Enhanced Visible Light Active WO3 Thin Films Toward Air Purification: Effect of the Synthesis Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
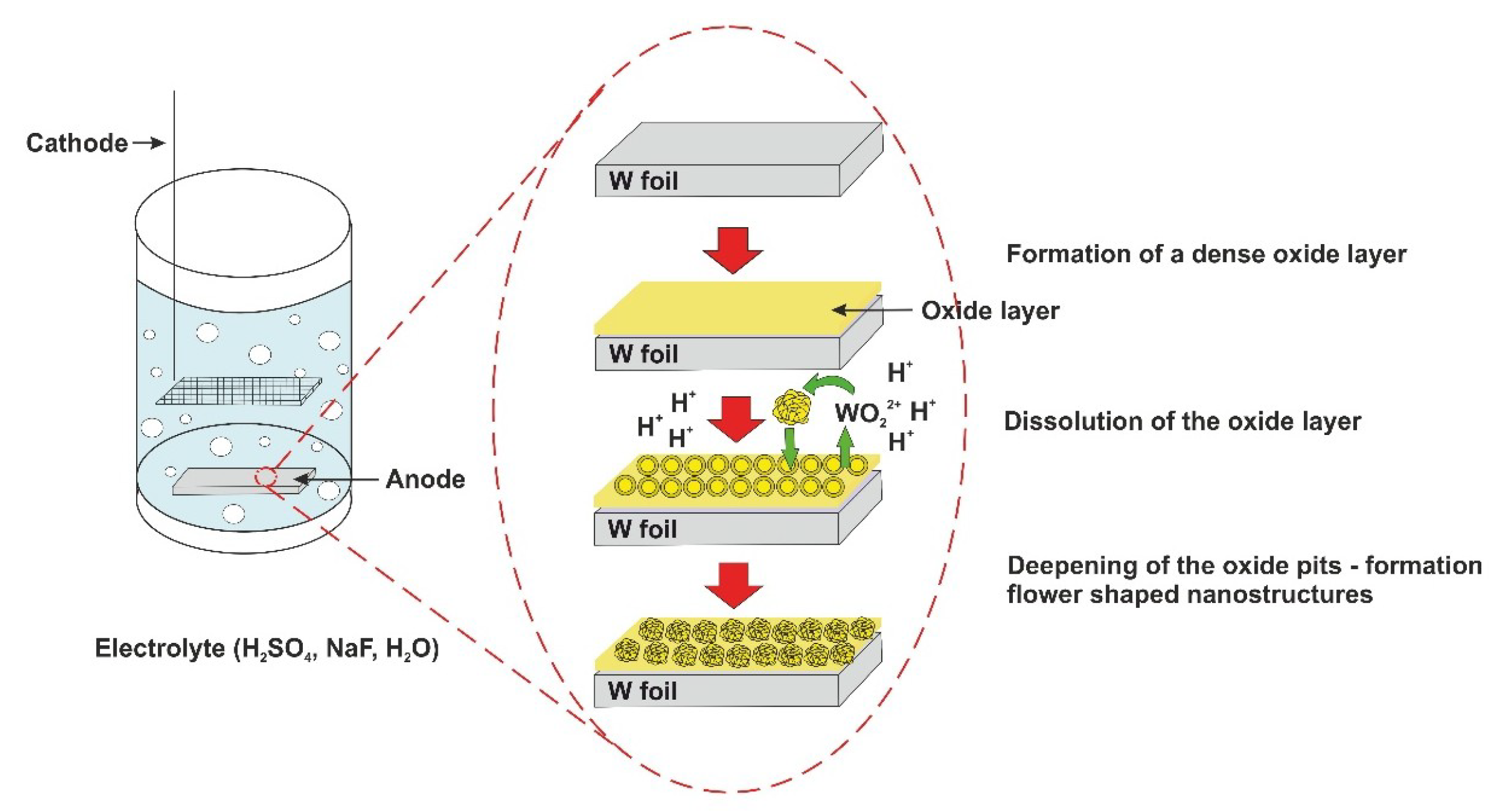
2.2. Synthesis of WO3 Nanoflowers
2.3. Material Characterization
2.4. Photocatalytic Performance
2.5. Photoelectrochemical Activity
3. Results
3.1. Morphology
3.1.1. Effect of the Anodization Potential
3.1.2. Effect of the Anodization Time
3.1.3. Effect of the Sulfuric Acid Concentration
3.1.4. Effect of the Sodium Fluoride Concentration
3.2. Crystallographic Structure
3.3. Optical and Photoluminescence Properties
3.4. Photocatalytic Activity
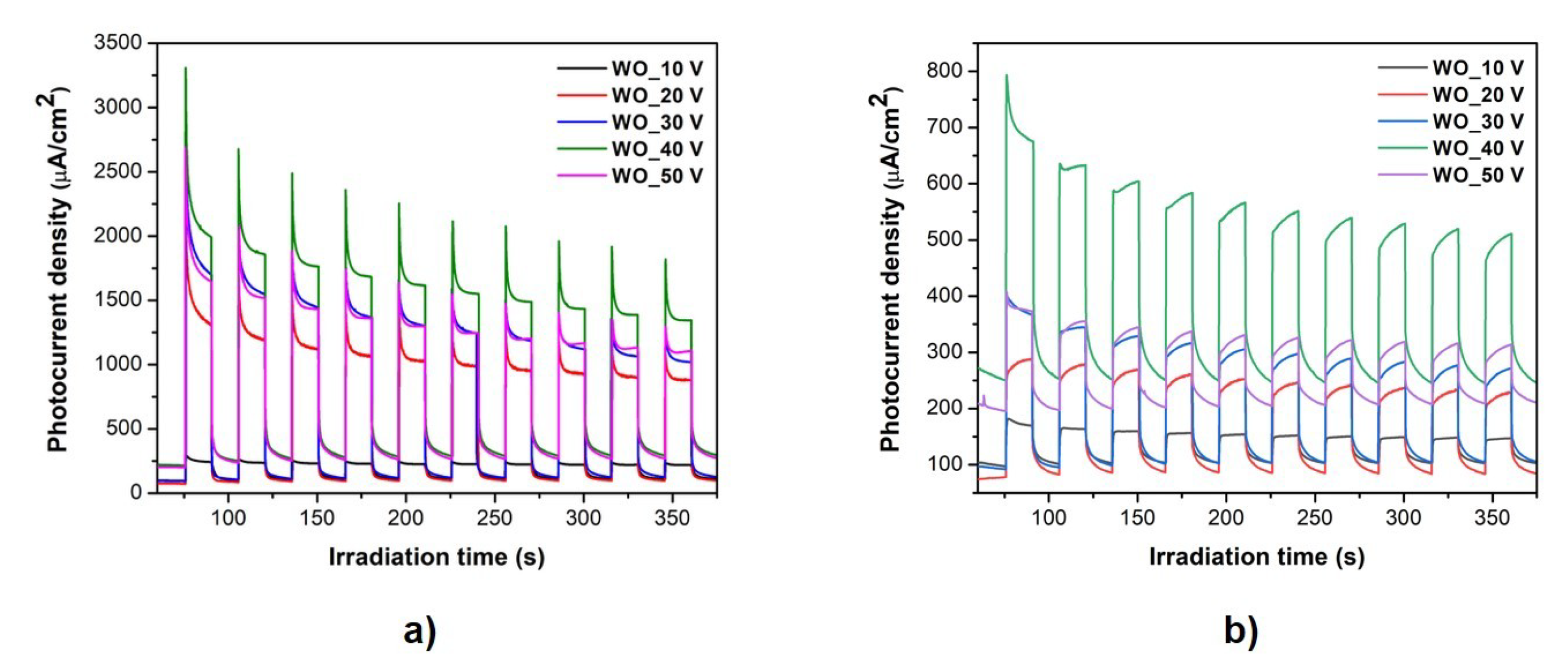
3.5. Photoelectrochemical Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Available online: https://www.who.int/gho/phe/outdoor_air_pollution/burden/en/ (accessed on 16 July 2020).
- Ghime, D.; Ghosh, P. Advanced Oxidation Processes: A Powerful Treatment Option for the Removal of Recalcitrant Organic Compounds. Adv. Oxid. Process. Appl. Trends Prospect 2020, 1–12. [Google Scholar] [CrossRef]
- Dewil, R.; Mantzavinos, D.; Poulios, I.; Rodrigo, M.A. New perspectives for Advanced Oxidation Processes. J. Environ. Manage. 2017, 195, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment–A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemannt, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 69–96. [Google Scholar] [CrossRef]
- Maira, A.J.; Yeung, K.L.; Soria, J.; Coronado, J.M.; Belver, C.; Lee, C.Y.; Augugliaro, V. Gas-phase photo-oxidation of toluene using nanometer-size TiO2 catalysts. Appl. Catal. B Environ. 2001, 29, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Weon, S.; He, F.; Choi, W. Status and challenges in photocatalytic nanotechnology for cleaning air polluted with volatile organic compounds: Visible light utilization and catalyst deactivation. Environ. Sci. Nano 2019, 6, 3185–3214. [Google Scholar] [CrossRef]
- Venkata Laxma Reddy, P.; Kim, K.H.; Kim, Y.H. A review of photocatalytic treatment for various air pollutants. Asian J. Atmos. Environ. 2011, 5, 181–188. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Kubacka, A.; Fernández-García, M.; Colón, G. Advanced nanoarchitectures for solar photocatalytic applications. Chem. Rev. 2012, 112, 1555–1614. [Google Scholar] [CrossRef]
- Nah, Y.C.; Paramasivam, I.; Schmuki, P. Doped TiO2 and TiO2 nanotubes: Synthesis and applications. ChemPhysChem 2010, 11, 2698–2713. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, H.; Hao, Y.; Ma, J.; Li, W.; Cai, S. Photoelectrochemical properties of metal-ion-doped TiO2 nanocrystalline electrodes. Thin Solid Film. 1999, 349, 120–125. [Google Scholar] [CrossRef]
- Guo, Y.; Quan, X.; Lu, N.; Zhao, H.; Chen, S. High photocatalytic capability of self-assembled nanoporous WO3 with preferential orientation of (002) planes. Environ. Sci. Technol. 2007, 41, 4422–4427. [Google Scholar] [CrossRef] [PubMed]
- Bamwenda, G.R.; Arakawa, H. Visible light induced photocatalytic activity of tungsten trioxide powders. Appl. Catal. A Gen. 2001, 210, 181–191. [Google Scholar] [CrossRef]
- Gondal, M.A.; Dastageer, M.A.; Khalil, A. Synthesis of nano-WO3 and its catalytic activity for enhanced antimicrobial process for water purification using laser induced photo-catalysis. Catal. Commun. 2009, 11, 214–219. [Google Scholar] [CrossRef]
- Waldner, G.; Brüger, A.; Gaikwad, N.S.; Neumann-Spallart, M. WO3 thin films for photoelectrochemical purification of water. Chemosphere 2007, 67, 779–784. [Google Scholar] [CrossRef]
- Su, X.; Li, Y.; Jian, J.; Wang, J. In situ etching WO3 nanoplates: Hydrothermal synthesis, photoluminescence and gas sensor properties. Mater. Res. Bull. 2010, 45, 1960–1963. [Google Scholar] [CrossRef]
- An, S.; Park, S.; Ko, H.; Lee, C. Fabrication of WO3 nanotube sensors and their gas sensing properties. Ceram. Int. 2014, 40, 1423–1429. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Song, L.; Gong, H.; Niu, Q. Ionic liquid-assisted synthesis of WO3 particles with enhanced gas sensing properties. J. Mater. Chem. A 2013, 1, 15377. [Google Scholar] [CrossRef]
- Miseki, Y.; Kusama, H.; Sugihara, H.; Sayama, K. Cs-modified WO3 photocatalyst showing efficient solar energy conversion for O2 production and Fe (III) ion reduction under visible light. J. Phys. Chem. Lett. 2010, 1, 1196–1200. [Google Scholar] [CrossRef]
- Miseki, Y.; Kusama, H.; Sayama, K. Photocatalytic energy storage over surface-modified WO3 using V 5+/V4+ redox mediator. Chem. Lett. 2012, 41, 1489–1491. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.G.; Sakai, G.; Shimanoe, K.; Teraoka, Y.; Miura, N.; Yamazoe, N. Preparation of size and habit-controlled nano crystallites of tungsten oxide. Sens. Actuators B Chem. 2003, 93, 486–494. [Google Scholar] [CrossRef]
- Santato, C.; Odziemkowski, M.; Ulmann, M.; Augustynski, J. Crystallographically oriented mesoporous WO3 films: Synthesis, characterization, and applications. J. Am. Chem. Soc. 2001, 123, 10639–10649. [Google Scholar] [CrossRef] [PubMed]
- Badilescu, S.; Ashrit, P.V. Study of sol-gel prepared nanostructured WO3 thin films and composites for electrochromic applications. Solid State Ion. 2003, 158, 187–197. [Google Scholar] [CrossRef]
- Yu, Z.; Jia, X.; Du, J.; Zhang, J. Electrochromic WO3 films prepared by a new electrodeposition method. Sol. Energy Mater. Sol. Cells 2000, 64, 55–63. [Google Scholar] [CrossRef]
- Brescacin, E.; Basato, M.; Tondello, E. Amorphous WO3 films via chemical vapor deposition from metallorganic precursors containing phosphorus dopant. Chem. Mater. 1999, 11, 314–323. [Google Scholar] [CrossRef]
- Palgrave, R.G.; Parkin, I.P. Aerosol assisted chemical vapour deposition of photochromic tungsten oxide and doped tungsten oxide thin films. J. Mater. Chem. 2004, 14, 2864–2867. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Macak, J.M.; Sieber, I.; Taveira, L.; Ghicov, A.; Sirotna, K.; Schmuki, P. Self-organized porous WO3 formed in NaF electrolytes. Electrochem. Commun. 2005, 7, 295–298. [Google Scholar] [CrossRef]
- Ng, C.; Ye, C.; Ng, Y.H.; Amal, R. Flower-shaped tungsten oxide with inorganic fullerene-like structure: Synthesis and characterization. Cryst. Growth Des. 2010, 10, 3794–3801. [Google Scholar] [CrossRef]
- Srichaiyaperk, T.; Aiempanakit, K.; Horprathum, M.; Eiamchai, P.; Chananonnawathorn, C.; Limwichean, S.; Chindaudom, P. Effects of annealing treatment on WO3 thin films prepared by DC reactive magnetron sputtering. Adv. Mater. Res. 2014, 979, 248–250. [Google Scholar] [CrossRef]
- Liang, Y.C.; Chang, C.W. Preparation of orthorhombic WO3 thin films and their crystal quality-dependent dye photodegradation ability. Coatings 2019, 9, 90. [Google Scholar] [CrossRef] [Green Version]
- Marszalek, K. Magnetron-sputtered WO3 films for electrochromic devices. Thin Solid Film. 1989, 175, 227–233. [Google Scholar] [CrossRef]
- Babu, M.B.; Madhuri, K.V. Structural, morphological and optical properties of electron beam evaporated WO3 thin films. J. Taibah Univ. Sci. 2017, 11, 1232–1237. [Google Scholar] [CrossRef] [Green Version]
- Sivakumar, R.; Gopalakrishnan, R.; Jayachandran, M.; Sanjeeviraja, C. Preparation and characterization of electron beam evaporated WO3 thin films. Opt. Mater. (Amst) 2007, 29, 679–687. [Google Scholar] [CrossRef]
- Malm, J.; Sajavaara, T.; Karppinen, M. Atomic layer deposition of WO3 thin films using W(CO)6 and O3 precursors. Chem. Vap. Depos. 2012, 18, 245–248. [Google Scholar] [CrossRef]
- Li, B.; Gao, X.; Zhang, H.C.; Yuan, C. Energy modeling of electrochemical anodization process of titanium dioxide nanotubes. ACS Sustain. Chem. Eng. 2014, 2, 404–410. [Google Scholar] [CrossRef]
- Berger, S.; Tsuchiya, H.; Ghicov, A.; Schmuki, P. High photocurrent conversion efficiency in self-organized porous WO3. Appl. Phys. Lett. 2006, 88, 88–91. [Google Scholar] [CrossRef]
- Caramori, S.; Cristino, V.; Meda, L.; Tacca, A.; Argazzi, R.; Bignozzi, C.A. Efficient anodically grown WO3 for photoelectrochemical water splitting. Energy Procedia 2012, 22, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Nah, Y.C.; Ghicov, A.; Kim, D.; Schmuki, P. Enhanced electrochromic properties of self-organized nanoporous WO3. Electrochem. Commun. 2008, 10, 1777–1780. [Google Scholar] [CrossRef]
- Ou, J.Z.; Rani, R.A.; Balendhran, S.; Zoolfakar, A.S.; Field, M.R.; Zhuiykov, S.; O’Mullane, A.P.; Kalantar-Zadeh, K. Anodic formation of a thick three-dimensional nanoporous WO3 film and its photocatalytic property. Electrochem. Commun. 2013, 27, 128–132. [Google Scholar] [CrossRef]
- Ou, J.Z.; Balendhran, S.; Field, M.R.; McCulloch, D.G.; Zoolfakar, A.S.; Rani, R.A.; Zhuiykov, S.; O’Mullane, A.P.; Kalantar-Zadeh, K. The anodized crystalline WO3 nanoporous network with enhanced electrochromic properties. Nanoscale 2012, 4, 5980–5988. [Google Scholar] [CrossRef]
- Mukherjee, N.; Paulose, M.; Varghese, O.K.; Mor, G.K.; Grimes, C.A. Fabrication of nanoporous tungsten oxide by galvanostatic anodization. J. Mater. Res. 2003, 18, 2296–2299. [Google Scholar] [CrossRef]
- Yu, L.; Kalantar-zadeh, K.; Zhu, J.; Latham, K.; Wlodarski, W.; Hu, Z.; Sadek, A.Z.; Breedon, M.; Zheng, H.; Bhargava, S.K.; et al. High-Temperature Anodized WO3 Nanoplatelet Films for Photosensitive Devices. Langmuir 2009, 25, 9545–9551. [Google Scholar] [CrossRef]
- Ng, C.; Ng, Y.H.; Iwase, A.; Amal, R. Influence of annealing temperature of WO3 in photoelectrochemical conversion and energy storage for water splitting. ACS Appl. Mater. Interfaces 2013, 5, 5269–5275. [Google Scholar] [CrossRef] [PubMed]
- Nischk, M.; Mazierski, P.; Gazda, M.; Zaleska, A. Ordered TiO2 nanotubes: The effect of preparation parameters on the photocatalytic activity in air purification process. Appl. Catal. B Environ. 2014, 144, 674–685. [Google Scholar] [CrossRef]
- Macak, J.M.; Schmuki, P. Anodic growth of self-organized anodic TiO2 nanotubes in viscous electrolytes. Electrochim. Acta 2006, 52, 1258–1264. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of electrochemical equilibria in aqueous solutions 2 English ed. Natl. Assoc. Corros. Eng. Houst. TX 1974. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 nanotubes: Self-organized electrochemical formation, properties and applications. Curr. Opin. Solid State Mater. Sci. 2007, 11, 3–18. [Google Scholar] [CrossRef]
- Chai, Y.; Tam, C.W.; Beh, K.P.; Yam, F.K.; Hassan, Z. Porous WO3 formed by anodization in oxalic acid. J. Porous Mater. 2013, 20, 997–1002. [Google Scholar] [CrossRef]
- Anik, M.; Osseo-Asare, K. Effect of pH on the Anodic Behavior of Tungsten. J. Electrochem. Soc. 2002, 149, B224. [Google Scholar] [CrossRef]
- Macdonald, D.D.; Sikora, E.; Sikora, J. The kinetics of growth of the passive film on tungsten in acidic phosphate solutions. Electrochim. Acta 1998, 43, 2851–2861. [Google Scholar] [CrossRef]
- Lai, C.W. Photocatalysis and photoelectrochemical properties of tungsten trioxide nanostructured films. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- El-Basiouny, M.S.; Hassan, S.A.; Hefny, M.M. On the electrochemical behaviour of tungsten: The formation and dissolution of tungsten oxide in sulphuric acid solutions. Corros. Sci. 1980, 20, 909–917. [Google Scholar] [CrossRef]
- Baserga, A.; Russo, V.; Di Fonzo, F.; Bailini, A.; Cattaneo, D.; Casari, C.S.; Li Bassi, A.; Bottani, C.E. Nanostructured tungsten oxide with controlled properties: Synthesis and Raman characterization. Thin Solid Film. 2007, 515, 6465–6469. [Google Scholar] [CrossRef]
- Daniel, M.F.; Desbat, B.; Lassegues, J.C.; Gerand, B.; Figlarz, M. Infrared and Raman study of WO3 tungsten trioxides and WO3xH2O tungsten trioxide tydrates. J. Solid State Chem. 1987, 67, 235–247. [Google Scholar] [CrossRef]
- Butler, M.A. Photoelectrolysis and physical properties of the semiconducting electrode WO2. J. Appl. Phys. 1977, 48, 1914–1920. [Google Scholar] [CrossRef]
- Teoh, L.G.; Shieh, J.; Lai, W.H.; Hung, I.M.; Hon, M.H. Structure and optical properties of mesoporous tungsten oxide. J. Alloys Compd. 2005, 396, 251–254. [Google Scholar] [CrossRef]
- Fujihara, S.; Ogawa, Y.; Kasai, A. Tunable visible photoluminescence from ZnO thin films through Mg-doping and annealing. Chem. Mater. 2004, 16, 2965–2968. [Google Scholar] [CrossRef]
- Sreekantan, S.; Saharudin, K.A.; Lockman, Z.; Tzu, T.W. Fast-rate formation of TiO2 nanotube arrays in an organic bath and their applications in photocatalysis. Nanotechnology 2010, 21. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Lin, S.; Liu, L.; Hu, J.; Cui, W. Oil-in-water self-assembled Ag@AgCl QDs sensitized Bi2WO6: Enhanced photocatalytic degradation under visible light irradiation. Appl. Catal. B Environ. 2015, 164, 192–203. [Google Scholar] [CrossRef]
- Zhu, Z.; Yan, Y.; Li, J. Preparation of flower-like BiOBr-WO3-Bi2WO6 ternary hybrid with enhanced visible-light photocatalytic activity. J. Alloy. Compd. 2015, 651, 184–192. [Google Scholar] [CrossRef]
- Kalantar-zadeh, K.; Sadek, A.Z.; Zheng, H.; Bansal, V.; Bhargava, S.K.; Wlodarski, W.; Zhu, J.; Yu, L.; Hu, Z. Nanostructured WO3 films using high temperature anodization. Sens. Actuators B Chem. 2009, 142, 230–235. [Google Scholar] [CrossRef]
- Zhuang, H.; Lin, C.; Lai, Y.; Sun, L.; Li, J. Some Critical Structure Factors of Titanium Oxide Nanotube Array in Its Photocatalytic Activity. Environ. Sci. Technol. 2007, 41, 4735–4740. [Google Scholar] [CrossRef] [PubMed]
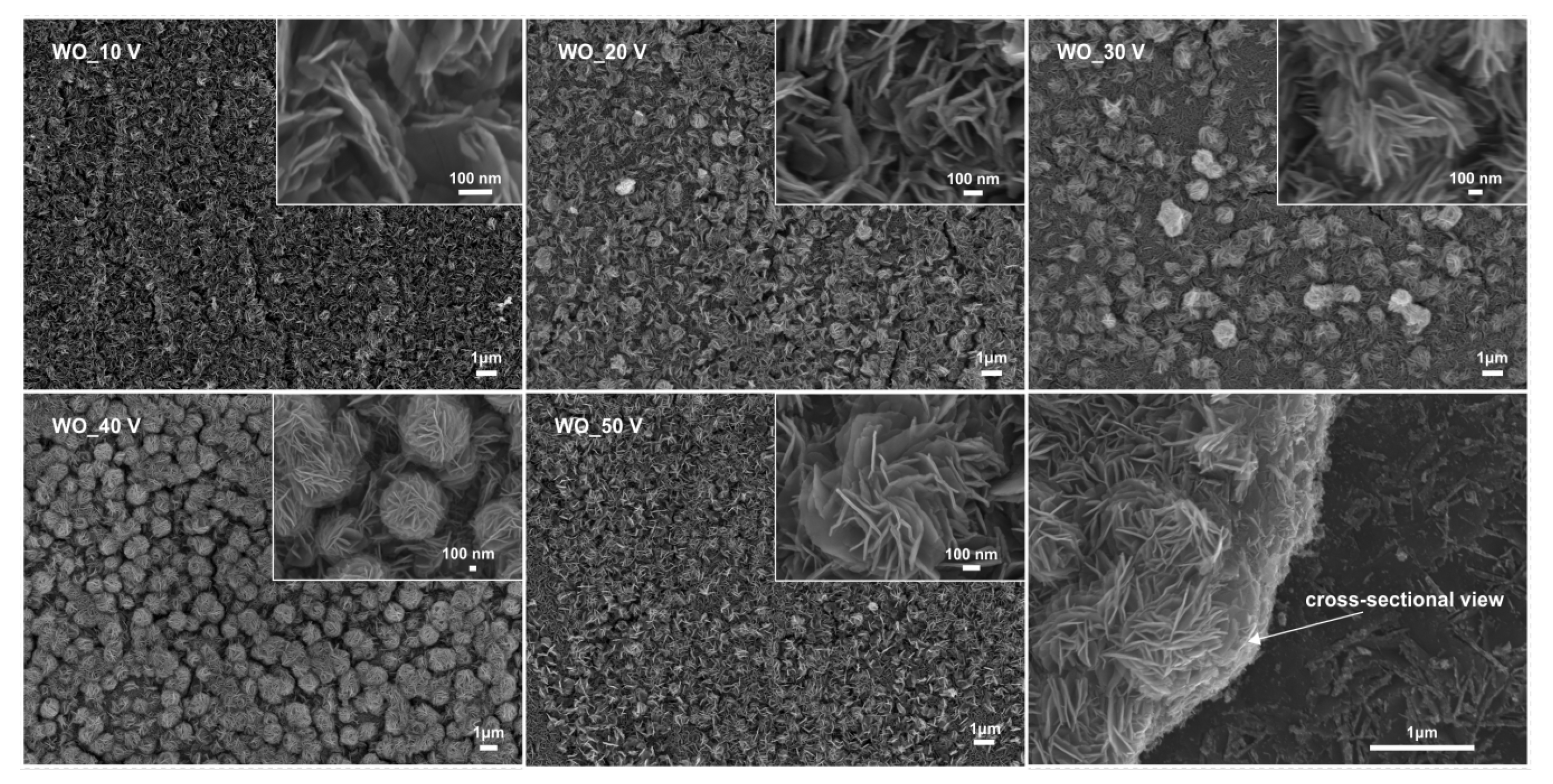

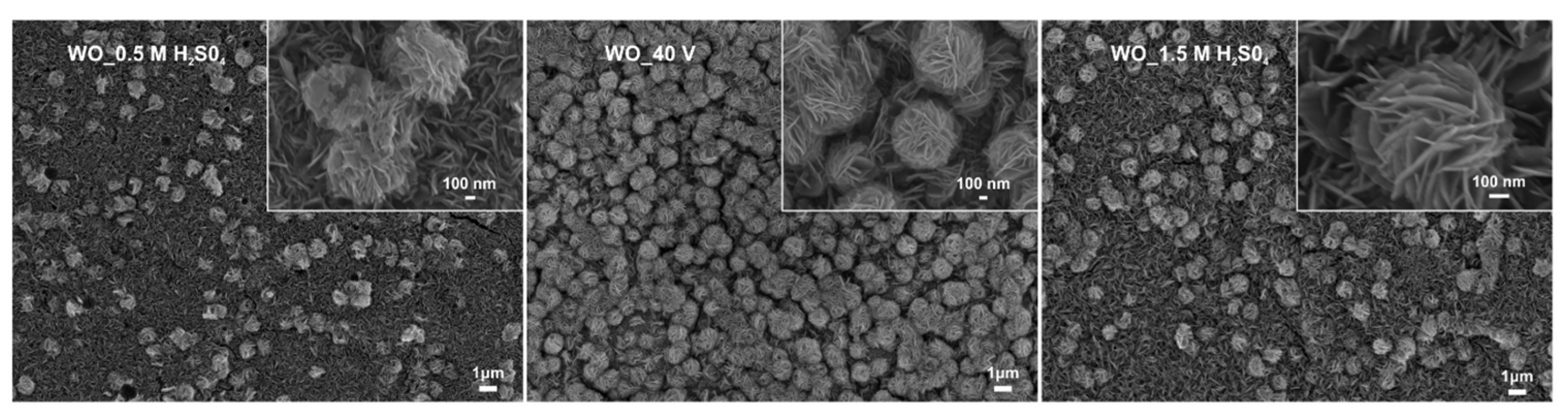
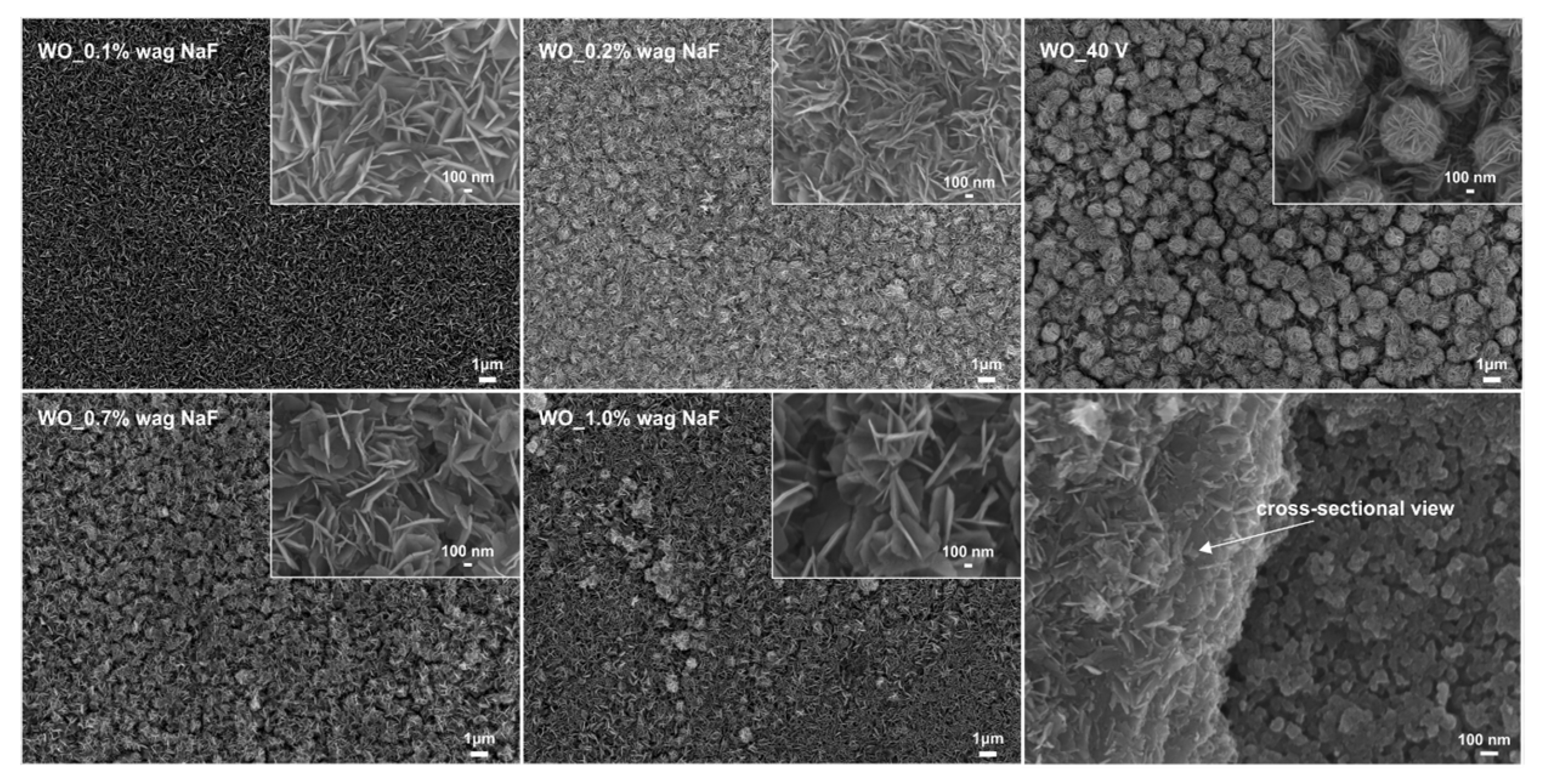

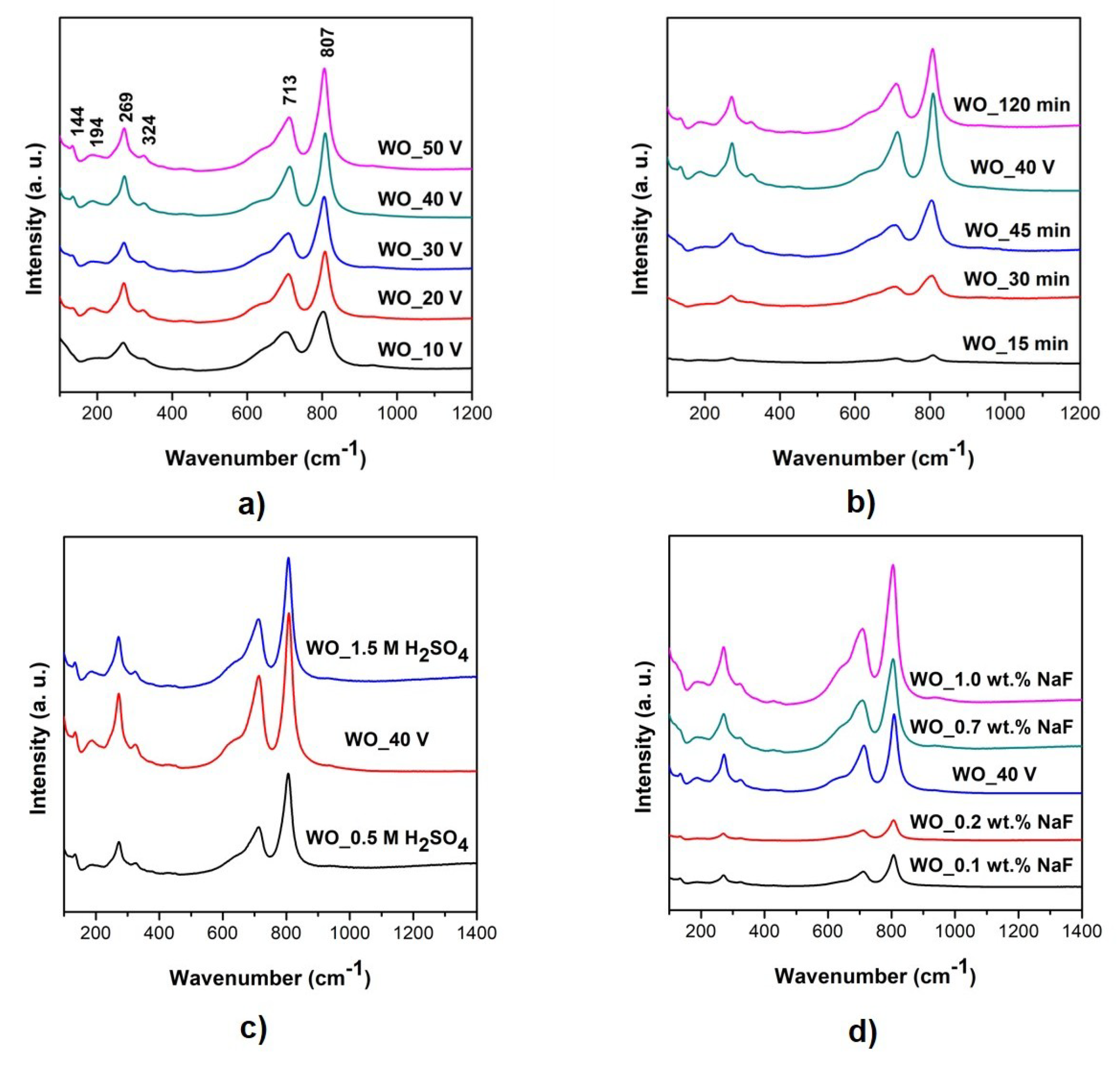
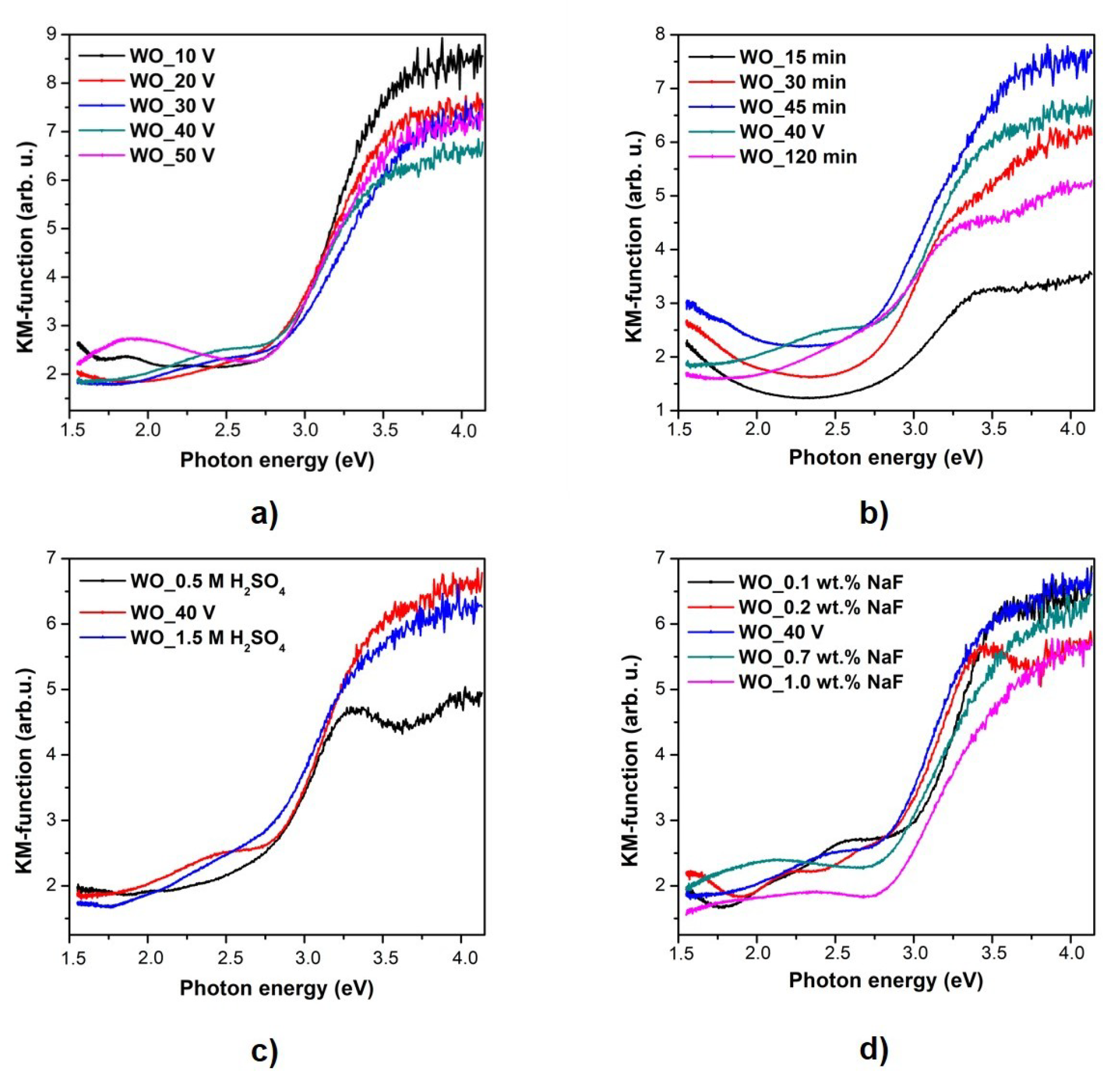
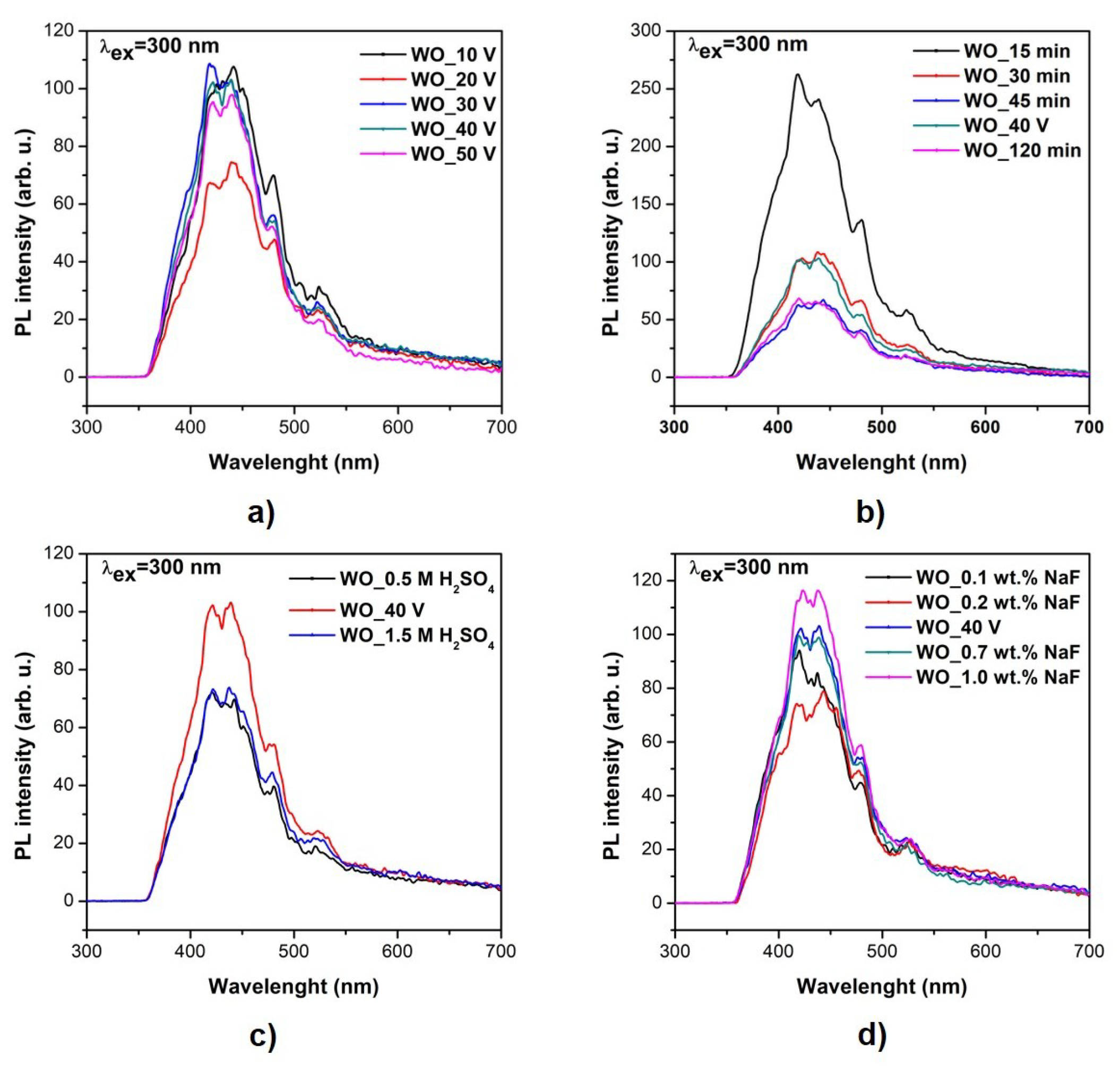
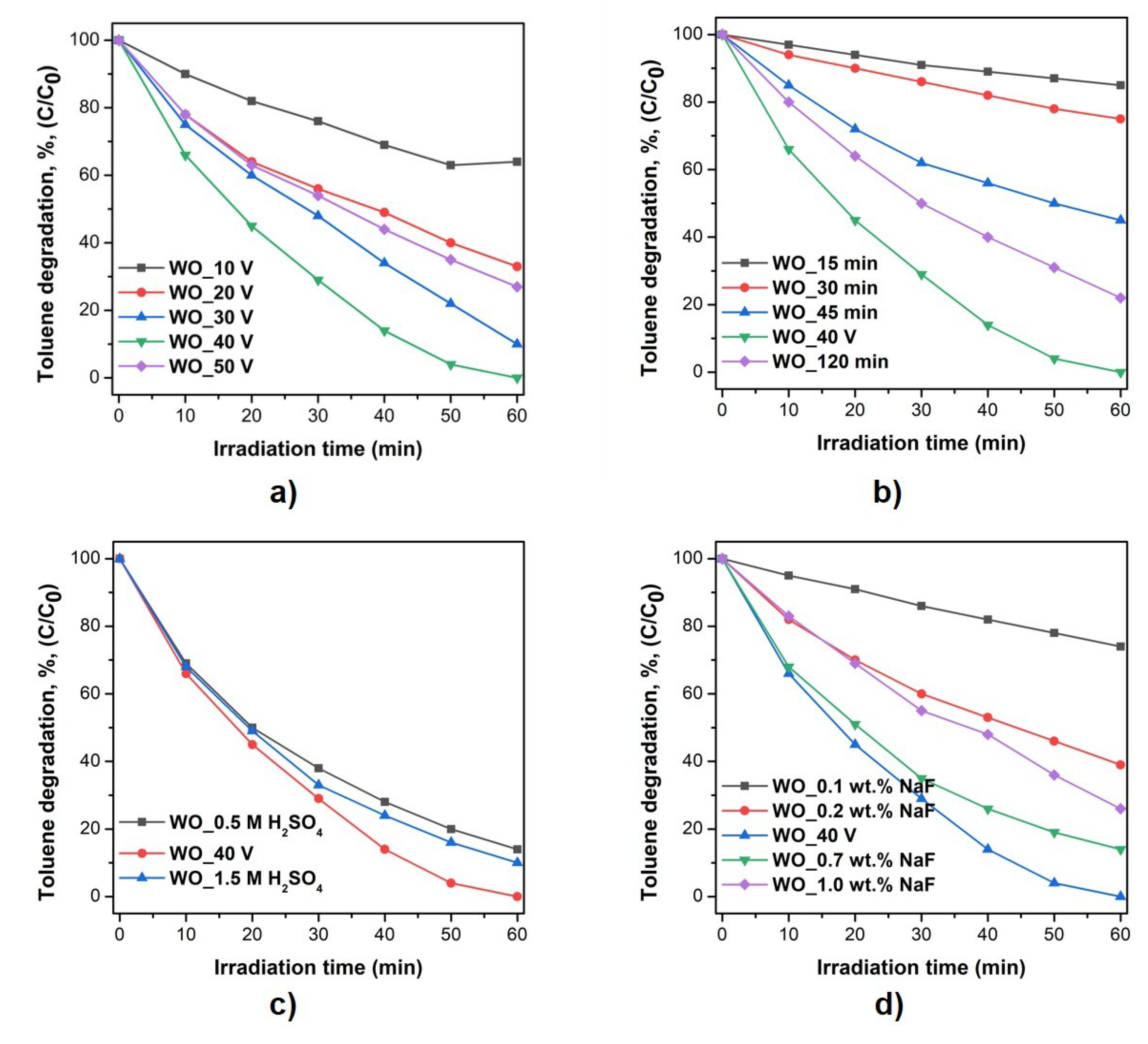
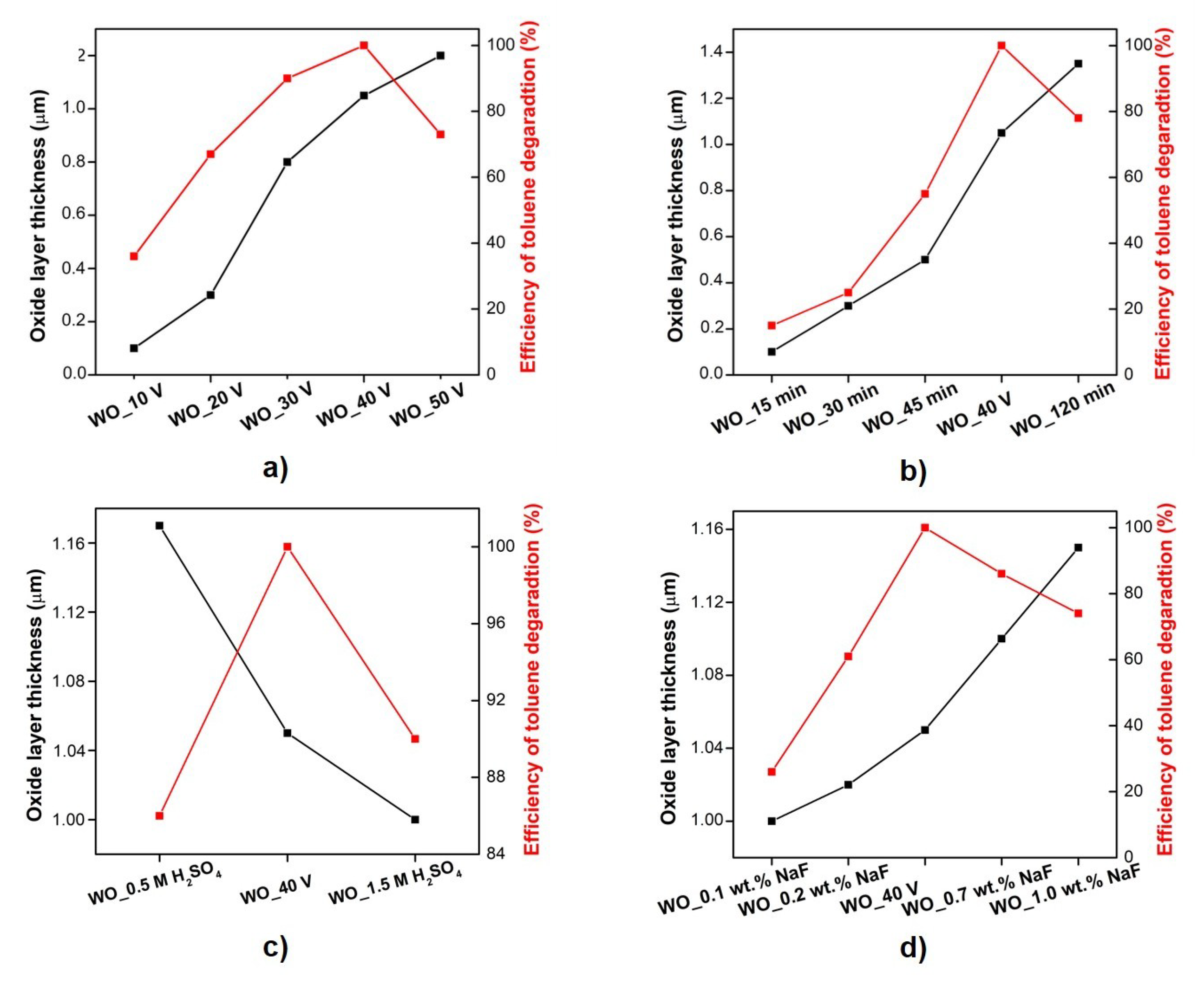
| Sample Label | Preparation Conditions (Electrolyte Composition and Anodization Parameters) | Flower Diameter (nm) | Oxide Layer Thickness (µm) | Platelets Thickness (nm) | Optical Band Gap (eV) | Efficiency of Toluene Degradation after 60 min of Irradiation (%) | |
|---|---|---|---|---|---|---|---|
| Constant Parameters | Variable Parameters | ||||||
| WO_10 V | 1.0 M H2SO4, 0.5 wt.% NaF, 90 min | 10 V | - ** | 0.1 ± 0.1 | 11.8 ± 0.1 | 2.75 | 36 |
| WO_20 V | 1.0 M H2SO4, 0.5 wt.% NaF, 90 min | 20 V | - ** | 0.3 ± 0.1 | 12.8 ± 0.2 | 2.61 | 67 |
| WO_30 V | 1.0 M H2SO4, 0.5 wt.% NaF, 90 min | 30 V | 0.72 ± 0.1 | 0.8 ± 0.1 | 13.6 ± 0.2 | 2.65 | 90 |
| WO_40 V * | 1.0 M H2SO4, 0.5 wt.% NaF, 90 min | 40 V | 1.21 ± 0.1 | 1.05 ± 0.1 | 14.9 ± 0.3 | 2.52 | 100 |
| WO_50 V | 1.0 M H2SO4, 0.5 wt.% NaF, 90 min | 50 V | 0.81 ± 0.2 | 1.2 ± 0.1 | 16.9 ± 0.3 | 2.65 | 73 |
| WO_15 min | 1.0 M H2SO4, 0.5 wt.% NaF, 40 V | 15 min | - ** | 0.1 ± 0.02 | 11.2 ± 0.1 | 2.66 | 15 |
| WO_30 min | 1.0 M H2SO4, 0.5 wt.% NaF, 40 V | 30 min | 0.91 ± 0.1 | 0.3 ± 0.05 | 12.3 ± 0.1 | 2.64 | 25 |
| WO_45 min | 1.0 M H2SO4, 0.5 wt.% NaF, 40 V | 45 min | 1.04 ± 0.2 | 0.5 ± 0.1 | 13.1 ± 0.2 | 2.56 | 55 |
| WO_40 V * | 1.0 M H2SO4, 0.5 wt.% NaF, 40 V, | 90 min | 1.21 ± 0.1 | 1.05 ± 0.2 | 14.9 ± 0.2 | 2.52 | 100 |
| WO_120 min | 1.0 M H2SO4, 0.5 wt.% NaF, 40 V | 120 min | 1.52 ± 0.2 | 1.35 ± 0.1 | 15.6 ± 0.2 | 2.40 | 78 |
| WO_0.5 M H2SO4 | 0.5 wt.% NaF, 40 V, 90 mi | 0.5 M H2SO4 | 1.15 ± 0.1 | 1.17 ± 0.1 | 13.4 ± 0.2 | 2.63 | 86 |
| WO_40 V * | 0.5 wt.% NaF, 40 V, 90 min | 1.0 M H2SO4 | 1.21 ± 0.1 | 1.05 ± 0.1 | 14.9 ± 0.2 | 2.52 | 100 |
| WO_1.5 M H2SO4 | 0.5 wt.% NaF, 40 V, 90 min | 1.5 M H2SO4 | 1.10 ± 0.1 | 1.0 ± 0.1 | 15.3 ± 0.3 | 2.55 | 90 |
| WO_0.1 wt.% NaF | 1.0 M H2SO4, 40 V, 90 min | 0.1 wt.% NaF | - ** | 1.0 ± 0.01 | 11.3 ± 0.1 | 2.79 | 26 |
| WO_0.2 wt.% NaF | 1.0 M H2SO4, 40 V, 90 min | 0.2 wt.% NaF | - ** | 1.02 ± 0.1 | 12.1 ± 0.2 | 2.62 | 61 |
| WO_40 V * | 1.0 M H2SO4, 40 V, 90 min | 0.5 wt.% NaF | 1.21 ± 0.1 | 1.05 ± 0.01 | 14.9 ± 0.3 | 2.52 | 100 |
| WO_0.7 wt.% NaF | 1.0 M H2SO4, 40 V, 90 min | 0.7 wt.% NaF | - ** | 1.1 ± 0.01 | 15.9 ± 0.3 | 2.55 | 86 |
| WO_1.0 wt.% NaF | 1.0 M H2SO4, 40 V, 90 min | 1.0 wt.% NaF | - ** | 1.15 ± 0.01 | 17.8 ± 0.3 | 2.66 | 74 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pancielejko, A.; Rzepnikowska, M.; Zaleska-Medynska, A.; Łuczak, J.; Mazierski, P. Enhanced Visible Light Active WO3 Thin Films Toward Air Purification: Effect of the Synthesis Conditions. Materials 2020, 13, 3506. https://doi.org/10.3390/ma13163506
Pancielejko A, Rzepnikowska M, Zaleska-Medynska A, Łuczak J, Mazierski P. Enhanced Visible Light Active WO3 Thin Films Toward Air Purification: Effect of the Synthesis Conditions. Materials. 2020; 13(16):3506. https://doi.org/10.3390/ma13163506
Chicago/Turabian StylePancielejko, Anna, Marta Rzepnikowska, Adriana Zaleska-Medynska, Justyna Łuczak, and Paweł Mazierski. 2020. "Enhanced Visible Light Active WO3 Thin Films Toward Air Purification: Effect of the Synthesis Conditions" Materials 13, no. 16: 3506. https://doi.org/10.3390/ma13163506
APA StylePancielejko, A., Rzepnikowska, M., Zaleska-Medynska, A., Łuczak, J., & Mazierski, P. (2020). Enhanced Visible Light Active WO3 Thin Films Toward Air Purification: Effect of the Synthesis Conditions. Materials, 13(16), 3506. https://doi.org/10.3390/ma13163506









