A Hybrid Model for Predicting Bone Healing around Dental Implants
Abstract
:1. Introduction
2. Materials and Methods
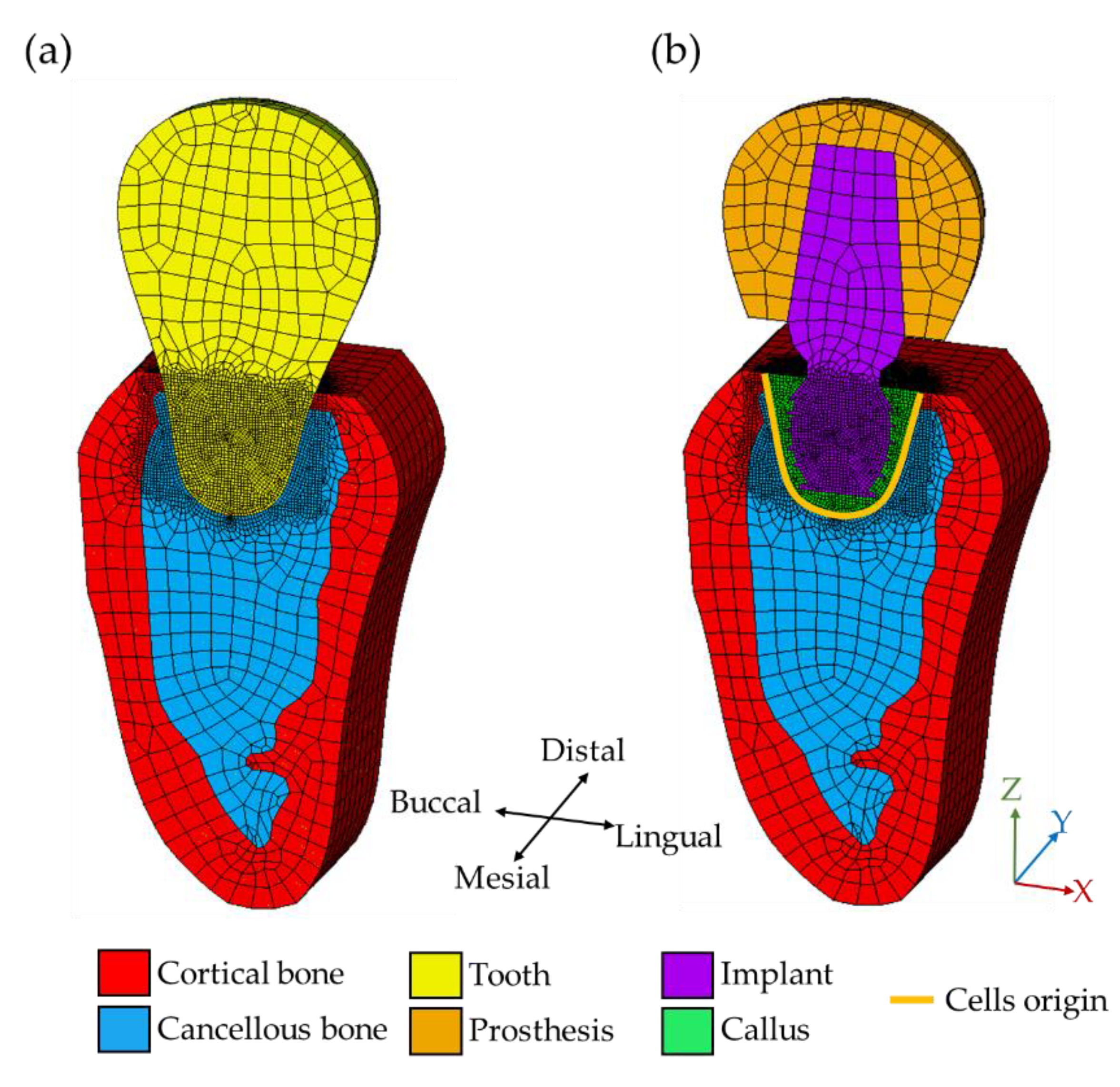
2.1. Three-Dimensional FEM Model
2.2. Mechano-Regulation Algorithm
2.3. Bone Remodeling Algorithm
3. Results
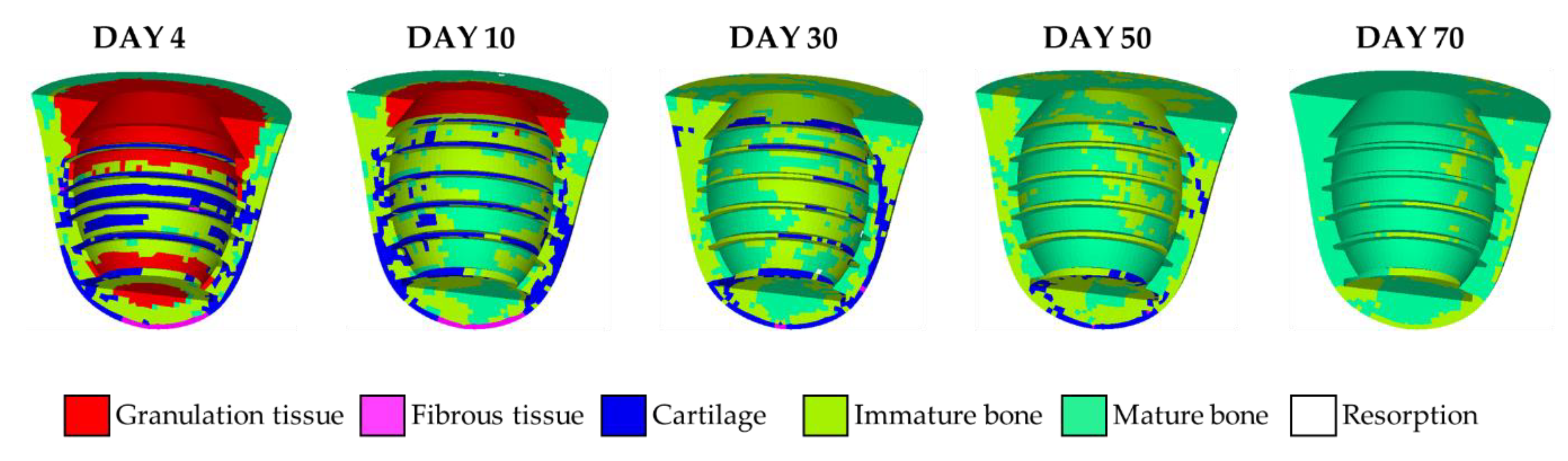
3.1. Short-Term Healing and Tissue Differentiation
3.2. Bone Remodeling
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meredith, N. Assessment of implant stability as a prognostic determinant. Int. J. Prosthodont. 1998, 11, 491–501. [Google Scholar] [PubMed]
- Ammon, C.; Kreutz, M.; Rehli, M.; Krause, S.W.; Andreesen, R. Platelets induce monocyte differentiation in serum-free coculture. J. Leukoc. Biol. 1998, 63, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Spisani, S.; Giuliani, A.L.; Cavalletti, T.; Zaccarini, M.; Milani, L.; Gavioli, R.; Traniello, S. Modulation of neutrophil functions by activated platelet release factors. Inflammation 1992, 16, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wu, X.; Lei, W.; Pang, L.; Wan, C.; Shi, Z.; Zhao, L.; Nagy, T.R.; Peng, X.; Hu, J.; et al. TGF-β1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat. Med. 2009, 15, 757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacroix, D.; Prendergast, P.J. A mechano-regulation model for tissue differentiation during fracture healing: Analysis of gap size and loading. J. Biomech. 2002, 35, 1163–1171. [Google Scholar] [CrossRef]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wollert, K.C.; Meyer, G.P.; Lotz, J.; Lichtenberg, S.R.; Lippolt, P.; Breidenbach, C.; Fichtner, S.; Korte, T.; Hornig, B.; Messinger, D.; et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: The BOOST randomised controlled clinical trial. Lancet 2004, 364, 141–148. [Google Scholar] [CrossRef]
- Ashton, B.A.; Allen, T.D.; Howlett, C.R.; Eaglesom, C.C.; Hattori, A.; Owen, M. Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin. Orthop. Relat. Res. 1980, 151, 294–307. [Google Scholar] [CrossRef]
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef]
- Frost, H.M. Tetracycline-based histological analysis of bone remodeling. Calcif. Tissue Int. 1969, 3, 211–237. [Google Scholar] [CrossRef]
- Wolff, J. The Law of Bone Remodelling, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1986; ISBN 978-3-642-71031-5. [Google Scholar]
- Ghiasi, M.S.; Chen, J.; Vaziri, A.; Rodriguez, E.K.; Nazarian, A. Bone fracture healing in mechanobiological modeling: A review of principles and methods. Bone Rep. 2017, 6, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Mohaghegh, K.; Pérez, M.A.; García-Aznar, J.M. Accelerating numerical simulations of strain-adaptive bone remodeling predictions. Comput. Methods Appl. Mech. Eng. 2014, 273, 255–272. [Google Scholar] [CrossRef]
- Pauwels, F. A new theory on the influence of mechanical stimuli on the differentiation of supporting tissue. The tenth contribution to the functional anatomy and causal morphology of the supporting structure. Z. Anat. Entwicklungsgesch. 1960, 121, 478–515. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.R.; Fyhrie, D.P.; Whalen, R.T. Trabecular bone density and loading history: Regulation of connective tissue biology by mechanical energy. J. Biomech. 1987, 20, 785–794. [Google Scholar] [CrossRef]
- Prendergast, P.J.; Huiskes, R.; Søballe, K. Biophysical stimuli on cells during tissue differentiation at implant interfaces. J. Biomech. 1997, 30, 539–548. [Google Scholar] [CrossRef] [Green Version]
- Chou, H.Y.; Müftü, S. Simulation of peri-implant bone healing due to immediate loading in dental implant treatments. J. Biomech. 2013, 46, 871–878. [Google Scholar] [CrossRef]
- Sotto-maior, B.S.; Graciliano, E.; Mercuri, F.; Mendes, P.; Maria, N.; Picorelli, S.; Francischone, C.E.; Maria, N.; Picorelli, S.; Francischone, C.E.; et al. Computer Methods in Biomechanics and Biomedical Engineering Evaluation of bone remodeling around single dental implants of different lengths: A mechanobiological numerical simulation and validation using clinical data. Comput. Methods Biomech. Biomed. Engin. 2016, 19, 699–706. [Google Scholar] [CrossRef]
- Fernández, J.R.; García-Aznar, J.M.; Martínez, R.; Viaño, J.M. Numerical analysis of a strain-adaptive bone remodelling problem. Comput. Methods Appl. Mech. Eng. 2010, 199, 1549–1557. [Google Scholar] [CrossRef]
- Cowin, S.C.; Hegedus, D.H. Bone remodeling I: Theory of adaptive elasticity. J. Elast. 1976, 6, 313–326. [Google Scholar] [CrossRef]
- Frost, H.M. Bone “mass” and the “mechanostat”: A proposal. Anat. Rec. 1987, 219, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, S.; Shin, K. Effect of cycloid movement on plate-to-roll gravure offset printing. Microsyst. Technol. 2016, 22, 357–365. [Google Scholar] [CrossRef]
- Hegedus, D.H.; Cowin, S.C. Bone remodeling II: Small strain adaptive elasticity. J. Elast. 1976, 6, 337–352. [Google Scholar] [CrossRef]
- Hart, R.T.; Davy, D.T.; Heiple, K.G. A computational method for stress analysis of adaptive elastic materials with a view toward applications in strain-induced bone remodeling. J. Biomech. Eng. 1984, 106, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.R. Mechanical loading histories and cortical bone remodeling. Calcif. Tissue Int. 1984, 36, S19–S24. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.R.; Hayes, W.C. The compressive behavior of bone as a two-phase porous structure. J. Bone Jt. Surg. Am. 1977, 59, 954–962. [Google Scholar] [CrossRef]
- Huiskes, R.; Weinans, H.; Grootenboer, H.J.; Dalstra, M.; Fudala, B.; Slooff, T.J. Adaptive bone-remodeling theory applied to prosthetic-design analysis. J. Biomech. 1987, 20, 1135–1150. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Li, Q.; Li, W.; Zhou, S.; Swain, M.V. Design optimization of functionally graded dental implant for bone remodeling. Compos. Part B Eng. 2009, 40, 668–675. [Google Scholar] [CrossRef]
- Chou, H.-Y.; Romanos, G.; Müftü, A.; Müftü, S. Peri-implant bone remodeling around an extraction socket: Predictions of bone maintenance by finite element method. Int. J. Oral Maxillofac. Implants 2012, 27, 39–48. [Google Scholar]
- Crupi, V.; Guglielmino, E.; LaRosa, G.; VanderSloten, J.; VanOosterwyck, H. Numerical analysis of bone adaptation around an oral implant due to overload stress. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2004, 218, 407–415. [Google Scholar] [CrossRef]
- Lian, Z.; Guan, H.; Ivanovski, S.; Loo, Y.-C.; Johnson, N.W.; Zhang, H. Effect of bone to implant contact percentage on bone remodelling surrounding a dental implant. Int. J. Oral Maxillofac. Surg. 2010, 39, 690–698. [Google Scholar] [CrossRef] [Green Version]
- Reina, J.M.; García-Aznar, J.M.; Domínguez, J.; Doblaré, M. Numerical estimation of bone density and elastic constants distribution in a human mandible. J. Biomech. 2007, 40, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Li, Q.; Li, W.; Swain, M. Bone remodeling induced by dental implants of functionally graded materials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 92, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, H.; Shi, L.; Fok, A.S.L.; Ucer, C.; Devlin, H.; Horner, K.; Silikas, N. A mathematical model for simulating the bone remodeling process under mechanical stimulus. Dent. Mater. 2007, 23, 1073–1078. [Google Scholar] [CrossRef]
- Lacroix, D.; Prendergast, P.J. A homogenization procedure to prevent numerical instabilities in poroelastic tissue differentiation models. In Proceedings of the Eighth Annual Symposium: Computational Methods in Orthopaedic Biomechanics, Orlando, FL, USA, 11 March 2000. [Google Scholar]
- Barbier, L.; VanderSloten, J.; Krzesinski, G.; Schepers, E.; Van DerPerre, G. Finite element analysis of non-axial versus axial loading of oral implants in the mandible of the dog. J. Oral Rehabil. 1998, 25, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Jansen, V.K.; Conrads, G.; Richter, E.J. Microbial leakage and marginal fit of the implant-abutment interface. Int. J. Oral Maxillofac. Implants 1997, 12, 23. [Google Scholar]
- Streckbein, P.; Streckbein, R.G.; Wilbrand, J.F.; Malik, C.Y.; Schaaf, H.; Howaldt, H.P.; Flach, M. Non-linear 3D evaluation of different oral implant-abutment connections. J. Dent. Res. 2012, 91, 1184–1189. [Google Scholar] [CrossRef]
- Zohar, R.; Cheifetz, S.; McCulloch, C.A.G.; Sodek, J. Analysis of intracellular osteopontin as a marker of osteoblastic cell differentiation and mesenchymal cell migration. Eur. J. Oral Sci. 1998, 106, 401–407. [Google Scholar] [CrossRef]
- Iwaki, A.; Jingushi, S.; Oda, Y.; Izumi, T.; Shida, J.I.; Tsuneyoshi, M.; Sugioka, Y. Localization and quantification of proliferating cells during rat fracture repair: Detection of proliferating cell nuclear antigen by immunohistochemistry. J. Bone Miner. Res. 1997, 12, 96–102. [Google Scholar] [CrossRef]
- Monjo, M.; Lamolle, S.F.; Lyngstadaas, S.P.; Rønold, H.J.; Ellingsen, J.E. In vivo expression of osteogenic markers and bone mineral density at the surface of fluoride-modified titanium implants. Biomaterials 2008, 29, 3771–3780. [Google Scholar] [CrossRef]
- Papalexiou, V.; Novaes, A.B., Jr.; Grisi, M.F.M.; Souza, S.S.L.S.; Taba, M., Jr.; Kajiwara, J.K. Influence of implant microstructure on the dynamics of bone healing around immediate implants placed into periodontally infected sites: A confocal laser scanning microscopic study. Clin. Oral Implants Res. 2004, 15, 44–53. [Google Scholar] [CrossRef]
- JR, S.A.; Allegrini, M.R.F.; Yoshimoto, M.; JR, B.K.; Mai, R.; Fanghanel, J.; Gedrange, T. Soft tissue integration in the neck area of titanium implants--an animal trial. J. Physiol. Pharmacol. 2008, 59, 117–132. [Google Scholar]
- Huiskes, R.; Weinans, H.; VanRietbergen, B. The relationship between stress shielding and bone resorption around total hip stems and the effects of flexible materials. Clin. Orthop. Relat. Res. 1992, 124–134. [Google Scholar] [CrossRef] [Green Version]
- Turner, C.H.; Anne, V.; Pidaparti, R.M. VA uniform strain criterion for trabecular bone adaptation: Do continuum-level strain gradients drive adaptation? J. Biomech. 1997, 30, 555–563. [Google Scholar] [CrossRef]
- Turner, C.H. Three rules for bone adaptation to mechanical stimuli. Bone 1998, 23, 399–407. [Google Scholar] [CrossRef]
- Weinans, H.; Huiskes, R.; Grootenboer, H.J. The behavior of adaptive bone-remodeling simulation models. J. Biomech. 1992, 25, 1425–1441. [Google Scholar] [CrossRef] [Green Version]
- Frost, H.M. Bone’s mechanostat: A 2003 update. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2003, 275, 1081–1101. [Google Scholar] [CrossRef]
- Akca, K.; Cehreli, M.C. Biomechanical consequences of progressive marginal bone loss around oral implants: A finite element stress analysis. Med. Biol. Eng. Comput. 2006, 44, 527–535. [Google Scholar] [CrossRef]
- Esposito, M.; Ekestubbe, A.; Gröndahl, K. Radiological evaluation of marginal bone loss at tooth surfaces facing single Brånemark implants. Clin. Oral Implants Res. 1993, 4, 151–157. [Google Scholar] [CrossRef]
- Arifin, A.; Sulong, A.B.; Muhamad, N.; Syarif, J.; Ramli, M.I. Material processing of hydroxyapatite and titanium alloy (HA/Ti) composite as implant materials using powder metallurgy: A review. Mater. Des. 2014, 55, 165–175. [Google Scholar] [CrossRef]
- Miyaura, K.; Matsuka, Y.; Morita, M.; Yamashita, A.; Watanabe, T. Comparison of biting forces in different age and sex groups: A study of biting efficiency with mobile and non-mobile teeth. J. Oral Rehabil. 1999, 26, 223–227. [Google Scholar] [CrossRef]
- Moy, P.K.; Medina, D.; Shetty, V.; Aghaloo, T.L. Dental implant failure rates and associated risk factors. Int. J. Oral. Maxillofac. Implant. 2005, 20, 569–577. [Google Scholar]
- Pérez, M.A.; Prendergast, P.J. Random-walk models of cell dispersal included in mechanobiological simulations of tissue differentiation. J. Biomech. 2007, 40, 2244–2253. [Google Scholar] [CrossRef]
- Toms, S.R.; Lemons, J.E.; Bartolucci, A.A.; Eberhardt, A.W. Nonlinear stress-strain behavior of periodontal ligament under orthodontic loading. Am. J. Orthod. Dentofac. Orthop. 2002, 122, 174–179. [Google Scholar] [CrossRef]
- Vollmer, D.; Bourauel, C.; Maier, K.; Jäger, A. Determination of the centre of resistance in an upper human canine and idealized tooth model. Eur. J. Orthod. 1999, 21, 633–648. [Google Scholar] [CrossRef] [Green Version]
- Provatidis, C.G. A comparative FEM-study of tooth mobility using isotropic and anisotropic models of the periodontal ligament. Med. Eng. Phys. 2000, 22, 359–370. [Google Scholar] [CrossRef]






| Materials | Young’s Modulus (GPa) | Poisson’s Ratio | Permeability (m4/Ns) |
|---|---|---|---|
| Ti6Al4V | 113.8 | 0.34 | N/A |
| Tooth | 20 | 0.3 | N/A |
| Prosthesis | 80 | 0.3 | N/A |
| Bone graft | 2 | 0.3 | N/A |
| Cortical bone | 13.7 | 0.3 | 10−17 |
| Cancellous bone | 2 | 0.3 | 3.7 × 10−13 |
| Granulation tissue | 0.001 | 0.17 | 10−14 |
| Fibrous tissue | 0.002 | 0.17 | 10−14 |
| Cartilage | 0.01 | 0.17 | 5 × 10−15 |
| Immature bone | 1 | 0.3 | 10−13 |
| Mature bone | 6 | 0.3 | 3.7 × 10−13 |
| Tissue Phenotypes | |||
|---|---|---|---|
| 3< | Fibrous tissue | ||
| 1< | 3 | Cartilage | |
| 0.266< | 1 | Immature bone | |
| 0.010< | 0.266 | Mature bone | |
| 0.010 | Initial resorption |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kung, P.-C.; Chien, S.-S.; Tsou, N.-T. A Hybrid Model for Predicting Bone Healing around Dental Implants. Materials 2020, 13, 2858. https://doi.org/10.3390/ma13122858
Kung P-C, Chien S-S, Tsou N-T. A Hybrid Model for Predicting Bone Healing around Dental Implants. Materials. 2020; 13(12):2858. https://doi.org/10.3390/ma13122858
Chicago/Turabian StyleKung, Pei-Ching, Shih-Shun Chien, and Nien-Ti Tsou. 2020. "A Hybrid Model for Predicting Bone Healing around Dental Implants" Materials 13, no. 12: 2858. https://doi.org/10.3390/ma13122858





