Microstructure-Based Relative Humidity in Cementitious System Due to Self-Desiccation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Sample Preparation
2.3. Measurement
2.3.1. Measurement of Relative Humidity
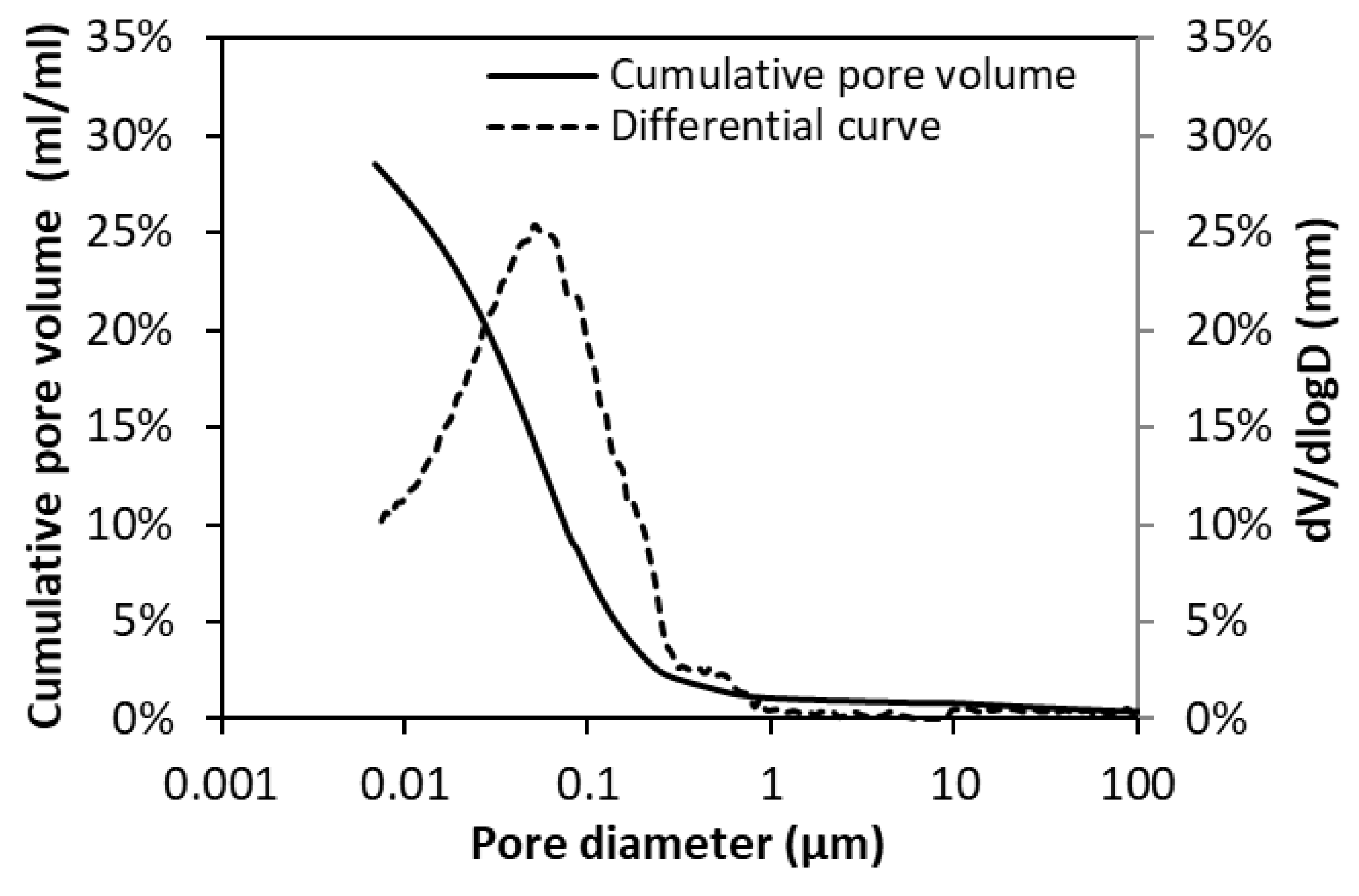
2.3.2. Measurement of Pore Structure
2.3.3. Thermogravimetric Analysis
2.3.4. X-ray Diffraction
3. Results and Discussion
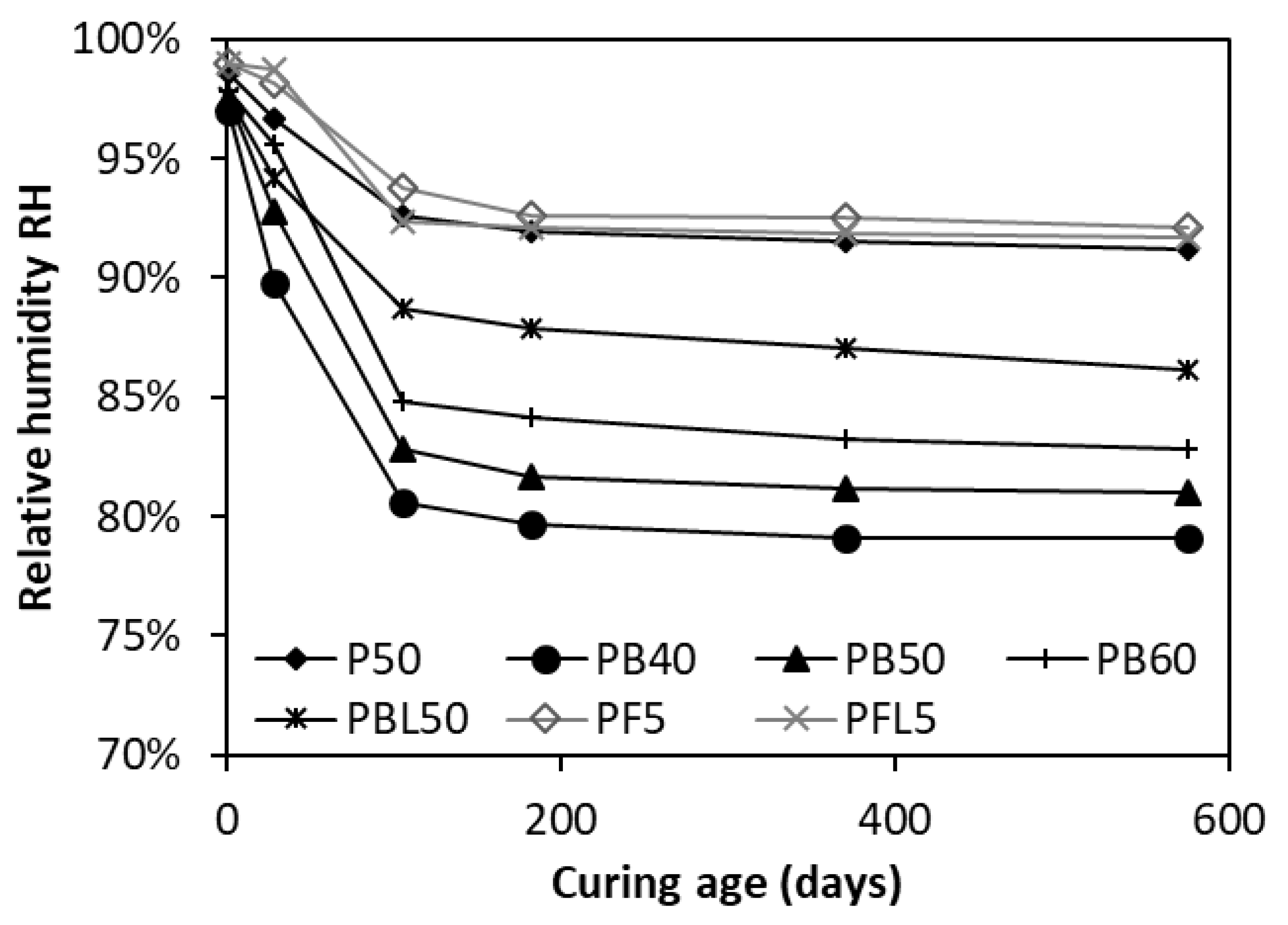
3.1. Relative Humidity RH of Cementitious Pastes
3.1.1. RH in OPC Cement Paste
3.1.2. Effect of SCMs on RH
3.2. Total Porosity and RH
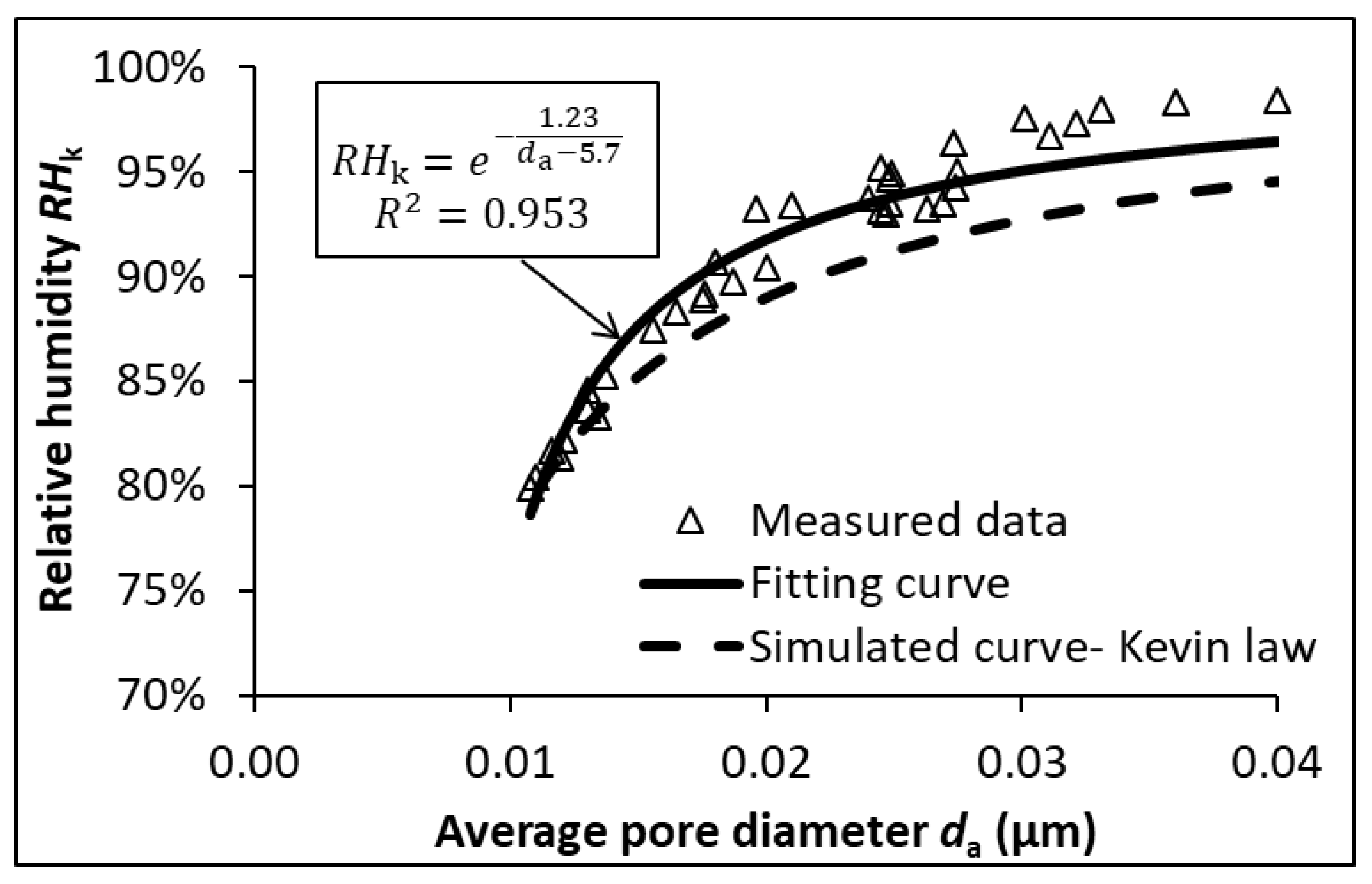
3.3. Average Pore Diameter and RH
3.4. Non-Evaporable Water/Evaporable Water and RH
4. Conclusions
- ■
- In the case of self-desiccation, the RH declines with age. A significant decrease occurs in the first 105 days, followed by a gradual decrease.
- ■
- The inclusion of slag decreases the RH, while FA or LP addition increases the RH. In the FA-OPC system, further addition of 5 wt.% LP slightly decreases the RH. Whereas in the slag-OPC system, further addition of LP 5 wt.% greatly increases the RH. A combination of alumina-rich FA and LP shows a synergistic effect, which results in the formation of carboaluminate and stabilization of water-rich bulky ettringite.
- ■
- The pastes blended with FA or slag exhibit lower RH than the OPC pastes of a given total porosity.
- ■
- There is an inherent relationship between RH and the average pore diameter in cementitious systems, regardless of cement type, w/b ratio or age.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Snyder, K.A.; Bentz, D.P. Suspended hydration and loss of freezable water in cement pastes exposed to 90% relative humidity. Cem. Concr. Res. 2004, 34, 2045–2056. [Google Scholar] [CrossRef]
- Flatt, R.J.; Scherer, G.W.; Bullard, J.W. Why alite stops hydrating below 80% relative humidity. Cem. Concr. Res. 2011, 41, 987–992. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.Z. Transport properties in unsaturated cement-based materials—A review. Constr. Build. Mater. 2014, 72, 367–379. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, G. A model for predicting the relative chloride diffusion coefficient in unsaturated cementitious materials. Cem. Concr. Res. 2019, 115, 133–144. [Google Scholar] [CrossRef]
- Huang, H.L.; Ye, G.; Denis, D. Effect of blast furnace slag on self-healing of microcracks in cementitious materials. Cem. Concr. Res. 2014, 60, 66–82. [Google Scholar] [CrossRef]
- Ma, Y.; Ye, G. The shrinkage of alkali activated fly ash. Cem. Concr. Res. 2015, 68, 75–82. [Google Scholar] [CrossRef]
- Parrott, L.J.; Hong, C.Z. Some factors influencing air permeation measurements in cover concrete. Mater. Struct. 1991, 24, 403–408. [Google Scholar] [CrossRef]
- Lura, P.; Jensen, O.M.; van Breugel, K. Autogenous shrinkage in high-performance cement paste: An evaluation of basic mechanisms. Cem. Concr. Res. 2003, 33, 223–232. [Google Scholar] [CrossRef]
- Köhler, H. The nucleus in and the growth of hygroscopic droplets. Trans. Farad. Soc. 1936, 32, 1152–1161. [Google Scholar] [CrossRef]
- Jensen, O.M. Autogenous phenomena in cement-based materials (in Danish). Ph.D. Thesis, The Technical University of Denmark, Lyngby, Denmark, December 2015. [Google Scholar]
- Neimark, A.V.; Ravikovitch, P.I.; Vishnyakov, A. Bridging scales from molecular simulations to classical thermodynamics: density functional theory of capillary condensation in nanopores. J. Phys. Condens. Matter. 2003, 15, 347–365. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.Z.; Ye, G. Influence of moisture condition on chloride diffusion in partially saturated ordinary Portland cement mortar. Mater. Struct. 2018, 51, 36. [Google Scholar] [CrossRef] [Green Version]
- Willis, K.L.; Abell, A.B.; Lange, D.A. Image-based characterization of cement pore structure using Wood’s metal intrusion. Cem. Concr. Res. 1998, 28, 1695–1706. [Google Scholar] [CrossRef]
- Zhang, Y. Non-saturated Chloride Diffusion in Sustainable Cementitious Materials. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, November 2018. [Google Scholar]
- Marsh, B.K.; Day, R.L. Pozzolanic and cementitious reactions of fly ash in blended cement pastes. Cem. Concr. Res. 1988, 18, 301–310. [Google Scholar] [CrossRef]
- Lothenbach, B.; Le Saout, G.; Gallucci, E.; Scrivener, K. Influence of limestone on the hydration of Portland cements. Cem. Concr. Res. 2008, 38, 848–860. [Google Scholar] [CrossRef]
- Baquerizo, L. Impact of water activity on the mineralogy of hydrated cement. Ph.D. Thesis, Swiss Federal Institute of Technology, Lausanne, Switzerland, January 2015. [Google Scholar]
- Persson, B. Self-desiccation and its importance in concrete technology. Mater. Struct. 1997, 30, 293. [Google Scholar] [CrossRef]
- Robins, P.J.; Austin, S.A.; Issaad, A. Suitability of GGBFS as a cement replacement for concrete in hot arid climates. Mater. Struct. 1992, 25, 598–612. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, G. Effect of Limestone Powder on Microstructure of Ternary Cementitious System. Proceedings of SusCom 2012: 2nd International Conference on Sustainable Construction Materials: Design, Performance and Application, Wuhan, China, 18–22 October 2012. [Google Scholar]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. The role of calcium carbonate in cement hydration. Cem. Concr. Res. 2007, 37, 551–558. [Google Scholar] [CrossRef]
- Kuzel, H.J.; Pollmann, H. Hydration of C3A in the presence of Ca(OH)2, CaSO4·2H2O and CaCO3. Cem. Concr. Res. 1991, 21, 885–895. [Google Scholar] [CrossRef]
- Pöllmann, H. Characterization of Different Water Contents of Ettringite and Kuzelite. Proceedings of 12th International Congress on the Chemistry of Cement, Montreal, QC, Canada, 8–13 July 2007. [Google Scholar]
- Renaudin, G.; Francois, M.; Evrard, O. Order and disorder in the lamellar hydrated tetracalcium monocarboaluminate compound. Cem. Concr. Res. 1999, 29, 63–69. [Google Scholar] [CrossRef]
- Desgranges, L.; Grebille, D.; Calvarin, G.; Chevrier, G.; Floquet, N.; Niepce, J.C. Hydrogen thermal motion in calcium hydroxide: Ca(OH)2. Acta Crystallographica. 1993, 49, 812–817. [Google Scholar] [CrossRef]
- Yu, Z.Q.; Ye, G. The pore structure of cement paste blended with fly ash. Constr. Build. Mater. 2013, 45, 30–35. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Jaturapitakkul, C.; Sinsiri, T. Effect of fly ash fineness on compressive strength and pore size of blended cement paste. Cem. Concr. Compos. 2005, 27, 425–428. [Google Scholar] [CrossRef]
- De Weerdt, K.; Kjellsen, K.O.; Sellevold, E.J.; Justnes, H. Synergistic effect between fly ash and limestone powder in ternary cements. Cem. Concr. Compos. 2011, 33, 30–38. [Google Scholar] [CrossRef]
- Snyder, K.A.; Feng, X.; Keen, B.D.; Mason, T.O. Estimating the electrical conductivity of cement paste pore solutions from OH−, K+ and Na+ concentrations. Cem. Concr. Res. 2003, 33, 793–798. [Google Scholar] [CrossRef]









| Items | Raw Materials | |||
|---|---|---|---|---|
| OPC | FA | BFS | LP | |
| CaO | 64.495 | 5.537 | 41.398 | - |
| SiO2 | 18.875 | 50.554 | 34.015 | 0.737 |
| Al2O3 | 4.481 | 30.743 | 11.117 | 0.180 |
| Fe2O3 | 3.686 | 6.301 | 0.529 | 0.073 |
| MgO | 2.012 | 1.009 | 8.284 | 0.523 |
| K2O | 0.508 | 1.109 | 0.398 | 0.026 |
| Na2O | 0.341 | 0.284 | 0.205 | 0.010 |
| SO3 | 2.625 | 0.785 | 2.430 | 0.082 |
| TiO2 | 0.319 | 2.362 | 1.027 | 0.020 |
| CaCO3 | 1.185 | - | - | 98.316 |
| Others | 3.824 | 1.316 | 0.597 | 0.033 |
| LOI% | 3.04% | 2.66% | 0.38% | 43.26% |
| Specific gravity (g/cm3) | 3.12 | 2.26 | 2.87 | 3.08 |
| Mixtures | OPC | FA | BFS | LP | Water-Binder-Ratio (w/b) |
|---|---|---|---|---|---|
| P35 | 100% | - | - | - | 0.35 |
| P40 | 100% | - | - | - | 0.4 |
| P50 | 100% | - | - | - | 0.5 |
| P60 | 100% | - | - | - | 0.6 |
| PF50 | 70% | 30% | - | - | 0.5 |
| PFL50 | 65% | 30% | - | 5% | 0.5 |
| PB40 | 30% | - | 70% | - | 0.4 |
| PB50 | 30% | - | 70% | - | 0.5 |
| PB60 | 30% | - | 70% | - | 0.6 |
| PBL50 | 25% | - | 70% | 5% | 0.5 |
| Mixtures | Water-Cement-Ratio | A | n | RH0 | R2 |
|---|---|---|---|---|---|
| P35 | 0.35 | 0.154 | 36.87 | 0.82 | 0.9925 |
| P40 | 0.4 | 0.118 | 42.47 | 0.86 | 0.9924 |
| P50 | 0.5 | 0.075 | 68.75 | 0.91 | 0.9893 |
| P60 | 0.6 | 0.044 | 106.76 | 0.94 | 0.9657 |
| Paste Mixtures | P50 | PF50 | PB50 | PFL50 | PBL50 | Density (kg/m3) |
|---|---|---|---|---|---|---|
| Ettringite | 0.1% | 2.3% | 0.2% | 4.5% | 0.2% | 1778 [23] |
| Mc | 4.3% | 0.2% | 0.2% | 8.6% | 3.1% | 2175 [24] |
| CH | 20.1% | 8.2% | 2.3% | 10.5% | 1.1% | 2251 [25] |
| Mixtures | By Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) | By Calculation | ||||||
|---|---|---|---|---|---|---|---|---|
| Na | K | Ca | Mg | Al | Si | Fe | OH | |
| P35 | 0.272 | 0.478 | 0.0055 | 1.88 × 10−4 | 1.42 × 10−3 | 0.0095 | 1.21 × 10−4 | 0.750 |
| P40 | 0.257 | 0.428 | 0.0013 | <8.75 × 10−5 | 1.07 × 10−4 | 0.0011 | <37.5 × 10−5 | 0.685 |
| P50 | 0.197 | 0.27 | 0.0037 | 1.00 × 10−4 | 2.63 × 10−4 | 0.0018 | 6.79 × 10−5 | 0.467 |
| P60 | 0.155 | 0.211 | 0.0023 | 4.50 × 10−5 | 4.01 × 10−4 | 0.0007 | <19.6 × 10−5 | 0.366 |
| PF50 | 0.172 | 0.181 | 0.0051 | 1.29 × 10−4 | 3.44 × 10−4 | 0.0038 | 9.64 × 10−5 | 0.353 |
| PFL50 | 0.142 | 0.154 | 0.0006 | <4.58 × 10−5 | 5.56 × 10−5 | 0.0005 | <19.6 × 10−5 | 0.296 |
| PB40 | 0.107 | 0.119 | 0.0009 | <21.20 × 10−5 | 4.89 × 10−4 | 0.0018 | <9.1 × 10−5 | 0.226 |
| PB50 | 0.089 | 0.097 | 0.0048 | 7.71 × 10−4 | 1.29 × 10−3 | 0.0034 | 7.14 × 10−5 | 0.186 |
| PB60 | 0.085 | 0.091 | 0.0011 | <5.00 × 10−5 | 4.52 × 10−4 | 0.0007 | <2.14 × 10−5 | 0.168 |
| PBL50 | 0.081 | 0.087 | 0.0015 | <4.6 × 10−5 | 1.19 × 10−4 | 0.0003 | <1.9 × 10−5 | 0.168 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Ouyang, X.; Yang, Z. Microstructure-Based Relative Humidity in Cementitious System Due to Self-Desiccation. Materials 2019, 12, 1214. https://doi.org/10.3390/ma12081214
Zhang Y, Ouyang X, Yang Z. Microstructure-Based Relative Humidity in Cementitious System Due to Self-Desiccation. Materials. 2019; 12(8):1214. https://doi.org/10.3390/ma12081214
Chicago/Turabian StyleZhang, Yong, Xiaowei Ouyang, and Zhengxian Yang. 2019. "Microstructure-Based Relative Humidity in Cementitious System Due to Self-Desiccation" Materials 12, no. 8: 1214. https://doi.org/10.3390/ma12081214




