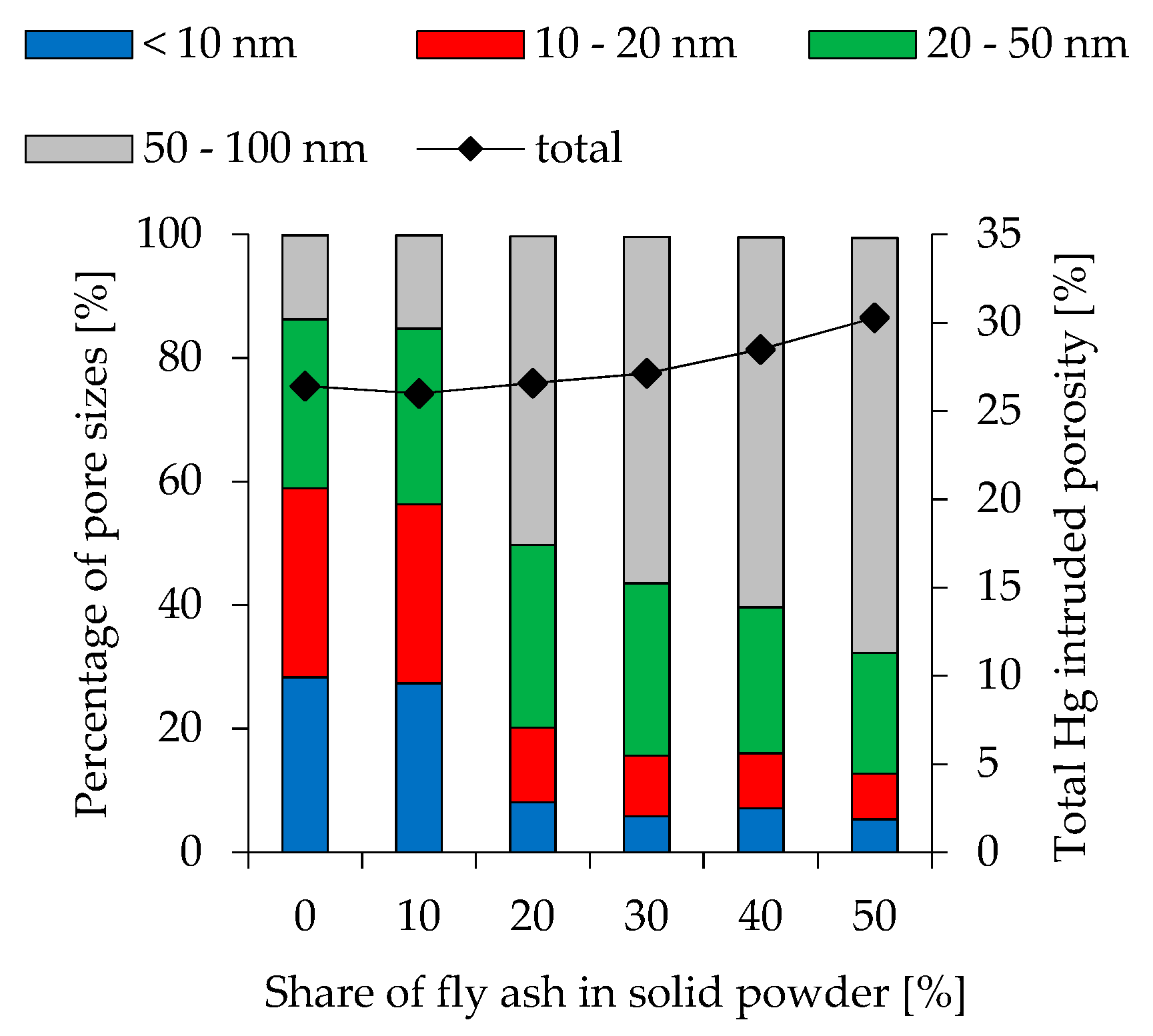
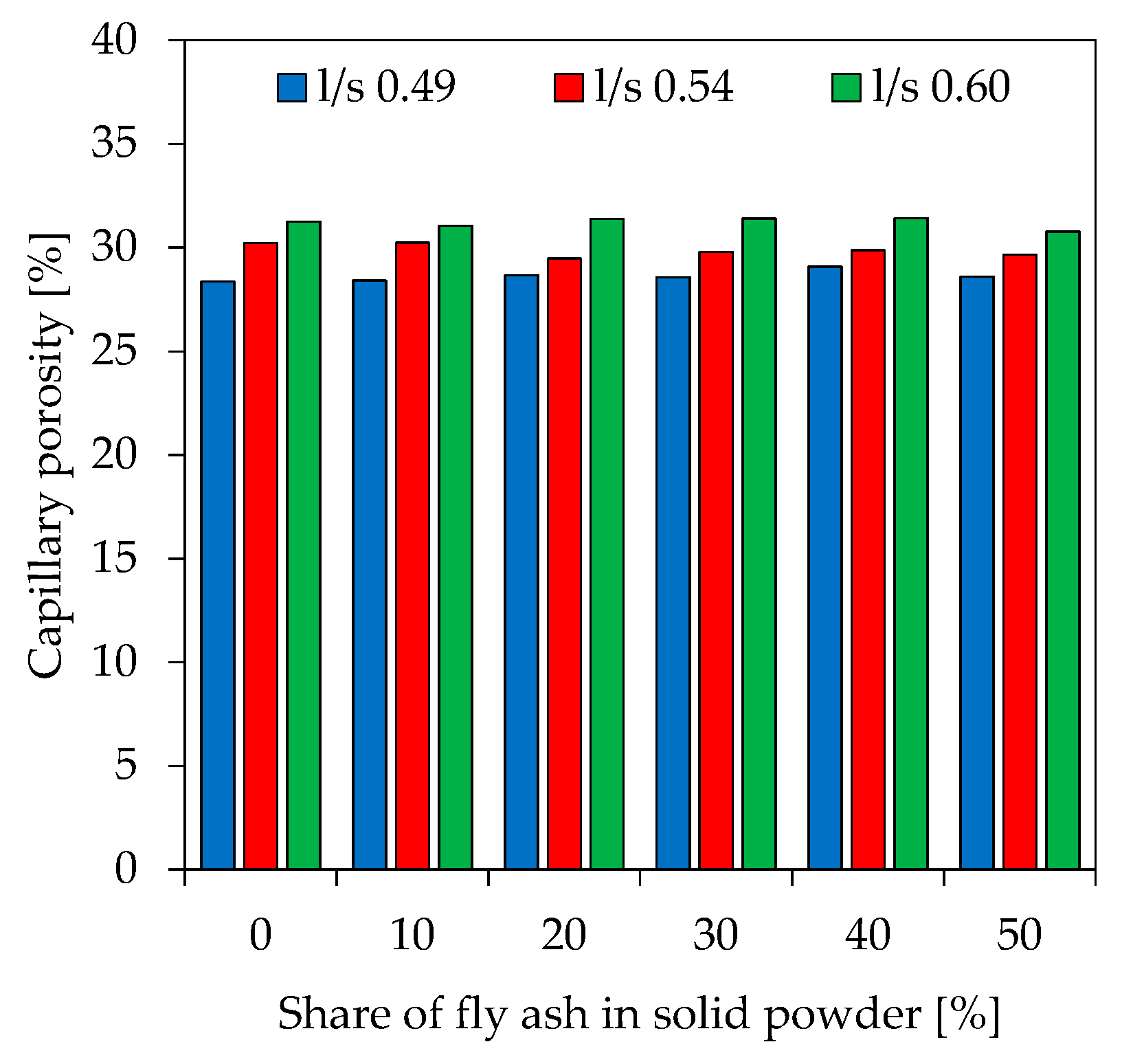
3.7. Porosity
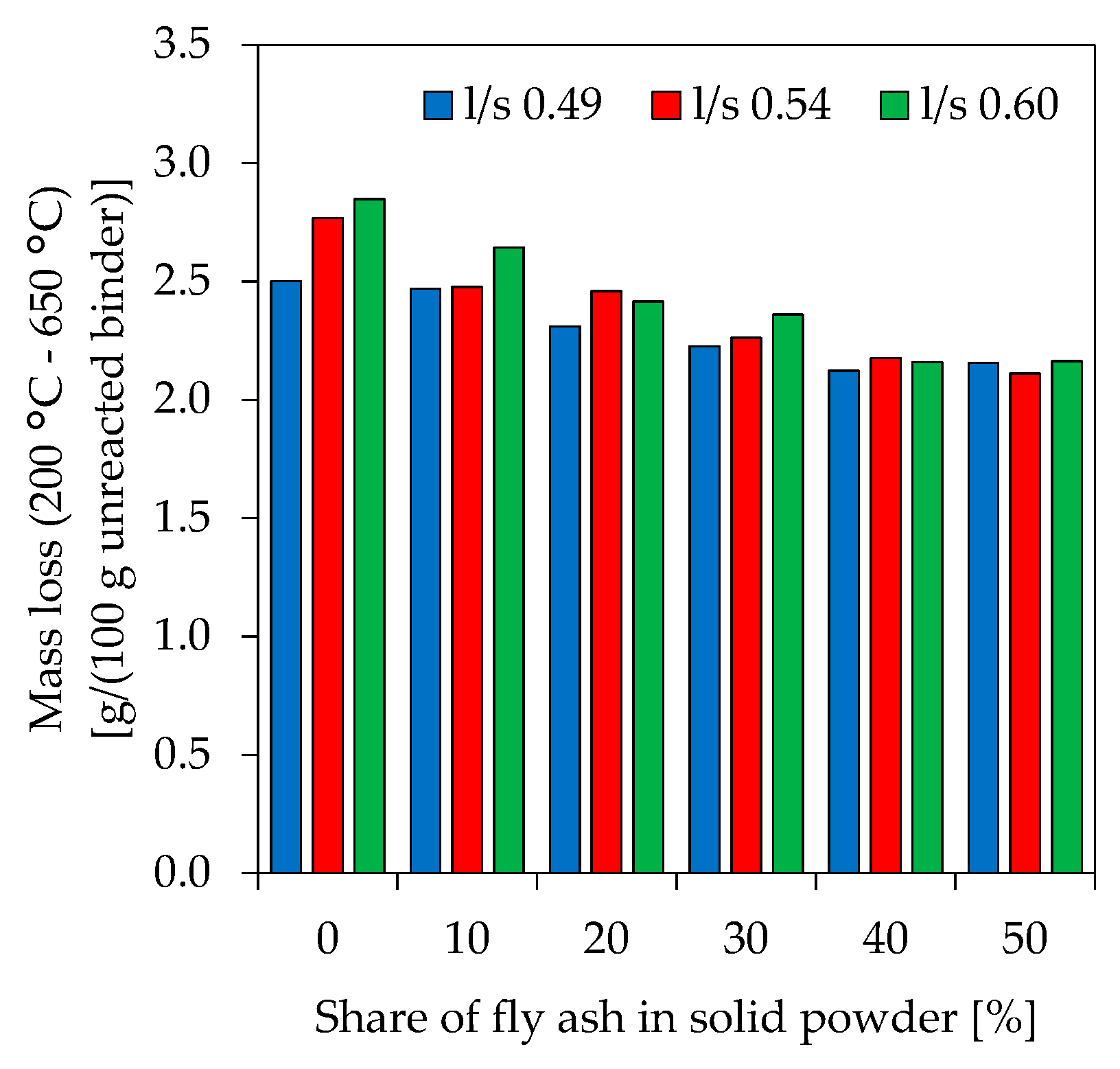
3.8. Thermogravimetric Analysis-Differential Scanning Calorimeter (TGA-DSC)
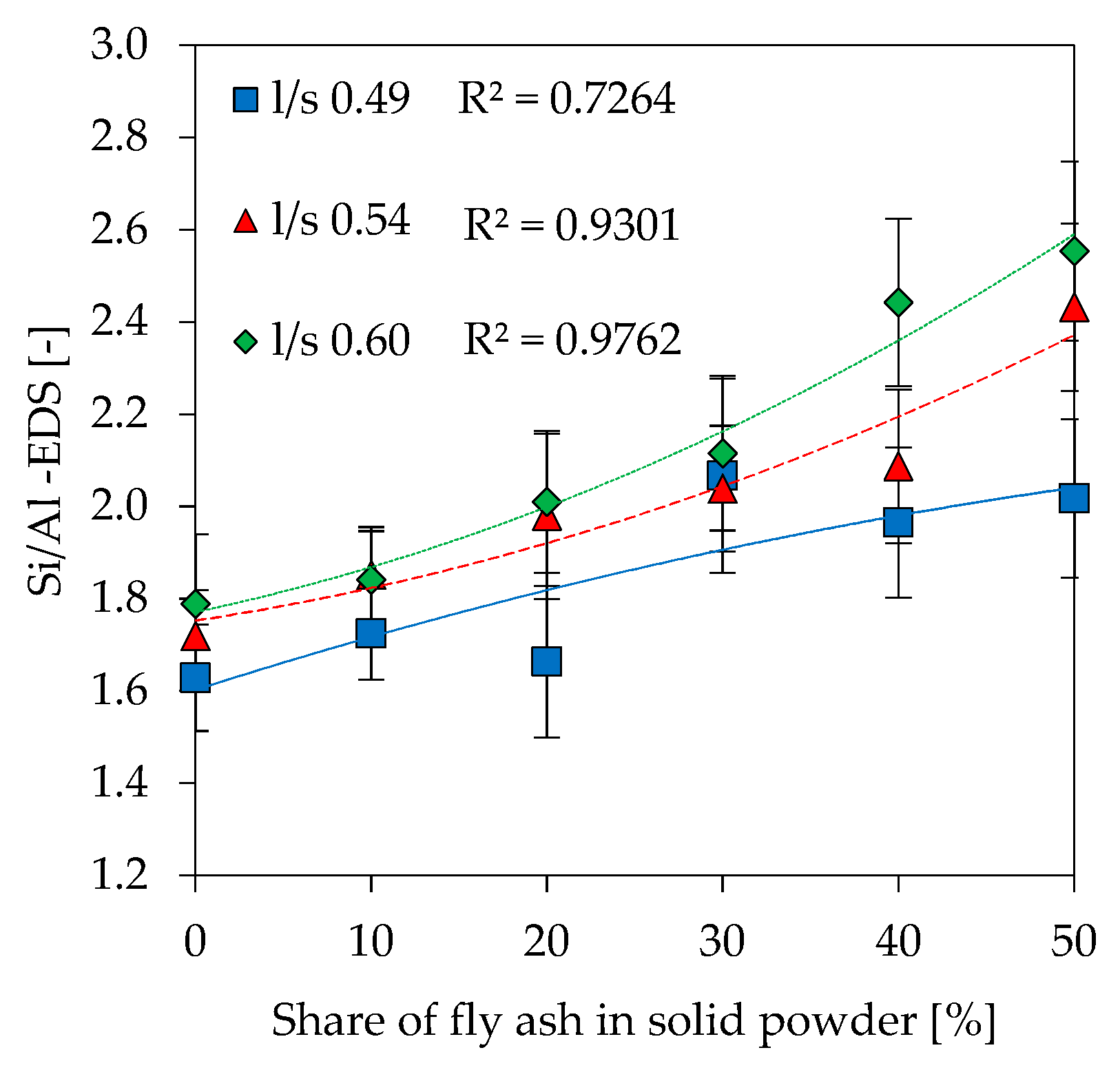
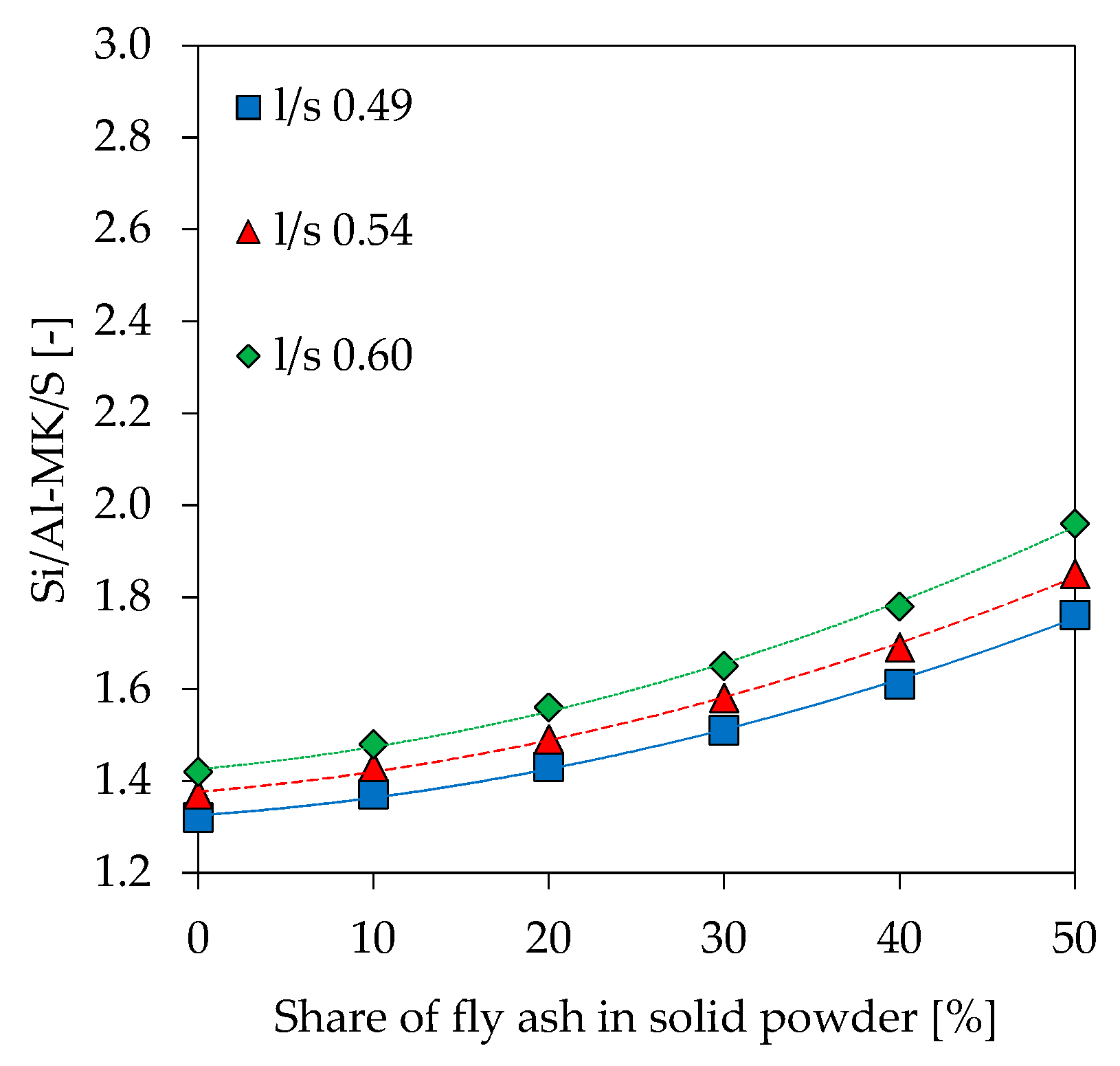
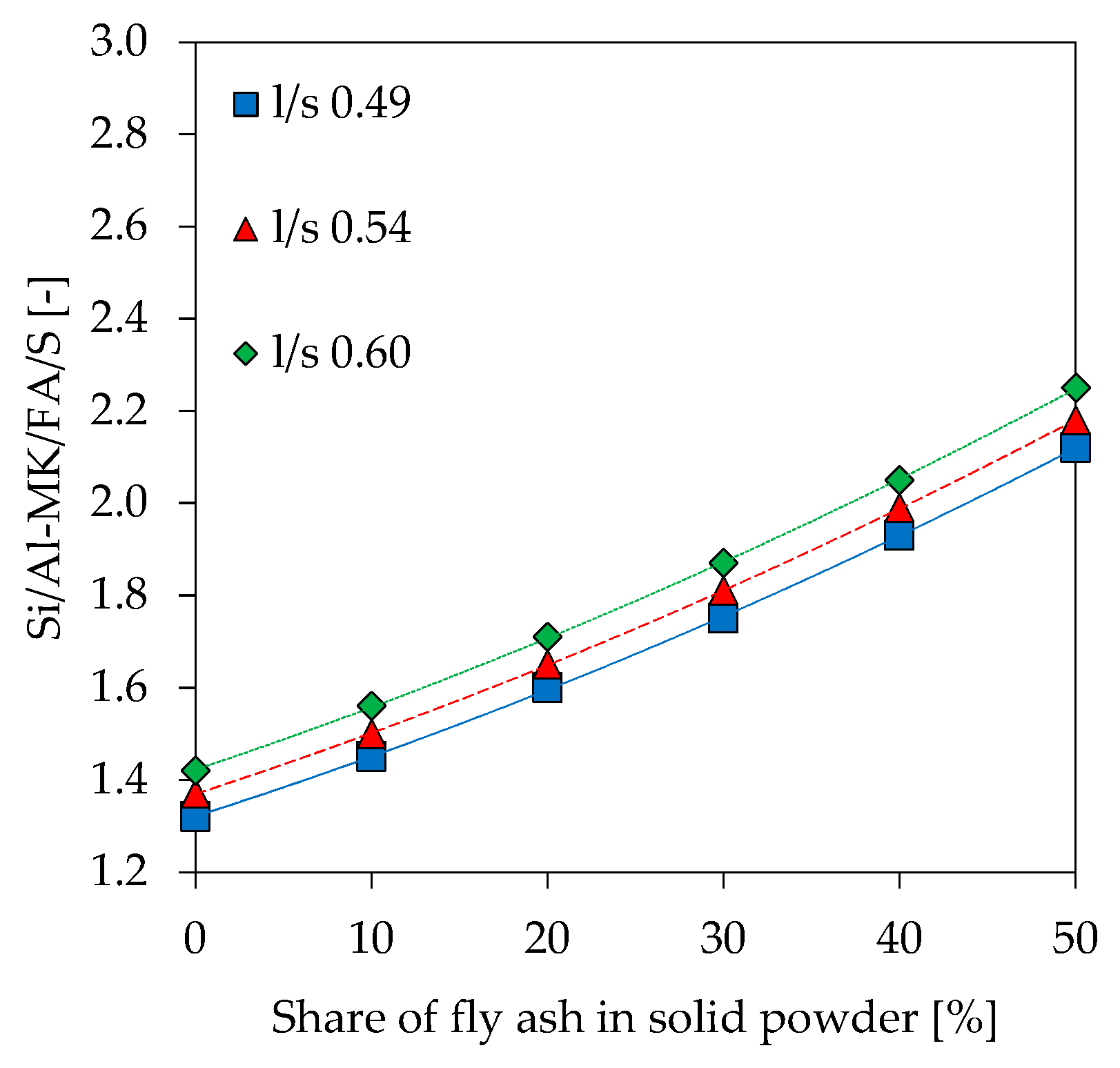
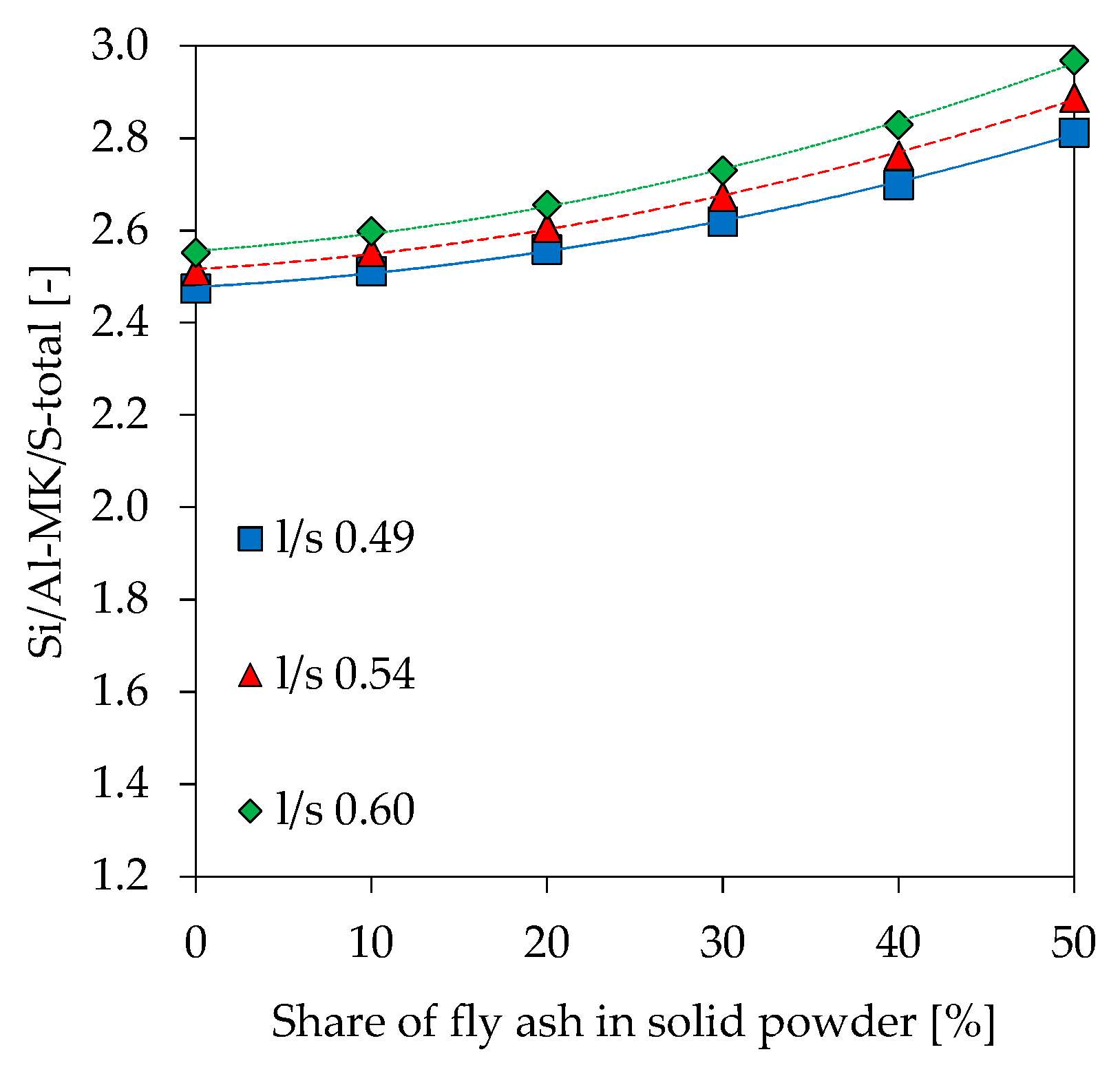
3.9. Scanning Electron Microscopy with Energy Dispersive Spectroscopy (SEM-EDS)
4. Discussion
5. Conclusion
- Substituting an impure metakaolin by 10% of fly ash increases workability and retards initial and final setting without significantly affecting the strength and microstructure of the geopolymer, especially at higher l/s ratio.
- Comparable pore size distribution (mercury intrusion porosimetry) between metakaolin geopolymer (0% fly ash content) and geopolymer with 10% fly ash are detected only at the highest l/s ratio (0.60). Lower l/s ratios show a significant differences between the two geopolymer formulations.
- Above 20% fly ash content there are significant differences with regard to strength and porosity, whereby the change in pore size distribution (mercury intrusion porosimetry) is most pronounced.
- Due to lower amounts of Al in geopolymers, total amount of heat evolved within the first 24 h of reaction decreases at higher l/s ratios and higher amounts of fly ash.
- Si/Al ratios obtained by SEM-EDS are between the calculated extreme cases (full and partial/amorphous reactivity), and are in agreement with results from reactivity of the precursors tested in diluted KOH.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pacheco-Torgal, F. Introduction to handbook of alkali-activated cements, mortars and concretes. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–16. [Google Scholar]
- Davidovits, J. Geopolymer Chemistry and Applications; Geopolymer Institute: Saint-Quentin, France, 2008. [Google Scholar]
- Buchwald, A. What are geopolymers? Current state of research and technology, the opportunities they offer, and their significance for the precast industry. Betonw. Fert. Tech. 2006, 72, 42–49. [Google Scholar]
- Silva, P.D.; Sagoe-Crenstil, K.; Sirivivatnanon, V. Kinetics of geopolymerization: Role of Al2O3 and SiO2. Cem. Concr. Res. 2007, 37, 512–518. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; van Deventer, J.S.J. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Duxson, P.; Mallicoat, S.; Lukey, G.C.; Kriven, W.M.; van Deventer, J.S. Microstructural characterisation of metakaolin-based geopolymers. Adv. Ceram. Matrix Compos. X 2006, 165, 71–85. [Google Scholar]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Ukrainczyk, N.; Muthu, M.; Vogt, O.; Koenders, E. Geopolymer, calcium aluminate, and Portland cement-based mortars: Comparing degradation using acetic acid. Materials 2019, 12, 3115. [Google Scholar] [CrossRef]
- Koenig, A.; Herrmann, A.; Overmann, S.; Dehn, F. Resistance of alkali-activated binders to organic acid attack: Assessment of evaluation criteria and damage mechanisms. Constr. Build. Mater. 2017, 151, 405–413. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; García-Lodeiro, I.; Palomo, A. Durability of alkali-activated fly ash cementitious materials. J. Mater. Sci. 2007, 42, 3055–3065. [Google Scholar] [CrossRef]
- Druga, B.; Ukrainczyk, N.; Weise, K.; Lackner, S.; Koenders, E.A.B. Interaction between wastewater microorganisms and geopolymer or cementitious materials: Biofilm characterization and deterioration characteristics of mortars. Int. Biodeterior. Biodegrad. 2019, 134, 58–67. [Google Scholar] [CrossRef]
- Okoye, F.N.; Prakash, S.; Singh, N.B. Durability of fly ash based geopolymer concrete in the presence of silica fume. J. Clean. Prod. 2017, 149, 1062–1067. [Google Scholar] [CrossRef]
- Buchwald, A.; Zellmann, H.-D.; Kaps, C. Condensation of aluminosilicate gels—Model system for geopolymer binders. J. Non Cryst. Solids 2011, 357, 1376–1382. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, Y.; Lin, W.; Liu, Z. In situ monitoring of the hydration process of K-PS geopolymer cement with ESEM. Cem. Concr. Res. 2004, 34, 935–940. [Google Scholar] [CrossRef]
- Xu, H.; van Deventer, J.S. Microstructural characterisation of geopolymers synthesised from kaolinite/stilbite mixtures using XRD, MAS-NMR, SEM/EDX, TEM/EDX, and HREM. Cem. Concr. Res. 2002, 32, 1705–1716. [Google Scholar] [CrossRef]
- Barbosa, V.F.F.; MacKenzie, K.J.D. Synthesis and thermal behaviour of potassium sialate geopolymers. Mater. Lett. 2003, 57, 1477–1482. [Google Scholar] [CrossRef]
- Ruiz-Santaquiteria, C.; Fernández-Jiménez, A.; Skibsted, J.; Palomo, A. Clay reactivity: Production of alkali activated cements. Appl. Clay Sci. 2013, 73, 11–16. [Google Scholar] [CrossRef]
- Provis, J.L.; Duxson, P.; van Deventer, J.S. The role of particle technology in developing sustainable construction materials. Adv. Powder Technol. 2010, 21, 2–7. [Google Scholar] [CrossRef]
- Fernandez, R.; Martirena, F.; Scrivener, K.L. The origin of the pozzolanic activity of calcined clay minerals: A comparison between kaolinite, illite and montmorillonite. Cem. Concr. Res. 2011, 41, 113–122. [Google Scholar] [CrossRef]
- Cassagnabere, F.; Diederich, P.; Mouret, M.; Escadeillas, G.; Lachemi, M. Impact of metakaolin characteristics on the rheological properties of mortar in the fresh state. Cem. Concr. Compos. 2013, 37, 95–107. [Google Scholar] [CrossRef]
- Pouhet, R. Formulation and Durability of Metakaolin-Based Geopolymers. Ph.D. Thesis, University Paul Sabatier-Toulouse III, Toulouse, France, 2015. [Google Scholar]
- Palomo, A.; Fernández-Jiménez, A.; Criado, M. Geopolymers: Same basic chemistry, different microstructures. Mater. Constr. 2004, 54, 77–91. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Composition and microstructure of alkali activated fly ash binder: Effect of the activator. Cem. Concr. Res. 2005, 35, 1984–1992. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Sobrados, I.; Sanz, J. The role played by the reactive alumina content in the alkaline activation of fly ashes. Microporous Mesoporous Mater. 2006, 91, 111–119. [Google Scholar] [CrossRef]
- Palomo, A.; Grutzeck, M.; Blanco, M. Alkali-activated fly ashes: A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Bakharev, T. Geopolymeric materials prepared using Class F fly ash and elevated temperature curing. Cem. Concr. Res. 2005, 35, 1224–1232. [Google Scholar] [CrossRef]
- Criado, M.; Palomo, A.; Fernández-Jiménez, A. Alkali activation of fly ashes. Part 1: Effect of curing conditions on the carbonation of the reaction products. Fuel 2005, 84, 2048–2054. [Google Scholar] [CrossRef]
- Criado, M.; Fernández-Jiménez, A.; de la Torre, A.; Aranda, M.; Palomo, A. An XRD study of the effect of the SiO2/Na2O ratio on the alkali activation of fly ash. Cem. Concr. Res. 2007, 37, 671–679. [Google Scholar] [CrossRef]
- Palomo, A.; Alonso, S.; Fernandez-Jiménez, A.; Sobrados, I.; Sanz, J. Alkaline activation of fly ashes: NMR study of the reaction products. J. Am. Ceram. Soc. 2004, 87, 1141–1145. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Alex, T.; Bandopadhyay, A.; Mehrotra, S. Influence of reactivity of fly ash on geopolymerisation. Adv. Appl. Ceram. 2007, 106, 120–127. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, S.; Mehrotra, S. Towards sustainable solutions for fly ash through mechanical activation. Resour. Conserv. Recycl. 2007, 52, 157–179. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R. Mechanical activation of fly ash: Effect on reaction, structure and properties of resulting geopolymer. Ceram. Int. 2011, 37, 533–541. [Google Scholar] [CrossRef]
- Marjanović, N.; Komljenović, M.; Baščarević, Z.; Nikolić, V. Improving reactivity of fly ash and properties of ensuing geopolymers through mechanical activation. Constr. Build. Mater. 2014, 57, 151–162. [Google Scholar] [CrossRef]
- Van Deventer, J.S.J.; Provis, J.L.; Duxson, P.; Lukey, G.C. Reaction mechanisms in the geopolymeric conversion of inorganic waste to useful products. J. Hazard. Mater. 2007, 139, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, S.V.; Vassileva, C.G. A new approach for the combined chemical and mineral classification of the inorganic matter in coal. 1. Chemical and mineral classification systems. Fuel 2009, 88, 235–245. [Google Scholar] [CrossRef]
- Komljenović, M.; Petrašinović-Stojkanović, L.; Baščarević, Z.; Jovanović, N.; Rosić, A. Fly ash as the potential raw mixture component for Portland cement clinker synthesis. J. Therm. Anal. Calorim. 2009, 96, 363–368. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Barbosa, V.F.; MacKenzie, K.J. Thermal behaviour of inorganic geopolymers and composites derived from sodium polysialate. Mater. Res. Bull. 2003, 38, 319–331. [Google Scholar] [CrossRef]
- Kong, D.L.; Sanjayan, J.G.; Sagoe-Crentsil, K. Comparative performance of geopolymers made with metakaolin and fly ash after exposure to elevated temperatures. Cem. Concr. Res. 2007, 37, 1583–1589. [Google Scholar] [CrossRef]
- Rashad, A.M. Alkali-activated metakaolin: A short guide for civil Engineer—An overview. Constr. Build. Mater. 2013, 41, 751–765. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Zhu, Y.; Reid, A.; Provis, J.L.; Bullen, F. Using fly ash to partially substitute metakaolin in geopolymer synthesis. Appl. Clay Sci. 2014, 88, 194–201. [Google Scholar] [CrossRef]
- Xie, J.; Kayali, O. Effect of initial water content and curing moisture conditions on the development of fly ash-based geopolymers in heat and ambient temperature. Constr. Build. Mater. 2014, 67, 20–28. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Zhou, W. Influence of partial replacement of fly ash by metakaolin on mechanical properties and microstructure of fly ash geopolymer paste exposed to sulfate attack. Ceram. Int. 2016, 42, 3504–3517. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Kodur, V.; Qi, S.L.; Cao, L.; Wu, B. Development of metakaolin—Fly ash based geopolymers for fire resistance applications. Constr. Build. Mater. 2014, 55, 38–45. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Kodur, V.; Qi, S.L.; Wu, B. Characterizing the bond strength of geopolymers at ambient and elevated temperatures. Cem. Concr. Compos. 2015, 58, 40–49. [Google Scholar] [CrossRef]
- Louati, S.; Baklouti, S.; Samet, B. Geopolymers based on phosphoric acid and illito-kaolinitic clay. Adv. Mater. Sci. Eng. 2016, 2016, 2359759. [Google Scholar] [CrossRef]
- Yan, S.; He, P.; Jia, D.; Duan, X.; Yang, Z.; Wang, S.; Zhou, Y. Effects of graphene oxide on the geopolymerization mechanism determined by quenching the reaction at intermediate states. RSC Adv. 2017, 7, 13498–13508. [Google Scholar] [CrossRef] [Green Version]
- Ward, C.R.; French, D. Determination of glass content and estimation of glass composition in fly ash using quantitative X-ray diffractometry. Fuel 2006, 85, 2268–2277. [Google Scholar] [CrossRef]
- Winburn, R.S.; Grier, D.G.; McCarthy, G.J.; Peterson, R.B. Rietveld quantitative X-ray diffraction analysis of NIST fly ash standard reference materials. Powder Diffr. 2000, 15, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.P.; van Riessen, A. Determination of the reactive component of fly ashes for geopolymer production using XRF and XRD. Fuel 2010, 89, 3683–3692. [Google Scholar] [CrossRef]
- Douiri, H.; Kaddoussi, I.; Baklouti, S.; Arous, M.; Fakhfakh, Z. Water molecular dynamics of metakaolin and phosphoric acid-based geopolymers investigated by impedance spectroscopy and DSC/TGA. J. Non Cryst. Solids 2016, 445, 95–101. [Google Scholar] [CrossRef]
- Assaedi, H.; Shaikh, F.; Low, I.M. Effect of nano-clay on mechanical and thermal properties of geopolymer. J. Asian Ceram. Soc. 2016, 4, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Buchwald, A. Der Einfluss des Kalziums auf die Kondensation von (Alumo-) Silikaten in alkali-aktivierten Bindern. Ph.D. Thesis, Bauhaus-Universität Weimar, Weimar, Germany, 2014. [Google Scholar]
- Zhang, Z.; Provis, J.L.; Wang, H.; Bullen, F.; Reid, A. Quantitative kinetic and structural analysis of geopolymers. Part 2. Thermodynamics of sodium silicate activation of metakaolin. Thermochim. Acta 2013, 565, 163–171. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Provis, J.L.; Bullen, F.; Reid, A.; Zhu, Y. Quantitative kinetic and structural analysis of geopolymers. Part 1. The activation of metakaolin with sodium hydroxide. Thermochim. Acta 2012, 539, 23–33. [Google Scholar] [CrossRef]
- White, C.E.; Page, K.; Henson, N.J.; Provis, J.L. In situ synchrotron X-ray pair distribution function analysis of the early stages of gel formation in metakaolin-based geopolymers. Appl. Clay Sci. 2013, 73, 17–25. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Provis, J.L.; van Deventer, J.S. Effect of alumina release rate on the mechanism of geopolymer gel formation. Chem. Mater. 2010, 22, 5199–5208. [Google Scholar] [CrossRef]
- Sagoe-Crentsil, L.W.K. Dissolution processes, hydrolysis and condensation reactions during geopolymer synthesis: Part I—Low Si/Al ratio systems. J. Mater. Sci. 2007, 42, 2997–3006. [Google Scholar] [CrossRef]
- Kuenzel, C.; Neville, T.; Donatello, S.; Vandeperre, L.; Boccaccini, A.; Cheeseman, C. Influence of metakaolin characteristics on the mechanical properties of geopolymers. Appl. Clay Sci. 2013, 83, 308–314. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, Z.; Zhu, H.; Chen, Y. Geopolymerization process of alkali–Metakaolinite characterized by isothermal calorimetry. Thermochim. Acta 2009, 493, 49–54. [Google Scholar] [CrossRef]
- De Jong, B.; Brown, G.E., Jr. Polymerization of silicate and aluminate tetrahedra in glasses, melts, and aqueous solutions—I. Electronic structure of H6Si2O7, H6AlSiO71−, and H6Al2O72−. Geochim. Cosmochim. Acta 1980, 44, 491–511. [Google Scholar] [CrossRef]
- Rowles, M.; O’connor, B. Chemical optimisation of the compressive strength of aluminosilicate geopolymers synthesised by sodium silicate activation of metakaolinite. J. Mater. Chem. 2003, 13, 1161–1165. [Google Scholar] [CrossRef]
- Yunsheng, Z.; Wei, S.; Zongjin, L. Composition design and microstructural characterization of calcined kaolin-based geopolymer cement. Appl. Clay Sci. 2010, 47, 271–275. [Google Scholar] [CrossRef]
- Wan, Q.; Rao, F.; Song, S.; García, R.E.; Estrella, R.M.; Patiño, C.L.; Zhang, Y. Geopolymerization reaction, microstructure and simulation of metakaolin-based geopolymers at extended Si/Al ratios. Cem. Concr. Compos. 2017, 79, 45–52. [Google Scholar] [CrossRef]
- Steveson, M.; Sagoe-Crentsil, K. Relationships between composition, structure and strength of inorganic polymers. J. Mater. Sci. 2005, 40, 2023–2036. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, M.; Zhang, G.; El-Korchi, T.; Tao, M. A multiscale investigation of reaction kinetics, phase formation, and mechanical properties of metakaolin geopolymers. Cem. Concr. Compos. 2017, 78, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Ozer, I.; Soyer-Uzun, S. Relations between the structural characteristics and compressive strength in metakaolin based geopolymers with different molar Si/Al ratios. Ceram. Int. 2015, 41, 10192–10198. [Google Scholar] [CrossRef]
- Casarez, R.; Antonio, C.; Soberón, G.; Vicente, J.M.; Sánchez, A.; Luis, J.; Ramón, C.H.; Chinchillas, C.; de Jesús, M.; Agüero, A.; et al. Experimental study of XRD, FTIR and TGA techniques in geopolymeric materials. In Proceedings of the International Conference on Advances in Civil and Structural Engineering (CSE), Kuala Lumpur, Malaysia, 2–3 August 2014; pp. 25–30. [Google Scholar]
- Škvára, F.; Kopeckỳ, L.; Šmilauer, V.; Bittnar, Z. Material and structural characterization of alkali activated low-calcium brown coal fly ash. J. Hazard. Mater. 2009, 168, 711–720. [Google Scholar] [CrossRef]
- Weng, L.; Sagoe-Crentsil, K.; Brown, T.; Song, S. Effects of aluminates on the formation of geopolymers. Mater. Sci. Eng. B 2005, 117, 163–168. [Google Scholar] [CrossRef]
- Williams, R.P.; Hart, R.D.; van Riessen, A. Quantification of the extent of reaction of metakaolin-based geopolymers using X-Ray diffraction, scanning electron microscopy, and energy-dispersive spectroscopy. J. Am. Ceram. Soc. 2011, 94, 2663–2670. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. Geopolymerisation kinetics. 2. Reaction kinetic modelling. Chem. Eng. Sci. 2007, 62, 2318–2329. [Google Scholar] [CrossRef]
- Tchakoute, H.K.; Rüscher, C.H.; Djobo, J.N.Y.; Kenne, B.B.D.; Njopwouo, D. Influence of gibbsite and quartz in kaolin on the properties of metakaolin-based geopolymer cements. Appl. Clay Sci. 2015, 107, 188–194. [Google Scholar] [CrossRef]
- Schmücker, M.; MacKenzie, K.J.D. Microstructure of sodium polysialate siloxo geopolymer. Ceram. Int. 2005, 31, 433–437. [Google Scholar] [CrossRef]
- Autef, A.; Joussein, E.; Poulesquen, A.; Gasgnier, G.; Pronier, S.; Sobrados, I.; Sanz, J.; Rossignol, S. Influence of metakaolin purities on potassium geopolymer formulation: The existence of several networks. J. Colloid Interface Sci. 2013, 408, 43–53. [Google Scholar] [CrossRef]
- Autef, A.; Joussein, E.; Gasgnier, G.; Pronier, S.; Sobrados, I.; Sanz, J.; Rossignol, S. Role of metakaolin dehydroxylation in geopolymer synthesis. Powder Technol. 2013, 250, 33–39. [Google Scholar] [CrossRef]
- Lee, W.K.W.; van Deventer, J.S.J. Chemical interactions between siliceous aggregates and low-Ca alkali-activated cements. Cem. Concr. Res. 2007, 37, 844–855. [Google Scholar] [CrossRef]





| amor. | Quartz | Muscovite | Mullite | Calcite | Diaoyudaoite | Other (<0.5%) | LOI | |
|---|---|---|---|---|---|---|---|---|
| MK | 46.0 | 39.6 | 10.0 | 0.2 | 2.0 | 1.0 | 1.2 | 0.8 |
| FA | 72.0 | 9.5 | 0.0 | 16.0 | 0.0 | 0.0 | 2.5 | 4.5 |
| Si [wt.%] | Al [wt.%] | SiO2 [wt.%] | Al2O3 [wt.%] | Si/Al (molar) [-] | |
|---|---|---|---|---|---|
| MK | 10.63 | 11.50 | 22.74 | 21.73 | 0.89 |
| FA | 18.44 | 6.74 | 39.46 | 12.74 | 2.63 |
| l/s 0.49 | l/s 0.54 | l/s 0.60 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fly ash [%] | 0 | 10 | 30 | 50 | 0 | 10 | 30 | 50 | 0 | 10 | 30 | 50 | |
| Setting | |||||||||||||
| Initial | 92 | 115 | 162 | 232 | 127 | 165 | 207 | 270 | 160 | 197 | 245 | 327 | |
| Final | 125 | 157 | 177 | 267 | 147 | 180 | 237 | 295 | 190 | 220 | 270 | 362 | |
| l/s 0.49 | l/s 0.54 | l/s 0.60 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fly ash [%] | 0 | 10 | 30 | 50 | 0 | 10 | 30 | 50 | 0 | 10 | 30 | 50 | |
| Porosity | |||||||||||||
| Hg intruded [%] | 23.5 | 24.7 | 25.5 | 25.9 | 25.6 | 25.6 | 25.7 | 26.5 | 26.4 | 26.0 | 27.1 | 30.3 | |
| Capillary [%] | 28.4 | 28.4 | 28.6 | 28.6 | 30.2 | 30.2 | 29.8 | 29.6 | 31.3 | 31.0 | 31.4 | 30.8 | |
| Designation | Range [°C] | Reference |
|---|---|---|
| Physically adsorbed an interstitial evaporable water | 0–120 | Casarez et al. [68] |
| 0–150 | Assaedi et al. [52] | |
| 0–200 | Douiri et al. [51] | |
| Water from alumosilicate network | 120–200 | Casarez et al. [68] |
| Interstitial water | 150–300 | Assaedi et al. [52] |
| Water in nano pores | 180–600 | Škvára et al. [69] |
| Structural water | 200–400 | Douiri et al. [51] |
| Dihydroxylation of chemically bound water | 300–600 | Assaedi et al. [52] |
| Carbonation process | 450–800 | Casarez et al. [68] |
| Carbon remnants in fly ash | 600–700 | Assaedi et al. [52] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogt, O.; Ukrainczyk, N.; Ballschmiede, C.; Koenders, E. Reactivity and Microstructure of Metakaolin Based Geopolymers: Effect of Fly Ash and Liquid/Solid Contents. Materials 2019, 12, 3485. https://doi.org/10.3390/ma12213485
Vogt O, Ukrainczyk N, Ballschmiede C, Koenders E. Reactivity and Microstructure of Metakaolin Based Geopolymers: Effect of Fly Ash and Liquid/Solid Contents. Materials. 2019; 12(21):3485. https://doi.org/10.3390/ma12213485
Chicago/Turabian StyleVogt, Oliver, Neven Ukrainczyk, Conrad Ballschmiede, and Eddie Koenders. 2019. "Reactivity and Microstructure of Metakaolin Based Geopolymers: Effect of Fly Ash and Liquid/Solid Contents" Materials 12, no. 21: 3485. https://doi.org/10.3390/ma12213485






