3D Printing of Silk Fibroin for Biomedical Applications
Abstract
:1. Introduction
2. Silk Fibroin Bioink
2.1. Processing of SF Bioink
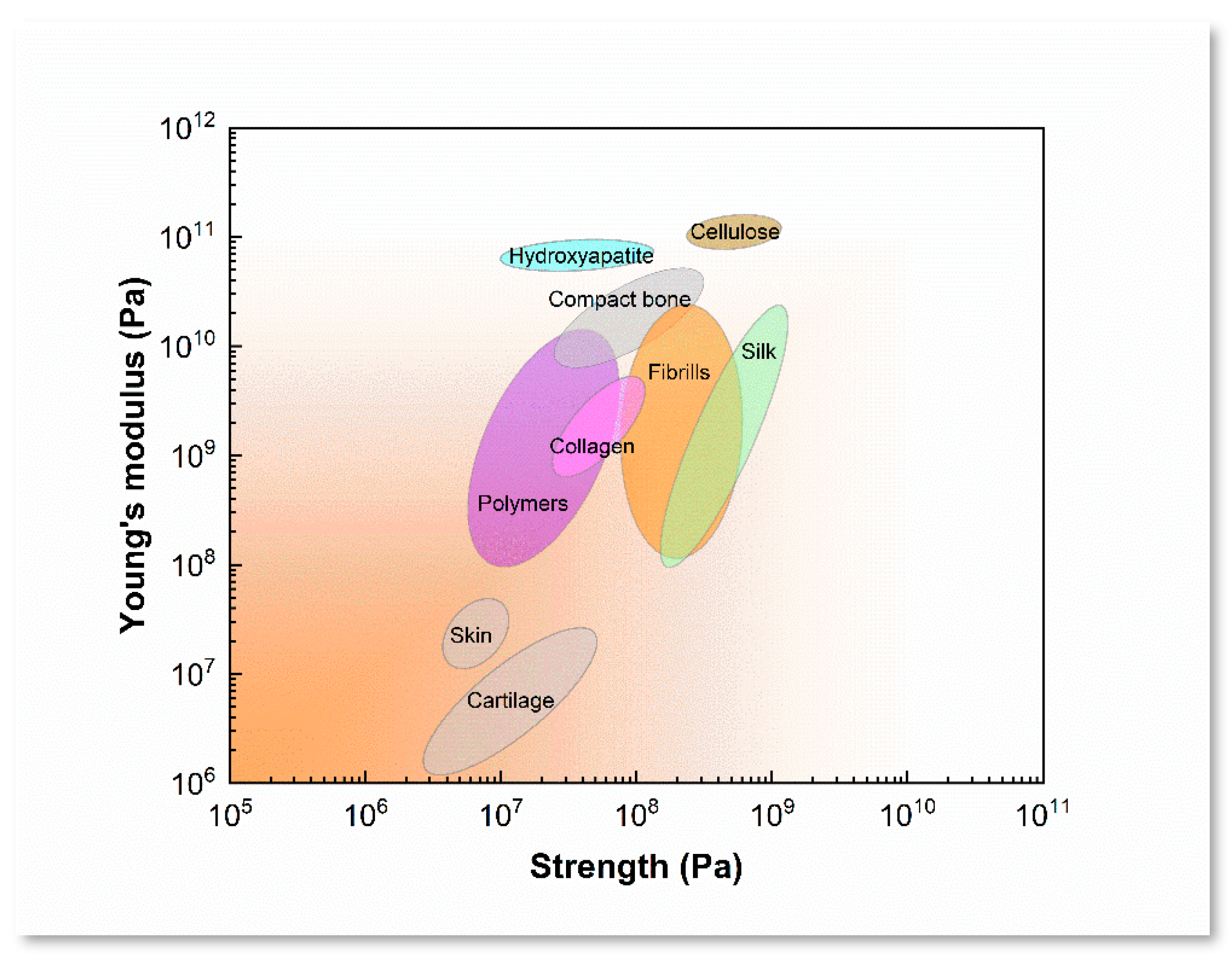
2.2. SF Bioink Design
3. Evaluation of Cell Viability with SF Based 3D Printing Scaffolds
4. SF Bioink for Biomedical Applications
4.1. Skin Tissue
4.2. Cartilage Tissue
4.3. Bone Tissue
4.4. Blood Vessel
5. Summary and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Wu, C.; Wang, B.; Zhang, C.; Wysk, R.A.; Chen, Y.W. Printing: An assessment based on manufacturing readiness levels. Crit. Rev. Biotechnol. 2017, 37, 333–354. [Google Scholar] [CrossRef] [PubMed]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D printing for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Jung, C.S.; Min, B.-H. Advances in three-dimensional printing for hard tissue engineering. Tissue Eng. Regen. Med. 2016, 13, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Guvendiren, M.; Molde, J.; Soares, R.M.; Kohn, J. Designing Biomaterials for 3D Printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, X.; Li, X.; Chen, Z.; Zhou, C.; Zhou, Q.; Chen, Y. Recent Progress in Biomimetic Additive Manufacturing Technology: From Materials to Functional Structures. Adv. Mater. 2018, e1706539. [Google Scholar] [CrossRef] [PubMed]
- DeSimone, E.; Schacht, K.; Pellert, A.; Scheibel, T. Recombinant spider silk-based bioinks. Biofabrication 2017, 9, 044104. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.X.; Eyster, T.W.; Doleyres, Y. Tissue Engineering Biomaterials. In Encyclopedia of Polymer Science and Technology; Wiley: Hoboken, NJ, USA, 2016; pp. 1–47. [Google Scholar] [CrossRef]
- Ali Khademhosseini, R.L.; Borenstein, J.; Vacanti, J.P. Microscale technologies for tissue engineering and biology. Proc. Natl. Acad. Sci. USA 2005, 103, 2480–2487. [Google Scholar] [CrossRef]
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018, 22, 1–15. [Google Scholar] [CrossRef]
- Freeman, F.E.; Kelly, D.J. Tuning Alginate Bioink Stiffness and Composition for Controlled Growth Factor Delivery and to Spatially Direct MSC Fate within Bioprinted Tissues. Sci. Rep. 2017, 7, 17042. [Google Scholar] [CrossRef]
- Norotte, C.; Marga, F.S.; Niklason, L.E.; Forgacs, G. Scaffold-free vascular tissue engineering using printing. Biomaterials 2009, 30, 5910–5917. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.N.; Kim, B.; Walma, A.M.; Choi, S.C.; Wu, H.; Mao, J.J.; Jun, H.W.; Cheon, K. Biomimetic microenvironments for regenerative endodontics. Biomater. Res. 2016, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Holzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D printing. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef] [PubMed]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef] [PubMed]
- Vepari, C.; Kaplan, D.L. Silk as a Biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Dong, S.; Lu, Q.; Hu, X.; Kaplan, D.L.; Zhang, B.; Zhu, H. Salt-leached silk scaffolds with tunable mechanical properties. Biomacromolecules 2012, 13, 3723–3729. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.H.; Ohgo, K.; Asakura, T. Preparation and characterization of regenerated Bombyx mori silk fibroin fiber with high strength. Express Polym. Lett. 2008, 2, 885–889. [Google Scholar] [CrossRef]
- Liang, H.C.; Chang, W.H.; Liang, H.F.; Lee, M.H.; Sung, H.W. Crosslinking structures of gelatin hydrogels crosslinked with genipin or a water-soluble carbodiimide. J. Appl. Polym. Sci. 2004, 91, 4017–4026. [Google Scholar] [CrossRef]
- Gruene, M.; Pflaum, M.; Hess, C.; Diamantouros, S.; Schlie, S.; Deiwick, A.; Koch, L.; Wilhelmi, M.; Jockenhoevel, S.; Haverich, A.; et al. Laser printing of three-dimensional multicellular arrays for studies of cell-cell and cell-environment interactions. Tissue Eng. Part C Methods 2011, 17, 973–982. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Y.; Chen, H.; Ozbolat, I.T. Characterization of printable cellular micro-fluidic channels for tissue engineering. Biofabrication 2013, 5, 025004. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Y.; Ozbolat, I.T. Direct Printing of Vessel-Like Tubular Microfluidic Channels. J. Nanotechnol. Eng. Med. 2013, 4, 2. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- Moon, B.M.; Choi, M.J.; Sultan, M.T.; Yang, J.W.; Ju, H.W.; Lee, J.M.; Park, H.J.; Park, Y.R.; Kim, S.H.; Kim, D.W.; et al. Novel fabrication method of the peritoneal dialysis filter using silk fibroin with urease fixation system. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 2136–2144. [Google Scholar] [CrossRef]
- Vasconcelos, A.; Gomes, A.C.; Cavaco-Paulo, A. Novel silk fibroin/elastin wound dressings. Acta Biomater. 2012, 8, 3049–3060. [Google Scholar] [CrossRef]
- Gao, F.; Xu, Z.; Liang, Q.; Liu, B.; Li, H.; Wu, Y.; Zhang, Y.; Lin, Z.; Wu, M.; Ruan, C.; et al. Direct 3D Printing of High Strength Biohybrid Gradient Hydrogel Scaffolds for Efficient Repair of Osteochondral Defect. Adv. Funct. Mater. 2018, 28, 1706644. [Google Scholar] [CrossRef]
- Garcia-Fuentes, M.; Meinel, A.J.; Hilbe, M.; Meinel, L.; Merkle, H.P. Silk fibroin/hyaluronan scaffolds for human mesenchymal stem cell culture in tissue engineering. Biomaterials 2009, 30, 5068–5076. [Google Scholar] [CrossRef]
- Porter, D.; Vollrath, F. Silk as a Biomimetic Ideal for Structural Polymers. Adv. Mater. 2009, 21, 487–492. [Google Scholar] [CrossRef]
- Brenckle, M.A.; Tao, H.; Kim, S.; Paquette, M.; Kaplan, D.L.; Omenetto, F.G. Protein-protein nanoimprinting of silk fibroin films. Adv. Mater. 2013, 25, 2409–2414. [Google Scholar] [CrossRef]
- Guo, N.; Leu, M.C. Additive manufacturing: Technology, applications and research needs. Front. Mech. Eng. 2013, 8, 215–243. [Google Scholar] [CrossRef]
- Kruth, J.P.; Leu, M.C.; Nakagawa, T. Progress in Additive Manufacturing and Rapid Prototyping. Ann. ClRP 1998, 47, 525–540. [Google Scholar] [CrossRef]
- Webb, P.A. A review of rapid prototyping (RP) techniques in the medical and biomedical sector. J. Med. Eng. Technol. 2000, 24, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Jose, R.R.; Brown, J.E.; Polido, K.E.; Omenetto, F.G.; Kaplan, D.L. Polyol-Silk Bioink Formulations as Two-Part Room-Temperature Curable Materials for 3D Printing. ACS Biomater. Sci. Eng. 2015, 1, 780–788. [Google Scholar] [CrossRef]
- Sommer, M.R.; Schaffner, M.; Carnelli, D.; Studart, A.R. 3D Printing of Hierarchical Silk Fibroin Structures. ACS Appl. Mater. Interfaces 2016, 8, 34677–34685. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.J.; Brown, J.; Giordano, J.; Lin, S.J.; Omenetto, F.G.; Kaplan, D.L. Silk based bioinks for soft tissue reconstruction using 3-dimensional (3D) printing with in vitro and in vivo assessments. Biomaterials 2017, 117, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Yeon, Y.K.; Lee, J.M.; Chao, J.R.; Lee, Y.J.; Seo, Y.B.; Sultan, M.T.; Lee, O.J.; Lee, J.S.; Yoon, S.I.; et al. Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat. Commun. 2018, 9, 1620. [Google Scholar]
- Rockwood, D.N.; Preda, R.C.; Yucel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Zhang, Q.; Kaplan, D.L.; Omenetto, F.; Buehler, M.J.; Qin, Z. Printing of stretchable silk membranes for strain measurements. Lab Chip 2016, 16, 2459–2466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; You, X.; Dou, H.; Liu, Z.; Zuo, B.; Zhang, X. Facile fabrication of robust silk nanofibril films via direct dissolution of silk in CaCl2-formic acid solution. ACS Appl. Mater. Interfaces 2015, 7, 3352–3361. [Google Scholar] [CrossRef] [PubMed]
- Chameettachal, S.; Midha, S.; Ghosh, S. Regulation of Chondrogenesis and Hypertrophy in Silk Fibroin-Gelatin-Based 3D Bioprinted Constructs. ACS Biomater. Sci. Eng. 2016, 2, 1450–1463. [Google Scholar] [CrossRef]
- Yeo, M.; Lee, J.S.; Chun, W.; Kim, G.H. An Innovative Collagen-Based Cell-Printing Method for Obtaining Human Adipose Stem Cell-Laden Structures Consisting of Core-Sheath Structures for Tissue Engineering. Biomacromolecules 2016, 17, 1365–1375. [Google Scholar] [CrossRef]
- Diamantides, N.; Wang, L.; Pruiksma, T.; Siemiatkoski, J.; Dugopolski, C.; Shortkroff, S.; Kennedy, S.; Bonassar, L.J. Correlating rheological properties and printability of collagen bioinks: The effects of riboflavin photocrosslinking and pH. Biofabrication 2017, 9, 034102. [Google Scholar] [CrossRef] [PubMed]
- Midha, S.; Murab, S.; Ghosh, S. Osteogenic signaling on silk-based matrices. Biomaterials 2016, 97, 133–153. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ogiso, M.; Minoura, N. Enzymatic degradation behavior of porous silk fibroin sheets. Biomaterials 2003, 24, 357–365. [Google Scholar] [CrossRef]
- Mobini, S.; Hoyer, B.; Solati-Hashjin, M.; Lode, A.; Nosoudi, N.; Samadikuchaksaraei, A.; Gelinsky, M. Fabrication and characterization of regenerated silk scaffolds reinforced with natural silk fibers for bone tissue engineering. J. Biomed. Mater. Res. A 2013, 101, 2392–2404. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yan, S.; Qu, J.; Li, M.; Ye, D.; You, R.; Zhang, Q.; Wang, D. Soft freezing-induced self-assembly of silk fibroin for tunable gelation. Int. J. Biol. Macromol. 2018, 117, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Rossle, M.; Panine, P.; Urban, V.S.; Riekel, C. Structural evolution of regenerated silk fibroin under shear: Combined wide- and small-angle X-ray scattering experiments using synchrotron radiation. Biopolymers 2004, 74, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Im, D.S.; Kim, M.H.; Yoon, Y.I.; Park, W.H. Gelation Behaviors and Mechanism of Silk Fibroin According to the Addition of Nitrate Salts. Int. J. Mol. Sci. 2016, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Terry, A.E.; Knight, D.P.; Porter, D.; Vollrath, F. pH Induced Changes in the Rheology of Silk Fibroin Solution from the Middle Division of Bombyx mori Silkworm. Biomacromolecules 2004, 5, 768–772. [Google Scholar] [CrossRef]
- Das, S.; Pati, F.; Choi, Y.J.; Rijal, G.; Shim, J.H.; Kim, S.W.; Ray, A.R.; Cho, D.W.; Ghosh, S. Bioprintable, cell-laden silk fibroin-gelatin hydrogel supporting multilineage differentiation of stem cells for fabrication of three-dimensional tissue constructs. Acta Biomater. 2015, 11, 233–246. [Google Scholar] [CrossRef]
- Sun, L.; Parker, S.T.; Syoji, D.; Wang, X.; Lewis, J.A.; Kaplan, D.L. Direct-write assembly of 3D silk/hydroxyapatite scaffolds for bone co-cultures. Adv. Healthc. Mater. 2012, 1, 729–735. [Google Scholar] [CrossRef]
- Lee, H.; Yang, G.H.; Kim, M.; Lee, J.; Huh, J.; Kim, G. Fabrication of micro/nanoporous collagen/dECM/silk-fibroin biocomposite scaffolds using a low temperature 3D printing process for bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 84, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lin, Z.Y.; Wenger, A.C.; Tam, K.C.; Tang, X. 3D printing of liver-mimetic construct with alginate/cellulose nanocrystal hybrid bioink. Printing 2018, 9, 1–6. [Google Scholar]
- Wei, J.; Wang, J.; Su, S.; Wang, S.; Qiu, J.; Zhang, Z.; Christopher, G.; Ning, F.; Cong, W. 3D printing of an extremely tough hydrogel. RSC Adv. 2015, 5, 81324–81329. [Google Scholar] [CrossRef]
- Lee, J.; Yeo, M.; Kim, W.; Koo, Y.; Kim, G.H. Development of a tannic acid cross-linking process for obtaining 3D porous cell-laden collagen structure. Int. J. Biol. Macromol. 2018, 110, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Boland, T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials 2009, 30, 6221–6227. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martinez Avila, H.; Hagg, D.; Gatenholm, P. 3D Printing Human Chondrocytes with Nanocellulose-Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef]
- Markstedt, K.; Escalante, A.; Toriz, G.; Gatenholm, P. Biomimetic Inks Based on Cellulose Nanofibrils and Cross-Linkable Xylans for 3D Printing. ACS Appl. Mater. Interfaces 2017, 9, 40878–40886. [Google Scholar] [CrossRef]
- Ouyang, L.; Highley, C.B.; Rodell, C.B.; Sun, W.; Burdick, J.A. 3D Printing of Shear-Thinning Hyaluronic Acid Hydrogels with Secondary Cross-Linking. ACS Sustain. Chem. Eng. 2016, 2, 1743–1751. [Google Scholar] [CrossRef]
- Ting, H.; Chunquan, F.; Min, Z.; Yufang, Z.; Weizhong, Z.; Lei, L. 3D-printed scaffolds of biomineralized hydroxyapatite nanocomposite on silk fibroin for improving bone regeneration. Appl. Surf. Sci. 2018. [Google Scholar] [CrossRef]
- Wang, Q.; Xia, Q.; Wu, Y.; Zhang, X.; Wen, F.; Chen, X.; Zhang, S.; Heng, B.C.; He, Y.; Ouyang, H.W. 3D-Printed Atsttrin-Incorporated Alginate/Hydroxyapatite Scaffold Promotes Bone Defect Regeneration with TNF/TNFR Signaling Involvement. Adv. Healthc. Mater. 2015, 4, 1701–1708. [Google Scholar] [CrossRef]
- Kesti, M.; Muller, M.; Becher, J.; Schnabelrauch, M.; D’Este, M.; Eglin, D.; Zenobi-Wong, M. A versatile bioink for three-dimensional printing of cellular scaffolds based on thermally and photo-triggered tandem gelation. Acta Biomater. 2015, 11, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Gillispie, G.J.; Copus, J.S.; Pr, A.K.; Seol, Y.J.; Atala, A.; Yoo, J.J.; Lee, S.J. Optimization of gelatin-alginate composite bioink printability using rheological parameters: A systematic approach. Biofabrication 2018, 10, 034106. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.P.; Buehler, M.J. Nanomechanics of functional and pathological amyloid materials. Nat. Nanotechnol. 2011, 6, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Marelli, B.; Yang, M.; An, B.; Onses, M.S.; Rogers, J.A.; Kaplan, D.L.; Omenetto, F.G. Inkjet Printing of Regenerated Silk Fibroin: From Printable Forms to Printable Functions. Adv. Mater. 2015, 27, 4273–4279. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, T.; Chen, Y.; Bayat, A.; Yuan, X.F. Rheology and electrospinning of regenerated Bombyx mori silk fibroin aqueous solutions. Biomacromolecules 2014, 15, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zhang, Y.; Hang, Y.; Shao, H.; Hu, X.; Xu, Y.; Feng, C. Significantly reinforced composite fibers electrospun from silk fibroin/carbon nanotube aqueous solutions. Biomacromolecules 2012, 13, 2859–2867. [Google Scholar] [CrossRef]
- Laity, P.R.; Holland, C. Native Silk Feedstock as a Model Biopolymer: A Rheological Perspective. Biomacromolecules 2016, 17, 2662–2671. [Google Scholar] [CrossRef]
- Berman, B. 3-D printing: The new industrial revolution. Bus. Horiz. 2012, 55, 155–162. [Google Scholar] [CrossRef]
- Murphy, A.R.; Kaplan, D.L. Biomedical applications of chemically-modified silk fibroin. J. Mater. Chem. 2009, 19, 6443–6450. [Google Scholar] [CrossRef]
- Osman Rathore, D.Y.S. Nanostructure Formation through β-Sheet Self-Assembly in Silk-Based materials. Macromolecules 2001, 34, 1477–1486. [Google Scholar] [CrossRef]
- Murphy, A.R.; John, P.S.; Kaplan, D.L. Corrigendum to ‘Modification of silk fibroin using diazonium coupling chemistry and the effects on hMSC proliferation and differentiation’ [Biomaterials 29 (2008) 2829–2838]. Biomaterials 2008, 29, 4260. [Google Scholar] [CrossRef]
- Tamada, Y. Sulfation of silk fibroin by chlorosulfonic acid and the anticoagulant activity. Biomaterials 2004, 25, 377–383. [Google Scholar] [CrossRef]
- Freddi, G.; Anghileri, A.; Sampaio, S.; Buchert, J.; Monti, P.; Taddei, P. Tyrosinase-catalyzed modification of Bombyx mori silk fibroin: Grafting of chitosan under heterogeneous reaction conditions. J. Biotechnol. 2006, 125, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.B.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Fast Setting Silk Fibroin Bioink for Printing of Patient-Specific Memory-Shape Implants. Adv. Healthc. Mater. 2017, 6, 1701021. [Google Scholar] [CrossRef] [PubMed]
- Chimene, D.; Lennox, K.K.; Kaunas, R.R.; Gaharwar, A.K. Advanced Bioinks for 3D Printing: A Materials Science Perspective. Ann. Biomed. Eng. 2016, 44, 2090–2102. [Google Scholar] [CrossRef]
- Compaan, A.M.; Christensen, K.; Huang, Y. Inkjet Printing of 3D Silk Fibroin Cellular Constructs Using Sacrificial Alginate. ACS Biomater. Sci. Eng. 2016, 3, 1519–1526. [Google Scholar] [CrossRef]
- Xiong, S.; Zhang, X.; Lu, P.; Wu, Y.; Wang, Q.; Sun, H.; Heng, B.C.; Bunpetch, V.; Zhang, S.; Ouyang, H. A Gelatin-sulfonated Silk Composite Scaffold based on 3D Printing Technology Enhances Skin Regeneration by Stimulating Epidermal Growth and Dermal Neovascularization. Sci. Rep. 2017, 7, 4288. [Google Scholar] [CrossRef]
- Meinel, L.; Kaplan, D.L. Silk constructs for delivery of musculoskeletal therapeutics. Adv. Drug Deliv. Rev. 2012, 64, 1111–1122. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Bose, S.; Das, S. 3D printing of biomaterials. MRS Bull. 2015, 40, 108–115. [Google Scholar] [CrossRef]
- Shim, J.H.; Kim, J.Y.; Park, M.; Park, J.; Cho, D.W. Development of a hybrid scaffold with synthetic biomaterials and hydrogel using solid freeform fabrication technology. Biofabrication 2011, 3, 034102. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Sycks, D.; Chan, H.F.; Lin, S.; Lopez, G.P.; Guilak, F.; Leong, K.W.; Zhao, X. 3D Printing of Highly Stretchable and Tough Hydrogels into Complex, Cellularized Structures. Adv. Mater. 2015, 27, 4035–4040. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, S.; Kundu, S.C. Silk protein-based hydrogels: Promising advanced materials for biomedical applications. Acta Biomater. 2016, 31, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.H.; Yodmuang, S.; Wang, X.; Sun, L.; Kaplan, D.L.; Vunjak-Novakovic, G. Silk hydrogel for cartilage tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 95, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Sun, H.; Tang, P.; Li, P.; Xie, C.; Wang, M.; Wang, K.; Weng, J.; Tan, H.; Ren, F.; et al. Mussel-inspired graphene oxide nanosheet-enwrapped Ti scaffolds with drug-encapsulated gelatin microspheres for bone regeneration. Biomater. Sci. 2018, 6, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Sun, M.; Hu, X.; Ren, B.; Cheng, J.; Li, C.; Duan, X.; Fu, X.; Zhang, J.; Chen, H.; et al. Structurally and Functionally Optimized Silk-Fibroin-Gelatin Scaffold Using 3D Printing to Repair Cartilage Injury In Vitro and In Vivo. Adv. Mater. 2017, 29, 1701089. [Google Scholar] [CrossRef] [PubMed]
- Hong, N.; Yang, G.H.; Lee, J.; Kim, G. 3D printing and its in vivo applications. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 444–459. [Google Scholar] [CrossRef]
- Highley, C.B.; Rodell, C.B.; Burdick, J.A. Direct 3D Printing of Shear-Thinning Hydrogels into Self-Healing Hydrogels. Adv. Mater. 2015, 27, 5075–5079. [Google Scholar] [CrossRef]
- Li, Z.; Jia, S.; Xiong, Z.; Long, Q.; Yan, S.; Hao, F.; Liu, J.; Yuan, Z. 3D-printed scaffolds with calcified layer for osteochondral tissue engineering. J. Biosci. Bioeng. 2018, 126, 389–396. [Google Scholar] [CrossRef]
- Cui, X.; Boland, T.; D’Lima, D.; Lotz, M. Thermal Inkjet Printing in Tissue Engineering and Regenerative Medicine. Recent Pat. Drug Deliv. Formul. 2012, 6, 149–155. [Google Scholar] [CrossRef]
- Malda, J.; Visser, J.; Melchels, F.P.; Jungst, T.; Hennink, W.E.; Dhert, W.J.; Groll, J.; Hutmacher, D.W. 25th anniversary article: Engineering hydrogels for biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef]
- Rujiravanit, R.; Kruaykitanon, S.; Jamieson, A.M.; Tokura, S. Preparation of Crosslinked Chitosan/Silk Fibroin Blend Films for Drug Delivery System. Macromol. Biosci. 2003, 3, 604–611. [Google Scholar] [CrossRef]
- Chrisey, D.B.; Pique, A.; Fitz-Gerald, J.; Auyeung, R.C.Y.; McGill, R.A.; Wu, H.D.; Duignan, M. New approach to laser direct writing active and passive. Appl. Surf. Sci. 1999, 154, 593–600. [Google Scholar]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D printing of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. A 2013, 101, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D printing of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Wu, J.; Liu, M.; Wang, H.; Li, C.; Rodriguez, M.J.; Li, G.; Wang, X.; Kaplan, D.L. 3D Printing of Self-Standing Silk-Based Bioink. Adv. Healthc. Mater. 2018, 7, e1701026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Allardyce, B.J.; Rajkhowa, R.; Zhao, Y.; Dilley, R.J.; Redmond, S.L.; Wang, X.; Liu, X. 3D Printing of Silk Particle-Reinforced Chitosan Hydrogel Structures and Their Properties. ACS Biomater. Sci. Eng. 2018, 4, 3036–3046. [Google Scholar] [CrossRef]
- Rider, P.; Zhang, Y.; Tse, C.; Zhang, Y.; Jayawardane, D.; Stringer, J.; Callaghan, J.; Brook, I.M.; Miller, C.A.; Zhao, X.; et al. Biocompatible silk fibroin scaffold prepared by reactive inkjet printing. J. Mater. Sci. 2016, 51, 8625–8630. [Google Scholar] [CrossRef]
- Catros, S.; Guillemot, F.; Nandakumar, A.; Ziane, S.; Moroni, L.; Habibovic, P.; van Blitterswijk, C.; Rousseau, B.; Chassande, O.; Amedee, J.; et al. Layer-by-layer tissue microfabrication supports cell proliferation in vitro and in vivo. Tissue Eng. Part C Methods 2012, 18, 62–70. [Google Scholar] [CrossRef]
- Pati, F.; Ha, D.H.; Jang, J.; Han, H.H.; Rhie, J.W.; Cho, D.W. Biomimetic 3D tissue printing for soft tissue regeneration. Biomaterials 2015, 62, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, J.A.; Honeywell-Nguyen, P.L. Skin structure and mode of action of vesicles. Adv. Drug Deliv. Rev. 2002, 54, S41–S45. [Google Scholar] [CrossRef]
- Khalili, S.; Khorasani, S.N.; Razavi, S.M.; Hashemibeni, B.; Tamayol, A. Nanofibrous Scaffolds with Biomimetic Composition for Skin Regeneration. Appl. Biochem. Biotechnol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Yeo, I.S.; Oh, J.E.; Jeong, L.; Lee, T.S.; Lee, S.J.; Park, W.H.; Min, B.M. Collagen-Based Biomimetic Nanofibrous Scaffolds—Preparation andcharacterization of collagen&silk fibroin bicomponent nanofibrous structures. Biomacromolecules 2008, 9, 1106–1116. [Google Scholar] [PubMed]
- Loeser, R.F. Age-related changes in the musculoskeletal system and the development of osteoarthritis. Clin. Geriatr. Med. 2010, 26, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Do, A.V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef]
- Dababneh, A.B.; Ozbolat, I.T. Printing Technology: A Current State-of-the-Art Review. J. Manuf. Sci. Eng. 2014, 136, 061016. [Google Scholar] [CrossRef]
- Chawla, S.; Midha, S.; Sharma, A.; Ghosh, S. Silk-Based Bioinks for 3D Printing. Adv. Healthc. Mater. 2018, 7, e1701204. [Google Scholar] [CrossRef]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef]
- Stevens, M.M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Alehosseini, M.; Golafshan, N.; Kharaziha, M. Design and characterization of poly-ε-caprolactone electrospun fibers incorporated with α-TCP nanopowder as a potential guided bone regeneration membrane. Mater. Today Proc. 2018, 5, 15783–15789. [Google Scholar] [CrossRef]
- Shkarina, S.; Shkarin, R.; Weinhardt, V.; Melnik, E.; Vacun, G.; Kluger, P.; Loza, K.; Epple, M.; Ivlev, S.I.; Baumbach, T.; et al. 3D biodegradable scaffolds of polycaprolactone with silicate-containing hydroxyapatite microparticles for bone tissue engineering: High-resolution tomography and in vitro study. Sci. Rep. 2018, 8, 8907. [Google Scholar] [CrossRef]
- Gao, G.; Lee, J.H.; Jang, J.; Lee, D.H.; Kong, J.-S.; Kim, B.S.; Choi, Y.-J.; Jang, W.B.; Hong, Y.J.; Kwon, S.-M.; et al. Tissue Engineered Bio-Blood-Vessels Constructed Using a Tissue-Specific Bioink and 3D Coaxial Cell Printing Technique: A Novel Therapy for Ischemic Disease. Adv. Funct. Mater. 2017, 27, 1700798. [Google Scholar] [CrossRef]
- Adler-Abramovich, L.; Arnon, Z.A.; Sui, X.; Azuri, I.; Cohen, H.; Hod, O.; Kronik, L.; Shimon, L.J.W.; Wagner, H.D.; Gazit, E. Bioinspired Flexible and Tough Layered Peptide Crystals. Adv. Mater. 2018, 30, 1704551. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj, R.; Natasha, G.; Kirby, G.; Rajadas, J.; Mosahebi, A.; Seifalian, A.M.; Tan, A. Vascularisation in regenerative therapeutics and surgery. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 54, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Kwak, H.; Hyun, J. Melanin Nanoparticle-Incorporated Silk Fibroin Hydrogels for the Enhancement of Printing Resolution in 3D-Projection Stereolithography of Poly(ethylene glycol)-Tetraacrylate Bio-ink. ACS Appl. Mater. Interfaces 2018, 10, 23573–23582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Liu, W.; Cui, L.; Cao, Y. Tissue engineering of blood vessel. J. Cell Mol. Med. 2007, 11, 945–957. [Google Scholar] [CrossRef] [PubMed]
- Unger, R.E.; Ghanaati, S.; Orth, C.; Sartoris, A.; Barbeck, M.; Halstenberg, S.; Motta, A.; Migliaresi, C.; Kirkpatrick, C.J. The rapid anastomosis between prevascularized networks on silk fibroin scaffolds generated in vitro with cocultures of human microvascular endothelial and osteoblast cells and the host vasculature. Biomaterials 2010, 31, 6959–6967. [Google Scholar] [CrossRef]





| Materials | Advantages | Disadvantages | Crosslinking Methods |
|---|---|---|---|
| Silkworm silk |
| ||
| Alginate |
|
|
|
| Agarose |
|
|
|
| Collagen |
|
|
|
| Fibrin |
|
|
|
| Cellulose |
|
| |
| Hyaluronic acid |
|
|
|
| Hydroxyapatite |
|
|
|
| Bioink Formulation | Crosslink Method(gelation) | Cell Types & Density & Viability | Advantages (A) and Disadvantages (D) | Applications | Printing Method | Ref. |
|---|---|---|---|---|---|---|
| SF-Gelatin | Enzymatic/sonication | hTMSCs; BMSC 2.5 × 106 mL−1; 2 × 105 86% (30 days); enriched (21 days); | A: Enhances cell adhesion Good mechanical | Artificial Implant/Cartilage tissue engineering | Inject printing | [50,79,87] |
| SF-Collagen | Ethanol | BMSCs 2 × 107 cells 4 × 102 cell (13 days); | A: Comprehensive physical properties; support cell growth | Knee cartilage; Tissue engineering | Extrude printing | [88] |
| SF-Chitosan | hexamethylene diisocyanate/chlorohydrin/glutaraldehyde | BMSCs 2 × 107 mL−1 102 cells; | A: Produce high porosity with different structures; D: the cross-linking agent have cytotoxic | Tissue engineering Drug release | Extrude printing | [88] |
| Cartilage acellular matrix (CAM)-SF | Enzyme (EDC-NHS) | rBM-MSCs Seeding efficiency 65% >80%; | D: Poor shape fidelity; low precision of printing | Cartilage tissue engineering | Extrude printing | [89] |
| SF-Alginate | Horseradish peroxidase (HRP)-H2O2 | NIH3T3 5 × 105 mL−1 begin to decline slowly (42 days); | A: maintain long-term metabolic activity for bioink D: the compatibility of silk and alginate need to be improved. | Vascular tissue engineering | Inject printing | [78] |
| SF/polyethylene glycol (PEG) | Sonication | hMSCs 2.5 × 106 mL−1 50% (3 weeks); | A: maintain shape for a long time (6weeks); the crosslinker without damage cell viability; with a good mechanical and high shape fidelity | Cartilage tissue engineering | Inject printing | [90] |
| SF-glycidyl methacrylate | Photo-crosslink | NIH/3T3 1 × 106 mL−1 50% (4 weeks) | A: a gentle crosslink environment and friendly to cells growth; the mechanical properties improved with Sil-MA concentration increased. | Bone tissue engineering | Digital light printing | [36] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Han, G.; Yan, S.; Zhang, Q. 3D Printing of Silk Fibroin for Biomedical Applications. Materials 2019, 12, 504. https://doi.org/10.3390/ma12030504
Wang Q, Han G, Yan S, Zhang Q. 3D Printing of Silk Fibroin for Biomedical Applications. Materials. 2019; 12(3):504. https://doi.org/10.3390/ma12030504
Chicago/Turabian StyleWang, Qiusheng, Guocong Han, Shuqin Yan, and Qiang Zhang. 2019. "3D Printing of Silk Fibroin for Biomedical Applications" Materials 12, no. 3: 504. https://doi.org/10.3390/ma12030504
APA StyleWang, Q., Han, G., Yan, S., & Zhang, Q. (2019). 3D Printing of Silk Fibroin for Biomedical Applications. Materials, 12(3), 504. https://doi.org/10.3390/ma12030504





