Biomass-Templated Fabrication of Metallic Materials for Photocatalytic and Bactericidal Applications
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Metallic Materials
2.3. Optical Catalysis Evaluations
2.4. Antibacterial Test
3. Results and Discussion
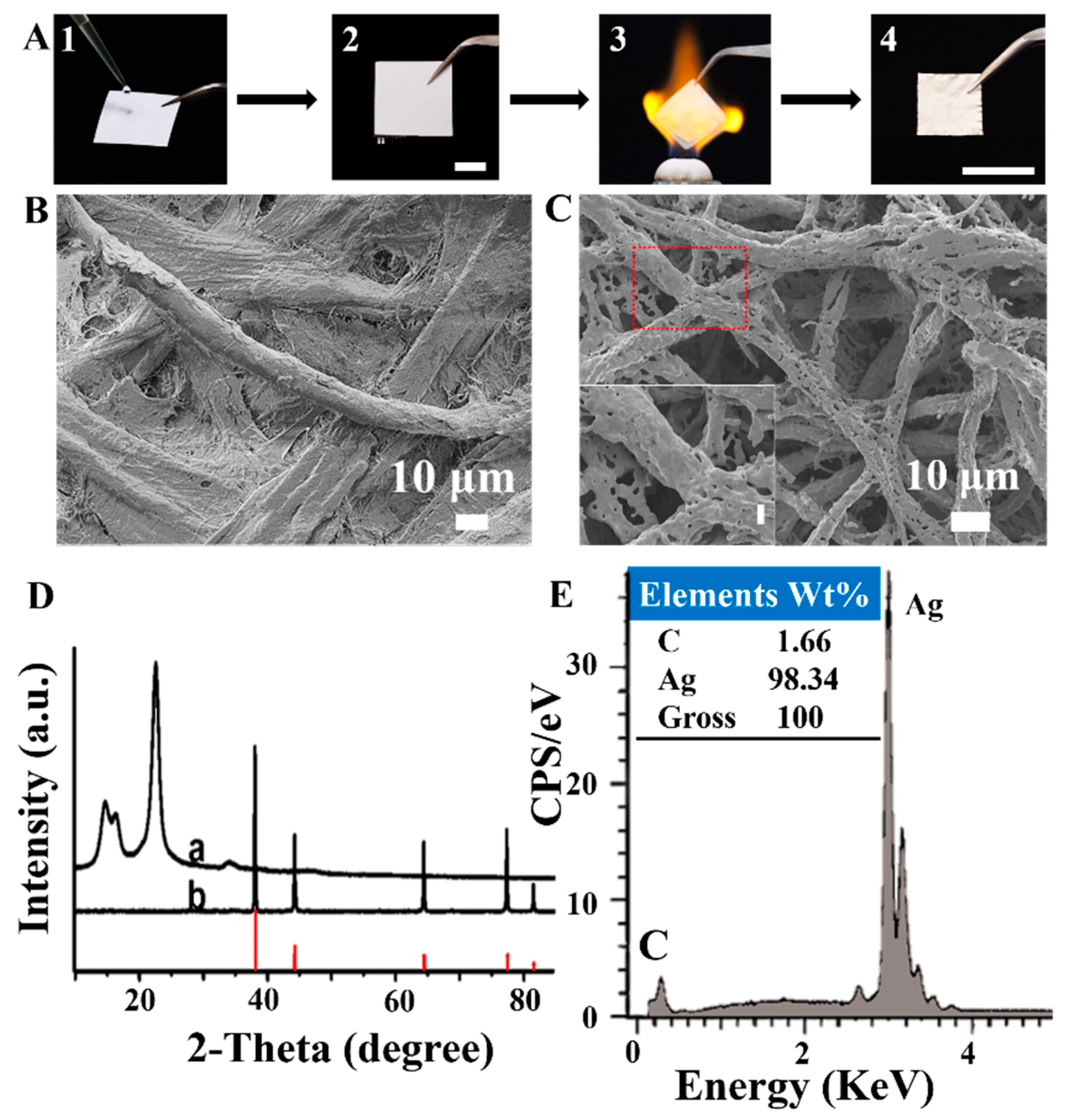
3.1. Paper-Templated Fabrication
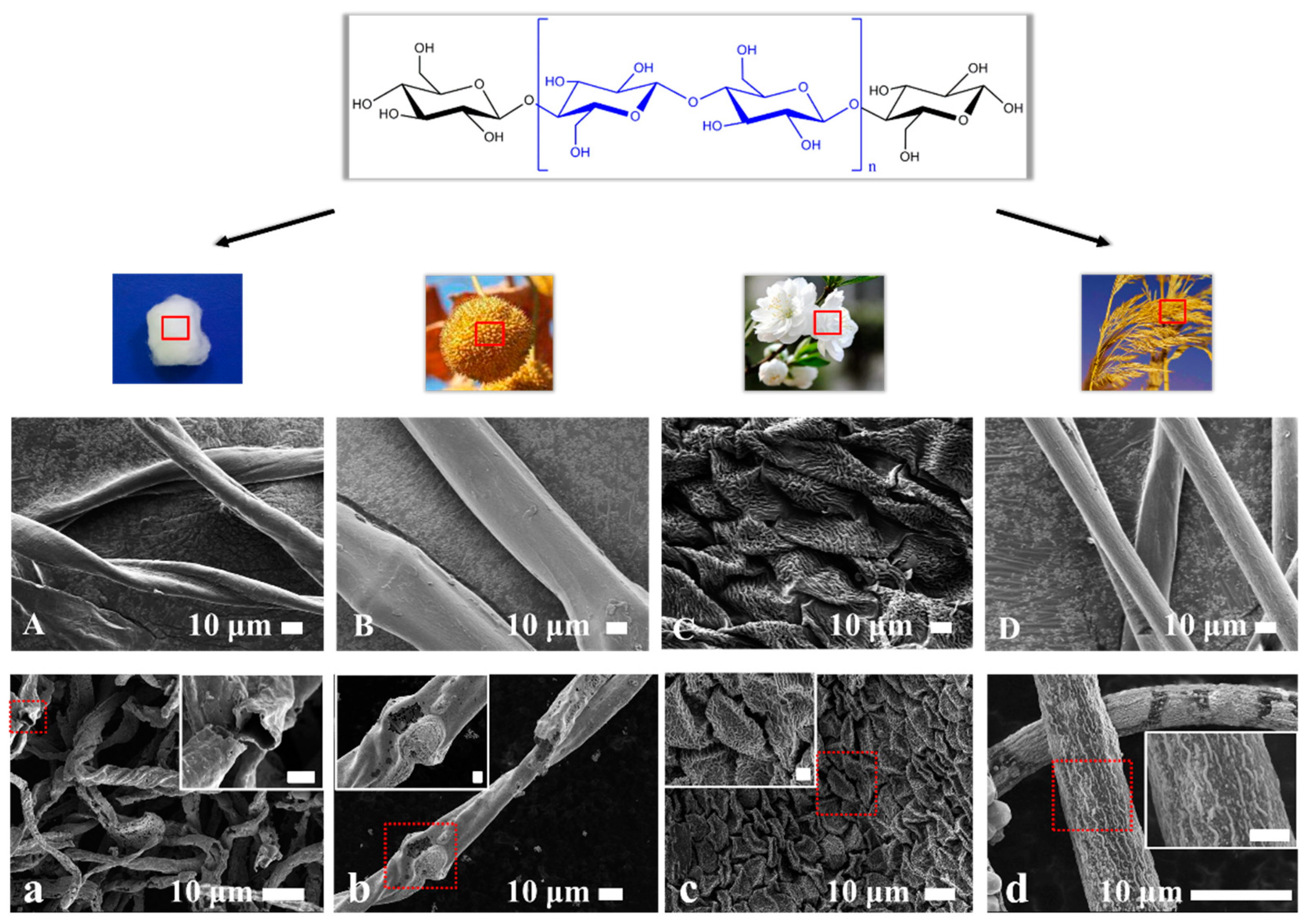
3.2. Biomass-Templated Fabrication
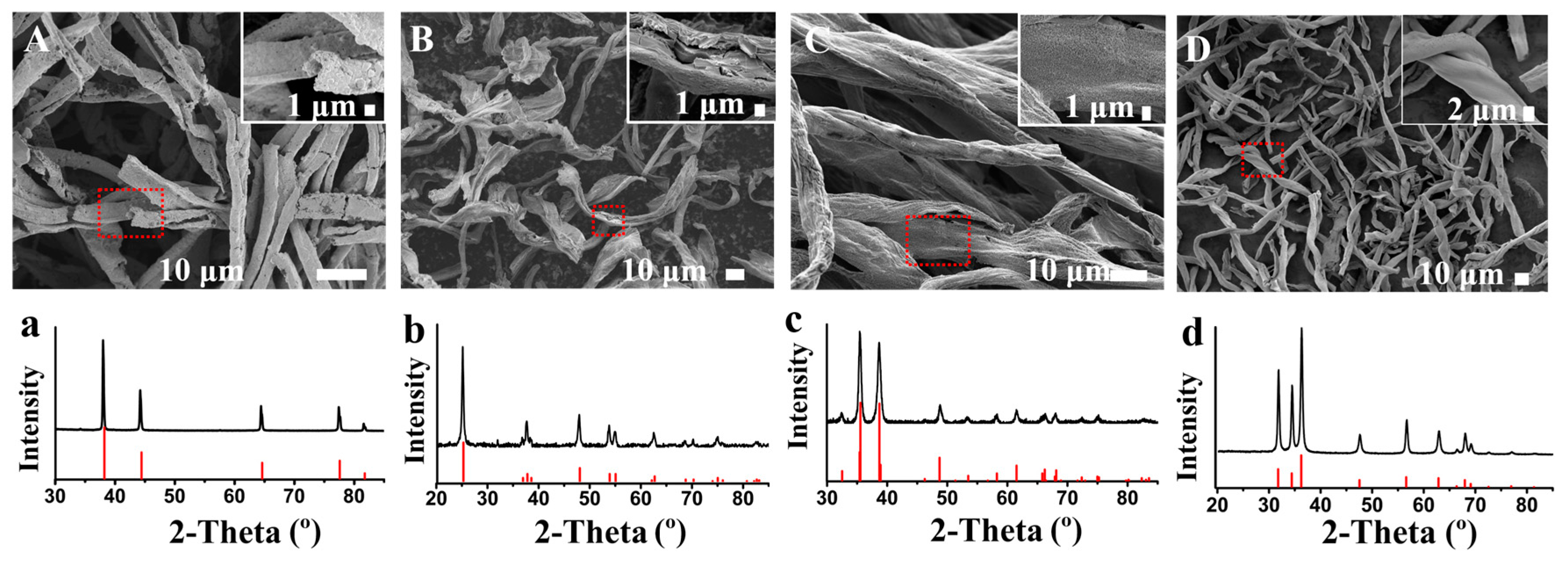
3.3. Fabrication of Other Metallic Materials
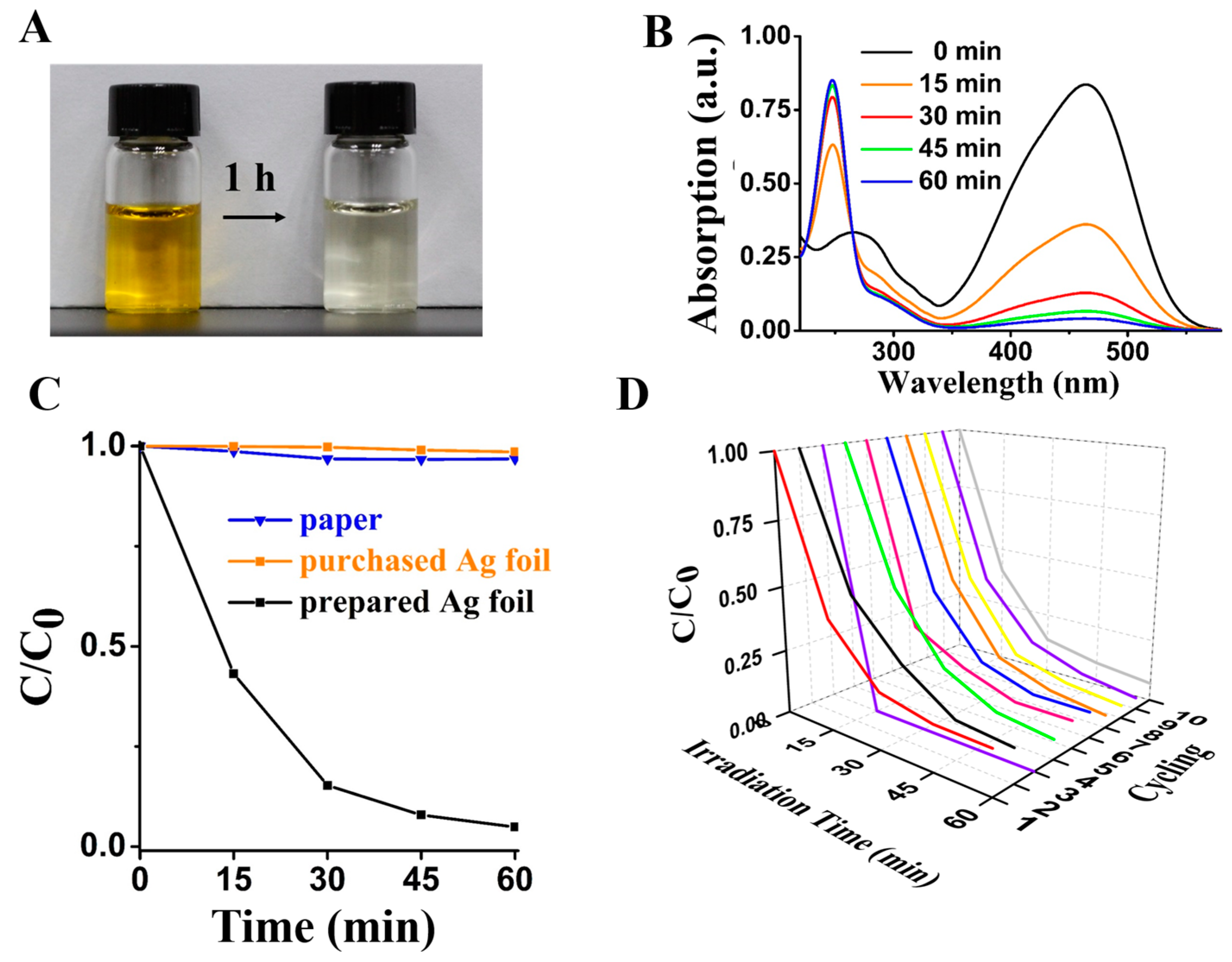
3.4. Photocatalytic and Bactericidal Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qin, J.; Chen, Q.; Yang, C.; Huang, Y. Research process on property and application of metal porous materials. J. Alloy. Compd. 2016, 654, 39–44. [Google Scholar] [CrossRef]
- Xue, Y.; Mou, Z.; Xiao, H. Nanocellulose as a sustainable biomass material: Structure, properties, present status and future prospects in biomedical applications. Nanoscale 2017, 9, 14758–14781. [Google Scholar] [CrossRef] [PubMed]
- Velev, O.D.; Tessier, P.M.; Lenhoff, A.M.; Kaler, E.W. Materials-A class of porous metallic nanostructures. Nature 1999, 401, 548. [Google Scholar] [CrossRef]
- Xi, Z.; Zhu, J.; Tang, H.; Ao, Q.; Zhi, H.; Wang, J.; Li, C. Progress of Application Researches of Porous Fiber Metals. Materials 2011, 4, 816–824. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Lian, X.; Xu, L.; Chen, M.; Yang, B.; Wu, C.-E.; Li, W.; Huang, B.; Hu, X. Designing and Fabricating Ordered Mesoporous Metal Oxides for CO2 Catalytic Conversion: A Review and Prospect. Materials 2019, 12, 276. [Google Scholar] [CrossRef]
- Luc, W.; Jiao, F. Synthesis of Nanoporous Metals, Oxides, Carbides, and Sulfides: Beyond Nanocasting. Acc. Chem. Res. 2016, 49, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, J. Cellulose-rich nanofiber-based functional nanoarchitectures. Adv. Mater. 2016, 28, 1143–1158. [Google Scholar] [CrossRef] [PubMed]
- Ben, H.; Huang, F.; Li, L.; Ragauskas, A.J. In situ upgrading of whole biomass to biofuel precursors with low average molecular weight and acidity by the use of zeolite mixture. RSC Adv. 2015, 5, 74821–74827. [Google Scholar] [CrossRef]
- Liu, H.-J.; Wang, X.-M.; Cui, W.-J.; Dou, Y.-Q.; Zhao, D.-Y.; Xia, Y.-Y. Highly ordered mesoporous carbon nanofiber arrays from a crab shell biological template and its application in supercapacitors and fuel cells. J. Mater. Chem. 2010, 20, 4223–4230. [Google Scholar] [CrossRef]
- Zimmerman, A.B.; Nelson, A.M.; Gillan, E.G. Titania and Silica Materials Derived from Chemically Dehydrated Porous Botanical Templates. Chem. Mater. 2012, 24, 4301–4310. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Zhang, L.; Ge, S.; Yan, M.; Yu, J. In-situ synthesized polypyrrole-cellulose conductive networks for potential-tunable foldable power paper. Nano Energy 2017, 31, 174–182. [Google Scholar] [CrossRef]
- Gu, Y.; Huang, J. Ultrathin cellulose film coating of porous alumina membranes for adsorption of superoxide dismutase. J. Mater. Chem. B 2013, 1, 5636–5643. [Google Scholar] [CrossRef]
- Gu, Y.; Huang, J. Fabrication of natural cellulose substance derived hierarchical polymeric materials. J. Mater. Chem. 2009, 19, 3764–3770. [Google Scholar] [CrossRef]
- Gu, Y.; Zhao, J.; Huang, J. Flame synthesis of hierarchical nanotubular rutile titania derived from natural cellulose substance. Chem. Commun. 2011, 47, 10551–10553. [Google Scholar]
- Luo, Y.; Li, J.; Huang, J. Bioinspired Hierarchical Nanofibrous Silver-Nanoparticle/Anatase–Rutile-Titania Composite as an Anode Material for Lithium-Ion Batteries. Langmuir 2016, 32, 12338–12343. [Google Scholar] [CrossRef]
- Kemell, M.; Pore, V.; Ritala, M.; Leskelä, M.; Lindén, M. Atomic Layer Deposition in Nanometer-Level Replication of Cellulosic Substances and Preparation of Photocatalytic TiO2/Cellulose Composites. J. Am. Chem. Soc. 2005, 127, 14178–14179. [Google Scholar] [CrossRef]
- Cook, G.; Timms, P.L.; Göltner-Spickermann, C.; Göltner-Spickermann, C. Exact Replication of Biological Structures by Chemical Vapor Deposition of Silica. Angew. Chem. Int. Ed. 2003, 42, 557–559. [Google Scholar] [CrossRef]
- Dong, Y.; Jia, B.; Fu, F.; Zhang, H.; Zhang, L.; Zhou, J. Fabrication of Hollow Materials by Fast Pyrolysis of Cellulose Composite Fibers with Heterogeneous Structures. Angew. Chem. Int. Ed. 2016, 55, 13504–13508. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Yao, B.; Hu, B.; Huo, K.; Chen, W.; Zhou, J. Polypyrrole-coated paper for flexible solid-state energy storage. Energy Environ. Sci. 2013, 6, 470–476. [Google Scholar] [CrossRef]
- Christodouleas, D.C.; Simeone, F.C.; Tayi, A.; Targ, S.; Weaver, J.C.; Jayaram, K.; Fernández-Abedul, M.T.; Whitesides, G.M. Fabrication of Paper-Templated Structures of Noble Metals. Adv. Mater. Technol. 2017, 2, 1600229. [Google Scholar] [CrossRef]
- Ko, J.W.; Chung, Y.J.; Lee, B.I.; Park, C.B. Carboxymethyl cellulose-templated synthesis of hierarchically structured metal oxides. Green Chem. 2015, 17, 4167–4172. [Google Scholar] [CrossRef]
- Saran, S.; Manjari, G.; Devipriya, S.P. Synergistic eminently active catalytic and recyclable Ag, Cu and Ag-Cu alloy nanoparticles supported on TiO2 for sustainable and cleaner environmental applications: A phytogenic mediated synthesis. J. Clean. Prod. 2018, 177, 134–143. [Google Scholar] [CrossRef]
- Pollini, M.; Russo, M.; Licciulli, A.A.; Sannino, A.; Maffezzoli, A. Characterization of antibacterial silver coated yarns. J. Mater. Sci. Mater. Med. 2009, 20, 2361–2366. [Google Scholar] [CrossRef] [PubMed]
- Carrilho, E.; Phillips, S.T.; Vella, S.J.; Martinez, A.W.; Whitesides, G.M. Paper microzone plates. Anal. Chem. 2009, 81, 5990–5998. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Kunitake, T.; Watanabe, T. Porous and nonporous Ag nanostructures fabricated using cellulose fiber as a template. Chem. Commun. 2005, 6, 795–796. [Google Scholar] [CrossRef] [PubMed]
- Ovais, M.; Khalil, A.T.; Raza, A.; Khan, M.A.; Ahmad, I.; Islam, N.U.; Saravanan, M.; Ubaid, M.F.; Ali, M.; Shinwari, Z.K. Green synthesis of silver nanoparticles via plant extracts: Beginning a new era in cancer theranostics. Nanomedicine 2016, 11, 3157–3177. [Google Scholar] [CrossRef]
- Tripathy, T.; Kolya, H.; Jana, S.; Senapati, M.; Tripathy, D.T. Green synthesis of Ag-Au bimetallic nanocomposites using a biodegradable synthetic graft copolymer; hydroxyethyl starch-g-poly (acrylamide-co-acrylic acid) and evaluation of their catalytic activities. Eur. Polym. J. 2017, 87, 113–123. [Google Scholar] [CrossRef]
- Dai, L.; Liu, R.; Hu, L.-Q.; Si, C.-L. Simple and green fabrication of AgCl/Ag-cellulose paper with antibacterial and photocatalytic activity. Carbohydr. Polym. 2017, 174, 450–455. [Google Scholar] [CrossRef]
- Patil, D.; Wasson, M.K.; Aravindan, S.; Vivekanandan, P.; Rao, P. Antibacterial and cytocompatibility study of modified Ti6Al4V surfaces through thermal annealing. Mater. Sci. Eng. C 2019, 99, 1007–1020. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef] [Green Version]
- Taheri, S.; Cavallaro, A.; Christo, S.N.; Smith, L.E.; Majewski, P.; Barton, M.; Hayball, J.D.; Vasilev, K. Substrate independent silver nanoparticle based antibacterial coatings. Biomaterials 2014, 35, 4601–4609. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, B.; Garmaroudi, F.S.; Hashemi, M.; Nezhad, H.; Nasrollahi, A.; Ardalan, S.; Ardalan, S. Comparison of the anti-bacterial activity on the nanosilver shapes: Nanoparticles, nanorods and nanoplates. Adv. Powder Technol. 2012, 23, 22–26. [Google Scholar] [CrossRef]
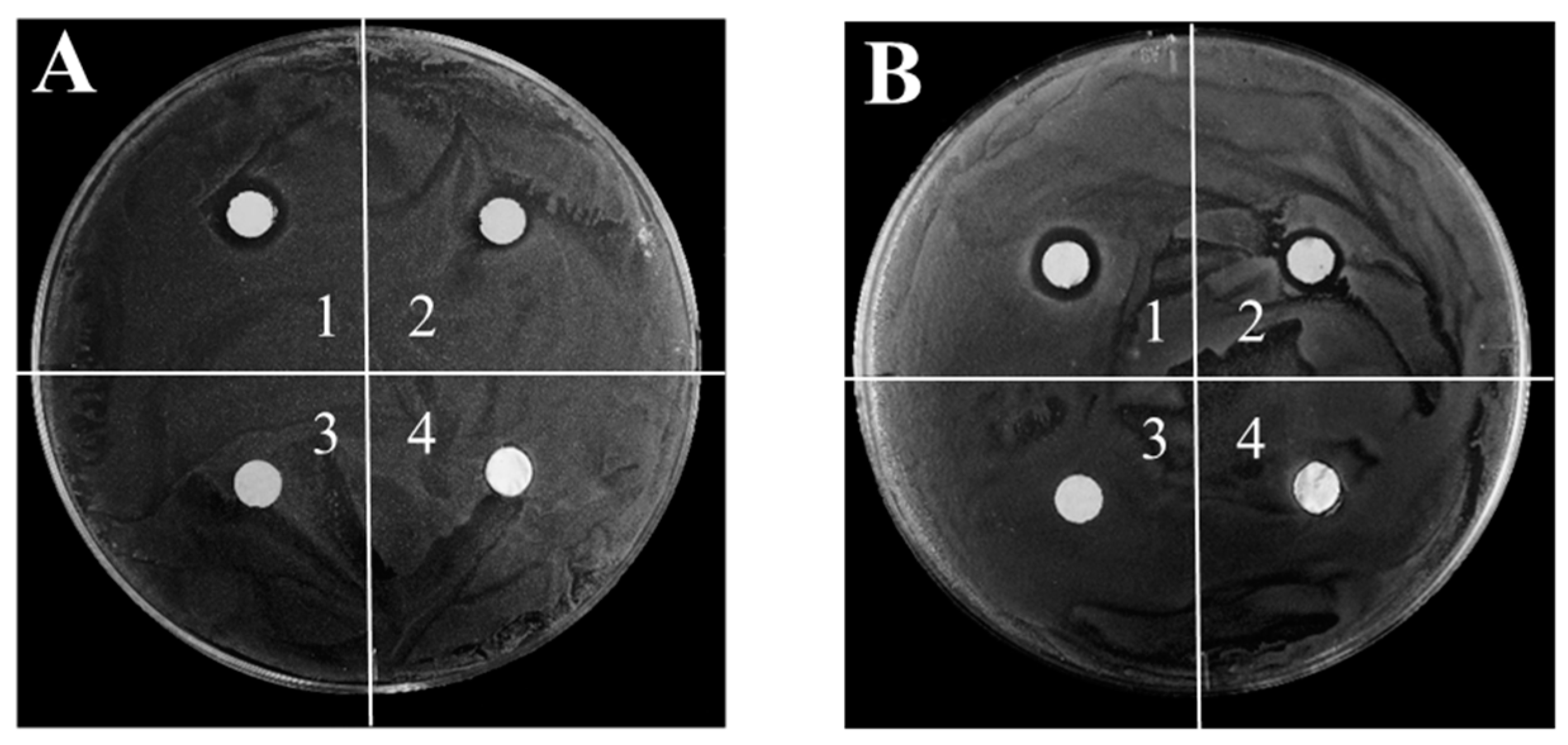
| Sample | Paper-Templated Silver (mm) | Paper-Templated Silver Used for Two Times (mm) | Paper (mm) | Pure Silver Foil (mm) | |
|---|---|---|---|---|---|
| The First Time | The Second Time | ||||
| MRSA | 3.6 ± 0.6 | 3.6 ± 0.7 | 1.4 ± 0.3 | 0 | 0 |
| E. coli | 3.6 ± 0.9 | 3.6 ± 0.8 | 1.3 ± 0.6 | 0 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Wang, Q.; Lai, Q.; Ouyang, Q.; Li, P.; Yu, H.-D.; Huang, W. Biomass-Templated Fabrication of Metallic Materials for Photocatalytic and Bactericidal Applications. Materials 2019, 12, 1271. https://doi.org/10.3390/ma12081271
Guo X, Wang Q, Lai Q, Ouyang Q, Li P, Yu H-D, Huang W. Biomass-Templated Fabrication of Metallic Materials for Photocatalytic and Bactericidal Applications. Materials. 2019; 12(8):1271. https://doi.org/10.3390/ma12081271
Chicago/Turabian StyleGuo, Xueying, Qianqian Wang, Qiongyu Lai, Qiran Ouyang, Peng Li, Hai-Dong Yu, and Wei Huang. 2019. "Biomass-Templated Fabrication of Metallic Materials for Photocatalytic and Bactericidal Applications" Materials 12, no. 8: 1271. https://doi.org/10.3390/ma12081271





