Low Resistance TiO2/p-Si Heterojunction for Tandem Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
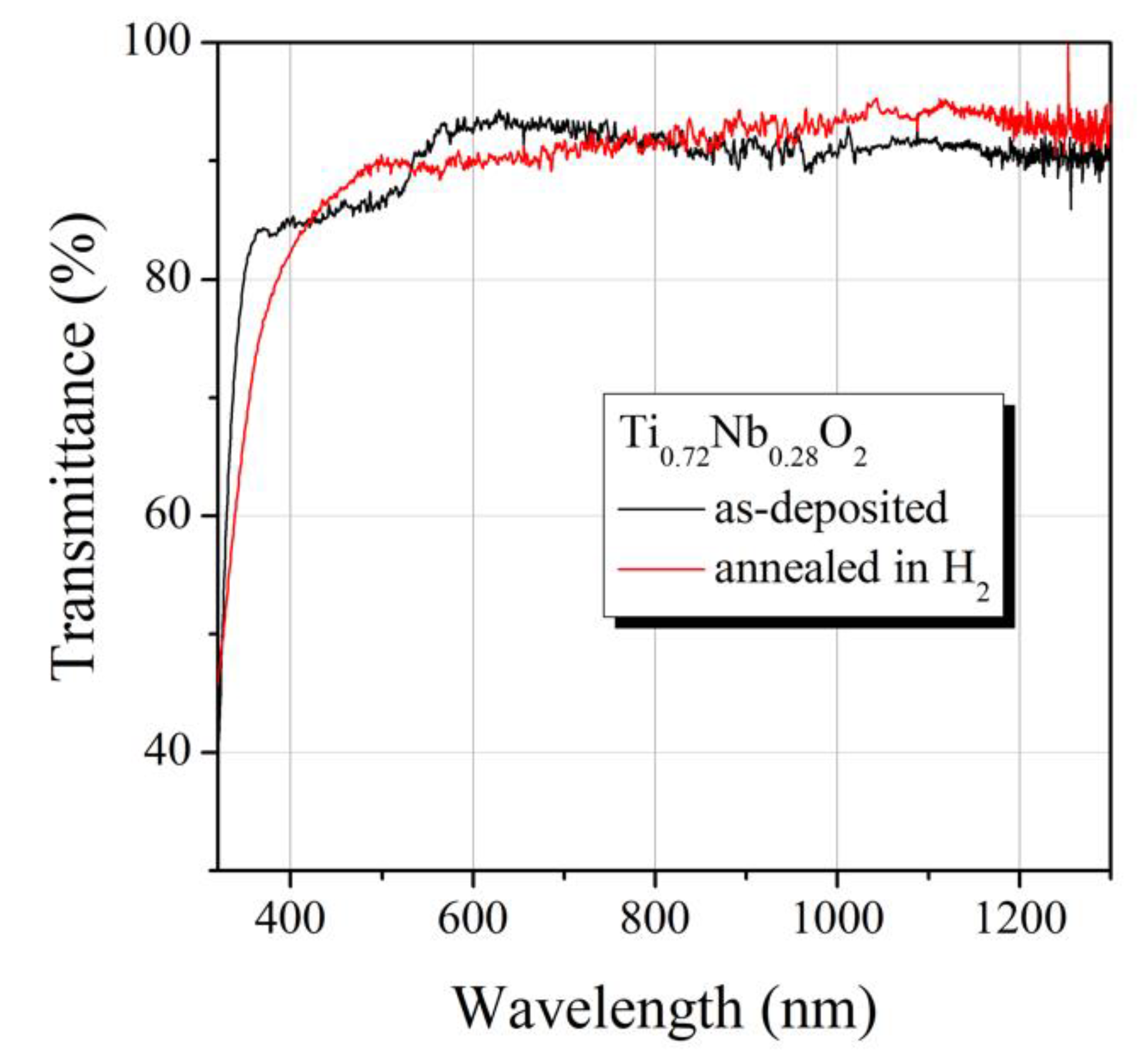
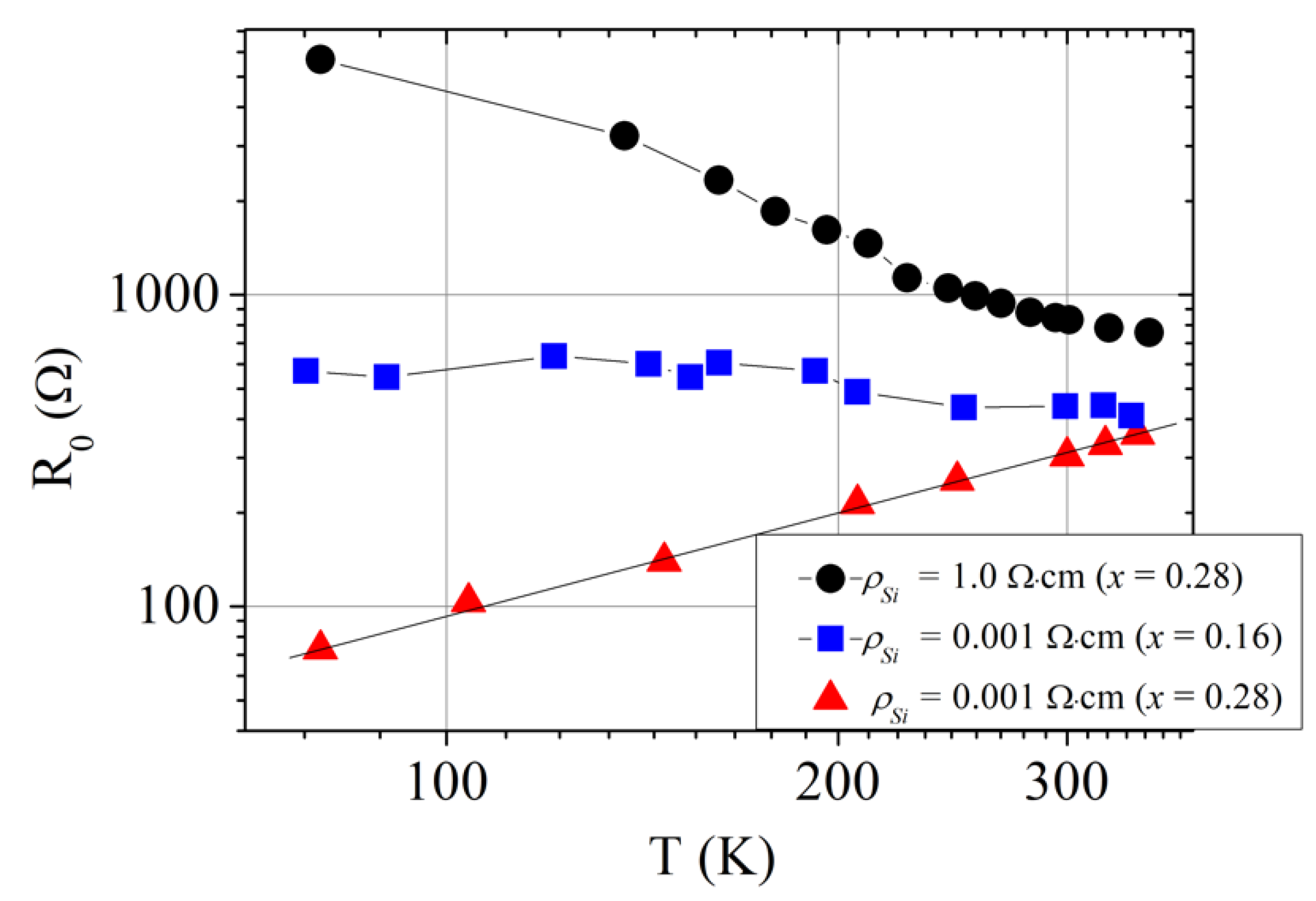
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mailoa, J.P.; Bailie, C.D.; Johlin, E.C.; Hoke, E.T.; Akey, A.J.; Nguyen, W.H.; McGehee, M.D.; Buonassisi, T. A 2-terminal perovskite/silicon multijunction solar cell enabled by a silicon tunnel junction. Appl. Phys. Lett. 2015, 106, 121105. [Google Scholar] [CrossRef] [Green Version]
- Asadpour, R.; Chavali, R.V.K.; Khan, M.R.; Alam, M.A. Bifacial Si heterojunction-perovskite organic-inorganic tandem to produce highly efficient (η*T ~33%) solar cell. Appl. Phys. Lett. 2015, 106, 243902. [Google Scholar] [CrossRef]
- Albrecht, S.; Saliba, M.; Baena, J.P.C.; Lang, F.; Kegelmann, L.; Mews, M.; Steier, L.; Abate, A.; Rappich, J.; Korte, L.; et al. Monolithic perovskite/silicon-heterojunction tandem solar cells processed at low temperature. Energy Environ. Sci. 2015, 9, 81–88. [Google Scholar] [CrossRef]
- Werner, J.; Weng, C.H.; Walter, A.; Fesquet, L.; Seif, J.P.; De Wolf, S.; Niesen, B.; Ballif, C. Efficient monolithic perovskite/silicon tandem solar cell with cell area > 1 cm2. Phys. Chem. Lett. 2016, 7, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.; Niesen, B.; Ballif, C. Perovskite/silicon tandem solar cells: Marriage of convenience or true love story?—An overview. Adv. Matter. Interf. 2017, 1700731. [Google Scholar] [CrossRef]
- Zheng, J.H.; Lau, C.F.J.; Mehrvarz, H.; Ma, F.J.; Jiang, Y.J.; Deng, X.F.; Soeriyadi, A.; Kim, J.; Zhang, M.; Hu, L.; et al. Large area efficient interface layer free monolithic perovskite/homo-junction-silicon tandem solar cell with over 20% efficiency. Energy Environ. Sci. 2018, 11, 2432–2443. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.P.; Omelchenko, S.T.; Jacobs, D.A.; Yalamanchili, S.; Wan, Y.; Yan, D.; Phang, P.; Duong, T.; Wu, Y.; Yin, Y.; et al. In situ recombination junction between p-Si and TiO2 enables high-efficiency monolithic perovskite/Si tandem cells. Sci. Adv. 2018, 4, eaau9711. [Google Scholar] [CrossRef] [Green Version]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [Green Version]
- Burschka, J.; Pellet, N.; Moon, S.J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, K.; Stranks, S.D.; Abate, A.; Sadoughi, G.; Sadhanala, A.; Kopidakis, N.; Rumbles, G.; Li, C.Z.; Friend, R.H.; Jen, A.K.Y.; et al. Heterojunction modification for highly efficient organic-inorganic perovskite solar cells. ACS NANO 2014, 8, 12701–12709. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Dyck, O.; Poplawsky, J.; Keum, J.; Puretzky, A.; Das, S.; Ivanov, I.; Rouleau, C.; Duscher, G.; Geohegan, D.; et al. Perovskite Solar Cells with Near 100% Internal quantum efficiency based on large single crystalline grains and vertical bulk heterojunctions. J. Am. Chem. Soc. 2015, 137, 9210–9213. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bi, D.; Yi, C.; Decoppet, J.D.; Luo, J.; Zakeeruddin, S.M.; Hagfeldt, A.; Grätzel, M. A vacuum flash-assisted solution process for high-efficiency large-area perovskite solar cells. Science 2016, 353, 58–62. [Google Scholar] [CrossRef]
- Yang, W.S.; Park, B.W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide-based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.-X.; Xue, D.-J.; Gao, D.; Han, Q.; Ge, Q.-Q.; Ma, J.-Y.; Ding, J.; Zhang, W.; Zhang, B.; Feng, Y.; et al. High-mobility hydrophobic conjugated polymer as effective interlayer for air-stable efficient perovskite solar cells. Sol. RRL 2018, 3, 1800232. [Google Scholar] [CrossRef] [Green Version]
- Ahiboz, D.; Nasser, H.; Aygün, E.; Bek, A.; Turan, R. Electrical response of electron selective atomic layer deposited TiO2-x heterocontacts on crystalline silicon substrates. Semicond. Sci. Technol. 2018, 33, 045013. [Google Scholar] [CrossRef]
- Anitha, V.C.; Banerjee, A.N.; Joo, S.W. Recent developments in TiO2 as n- and p-type transparent semiconductors: Synthesis, modification, properties, and energy-related applications. J. Mater. Sci. 2015, 50, 7495–7536. [Google Scholar] [CrossRef]
- Dueñas, S.; Castán, H.; García, H.; San Andrés, E.; Toledano-Luque, M.; Mártil, I.; González-Díaz, G.; Kukli, K.; Uustare, T.; Aarik, J. A comparative study of the electrical properties of TiO2 films grown by high-pressure reactive sputtering and atomic layer deposition. Semicond. Sci. Technol. 2005, 20, 1011–1051. [Google Scholar] [CrossRef]
- Nabatame, T.; Ohi, A.; Chikyo, T.; Kimura, M.; Yamada, H.; Ohishi, T. Electrical properties of anatase TiO2 films by atomic layer deposition and low annealing temperature. J. Vac. Sci. Technol. B 2014, 32, 03D121. [Google Scholar] [CrossRef]
- Nowotny, M.K.; Bak, T.; Nowotny, J. Electrical properties and defect chemistry of TiO2 single crystal. I. Electrical conductivity. J. Phys. Chem B 2006, 110, 16270–16282. [Google Scholar] [CrossRef] [PubMed]
- Bak, T.; Nowotny, J.; Nowotny, M.K. Defect disorder of titanium dioxide. J. Phys. Chem. B 2006, 110, 21560–21567. [Google Scholar] [CrossRef] [PubMed]
- Furubayashi, Y.; Hitosugi, T.; Yamamoto, Y.; Inaba, K.; Kinoda, G.; Hirose, Y.; Shimada, T.; Hasegawa, T. A transparent metal: Nb–doped anatase TiO2. Appl. Phys. Lett. 2005, 86, 252101. [Google Scholar] [CrossRef]
- Niemelä, J.-P.; Hirose, Y.; Shigematsu, K.; Sano, M.; Hasegawa, T.; Karppinen, M. Suppressed grain-boundary scattering in atomic layer deposited Nb:TiO2 thin films. Appl. Phys. Lett. 2015, 107, 192102. [Google Scholar] [CrossRef]
- Potlog, T.; Dimitriu, P.; Dobromir, M.; Manole, A.; Luca, D. Nb-doped TiO2 thin films for photovoltaic applications. Mater. Des. 2015, 85, 558–563. [Google Scholar] [CrossRef]
- Seeger, K. Semiconductor Physics, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1982; p. 462. [Google Scholar]
- Ašmontas, S.; Gradauskas, J.; Petkun, V.; Seliuta, D.; Sužiedėlis, A.; Urbelis, A. Hot electron effect in degenerate semiconductor tunnel junction. Acta Phys. Pol. 2005, 107, 198–202. [Google Scholar] [CrossRef]
- Nogawa, H.; Chikamatsu, A.; Hirose, Y.; Nakao, S.; Kumigashira, H.; Oshima, M.; Hasegawa, T. Carrier compensation mechanism in heavily Nb-doped anatase Ti1-xNbxO2+δ epitaxial thin films. J. Phys. D Appl. Phys. 2011, 44, 365–404. [Google Scholar] [CrossRef]
- Niemelä, J.-P.; Marin, G.; Karppinen, M. Titanium dioxide thin films by atomic layer deposition: A review. Semicond. Sci. Technol. 2017, 32, 093005. [Google Scholar] [CrossRef]
- Mostovyi, A.I.; Brus, V.V.; Maryanchuk, P.D. Charge transport mechanisms in anisotype n-TiO2/p-Si heterostructures. Semiconductors 2013, 47, 799–803. [Google Scholar] [CrossRef]
- Zide, J.M.O.; Kleiman-Shwarsctein, A.; Standwitz, N.C.; Zimmerman, J.D.; Steenblock-Smith, T.; Gossard, A.C.; Forman, A.; Ivanovskaya, A.; Stucky, G.D. Increased efficiency in multijunction solar cells through the incorporation of semimetallic ErAs nanoparticles into tunnel junction. Appl. Phys. Lett. 2006, 88, 162103. [Google Scholar] [CrossRef] [Green Version]
- Björk, M.T.; Schmid, H.; Bessire, C.D.; Moselund, K.E.; Ghoneim, H.; Karg, S.; Lörtscher, E.; Riel, H. Si-InAs heterojunction Esaki tunnel diodes with high current densities. Appl. Phys. Lett. 2010, 97, 163501. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ašmontas, S.; Anbinderis, M.; Gradauskas, J.; Juškėnas, R.; Leinartas, K.; Lučun, A.; Selskis, A.; Staišiūnas, L.; Stanionytė, S.; Sužiedėlis, A.; et al. Low Resistance TiO2/p-Si Heterojunction for Tandem Solar Cells. Materials 2020, 13, 2857. https://doi.org/10.3390/ma13122857
Ašmontas S, Anbinderis M, Gradauskas J, Juškėnas R, Leinartas K, Lučun A, Selskis A, Staišiūnas L, Stanionytė S, Sužiedėlis A, et al. Low Resistance TiO2/p-Si Heterojunction for Tandem Solar Cells. Materials. 2020; 13(12):2857. https://doi.org/10.3390/ma13122857
Chicago/Turabian StyleAšmontas, Steponas, Maksimas Anbinderis, Jonas Gradauskas, Remigijus Juškėnas, Konstantinas Leinartas, Andžej Lučun, Algirdas Selskis, Laurynas Staišiūnas, Sandra Stanionytė, Algirdas Sužiedėlis, and et al. 2020. "Low Resistance TiO2/p-Si Heterojunction for Tandem Solar Cells" Materials 13, no. 12: 2857. https://doi.org/10.3390/ma13122857
APA StyleAšmontas, S., Anbinderis, M., Gradauskas, J., Juškėnas, R., Leinartas, K., Lučun, A., Selskis, A., Staišiūnas, L., Stanionytė, S., Sužiedėlis, A., Šilėnas, A., & Širmulis, E. (2020). Low Resistance TiO2/p-Si Heterojunction for Tandem Solar Cells. Materials, 13(12), 2857. https://doi.org/10.3390/ma13122857





