Recent Advances in Barrier Layer of Cu Interconnects
Abstract
1. Introduction
2. Cu Interconnects and Diffusion Barrier Materials
3. Platinum Group Metals (PGM)-Based Materials
4. Two-Dimensional (2D) Materials
5. Self-Assembled Molecular Layers (SAMs)
6. High-Entropy Alloys (HEAs)
7. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Andricacos, P.C. Copper On-Chip Interconnections. Electrochem. Soc. Interface. 1999, 8, 32–37. [Google Scholar]
- Li, B.; Sullivan, T.D.; Lee, T.C.; Badami, D. Reliability Challenges for Copper Interconnects. Microelectron. Reliab. 2004, 44, 365–380. [Google Scholar] [CrossRef]
- Dang, R.L.M.; Shigyo, N. Coupling Capacitances for Two-Dimensional Wires. IEEE Electr. Device 1981, 2, 196–197. [Google Scholar] [CrossRef]
- Stamper, A.K.; Fuselier, M.B.; Tian, X. Advanced Wiring RC Delay Issues for Sub-0.25-Micron General CMOS. Proc. Int. Interconnet. Tech. Conf. 1998, 62–68. [Google Scholar] [CrossRef]
- Zuckerman, L. IBM to Make Smaller and Faster Chips. The New York Times, 22 September 1997; D1. [Google Scholar]
- Nitta, T.; Ohmi, T.; Otsuki, M.; Takewaki, T.; Shibata, T. Electrical Properties of Giant-Grain Copper Thin Films Formed by a Low Kinetic Energy Particle Process. J. Electrochem. Soc. 1992, 139, 922–927. [Google Scholar] [CrossRef]
- Hu, C.K.; Harper, J.M.E. Copper Interconnections and Reliability. Mater. Chem. Phys. 1998, 52, 5–16. [Google Scholar] [CrossRef]
- Zhao, B.; Feiler, D.; Ramanathan, V.; Liu, Q.Z.; Brongo, M.; Wu, J.; Zhang, H.; Kuei, J.C.; Young, D.; Brown, J.; et al. Dual Damascene Interconnect of Copper and Low Permittivity Dielectric for High Performance Integrated Circuits. Electrochem. Solid State Lett. 1998, 1, 276–278. [Google Scholar] [CrossRef]
- Andricacos, P.; Uzoh, C.; Dukovic, J.; Horkans, J.; Deligianni, H. Damascene Copper Electroplating for Chip Interconnections. IBM. J. Res. Dev. 1998, 42, 567–574. [Google Scholar] [CrossRef]
- Lizama-Tzec, F.I.; Canché-Canul, L.; Oskam, G. Electrodeposition of Copper into Trenches from a Citrate Plating Bath. Electrochim. Acta. 2011, 56, 9391–9396. [Google Scholar] [CrossRef]
- Akolkar, R. Current Status and Advances in Damascene Electrodeposition. Encycl. Interfacial Chem. Surf. Sci. Electrochem. Elsevier 2018, 24–31. [Google Scholar] [CrossRef]
- Chang, C.A. Outdiffusion of Cu through Au: Comparison of (100) and (111) Cu Flms Epitaxially Deposited on Si, and Effects of Annealing Ambients. Appl. Phys. Lett. 1989, 55, 2754–2756. [Google Scholar] [CrossRef]
- Holloway, K.; Fryer, P.M. Tantalum as a Diffusion Barrier between Copper and Silicon. Appl. Phys. Lett. 1990, 57, 1736–1738. [Google Scholar] [CrossRef]
- Holloway, K.; Fryer, P.M.; Cabral, C., Jr.; Harper, J.M.E.; Bailey, P.J.; Kelleher, K.H. Tantalum as a Diffusion Barrier between Copper and Silicon: Failure Mechanism and Effect of Nitrogen Additions. J. Appl. Phys. 1992, 71, 5433–5444. [Google Scholar] [CrossRef]
- Catania, P.; Doyle, J.P.; Cuomo, J.J. Low Resistivity Body-Centered Cubic Tantalum Thin Films as Diffusion Barriers between Copper and Silicon. J. Vac. Sci. Technol. 1992, 10, 3318–3321. [Google Scholar] [CrossRef]
- Shen, B.W.; Smith, G.C.; Anthony, J.M.; Matyi, R.J. Diffusion Barrier Properties of Thin Selective Chemical Vapor Deposited Tungsten Films. J. Vac. Sci. Technol. 1986, 4, 1369–1376. [Google Scholar] [CrossRef]
- Park, K.S.; Kim, S. Seedless Copper Electrodeposition onto Tungsten Diffusion Barrier. J. Electrochem. Soc. 2010, 157, D609–D613. [Google Scholar] [CrossRef]
- Pauleau, Y.; Dassapa, F.C.; Lami, P.; Oberlin, J.C.; Romagna, F. Silicide Formation in Metal/Si Structures and Diffusion Barrier Properties of CVD Tungsten Films. J. Mater. Res. 1989, 4, 156–162. [Google Scholar] [CrossRef]
- Ting, C.Y.; Wittmer, M. The Use of Titanium-Based Contact Barrier Layers in Silicon Technology. Thin Solid Films 1982, 96, 327–345. [Google Scholar] [CrossRef]
- Farahani, M.M.; Turner, T.E.; Barnes, J.J. Evaluation of Titanium as a Diffusion Barrier between Aluminum and Silicon for 1.2 μm CMOS Integrated Circuits. J. Electrochem. Soc. 1987, 134, 2835. [Google Scholar] [CrossRef]
- Cho, S.L.; Kim, K.B.; Min, S.H.; Shin, H.K.; Kimd, S.D. Diffusion Barrier Properties of Metallorganic Chemical Vapor Deposited Tantalum Nitride Films against Cu Metallization. J. Electrochem. Soc. 1999, 146, 3724. [Google Scholar] [CrossRef]
- Xie, Q.; Qu, X.P.; Tan, J.J.; Jiang, Y.L.; Zhou, M.; Chen, T.; Ru, G.P. Superior Thermal Stability of Ta/TaN Bi-Layer Structure for Copper Metallization. Appl. Surf. Sci. 2006, 253, 1666–1672. [Google Scholar] [CrossRef]
- Fréty, N.; Bernard, F.; Nazon, J.; Sarradin, J.; Tedenac, J.C. Copper Diffusion into Silicon Substrates through TaN and Ta/TaN Multilayer Barriers. J. Phase. Equilib. Diff. 2006, 27, 590–597. [Google Scholar] [CrossRef]
- Bryner, J.; Profunser, D.M.; Vollmann, J.; Mueller, E.; Dual, J. Characterization of Ta and TaN Diffusion Barriers beneath Cu Layers Using Picosecond Ultrasonics. Ultrasonics 2006, 44, e1269–e1275. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.Y.; Zhang, D.H.; Li, C.Y.; Foo, P.D. Comparative Study of Ta, TaN and Ta/TaN Bi-Layer Barriers for Cu Ultra Low-k Porous Polymer Integration. Thin Solid Films 2004, 462, 176–181. [Google Scholar] [CrossRef]
- Suh, B.S.; Lee, Y.J.; Hwang, J.S.; Park, C.O. Properties of Reactively Sputtered WNx as Cu Diffusion Barrier. Thin Solid Films 1999, 348, 299–303. [Google Scholar] [CrossRef]
- Uekubo, M.; Oku, T.; Nii, K.; Murakami, M.; Takahiro, K.; Yamaguchi, S.; Nakano, T.; Ohta, T. WNx Diffusion Barriers between Si and Cu. Thin Solid Films 1996, 286, 170–175. [Google Scholar] [CrossRef]
- Lee, B.H.; Yong, K. Diffusion Barrier Properties of Metalorganic Chemical Vapor Deposition-WNx Compared with Other Barrier Materials. J. Vac. Sci. Technol. 2004, 22, 2375–2379. [Google Scholar] [CrossRef]
- Rha, S.K.; Lee, W.J.; Lee, S.Y.; Hwang, Y.S.; Lee, Y.J.; Kim, D.I.; Kim, D.W.; Chun, S.S.; Park, C.O. Improved TiN Film as a Diffusion Barrier between Copper and Silicon. Thin Solid Films 1998, 320, 134–140. [Google Scholar] [CrossRef]
- Uhm, J.; Jeon, H. TiN Diffusion Barrier Grown by Atomic Layer Deposition Method for Cu Metallization. Jpn. J. Appl. Phys. 2001, 40, 4657–4660. [Google Scholar] [CrossRef]
- Gagnon, G.; Currie, J.F.; Brebner, J.L.; Darwall, T. Efficiency of TiN Diffusion Barrier between Al and Si Prepared by Reactive Evaporation and Rapid Thermal Annealing. J. Appl. Phys. 1996, 79, 7612–7620. [Google Scholar] [CrossRef]
- Wang, S.Q.; Raaijmakers, I.; Burrow, B.J.; Suthar, S.; Redkar, S.; Kim, K.B. Reactively Sputtered TiN as a Diffusion Barrier between Cu and Si. J. Appl. Phys. 1990, 68, 5176–5187. [Google Scholar] [CrossRef]
- Appelbaum, A.; Murarka, S.P. TiC as a Diffusion Barrier between Al and CoSi2. J. Vac. Sci. Technol. 1986, 4, 637–640. [Google Scholar] [CrossRef]
- Wang, S.J.; Tsai, H.Y.; Sun, S.C. Characterization of Sputtered Titanium Carbide Film as Diffusion Barrier for Copper Metallization. J. Electrochem. Soc. 2001, 148, C563–C568. [Google Scholar] [CrossRef][Green Version]
- Eizenberg, M.; Brener, R.; Murarka, S.P. Thermal Stability of the Aluminum/Titanium Carbide/Silicon Contact System. J. Appl. Phys. 1984, 55, 3799–3803. [Google Scholar] [CrossRef]
- Angyal, M.S.; Shacham-Diamand, Y.; Reid, J.S.; Nicolet, M.A. Performance of Tantalum-Silicon-Nitride Diffusion Barriers between Copper and Silicon Dioxide. Appl. Phys. Lett. 1995, 67, 2152–2154. [Google Scholar] [CrossRef]
- Hara, T.; Yoshida, Y.; Toida, H. Improved Barrier and Adhesion Properties in Sputtered TaSiN Layer for Copper Interconnects. Electrochem. Solid State Lett. 2002, 5, G36–G39. [Google Scholar] [CrossRef]
- Girll, A.; Jahnes, C.; Cabral, C. Layered TaSiN as an Oxidation Resistant Electrically Conductive Barrier. J. Mater. Res. 1999, 14, 1604–1609. [Google Scholar] [CrossRef]
- Klaus, J.W.; Ott, A.W.; Dillon, A.C.; George, S.M. Atomic Layer Controlled Growth of Si3N4 Films Using Sequential Surface Reactions. Surf. Sci. 1998, 418, L14–L19. [Google Scholar] [CrossRef]
- Raman, R.S.; Banerjee, P.C.; Lobo, D.E.; Gullapalli, H.; Sumandasa, M.; Kumar, A.; Choudhary, L.; Tkacz, R.; Ajayan, P.M.; Majumder, M. Protecting Copper from Electrochemical Degradation by Graphene Coating. Carbon 2012, 50, 4040–4045. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Lou, J. Investigation of Hexagonal Boron Nitride as an Atomically Thin Corrosion Passivation Coating in Aqueous Solution. Nanotechnology 2016, 27, 364004. [Google Scholar] [CrossRef]
- Zheng, S.J.; Cai, Z.B.; Pu, J.B.; Zeng, C.; Chen, S.Y.; Chen, R.; Wang, L.P. A Feasible Method for the Fabrication of VAlTiCrSi Amorphous High Entropy Alloy Film with Outstanding Anti-Corrosion Property. Appl. Surf. Sci. 2019, 483, 870–874. [Google Scholar] [CrossRef]
- Yang, W.J.; Li, T.Q.; Zhou, H.H.; Huang, Z.; Fu, C.P.; Chen, L.; Li, M.B.; Kuang, Y.F. Electrochemical and Anti-Corrosion Properties of Octadecanethiol and Benzotriazole Binary Self-Assembled Monolayers on Copper. Electrochim. Acta 2016, 220, 245–251. [Google Scholar] [CrossRef]
- Fugare, B.Y.; Lokhande, B.J. Study on Structural, Morphological Electrochemical and Corrosion Properties of Mesoporous RuO2 Thin Films Prepared by Ultrasonic Spray Pyrolysis for Supercapacitor Electrode Application. Mat. Sci. Semicon. Proc. 2017, 71, 121–127. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.B.; Zhang, X.; Li, Q.H.; Liu, Q.; Cheng, Y.; Zheng, Y.F.; Xi, T.F.; Wei, S.C. Surface Characteristics and Electrochemical Corrosion Behavior of NiTi Alloy Coated with IrO2. Mat. Sci. Eng. 2013, 33, 15–20. [Google Scholar] [CrossRef]
- Broekmann, P. Tailored Design of Suppressor Ensembles for Damascene and 3D-TSV Copper Plating. In Proceedings of the 12th International Fischer Symposium, Keil, Germany, 4 June 2012. [Google Scholar]
- Moore, G.E. Cramming More Components onto Integrated Circuits. Proc. IEEE 1998, 86, 82–85. [Google Scholar] [CrossRef]
- Fang, J.S.; Chiu, C.F.; Lin, J.H.; Lin, T.Y.; Chin, T.S. Failure Mechanism of 5 nm Thick Ta-Si-C Barrier Layers against Cu Penetration at 700–800 °C. J. Electrochem. Soc. 2009, 156, H147–H152. [Google Scholar] [CrossRef]
- Kouno, T.; Niwa, H.; Yamada, M. Effect of TiN Microstructure on Diffusion Barrier Properties in Cu Metallization. J. Electrochem. Soc. 1998, 145, 2164–2167. [Google Scholar] [CrossRef]
- Oku, T.; Kawakami, E.; Uecubo, M.; Takahiro, K.; Yamaguchi, S.; Murakami, M. Diffusion Barrier Property of TaN between Si and Cu. Appl. Surf. Sci. 1996, 99, 265–272. [Google Scholar] [CrossRef]
- Stavrev, M.; Fischer, D.; Preub, A.; Wenzel, C.; Mattern, N. Study of Nanocrystalline Ta(N,O) Diffusion Barriers for Use in Cu Metallization. Microelectron. Eng. 1997, 33, 269–275. [Google Scholar] [CrossRef]
- Bisang, J.M.; Kreysa, G. Study of the Effect of Electrode Resistance on Current Density Distribution in Cylindrical Electrochemical Reactors. J. Appl. Electrochem. 1988, 18, 422–430. [Google Scholar] [CrossRef]
- Lee, J.M.; McCrabb, H.; Taylor, E.J.; Carpio, R. Current Distribution for the Metallization of Resistive Wafer Substrates under Controlled Geometric Variations. J. Electrochem. Soc. 2006, 153, C265–C271. [Google Scholar] [CrossRef]
- Marshall, S.L.; Wolff, S.K. Analysis of Terminal Effects in Rectangular Electrochemical Cells. Electrochim. Acta 1998, 43, 405–415. [Google Scholar] [CrossRef]
- Armini, S.; Vereecken, P.M. Impact of “Terminal Effect” on Cu Plating: Theory and Experimental Evidence. ECS Trans. 2010, 25, 185–194. [Google Scholar] [CrossRef]
- Armini, S. Cu Electrodeposition on Resistive Substrates in Alkaline Chemistry: Effect of Current Density and Wafer RPM. J. Electrochem. Soc. 2011, 158, D390–D394. [Google Scholar] [CrossRef]
- Choi, J.W.; Guan, O.L.; Mao, Y.J.; Yusoff, H.B.M.; Xie, J.L.; Lan, C.C.; Loh, W.L.; Lau, B.L.; Hong, L.L.H.; Kian, L.G.; et al. TSV Cu Filling Failure Modes and Mechanisms Causing the Failures. IEEE Trans. Comp. Pack. Man. 2014, 4, 581–587. [Google Scholar] [CrossRef]
- Yang, L.; Atanasova, T.; Radisic, A.; Deconinck, J.; West, A.C.; Vereecken, P. Wafer-Scale Cu Plating Uniformity on Thin Cu Seed Layers. Electrochim. Acta 2013, 104, 242–248. [Google Scholar] [CrossRef]
- Matlosz, M.; Vallotton, P.H.; West, A.C.; Landolt, D. Nonuniform Current Distribution and Thickness during Electrodeposition onto Resistive Substrates. J. Electrochem. Soc. 1992, 139, 752–761. [Google Scholar] [CrossRef]
- Sukamto, J.H.; Webb, E.; Andryushchenko, T.; Reid, J. An Evaluation of Electrolytic Repair of Discontinuous PVD Copper Seed Layers in Damascene Vias. J. Appl. Electrochem. 2004, 34, 283–290. [Google Scholar] [CrossRef]
- Motoyama, K.; van der Straten, O.; Maniscalco, J.; He, M. PVD Cu Reflow Seed Process Optimization for Defect Reduction in Nanoscale Cu/Low-k Dual Damascene Interconnects. J. Electrochem. Soc. 2013, 160, D3211–D3215. [Google Scholar] [CrossRef]
- Lim, S.T.; Park, Y.C.; Yoo, S.J.; Lee, B.J. Customized Step Coverage of Copper Seed Layer Using Eni-PVD (Energetic Neutral and Ion Physical Vapor Deposition). Thin Solid Films 2009, 517, 3935–3937. [Google Scholar] [CrossRef]
- Wickramanayaka, S.; Nagahama, H.; Watanabe, E.; Sato, M.; Mizuno, S. Using I-PVD for Copper-Based Interconnects. (Deposition). Solid State Technol. 2002, 45, 67–72. [Google Scholar]
- Choi, K.K.; Rhee, S.W. Chemical Vapor Deposition of Copper Film from Hexafluoroacetyl-Acetonate Cu (I) Vinylcyclohexane. Thin Solid Films 2001, 397, 70–77. [Google Scholar] [CrossRef]
- Shim, K.C.; Lee, H.B.; Kwon, O.K.; Park, H.S.; Koh, W.; Kang, S.W. Bottom-Up Filling of Submicrometer Features in Catalyst-Enhanced Chemical Vapor Deposition of Copper. J. Electrochem. Soc. 2001, 149, G109–G113. [Google Scholar] [CrossRef]
- Kim, H.; Bhandari, H.B.; Xu, S.; Gordon, R.G. Ultrathin CVD Cu Seed Layer Formation Using Copper Oxynitride Deposition and Room Temperature Remote Hydrogen Plasma Reduction. J. Electrochem. Soc. 2008, 155, H496–H503. [Google Scholar] [CrossRef]
- Reynolds, S.K.; Smart, C.J.; Baran, E.F.; Baum, T.H.; Larson, C.E.; Brock, P.J. Chemical Vapor Deposition of Copper from 1,5-Cyclooctadiene Copper (I) Hexafluoroacetylacetonate. Appl. Phys. Lett. 1991, 59, 2332–2334. [Google Scholar] [CrossRef]
- Kröger, R.; Eizenberg, M.; Cong, D.; Yoshida, N.; Chen, L.Y.; Ramaswami, S.; Carl, D. Properties of Copper Films Prepared by Chemical Vapor Deposition for Advanced Metallization of Microelectronic Devices. J. Electrochem. Soc. 1999, 146, 3248. [Google Scholar] [CrossRef]
- Kwon, O.K.; Lee, H.B.; Kang, S.W.; Park, H.S. Enhancement of the Film Growth Rate by Promoting Iodine Adsorption in the Catalyst-Enhanced Chemical Vapor Deposition of Cu. J. Vac. Sci. Technol. 2002, 20, 408–412. [Google Scholar] [CrossRef]
- Solanke, R.; Pathangey, B. Atomic Layer Deposition of Copper Seed Layers. Electrochem. Solid State Lett. 2000, 3, 479–480. [Google Scholar] [CrossRef]
- Moon, D.Y.; Han, D.S.; Shin, S.Y.; Park, J.W.; Kim, B.M.; Kim, J.H. Effects of the Substrate Temperature on the Cu Seed Layer Formed Using Atomic Layer Deposition. Thin Solid Films 2011, 519, 3636–3640. [Google Scholar] [CrossRef]
- Li, Z.W.; Rahtu, A.; Gordon, R.G. Atomic Layer Deposition of Ultrathin Copper Metal Films from a Liquid Copper (I) Amidinate Precursor. J. Electrochem. Soc. 2006, 153, C787–C794. [Google Scholar] [CrossRef]
- Kalutarage, L.C.; Clendenning, S.B.; Winter, C.H. Low-Temperature Atomic Layer Deposition of Copper Films Using Borane Dimethylamine as the Reducing Co-Reagent. Chem. Mater. 2014, 26, 3731–3738. [Google Scholar] [CrossRef]
- Lee, C.H.; Hwang, S.; Kim, S.C.; Kim, J.J. Cu Electroless Deposition onto Ta Substrates: Application to Create a Seed Layer for Cu Electrodeposition. Electrochem. Solid State Lett. 2006, 9, C157–C160. [Google Scholar] [CrossRef]
- Aithal, R.K.; Yenamandra, S.; Gunasekaran, R.A.; Coane, P.; Varahramyan, K. Electroless Copper Deposition on Silicon with Titanium Seed Layer. Mater. Chem. Phys. 2006, 98, 95–102. [Google Scholar] [CrossRef]
- Chong, S.P.; Ee, Y.C.; Chen, Z.; Law, S.B. Electroless Copper Seed Layer Deposition on Tantalum Nitride Barrier Film. Surf. Coat. Technol. 2005, 198, 287–290. [Google Scholar] [CrossRef]
- Wang, Z.; Yaegashi, O.; Sakaue, H.; Takahagi, T.; Shingubara, S. Highly Adhesive Electroless Cu Layer Formation Using an Ultra-Thin Ionized Cluster Beam (ICB)-Pd Catalytic Layer for Sub-100 nm Cu Interconnections. Jpn. J. Appl. Phys. 2003, 42, L1223–L1225. [Google Scholar] [CrossRef]
- Hsu, H.H.; Teng, C.W.; Lin, S.J.; Yeh, J.W. Sn/Pd Catalyzation and Electroless Cu Deposition on TaN Diffusion Barrier Layers. J. Electrochem. Soc. 2002, 149, C143–C149. [Google Scholar] [CrossRef]
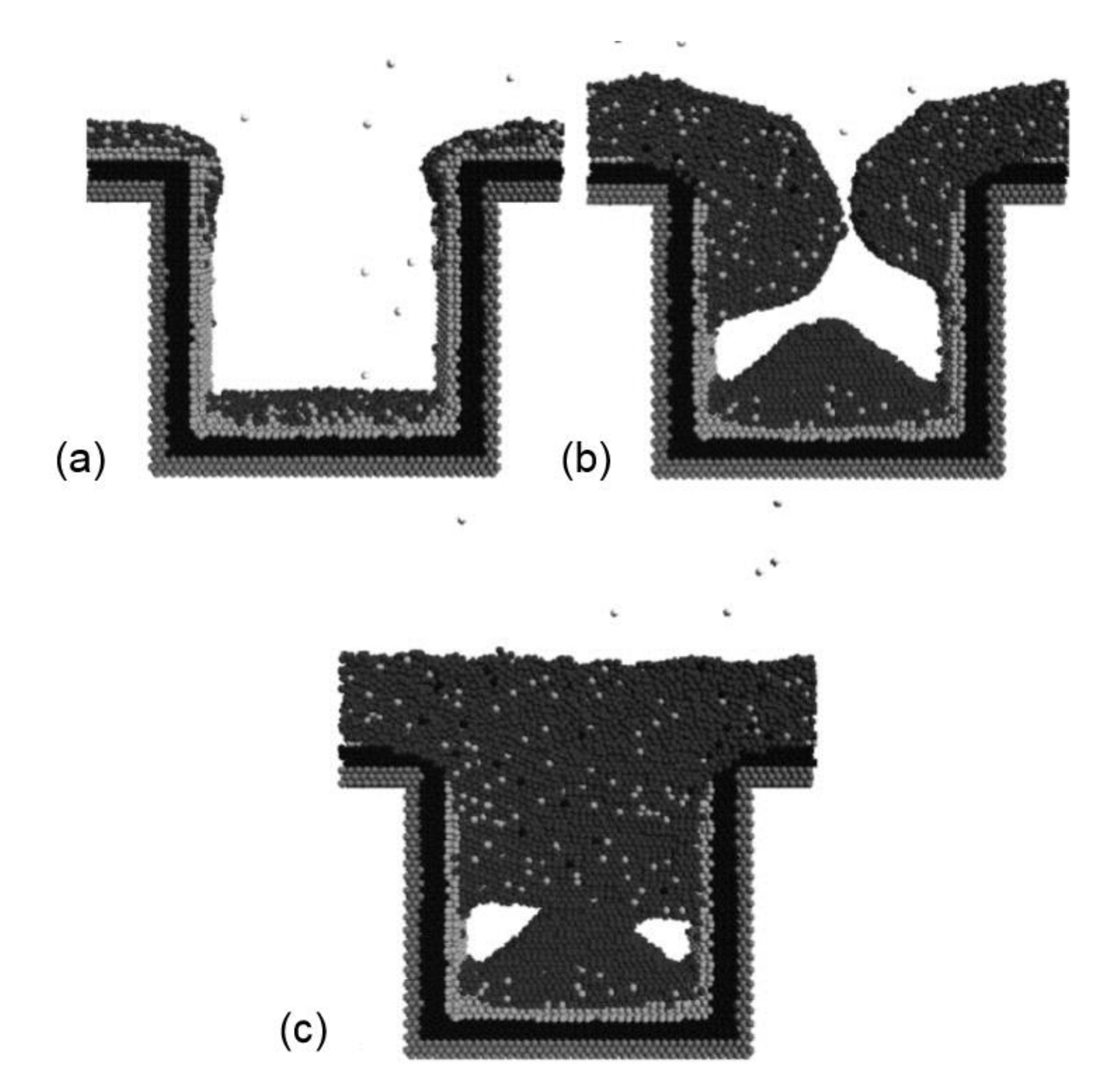
- Hong, R.T.; Huang, M.J.; Yang, J.Y. Molecular Dynamics Study of Copper Trench Filling in Damascene Process. Mat. Sci. Semicon. Proc. 2005, 8, 587–601. [Google Scholar] [CrossRef]
- Nicolet, M.A. Diffusion Barriers in Thin Films. Thin Solid Films 1978, 52, 415–443. [Google Scholar] [CrossRef]
- Pillai, K.S.M. Copper Electrodeposition on Ruthenium-Tantalum and Corrosion of Plasma Treated Copper in Post Etch Cleaning Solution; University of North Texas: Denton, TX, USA, 2011. [Google Scholar]
- Kim, H.; Koseki, T.; Ohba, T.; Ohta, T.; Kojima, Y.; Sato, H.; Shimogaki, Y. Cu Wettability and Diffusion Barrier Property of Ru Thin Film for Cu Metallization. J. Electrochem. Soc. 2005, 152, G594–G600. [Google Scholar] [CrossRef]
- Arunagiri, T.N.; Zhang, Y.; Chyan, O.; El-Bouanani, M.; Kim, M.J.; Chen, K.H.; Wu, C.T.; Chen, L.C. 5 nm Ruthenium Thin Film as a Directly Plateable Copper Diffusion Barrier. Appl. Phys. Lett. 2005, 86, 083104–083106. [Google Scholar] [CrossRef]
- Chyan, O.; Arunagiri, T.N.; Ponnuswamy, T. Electrodeposition of Copper Thin Film on Ruthenium: A Potential Diffusion Barrier for Cu Interconnects. J. Electrochem. Soc. 2003, 150, C347–C350. [Google Scholar] [CrossRef]
- Chan, R.; Arunagiri, T.N.; Zhang, Y.; Chyan, O.; Wallace, R.M.; Kim, M.J.; Hurd, T.Q. Diffusion Studies of Copper on Ruthenium Thin Film: A Plateable Copper Diffusion Barrier. Electrochem. Solid State Lett. 2004, 7, G154–G157. [Google Scholar] [CrossRef]
- Lim, Y.H.; Yoo, H.; Choi, B.H.; Lee, J.H.; Lee, H.N.; Lee, H.K. Atomic-Layer-Deposited Ir Thin Film as a Novel Diffusion Barrier Layer in Cu Interconnection. Phys. Status Solidi. 2011, 8, 891–894. [Google Scholar] [CrossRef]
- Choi, B.H.; Lee, J.H.; Lee, H.K.; Kim, J.H. Effect of Interface Layer on Growth Behavior of Atomic-Layer-Deposited Ir Thin Film as Novel Cu Diffusion Barrier. Appl. Surf. Sci. 2011, 257, 9654–9660. [Google Scholar] [CrossRef]
- Song, S.I.; Lee, J.H.; Choi, B.H.; Lee, H.K.; Shin, D.C.; Lee, J.W. Hydrogen-Plasma-Assisted Hybrid Atomic Layer Deposition of Ir Thin Film as Novel Cu Diffusion Barrier. Surf. Coat. Technol. 2012, 211, 14–17. [Google Scholar] [CrossRef]
- Josell, D.; Bonevich, J.; Moffat, T.; Aaltonen, T.; Ritala, M.; Leskelä, M. Iridium Barriers for Direct Copper Electrodeposition in Damascene Processing. Electrochem. Solid State Lett. 2006, 9, C48–C50. [Google Scholar] [CrossRef]
- Chow, K.M.; Ng, W.Y.; Yeung, L.K. Barrier Properties of Ni, Pd and Pd-Fe for Cu Diffusion. Surf. Coat. Technol. 1998, 105, 56–64. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, H.I.; Cho, J.H.; Seo, H.K.; Dar, M.A.; Shin, H.S.; Eyck, G.A.T.; Lu, T.M.; Senkevich, J.J. Electroless Copper on Refractory and Noble Metal Substrates with an Ultra-Thin Plasma-Assisted Atomic Layer Deposited Palladium Layer. Electrochim. Acta 2006, 51, 2400–2406. [Google Scholar] [CrossRef]
- Leu, L.C.; Norton, D.P.; McElwee-White, L.; Anderson, T.J. Ir/TaN as a Bilayer Diffusion Barrier for Advanced Cu Interconnects. Appl. Phys. Lett. 2008, 92, 111917–111919. [Google Scholar] [CrossRef]
- De Reus, R.; Koper, R.J.I.M.; Zeijlemaker, H.; Saris, F.W. Stability of Amorphous Ir-Ta Diffusion Barriers between Cu and Si. Mater. Lett. 1990, 9, 500–503. [Google Scholar] [CrossRef]
- Yang, C.C.; Cohen, S.; Shaw, T.; Wang, P.C.; Nogami, T.; Edelstein, D. Characterization of “Ultrathin-Cu”/Ru (Ta)/TaN Liner Stack for Copper Interconnects. IEEE Trans. Electr. Device 2010, 31, 722–724. [Google Scholar] [CrossRef]
- Kondati Natarajan, S.; Nies, C.L.; Nolan, M. Ru Passivated and Ru Doped ε-TaN Surfaces as Combined Barrier and Liner Material for Copper Interconnects: A First Principles Study. J. Mater. Chem. 2019, 7, 7959–7973. [Google Scholar] [CrossRef]
- Tan, J.J.; Qu, X.P.; Xie, Q.; Zhou, Y.; Ru, G.P. The Properties of Ru on Ta-Based Barriers. Thin Solid Films 2006, 504, 231–234. [Google Scholar] [CrossRef]
- Li, J.; Lu, H.S.; Wang, Y.W.; Qu, X.P. Sputtered Ru–Ti, Ru–N and Ru–Ti–N Films as Cu Diffusion Barrier. Microelectron. Eng. 2011, 88, 635–640. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Z.; Sun, T.; Zhang, L.; Jie, W.; Wang, X.; Xie, Y.; Tsang, Y.; Long, H.; Chai, Y. Mass Transport Mechanism of Cu Species at the Metal/Dielectric Interfaces with a Graphene Barrier. ACS Nano 2014, 8, 12601–12611. [Google Scholar] [CrossRef]
- Lee, H.C.; Jo, M.; Lim, H.; Yoo, M.S.; Lee, E.; Nguyen, N.N.; Han, S.Y.; Cho, K. Toward Near-Bulk Resistivity of Cu for Next-Generation Nano-Interconnects: Graphene-Coated Cu. Carbon 2019, 149, 656–663. [Google Scholar] [CrossRef]
- Zhao, L.; Lofrano, M.; Croes, K.; Van Besien, E.; Tőkei, Z.; Wilson, C.J.; Degraeve, R.; Kauerauf, T.; Beyer, G.P.; Claeys, C. Evaluations of Intrinsic Time Dependent Dielectric Breakdown of Dielectric Copper Diffusion Barriers. Thin Solid Films 2011, 520, 662–666. [Google Scholar] [CrossRef]
- Nies, C.L.; Nolan, M. DFT Calculations of the Structure and Stability of Copper Clusters on MoS2. Beilstein. J. Nanotech. 2020, 11, 391–406. [Google Scholar] [CrossRef]
- Jing, D.; Lii-Rosales, A.; Lai, K.C.; Li, Q.; Kim, J.; Tringides, M.C.; Evans, J.W.; Thiel, P.A. Non-Equilibrium Growth of Metal Clusters on a Layered Material: Cu on MoS2. New J. Phys. 2020, 22, 053033. [Google Scholar] [CrossRef]
- Ramanath, G.; Cui, G.; Ganesan, P.G.; Guo, X.; Ellis, A.V.; Stukowski, M.; Doppelt, P.; Lane, M. Self-Assembled Subnanolayers as Interfacial Adhesion Enhancers and Diffusion Barriers for Integrated Circuits. Appl. Phys. Lett. 2003, 83, 383–385. [Google Scholar] [CrossRef]
- Khaderbad, M.A.; Pandharipande, R.; Singh, V.; Madhu, S.; Ravikanth, M.; Rao, V.R. Porphyrin Self-Assembled Monolayer as a Copper Diffusion Barrier for Advanced CMOS Technologies. IEEE Trans. Electron. Dev. 2012, 59, 1963–1969. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Wu, S.; Liu, Z. Enhanced CVD of Copper Films on Self-Assembled Monolayers as Ultrathin Diffusion Barriers. J. Electrochem. Soc. 2006, 153, C142–C145. [Google Scholar] [CrossRef]
- Caro, A.M.; Armini, S.; Richard, O.; Maes, G.; Borghs, G.; Whelan, C.M.; Travaly, Y. Bottom-Up Engineering of Subnanometer Copper Diffusion Barriers Using NH2-Derived Self-Assembled Monolayers. Adv. Funct. Mater. 2010, 20, 1125–1131. [Google Scholar] [CrossRef]
- Kong, Z.; Wang, Q.; Ding, L.; Wu, T. Study on Chemical Vapor Deposited Copper Films on Cyano and Carboxylic Self-Assembled Monolayer Diffusion Barriers. Thin Solid Films 2010, 518, 4852–4859. [Google Scholar] [CrossRef]
- Tsai, M.H.; Wang, C.W.; Lai, C.H.; Yeh, J.W.; Gan, J.Y. Thermally Stable Amorphous (AlMoNbSiTaTiVZr)50N50 Nitride Film as Diffusion Barrier in Copper Metallization. Appl. Phys. Lett. 2008, 92, 052109. [Google Scholar] [CrossRef]
- Li, R.; Li, M.; Jiang, C.; Qiao, B.; Zhang, W.; Xu, J. Thermal Stability of AlCrTaTiZrMo-Nitride High Entropy Film as a Diffusion Barrier for Cu Metallization. J. Alloys Compd. 2019, 773, 482–489. [Google Scholar] [CrossRef]
- Chang, S.Y.; Wang, C.Y.; Li, C.E.; Huang, Y.C. 5 nm-Thick (AlCrTaTiZrRu)N0.5 Multi-Component Barrier Layer with High Diffusion Resistance for Cu Interconnects. Nanosci. Nanotech. Lett. 2011, 3, 289–293. [Google Scholar] [CrossRef]
- Chang, S.Y.; Chen, M.K.; Chen, D.S. Multiprincipal-Element AlCrTaTiZr-Nitride Nanocomposite Film of Extremely High Thermal Stability as Diffusion Barrier for Cu Metallization. J. Electrochem. Soc. 2009, 156, G37–G42. [Google Scholar] [CrossRef]
- Steeves, M.M. Electronic Transport Properties of Ruthenium and Ruthenium Dioxide Thin Films. In Electronic Theses and Dissertations; University of Maine: Orono, ME, USA, 2011; Available online: https://digitalcommons.library.umaine.edu/etd/262 (accessed on 15 October 2020).
- Park, C.; Bauer, E.; Poppa, H. A Re-Examination of the Cu/Ru (0001) System. Surf. Sci. 1987, 187, 86–97. [Google Scholar] [CrossRef]
- Chu, J.P.; Lin, C.H.; John, V.S. Cu Films Containing Insoluble Ru and RuNx on Barrierless Si for Excellent Property Improvements. Appl. Phys. Lett. 2007, 91, 132109–132111. [Google Scholar] [CrossRef]
- Kim, K.H.; Lim, T.; Park, K.J.; Koo, H.C.; Kim, M.J.; Kim, J.J. Investigation of Cu Growth Phenomena on Ru Substrate During Electroless Deposition Using Hydrazine as a Reducing Agent. Electrochim. Acta 2015, 151, 249–255. [Google Scholar] [CrossRef]
- Damayanti, M.; Sritharan, T.; Mhaisalkar, S.G.; Phoon, E.; Chan, L. Study of Ru Barrier Failure in the Cu/Ru/Si System. J. Mater. Res. 2007, 22, 2505–2511. [Google Scholar] [CrossRef]
- Damayanti, M.; Sritharan, T.; Mhaisalkar, S.G.; Gan, Z.H. Effects of Dissolved Nitrogen in Improving Barrier Properties of Ruthenium. Appl. Phys. Lett. 2006, 88, 044101–044103. [Google Scholar] [CrossRef]
- Shin, J.; Waheed, A.; Agapiou, K.; Winkenwerder, W.A.; Kim, H.W.; Jones, R.A.; Hwang, G.S.; Ekerdt, J.G. Growth of Ultrathin Films of Amorphous Ruthenium-Phosphorus Alloys Using a Single Source CVD Precursor. J. Am. Chem. Soc. 2006, 128, 16510–16511. [Google Scholar] [CrossRef]
- Perng, D.C.; Yeh, J.B.; Hsu, K.C. Phosphorous Doped Ru Film for Advanced Cu Diffusion Barriers. Appl. Surf. Sci. 2008, 254, 6059–6062. [Google Scholar] [CrossRef]
- Shin, J.; Kim, H.W.; Agapiou, K.; Jones, R.A.; Hwang, G.S.; Ekerdt, J.G. Effects of P on Amorphous Chemical Vapor Deposition Ru-P Alloy Films for Cu Interconnect Liner Applications. J. Vac. Sci. Technol. 2008, 26, 974–979. [Google Scholar] [CrossRef]
- McCarty, W.J.; Yang, X.; Anderson, L.J.D.; Jones, R.A. Chemical Vapour Deposition of Amorphous Ru (P) Thin Films from Ru Trialkylphosphite Hydride Complexes. Dalton 2012, 41, 13496–13503. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bost, D.E.; Kim, H.W.; Chou, C.Y.; Hwang, G.S.; Ekerdt, J.G. First-Principles Predictions of Ruthenium-Phosphorus and Ruthenium-Boron Glassy Structures and Chemical Vapor Deposition of Thin Amorphous Ruthenium-Boron Alloy Films. Thin Solid Films 2017, 622, 56–64. [Google Scholar] [CrossRef]
- Perng, D.C.; Yeh, J.B.; Hsu, K.C.; Wang, Y.C. 5 nm Amorphous Boron and Carbon Added Ru Film as a Highly Reliable Cu Diffusion Barrier. Electrochem. Solid-State Lett. 2010, 13, H290–H293. [Google Scholar] [CrossRef]
- Perng, D.C.; Hsu, K.C.; Tsai, S.W.; Yeh, J.B. Thermal and Electrical Properties of PVD Ru (P) Film as Cu Diffusion Barrier. Microelectron. Eng. 2010, 87, 365–369. [Google Scholar] [CrossRef]
- Chen, C.W.; Chen, J.S.; Jeng, J.S. Effectiveness of Ta Addition on the Performance of Ru Diffusion Barrier in Cu Metallization. J. Electrochem. Soc. 2008, 155, H1003–H1008. [Google Scholar] [CrossRef]
- Yeh, J.B.; Perng, D.C.; Hsu, K.C. Amorphous RuW Film as a Diffusion Barrier for Advanced Cu Metallization. J. Electrochem. Soc. 2010, 157, H810–H814. [Google Scholar] [CrossRef]
- Hsu, K.C.; Perng, D.C.; Yeh, J.B.; Wang, Y.C. Ultrathin Cr Added Ru Film as a Seedless Cu Diffusion Barrier for Advanced Cu Interconnects. Appl. Surf. Sci. 2012, 258, 7225–7230. [Google Scholar] [CrossRef]
- Sari, W.; Eom, T.K.; Jeon, C.W.; Sohn, H.; Kim, S.H. Improvement of the Diffusion Barrier Performance of Ru by Incorporating a WNx Thin Film for Direct-Plateable Cu Interconnects. Electrochem. Solid State Lett. 2009, 12, H248–H251. [Google Scholar] [CrossRef]
- Kim, S.W.; Kwon, S.H.; Jeong, S.J.; Kang, S.W. Improvement of Copper Diffusion Barrier Properties of Tantalum Nitride Films by Incorporating Ruthenium Using PEALD. J. Electrochem. Soc. 2008, 155, H885–H888. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H.T.; Yim, S.S.; Lee, D.J.; Kim, K.S.; Kim, H.M.; Kim, K.B.; Sohn, H. A Bilayer Diffusion Barrier of ALD-Ru/ALD-TaCN for Direct Plating of Cu. J. Electrochem. Soc. 2008, 155, H589–H594. [Google Scholar] [CrossRef]
- Burke, L.D.; Naser, N.S.; Sharna, R. The Oxide Electrochemistry of Ruthenium and Its Relevance to Trench Liner Applications in Damascene Copper Plating. J. Appl. Electrochem. 2008, 38, 377–384. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.; Jiang, D.; Zhang, Y.; Dubonos, S.A.; Grigorieva, I.; Firsov, A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.W.; Stormer, H.L.; Kim, P. Experimental Observation of the Quantum Hall Effect and Berry’s Phase in Graphene. Nature 2005, 438, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Brown, L.; Levendorf, M.; Cai, W.; Ju, S.Y.; Edgeworth, J.; Li, X.S.; Magnuson, C.; Velamaknni, A.; Piner, R.R.; et al. Oxidation Resistance of Graphene-Coated Cu and Cu/Ni Alloy. ACS Nano 2011, 5, 1321–1327. [Google Scholar] [CrossRef]
- Mehta, R.; Chugh, S.; Chen, Z. Transfer-Free Multi-Layer Graphene as a Diffusion Barrier. Nanoscale 2017, 9, 1827–1833. [Google Scholar] [CrossRef]
- Bong, J.H.; Yoon, S.J.; Yoon, A.; Hwang, W.S.; Cho, B.J. Ultrathin Graphene and Graphene Oxide Layers as a Diffusion Barrier for Advanced Cu Metallization. Appl. Phys. Lett. 2015, 106, 063112. [Google Scholar] [CrossRef]
- Roy, S.S.; Arnold, M.S. Improving Graphene Diffusion Barriers via Stacking Multiple Layers and Grain Size Engineering. Adv. Funct. Mater. 2013, 23, 3638–3644. [Google Scholar] [CrossRef]
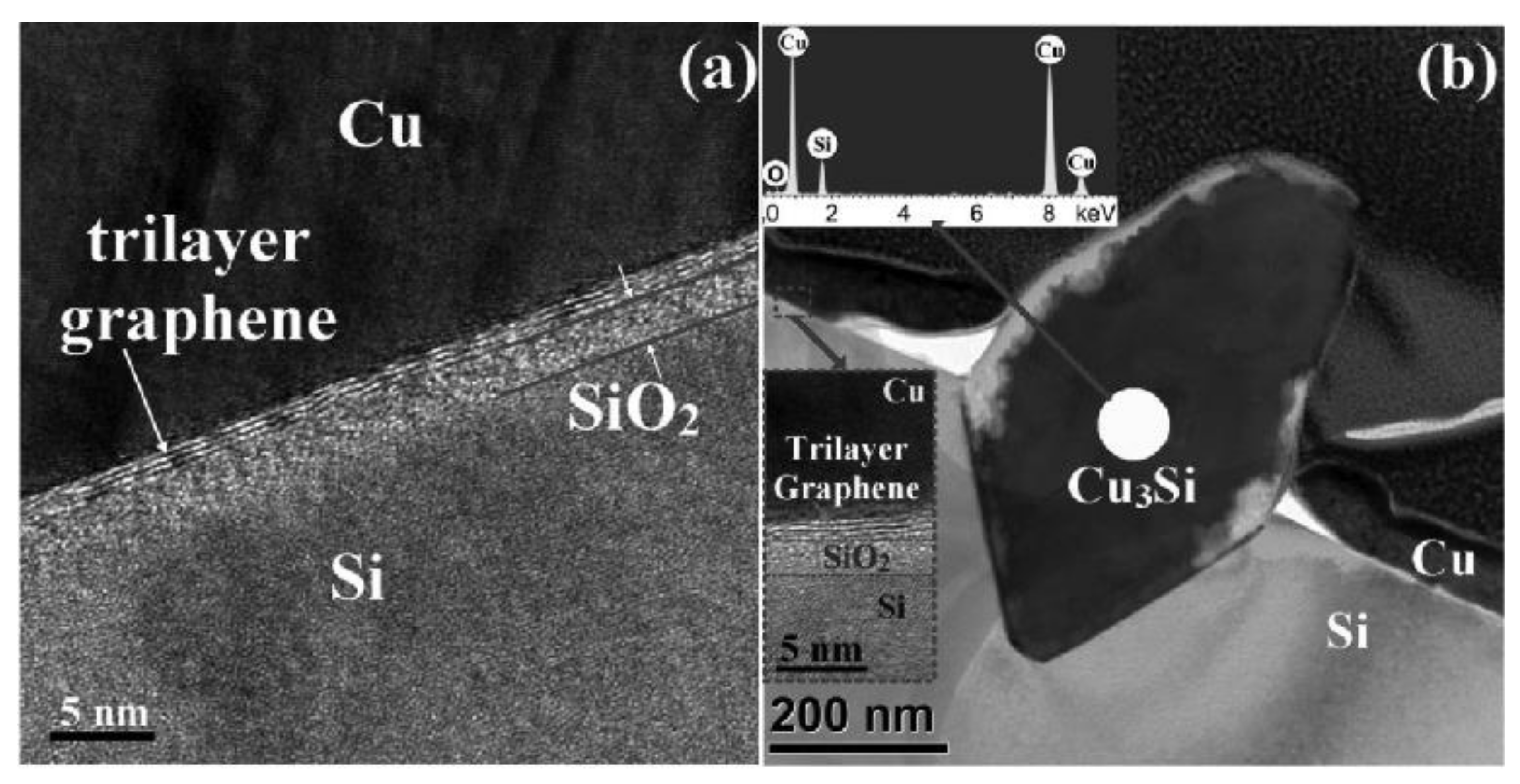
- Nguyen, B.S.; Lin, J.F.; Perng, D.C. 1-nm-Thick Graphene Tri-Layer as the Ultimate Copper Diffusion Barrier. Appl. Phys. Lett. 2014, 104, 082105. [Google Scholar] [CrossRef]
- Hong, J.; Lee, S.; Lee, S.; Han, H.; Mahata, C.; Yeon, H.W.; Koo, B.; Kim, S.I.; Nam, T.; Min, B.W.; et al. Graphene as an Atomically Thin Barrier to Cu Diffusion into Si. Nanoscale 2014, 6, 7503–7511. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gong, Y.; Zhou, W.; Ma, L.; Yu, J.; Idrobo, J.C.; Jung, J.; MacDonald, A.H.; Vajtai1, R.; Lou, J.; et al. Ultrathin High-Temperature Oxidation-Resistant Coatings of Hexagonal Boron Nitride. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Shen, L.; Zhao, Y.; Wang, Y.; Song, R.; Yao, Q.; Chen, S.; Chai, Y. A Long-Term Corrosion Barrier with an Insulating Boron Nitride Monolayer. J. Mater. Chem. 2016, 4, 5044–5050. [Google Scholar] [CrossRef]
- Ren, S.; Cui, M.; Pu, J.; Xue, Q.; Wang, L. Multilayer Regulation of Atomic Boron Nitride Films to Improve Oxidation and Corrosion Resistance of Cu. ACS Appl. Mater. Interfaces 2017, 9, 27152–27165. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.L.; Catalano, M.; Smithe, K.K.; Wang, L.; Zhang, S.; Pop, E.; Kim, M.J.; Chen, Z. Studies of Two-Dimensional h-BN and MoS2 for Potential Diffusion Barrier Application in Copper Interconnect Technology. NPJ 2D Mater. Appl. 2017, 1, 1–7. [Google Scholar] [CrossRef]
- Mertens, S.F.L. Copper Underpotential Deposition on Boron Nitride Nanomesh. Electrochim. Acta 2017, 246, 730–736. [Google Scholar] [CrossRef]
- Smithe, K.K.; Zhu, Z.; Bailey, C.S.; Pop, E.; Yoon, A. Investigation of Monolayer MX2 as Sub-Nanometer Copper Diffusion Barriers. Int. Reliab. Phys. Symp. 2018. [Google Scholar] [CrossRef]
- Lo, C.L.; Zhang, K.; Smith, R.S.; Shah, K.; Robinson, J.A.; Chen, Z. Large-Area, Single-Layer Molybdenum Disulfide Synthesized at BEOL Compatible Temperature as Cu Diffusion Barrier. IEEE Trans. Electr. Device 2018, 39, 873–876. [Google Scholar] [CrossRef]
- Mikami, N.; Hata, N.; Kikkawa, T.; Machida, H. Robust Self-Assembled Monolayer as Diffusion Barrier for Copper Metallization. Appl. Phys. Lett. 2003, 83, 5181–5183. [Google Scholar] [CrossRef]
- Yoshino, T.; Hata, N.; Muramoto, I.; Machida, H.; Kikkawa, T. Effect of Phosphorus Atom in Self-Assembled Monolayer as a Drift Barrier for Advanced Copper Interconnects. Appl. Phys. Express 2008, 1, 065003. [Google Scholar] [CrossRef]
- Krishnamoorthy, A.; Chanda, K.; Murarka, S.P.; Ramanath, G.; Ryan, J.G. Self-Assembled Near-Zero-Thickness Molecular Layers as Diffusion Barriers for Cu Metallization. Appl. Phys. Lett. 2001, 78, 2467–2469. [Google Scholar] [CrossRef]
- Ganesan, P.G.; Singh, A.P.; Ramanath, G. Diffusion Barrier Properties of Carboxyl-and Amine-Terminated Molecular Nanolayers. Appl. Phys. Lett. 2004, 85, 579–581. [Google Scholar] [CrossRef]
- Caro, A.M.; Maes, G.; Borghs, G.; Whelan, C.M. Screening Self-Assembled Monolayers as Cu Diffusion Barriers. Microelectron. Eng. 2008, 85, 2047–2050. [Google Scholar] [CrossRef]
- Chung, Y.; Lee, S.; Mahata, C.; Seo, J.; Lim, S.M.; Jeong, M.S.; Jung, H.; Joo, Y.C.; Park, Y.B.; Kim, H.; et al. Coupled Self-Assembled Monolayer for Enhancement of Cu Diffusion Barrier and Adhesion Properties. RSC Adv. 2014, 4, 60123–60130. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, M.; Rani, S.; Kumar, D. Deposition and Characterization of 3-Aminopropyltrimethoxysilane Monolayer Diffusion Barrier for Copper Metallization. Metall. Mater. Trans. 2015, 46, 928–932. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Yan, X.H.; Li, J.S.; Zhang, W.R.; Zhang, Y. A Brief Review of High-Entropy Films. Mater. Chem. Phys. 2018, 210, 12–19. [Google Scholar] [CrossRef]
- Yu, R.S.; Huang, C.J.; Huang, R.H.; Sun, C.H.; Shieu, F.S. Structure and Optoelectronic Properties of Multi-Element Oxide Thin Film. Appl. Surf. Sci. 2011, 257, 6073–6078. [Google Scholar] [CrossRef]
- Huang, P.K.; Yeh, J.W. Effects of Substrate Bias on Structure and Mechanical Properties of (AlCrNbSiTiV)N Coatings. J. Phys. D Appl. Phys. 2009, 42, 115401–115407. [Google Scholar] [CrossRef]
- Chang, Z.C.; Liang, S.C.; Han, S.; Chen, Y.K.; Shieu, F.S. Characteristics of TiVCrAlZr Multi-Element Nitride Films Prepared by Reactive Sputtering. Nucl. Instrum. Methods Phys. Res. 2010, 268, 2504–2509. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; He, Y.H. The Preparation of FeCoNiCrAl2Si High Entropy Alloy Coating by Laser Cladding. J. Metals Chin. 2011, 8, 1075–1079. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Y.; Shen, J.; Vilar, R. Thermal Stability and Oxidation Resistance of Laser Clad TiVCrAlSi High Entropy Alloy Coatings on Ti-6Al-4V Alloy. Surf. Coat. Technol. 2011, 206, 1389–1395. [Google Scholar] [CrossRef]
- Yao, C.Z.; Wei, F.H.; Zhang, P.; Lu, X.H.; Liu, P.; Tong, Y.X. Facile Preparation and Magnetic Study of Amorphous Tm-Fe-Co-Ni-Mn Multicomponent Alloy Nanofilm. J. Rare Earth. 2011, 29, 133–137. [Google Scholar] [CrossRef]
- Niu, X.L.; Wang, L.J.; Sun, D.; Dong, J.L.; Li, C.M. Research on Corrosion Resistance of Al-Fe-Co-Cr-Ni-Cu High-Entropy Alloy Coating by Electron Beam Evaporation Plating. J. Dalian Univ. Technol. 2013, 53, 689–694. [Google Scholar]
- Otto, F.; Yang, Y.; Bei, H.; George, E.P. Relative Effects of Enthalpy and Entropy on the Phase Stability of Equiatomic High-Entropy Alloys. Acta Mater. 2013, 61, 2628–2638. [Google Scholar] [CrossRef]
- Tasan, C.C.; Deng, Y.; Pradeep, K.G.; Yao, M.J.; Springer, H.; Raabe, D. Composition Dependence of Phase Stability, Deformation Mechanisms, and Mechanical Properties of the CoCrFeMnNi High-Entropy Alloy System. JOM-US 2014, 66, 1993–2001. [Google Scholar] [CrossRef]
- Beke, D.L.; Erdelyi, G. On the Diffusion in High-Entropy Alloys. Mater. Lett. 2016, 164, 111–113. [Google Scholar] [CrossRef]
- Kucza, W.; Dąbrowa, J.; Cieślak, G.; Berent, K.; Kulik, T.; Danielewski, M. Studies of “Sluggish Diffusion” Effect in Co-Cr-Fe-Mn-Ni, Co-Cr-Fe-Ni and Co-Fe-Mn-Ni High Entropy Alloys; Determination of Tracer Diffusivities by Combinatorial Approach. J. Alloys Compd. 2018, 731, 920–928. [Google Scholar] [CrossRef]
- Tsai, K.Y.; Tsaai, M.H.; Yeh, J.W. Sluggish Diffusion in Co–Cr–Fe–Mn–NiHigh-Entropy Alloys. Acta Mater. 2013, 61, 4887–4897. [Google Scholar] [CrossRef]
- Xu, X.D.; Liu, P.; Tang, Z.; Hirata, A.; Song, S.X.; Nieh, T.G.; Liaw, P.K.; Liu, C.T.; Chen, M.W. Transmission Electron Microscopy Characterization of Dislocation Structure in a Face-Centered Cubic High-Entropy Alloy Al0.1CoCrFeNi. Acta Mater. 2018, 144, 107–115. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Lei, Z.F.; Lu, Z.P.; Huang, J.C.; Nieh, T.G. A Simplified Model Connecting Lattice Distortion with Friction Stress of Nb-Based Equiatomic High-Entropy Alloys. Mater. Res. Lett. 2019, 7, 340–346. [Google Scholar] [CrossRef]
- Liu, W.H.; Lu, Z.P.; He, J.Y.; Luan, J.H.; Wang, Z.J.; Liu, B.; Liu, Y.; Chen, M.W.; Liu, C.T. Ductile CoCrFeNiMox High Entropy Alloys Strengthened by Hard Intermetallic Phases. Acta Mater. 2016, 116, 332–342. [Google Scholar] [CrossRef]
- Meyer, M.A.; Chawla, K.K. Mechanical Metallurgy-Principles and Applications; Prentice-Hall Inc.: Englewood Cliffs, NJ, USA, 1984; pp. 383–401. [Google Scholar]
- Zhou, Y.J.; Zhang, Y.; Wang, Y.L.; Chen, G.L. Solid Solution Alloys of AlCoCrFeNiTix with Excellent Room-Temperature Mechanical Properties. Appl. Phys. Lett. 2007, 90, 181904. [Google Scholar] [CrossRef]
- Ranganathan, S. Alloyed Pleasures: Multimetallic Cocktails. Curr. Sci. 2003, 85, 1404–1406. Available online: http://eprints.iisc.ac.in/id/eprint/6189 (accessed on 16 October 2020).
- Tsai, M.H.; Wang, C.W.; Tsai, C.W.; Shen, W.J.; Yeh, J.W.; Gan, J.Y.; Wu, W.W. Thermal Stability and Performance of NbSiTaTiZr High-Entropy Alloy Barrier for Copper Metallization. J. Electrochem. Soc. 2011, 158, H1161–H1165. [Google Scholar] [CrossRef]
- Chen, T.K.; Shun, T.T.; Yeh, J.W.; Wong, M.S. Nanostructured Nitride Films of Multi-Element High-Entropy Alloys by Reactive DC Sputtering. Surf. Coat. Technol. 2004, 188, 193–200. [Google Scholar] [CrossRef]
- Huang, Y.S.; Chen, L.; Lui, H.W.; Cai, M.H.; Yeh, J.W. Microstructure, Hardness, Resistivity and Thermal Stability of Sputtered Oxide Films of AlCoCrCu05NiFe High-Entropy Alloy. Mater. Sci. Eng. 2007, 457, 77–83. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yeh, J.W.; Gan, J.Y. Diffusion Barrier Properties of AlMoNbSiTaTiVZr High-Entropy Alloy Layer between Copper and Silicon. Thin Solid Films 2008, 516, 5527–5530. [Google Scholar] [CrossRef]
- Tung, C.C.; Yeh, J.W.; Shun, T.T.; Chen, S.K.; Huang, Y.S.; Chen, H.C. On the Elemental Effect of AlCoCrCuFeNi High-Entropy Alloy System. Mater. Lett. 2007, 61, 1–5. [Google Scholar] [CrossRef]
- Tong, C.J.; Chen, M.R.; Yeh, J.W.; Lin, S.J.; Chen, S.K.; Shun, T.T.; Chang, S.Y. Mechanical Performance of the AlxCoCrCuFeNi High-Entropy Alloy System with Multiprincipal Elements. Metall. Mater. Trans. 2005, 36, 1263–1271. [Google Scholar] [CrossRef]
- Lin, C.H.; Duh, J.G.; Yeh, J.W. Multi-Component Nitride Coatings Derived from Ti–Al–Cr–Si–V Target in RF Magnetron Sputter. Surf. Coat. Technol. 2007, 201, 6304–6308. [Google Scholar] [CrossRef]
- Chen, R.; Cai, Z.; Pu, J.; Lu, Z.; Chen, S.; Zheng, S.; Zeng, C. Effects of Nitriding on the Microstructure and Properties of VAlTiCrMo High-Entropy Alloy Coatings by Sputtering Technique. J. Alloys Compd. 2020, 827, 153836. [Google Scholar] [CrossRef]
- Jiang, C.X.; Li, R.B.; Wang, X.; Shang, H.L.; Zhang, Y.; Liaw, P.K. Diffusion Barrier Performance of AlCrTaTiZr/ AlCrTaTiZr-N High-Entropy Alloy Films for Cu/Si Connect System. Entropy 2020, 22, 234. [Google Scholar] [CrossRef]






| Barriers | Resistivity (μΩ·cm) | Melting Point (°C) | Deposition Method | Expected Thickness |
|---|---|---|---|---|
| Ta/TaN | Ta > 13 | Ta ~ 2996 | PVD or CVD | A few nm |
| PGMs | Ru ~ 7 Ir ~ 4.7 | Ru ~ 2334 Ir ~ 2454 | PVD, CVD, ALD, ED, electroless deposition | Few nm |
| 2D materials | Graphene ~ 1 | Graphene ~ 3652 | CVD | ~1 nm |
| SAMs | / | / | Solution immersion | Monolayer |
| HEAs | Poor | Normally > 1000 | Magnetron sputtering, laser cladding, ED, electron beam evaporation | Few nm |
| Alloys | Microstructure | FCC Lattice Constants (Å) | BCC Lattice Constants (Å) | Hardness (HV) |
|---|---|---|---|---|
| AlCoCrCuFeNi | FCC+BCC | 3.60 | 2.87 | 420 |
| Al0.5CoCrCuFeNi | FCC | 3.59 | - | 208 |
| AlCo0.5CrCuFeNi | FCC+BCC | 3.62 | 2.87 | 473 |
| AlCoCr0.5CuFeNi | FCC+BCC | 3.61 | 2.87 | 367 |
| AlCoCrCu0.5FeNi | BCC | - | 2.87 | 458 |
| AlCoCrCuFe0.5Ni | FCC+BCC | 3.61 | 2.87 | 418 |
| AlCoCrCuFeNi0.5 | FCC+BCC | 3.63 | 2.87 | 423 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Tian, Y.; Teng, C.; Cao, H. Recent Advances in Barrier Layer of Cu Interconnects. Materials 2020, 13, 5049. https://doi.org/10.3390/ma13215049
Li Z, Tian Y, Teng C, Cao H. Recent Advances in Barrier Layer of Cu Interconnects. Materials. 2020; 13(21):5049. https://doi.org/10.3390/ma13215049
Chicago/Turabian StyleLi, Zhi, Ye Tian, Chao Teng, and Hai Cao. 2020. "Recent Advances in Barrier Layer of Cu Interconnects" Materials 13, no. 21: 5049. https://doi.org/10.3390/ma13215049
APA StyleLi, Z., Tian, Y., Teng, C., & Cao, H. (2020). Recent Advances in Barrier Layer of Cu Interconnects. Materials, 13(21), 5049. https://doi.org/10.3390/ma13215049






