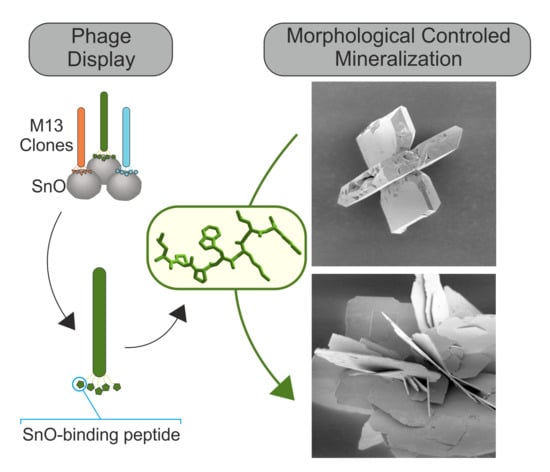
Peptide Controlled Shaping of Biomineralized Tin(II) Oxide into Flower-Like Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phage Display
2.2. Binding Strength Assay
2.3. Mineralization of Tin(II) Oxide Microstructures
2.4. Structural Analysis
2.5. Fourier-Transform Infrared Spectroscopy
3. Results and Discussion
3.1. Phage Display
3.2. Binding Assay
3.3. Mineralization of tin(II) oxide microstructures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ogo, Y.; Hiramatsu, H.; Nomura, K.; Yanagi, H.; Kamiya, T.; Kimura, M.; Hirano, M.; Hosono, H. Tin monoxide as an s-orbital-based p-type oxide semiconductor: Electronic structures and tft application. Phys. Status Solidi (a) 2009, 206, 2187–2191. [Google Scholar] [CrossRef]
- Aurbach, D.; Nimberger, A.; Markovsky, B.; Levi, E.; Sominski, E.; Gedanken, A. Nanoparticles of SnO produced by sonochemistry as anode materials for rechargeable lithium batteries. Chem. Mater. 2002, 14, 4155–4163. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Lemos Cardoso, A. Heterogeneous tin catalysts applied to the esterification and transesterification reactions. J. Catal. 2013, 2013, 11. [Google Scholar] [CrossRef]
- Han, Z.; Guo, N.; Li, F.; Zhang, W.; Zhao, H.; Qian, Y. Solvothermal preparation and morphological evolution of stannous oxide powders. Mater. Lett. 2001, 48, 99–103. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, J.; Li, J.; Cao, L.; Qi, H. Hydrothermal synthesis of shape-controlled SnO as anode material for li-ion batteries. IET Micro Nano Lett. 2018, 13, 257–260. [Google Scholar] [CrossRef]
- Liang, Y.; Zheng, H.; Fang, B. Synthesis and characterization of sno with controlled flowerlike microstructures. Mater. Lett. 2013, 108, 235–238. [Google Scholar] [CrossRef]
- Sakaushi, K.; Oaki, Y.; Uchiyama, H.; Hosono, E.; Zhou, H.; Imai, H. Aqueous solution synthesis of SnO nanostructures with tuned optical absorption behavior and photoelectrochemical properties through morphological evolution. Nanoscale 2010, 2, 2424–2430. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Qi, F.; Li, Y.; Wu, N.; Cao, J.; Zhang, S.; Wang, X.; Yi, G.; Bala, H.; Zhang, Z. Solvothermal synthesis and characterization of ultrathin sno nanosheets. Mater. Lett. 2014, 118, 69–71. [Google Scholar] [CrossRef]
- Xu, X.; Ge, M.; Ståhl, K.; Jiang, J.Z. Growth mechanism of cross-like SnO structure synthesized by thermal decomposition. Chem. Phys. Lett. 2009, 482, 287–290. [Google Scholar] [CrossRef]
- Kumar, B.; Lee, D.-H.; Kim, S.-H.; Yang, B.; Maeng, S.; Kim, S.-W. General route to single-crystalline SnO nanosheets on arbitrary substrates. J. Phys. Chem. C 2010, 114, 11050–11055. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, F.; Iqbal, M.Z.; Li, Y.; Toufiq, A.M.; Wang, Z.; Wang, Z.; Ali, S. Synthesis of nanoflakes-based self-assembling crossed structure of stannous oxide and photocatalysis property. Cryst. Res. Technol. 2015, 50, 210–214. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Wang, F.; Hussain, R.; Rafique, M.Y.; Ali, S.; Ali, I. Time-dependent growth and optical properties of stannous oxide architectures. Mater. Focus 2014, 3, 92–97. [Google Scholar] [CrossRef]
- Weiner, S.; Dove, P.M. An overview of biomineralization processes and the problem of the vital effect. Rev. Mineral. Geochem. 2003, 54, 1–29. [Google Scholar] [CrossRef]
- Rothenstein, D.; Facey, S.J.; Ploss, M.; Hans, P.; Melcher, M.; Srot, V.; van Aken, P.A.; Hauer, B.; Bill, J. Mineralization of gold nanoparticles using tailored m13 phages. Bioinspired Biomim. Nanobiomater. 2013, 2, 173–185. [Google Scholar] [CrossRef]
- Rothenstein, D.; Claasen, B.; Omiecienski, B.; Lammel, P.; Bill, J. Isolation of ZnO-binding 12-mer peptides and determination of their binding epitopes by nmr spectroscopy. J. Am. Chem. Soc. 2012, 134, 12547–12556. [Google Scholar] [CrossRef]
- Rothenstein, D.; Shopova-Gospodinova, D.; Bakradze, G.; Jeurgens, L.P.H.; Bill, J. Generation of luminescence in biomineralized zirconia by zirconia-binding peptides. Crystengcomm 2015, 17, 1783–1790. [Google Scholar] [CrossRef]
- Thota, V.; Perry, C.C. A review on recent patents and applications of inorganic material binding peptides. Recent Pat. Nanotechnol. 2017, 11, 168–180. [Google Scholar] [CrossRef]
- Naik, R.; Stone, M.; Carter, D. Peptide templates for nanoparticle synthesis obtained through PCR-driven phage display method. Google Patents US20060172282A1, 3 August 2006. [Google Scholar]
- Nakazawa, H.; Seta, Y.; Hirose, T.; Masuda, Y.; Umetsu, M. Use of a phage-display method to identify peptides that bind to a tin oxide nanosheets. Protein Pept. Lett. 2018, 25, 68–75. [Google Scholar] [CrossRef]
- NEB. Ph.D.™ phage display libraries manuale8100. Available online: www.neb-online.de (accessed on 23 October 2018).
- Togashi, T.; Yokoo, N.; Umetsu, M.; Ohara, S.; Naka, T.; Takami, S.; Abe, H.; Kumagai, I.; Adschiri, T. Material-binding peptide application—ZnO crystal structure control by means of a ZnO-binding peptide. J. Biosci. Bioeng. 2011, 111, 140–145. [Google Scholar] [CrossRef]
- Kosmulski, M. Surface Charging and Points of Zero Charge; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Lemloh, M.L.; Altintoprak, K.; Wege, C.; Weiss, I.M.; Rothenstein, D. Biogenic and synthetic peptides with oppositely charged amino acids as binding sites for mineralization. Materials 2017, 10, 119. [Google Scholar] [CrossRef]
- Pires, F.I.; Joanni, E.; Savu, R.; Zaghete, M.A.; Longo, E.; Varela, J.A. Microwave-assisted hydrothermal synthesis of nanocrystalline SnO powders. Mater. Lett. 2008, 62, 239–242. [Google Scholar] [CrossRef]
- Uchiyama, H.; Nakanishi, S.; Kozuka, H. Biomimetic synthesis of nanostructured SnO particles from Sn6O4(OH)4 in aqueous solution of gelatin. Crystengcomm 2015, 17, 628–632. [Google Scholar] [CrossRef]
- Izumi, F. Pattern-fitting structure refinement of tin(ii) oxide. J. Solid State Chem. 1981, 38, 381–385. [Google Scholar] [CrossRef]
- Wolfenden, R.; Andersson, L.; Cullis, P.M.; Southgate, C.C.B. Affinities of amino acid side chains for solvent water. Biochemistry 1981, 20, 849–855. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Xu, J.; Yang, H.; Lee, C.-S.; Rogach, A.L. Polyvinylpyrrolidone-assisted ultrasonic synthesis of SnO nanosheets and their use as conformal templates for tin dioxide nanostructures. Langmuir 2012, 28, 10597–10601. [Google Scholar] [CrossRef]
- Mudunkotuwa, I.A.; Minshid, A.A.; Grassian, V.H. Atr-ftir spectroscopy as a tool to probe surface adsorption on nanoparticles at the liquid–solid interface in environmentally and biologically relevant media. Analyst 2014, 139, 870–881. [Google Scholar] [CrossRef]
- Vreuls, C.; Genin, A.; Zocchi, G.; Boschini, F.; Cloots, R.; Gilbert, B.; Martial, J.; Van de Weerdt, C. Genetically engineered polypeptides as a new tool for inorganic nano-particles separation in water based media. J. Mater. Chem. 2011, 21, 13841–13846. [Google Scholar] [CrossRef]
- Williams, J.; Haq, S.; Raval, R. The bonding and orientation of the amino acid l-alanine on Cu{110} determined by rairs. Surf. Sci. 1996, 368, 303–309. [Google Scholar] [CrossRef]
- MacArthur, M.W.; Thornton, J.M. Influence of proline residues on protein conformation. J. Mol. Biol. 1991, 218, 397–412. [Google Scholar] [CrossRef]



| Peptide | Sequence | Frequency Fourth Round | Frequency Fifth Round | Isoelectric Point (pI) a | Charge, pH 7.5 (a.u.) b |
|---|---|---|---|---|---|
| 01 | LPPWKLK | 11/27 | 17/23 | 10.00 | 1.8 |
| 02 | WSLSELH | 4/27 | 2/23 | 5.5 | -1.1 |
| 03 | IGASVHR | 1/27 | 0/23 | 10.00 | 0.9 |
| 04 | AHHLKVS | 1/27 | 0/23 | 9.06 | 0.9 |
| 05 | NHPLYNR | 1/27 | 0/23 | 9.06 | 0.8 |
| 06 | ALEHTSR | 1/27 | 0/23 | 7.19 | -0.1 |
| 07 | HPAIRPP | 1/27 | 0/23 | 10.00 | 0.9 |
| 08 | LHRHANL | 2/27 | 1/23 | 10.00 | 0.9 |
| 09 | SSNQFHQ | 1/27 | 1/23 | 7.19 | -0.1 |
| 10 | KVPGHQQ | 1/27 | 0/23 | 9.06 | 0.8 |
| 11 | TLAPRTA | 1/27 | 0/23 | 10.00 | 0.8 |
| 12 | VGKTHAD | 0/27 | 1/23 | 7.19 | -0.2 |
| 13 | FPLHELR | 0/27 | 1/23 | 7.19 | -0.1 |
| Amino Acid | Multiplication Factor * | Type | Frequency |
|---|---|---|---|
| H | 3.11 | basic | enriched |
| L | 1.60 | nonpolar | |
| R | 1.54 | basic | |
| K | 1.53 | basic | |
| A | 1.20 | nonpolar | Not affected |
| Q | 1.20 | polar | |
| W | 1.15 | aromatic | |
| P | 1.11 | nonpolar | |
| N | 1.01 | polar | |
| F | 0.93 | aromatic | |
| S | 0.91 | polar | |
| I | 0.82 | nonpolar | |
| E | 0.74 | acidic | depleted |
| V | 0.74 | nonpolar | |
| G | 0.72 | nonpolar | |
| T | 0.38 | polar | |
| Y | 0.20 | aromatic | |
| D | 0.19 | acidic | |
| C | 0 | polar | |
| M | 0 | polar |
| Sample | Thickness (µm) * | Length (µm) * | Aspect Ratio (Length/Thickness) |
|---|---|---|---|
| Reference | 11.67 ± 0.55 | 61.10 ± 12.00 | 5.2 |
| WKLK | 5.13 ± 0.36 | 128.10 ±17.53 | 25.0 |
| LPPW | 0.93 ± 0.16 | 171.16 ± 22.80 | 183.5 |
| LPPWKLK | 0.51 ± 0.13 | 184.83 ± 22.95 ** | 364.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kilper, S.; Jahnke, T.; Wiegers, K.; Grohe, V.; Burghard, Z.; Bill, J.; Rothenstein, D. Peptide Controlled Shaping of Biomineralized Tin(II) Oxide into Flower-Like Particles. Materials 2019, 12, 904. https://doi.org/10.3390/ma12060904
Kilper S, Jahnke T, Wiegers K, Grohe V, Burghard Z, Bill J, Rothenstein D. Peptide Controlled Shaping of Biomineralized Tin(II) Oxide into Flower-Like Particles. Materials. 2019; 12(6):904. https://doi.org/10.3390/ma12060904
Chicago/Turabian StyleKilper, Stefan, Timotheus Jahnke, Katharina Wiegers, Vera Grohe, Zaklina Burghard, Joachim Bill, and Dirk Rothenstein. 2019. "Peptide Controlled Shaping of Biomineralized Tin(II) Oxide into Flower-Like Particles" Materials 12, no. 6: 904. https://doi.org/10.3390/ma12060904
APA StyleKilper, S., Jahnke, T., Wiegers, K., Grohe, V., Burghard, Z., Bill, J., & Rothenstein, D. (2019). Peptide Controlled Shaping of Biomineralized Tin(II) Oxide into Flower-Like Particles. Materials, 12(6), 904. https://doi.org/10.3390/ma12060904