Comparison of the Physicochemical Properties of Carboxylic and Phosphonic Acid Self-Assembled Monolayers Created on a Ti-6Al-4V Substrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Modification Procedures
2.2. Surface Characterization
3. Results and Discussion
3.1. Optimization of the Liquid Phase Deposition Method Parameters
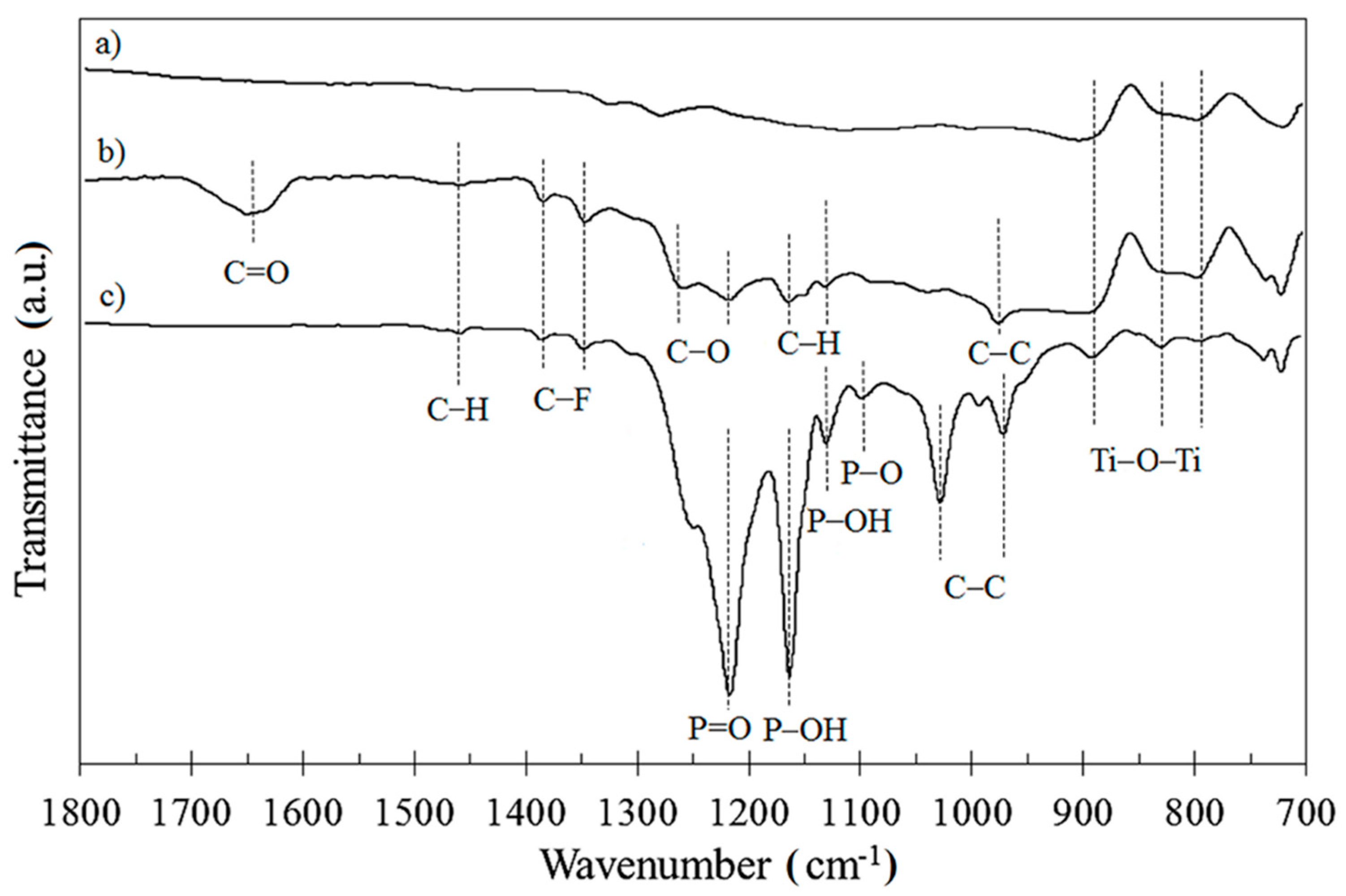
3.2. Surface Characterization Using XPS and FTIR Measurements
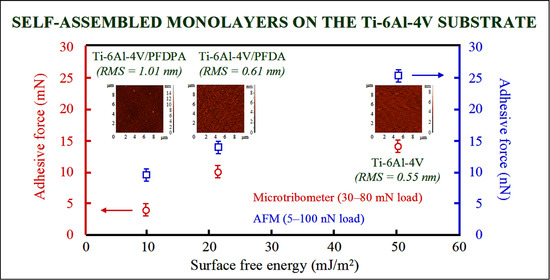
3.3. Surface Free Energy Measurements
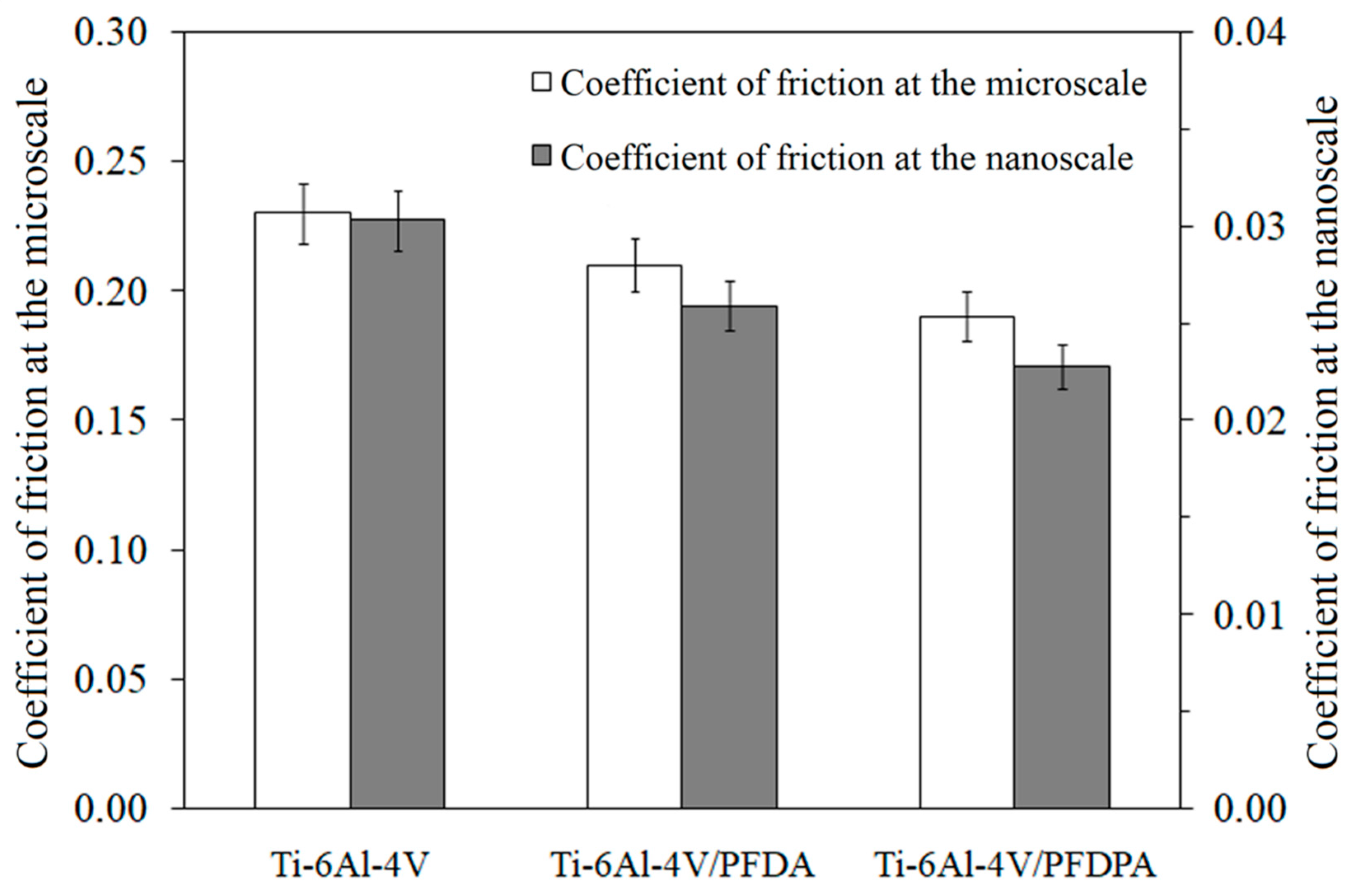
3.4. Micro- and Nanotribological Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New developments of Ti-based alloys for biomedical applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef] [Green Version]
- Abkowitz, A.; Burke, J.J.; Hiltz, B.H. Titanium in Industry; D. Van Nostrand: New York, NY, USA, 1995. [Google Scholar]
- Elias, C.N.; Lima, J.H.C.; Valiev, R.; Meyers, M.A. Biomedical Applications of Titanium and its Alloys. JOM 2008, 60, 46–49. [Google Scholar] [CrossRef]
- Sovak, G.; Gotman, I.; Weiss, A. Osseointegration of Ti–6Al–4V Alloy Implants with a Titanium Nitride Coating Produced by a PIRAC Nitriding Technique: A Long-Term Time Course Study in the Rat. Microsc. Microanal. 2015, 21, 179–189. [Google Scholar] [CrossRef]
- Anderson, C.; Mikaberidze, M.; Gordeziani, G.; Gozalishvili, E.; Akhvlediani, L.; Ramazashvili, D. Corrosion Resistant Titanium Alloys for Medical Tools and Implants. J. Powder Metall. Min. 2013, 2, 000110. [Google Scholar]
- Geetha, M.; Singh, A.; Asokamani, R.; Gogia, A. Ti based biomaterials, the ultimate choice for orthopedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Guleryuz, H.; Cimenoglu, H. Surface modification of a Ti-6Al-4V alloy by thermal oxidation. Surf. Coat. Technol. 2005, 192, 164–170. [Google Scholar] [CrossRef]
- Sansone, V.; Pagani, D.; Melato, M. The effects on bone cells of metal ions released from orthopedic implants. A review. Clin. Cases Miner. Bone Metab. 2013, 10, 34–40. [Google Scholar] [PubMed]
- Vadiraj, A.; Kamaraj, M. Effect of surface treatments on fretting fatigue damage of biomedical titanium alloys. Tribol. Int. 2007, 40, 82–88. [Google Scholar] [CrossRef]
- Antoniou, R.A.; Radtke, T.C. Mechanism of fretting fatigue of titanium alloys. Mater. Sci. Eng. 1997, 237, 229–240. [Google Scholar] [CrossRef]
- Dong, H.; Bell, T. Enhanced wear resistance of titanium surfaces by a new thermal oxidation treatment. Wear 2000, 238, 131–137. [Google Scholar] [CrossRef]
- Fridrici, V.; Fouvry, S.; Kapsa, P. Effect of shot peening on the fretting wear of Ti–6Al–4V. Wear 2001, 250, 642–649. [Google Scholar] [CrossRef]
- Dudek, M.; Fouvry, S.; Wendler, B.; Kapsa, P.; Liskiewicz, T. The effect of diffusion treatments in a glow-discharge plasma in Ar+O2 atmosphere on friction and wear of Ti–6Al–4V alloy. Vacuum 2003, 70, 187–191. [Google Scholar] [CrossRef]
- Jedrzejczak, A.; Kolodziejczyk, L.; Szymanski, W.; Piwonski, I.; Cichomski, M.; Kisielewska, A.; Dudek, M.; Batory, D. Friction and wear of a-C:H:SiOx coatings in combination with AISI 316L and ZrO2 counterbodies. Tribol. Int. 2017, 112, 155–162. [Google Scholar] [CrossRef]
- Bhushan, B.; Cichomski, M.; Tao, Z.; Tran, N.T.; Ethen, T.; Merton, C.; Jewett, R.E. Nanotribological Characterization and Lubricant Degradation Studies of Metal-Film Magnetic Tapes Using Novel Lubricants. J. Tribol. 2007, 129, 621–627. [Google Scholar] [CrossRef]
- Hahner, G.; Hofer, R.; Klingenfuss, I. Order and Orientation in Self-Assembled Long Chain Alkanephosphate Monolayers Adsorbed on Metal Oxide Surfaces. Langmuir 2001, 17, 7047–7052. [Google Scholar] [CrossRef]
- Taffa, D.H.; Kathiresan, M.; Walder, L. Tuning the Hydrophilic, Hydrophobic, and Ion Exchange Properties of Mesoporous TiO2. Langmuir 2009, 25, 5371–5379. [Google Scholar] [CrossRef]
- Hoque, E.; DeRose, J.A.; Hoffmann, P.; Mathieu, H.J.; Bhushan, B.; Cichomski, M. Phosphonate self-assembled monolayers on aluminum surfaces. J. Chem. Phys. 2006, 124, 174710. [Google Scholar] [CrossRef]
- Bhushan, B.; Cichomski, M.; Hoque, E.; DeRose, J.A.; Hoffmann, P.; Mathieu, H.J. Nanotribological characterization of perfluoroalkylphosphonate self-assembled monolayers deposited on aluminum-coated silicon substrates. Microsyst. Technol. 2006, 12, 588–596. [Google Scholar] [CrossRef]
- Ptak, A.; Makowski, M.; Cichomski, M. Characterization of nanoscale adhesion between a fluoroalkyl silane monolayer and a silicon AFM tip. Complex character of the interaction potential. Chem. Phys. Lett. 2010, 489, 54–58. [Google Scholar] [CrossRef]
- Krzykawska, A.; Ossowski, J.; Żaba, T.; Cyganik, P. Binding groups for highly ordered SAM formation: Carboxylic versus thiol. R. Soc. Chem. Chem. Commun. 2017, 53, 5748–5751. [Google Scholar] [CrossRef]
- Marcinko, S.; Fadeev, A.Y. Hydrolytic stability of organic monolayers supported on TiO2 and ZrO2. Langmuir 2004, 20, 2270–2273. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Sun, X.; He, T.; Sun, S. Formation of highly stable self-assembled alkyl phosphonic acid monolayers for the functionalization of titanium surfaces and protein patterning. Langmuir 2015, 31, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, G.; Alauzun, J.G.; Granier, M.; Laurencin, D.; Mutin, H. Phosphonate coupling molecules for the control of surface/interface properties and the synthesis of nanomaterials. R. Soc. Chem. Dalton Trans. 2013, 42, 12569–12585. [Google Scholar] [CrossRef] [PubMed]
- Sikora, A.; Bednarz, L.; Ekwinski, G.; Ekwinska, M. The determination of the spring constant of T-shaped cantilevers using calibration structures. Meas. Sci. Technol. 2014, 25, 044015. [Google Scholar] [CrossRef]
- Rozlosnik, N.; Gerstenberg, M.C.; Larsen, N.B. Effect of Solvents and Concentration on the Formation of a Self-Assembled Monolayer of Octadecylsiloxane on Silicon. Langmuir 2003, 19, 1182–1188. [Google Scholar] [CrossRef]
- Glaser, A.; Foisner, J.; Friedbacher, G.; Hoffmann, H. Low-temperature investigation of the growth mechanism of alkylsiloxane self-assembled monolayers. Anal. Bioanal. Chem. 2004, 379, 653–657. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, M.C.; Zhang, X.; Ng, H.W.; Gates, B.D. Optimizing the quality of monoreactive perfluoroalkylsilane-based self-assembled monolayers. Langmuir 2012, 28, 11790–11801. [Google Scholar] [CrossRef]
- Vallant, T.; Brunner, H.; Mayer, U.; Hoffmann, H.; Leitner, T.; Friedbacher, G. Formation of self-assembled octadecylsiloxane monolayers on mica and silicon surfaces studied by atomic force microscopy and infrared spectroscopy. J. Phys. Chem. B 1998, 102, 7190–7197. [Google Scholar] [CrossRef]
- Cichomski, M.; Prowizor, M.; Borkowska, E.; Piwoński, I.; Jędrzejczak, A.; Dudek, M.; Batory, D.; Wrońska, N.; Lisowska, K. Impact of perfluoro and alkylphosphonic self-assembled monoayers on tribological and antimicrobial properties of Ti-DLC coatings. Materials 2019, 12, 2365. [Google Scholar] [CrossRef] [Green Version]
- Cichomski, M. Tribological investigations of perfluoroalkylsilanes monolayers deposited on titanium surface. Mater. Chem. Phys. 2012, 136, 498–504. [Google Scholar] [CrossRef]
- Cichomski, M.; Kisielewska, A.; Prowizor, M.; Borkowska, E.; Piwoński, I.; Dudek, M.; Jędrzejczak, A.; Batory, D. The influence of self-assembled monolayers on tribological properties of Si-DLC coatings. Surf. Topogr. Metrol. Prop. 2019, 7, 045006. [Google Scholar] [CrossRef]
- Krafft, M.P. Fluorocarbons and fluorinated amphiphiles in drug delivery and biomedical research. Adv. Drug Deliv. Rev. 2001, 47, 209–228. [Google Scholar] [CrossRef]
- Krafft, M.P.; Riess, J.G. Chemistry, physical chemistry, and uses of molecular fluorocarbon−hydrocarbon diblocks, triblocks, and related compounds—Unique ‘Apolar’ components for self-assembled colloid and interface engineering. Chem. Rev. 2009, 109, 1714–1792. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, V.H.; Rossky, P.J. Molecular origins of fluorocarbon hydrophobicity. Proc. Natl. Acad. Sci. USA 2010, 107, 13603–13607. [Google Scholar] [CrossRef] [Green Version]
- Miller, T.M.; Bederson, B. Atomic and molecular polarizabilities-a review of recent advances. Adv. Aant. Mol. Opt. Phys. 1978, 13, 1–55. [Google Scholar]
- Dunitz, J.D. Organic fluorine: Odd man out. ChemBioChem 2004, 5, 614–621. [Google Scholar] [CrossRef]
- Helmy, R.; Fadeev, A.Y. Self-assembled monolayers supported on TiO2: Comparison of C18H37SiX3 (X=H, Cl, OCH3), C18H37Si(CH3)2Cl, and C18H37PO(OH)2. Langmuir 2002, 18, 8924–8928. [Google Scholar] [CrossRef]
- Wallace, R.M.; Chen, P.J.; Henck, S.A.; Webb, D.A. Adsorption of perfluorinated n-alkanoic acids on native aluminum oxide surfaces. J. Vac. Sci. Technol. A 1995, 13, 1345–1350. [Google Scholar] [CrossRef]
- DeRose, J.A.; Hoque, E.; Bhushan, B.; Mathieu, H.J. Characterization of perfluorodecanoate self-assembled monolayers on aluminum and comparison of stability with phosphonate and siloxy self-assembled monolayers. Surf. Sci. 2008, 602, 1360–1367. [Google Scholar] [CrossRef]
- Karlsson, J.; Snis, A.; Engqvist, H.; Lausmaa, J. Characterization and comparison of materials produced by Electron Beam Melting (EBM) of two different Ti-6Al-4V powder fractions. J. Mater. Process. Technol. 2013, 213, 2109–2118. [Google Scholar] [CrossRef]
- Cichomski, M.; Kośla, K.; Pawlak, W.; Kozłowski, W.; Szmaja, W. Stability and tribological investigations of 1H,1H,2H,2H-perfluoroalkyltrichlorosilane on titania surface. Tribol. Int. 2014, 77, 1–6. [Google Scholar] [CrossRef]
- Ting, G.G.; Acton, O.; Ma, H.; Ka, J.W.; Jen, A.K.Y. Study on the formation of self-assembled monolayers on sol-gel processed hafnium oxide as dielectric layers. Langmuir 2009, 25, 2140–2147. [Google Scholar] [CrossRef] [PubMed]
- Zuilhof, H.; Pujari, S.P.; Scheres, L.; Marcelis, A.T.M. Covalent Surface Modification of Oxide Surfaces. Angew. Chem. Int. Ed. 2014, 53, 2–36. [Google Scholar]
- Brodard-Severac, F.; Guerrero, G.; Maquet, J.; Florian, P.; Gervais, C.; Mutin, P.H. High-Field 17O MAS NMR Investigation of Phosphonic Acid Monolayers on Titania. Chem. Mater. 2008, 20, 5191–5196. [Google Scholar] [CrossRef]
- Textor, M.; Ruiz, L.; Hofer, R.; Rossi, A.; Feldman, K.; Hahner, G.; Spencer, N.D. Structural chemistry of self-assembled monolayers of octadecylphosphoric acid on tantalum oxide surfaces. Langmuir 2000, 16, 3257. [Google Scholar] [CrossRef]
- Davies, P.R.; Newton, N.G. The chemisorption of organophosphorus compounds at an Al(111) surface. Appl. Surf. Sci. 2001, 181, 296. [Google Scholar] [CrossRef]
- Korrapatia, V.K.; Scharnagl, N.; Letzig, D.; Zheludkevich, M.L. Self-assembled layers for the temporary corrosion protection of magnesium-AZ31 alloy. Corros. Sci. 2020, 179, 108619. [Google Scholar] [CrossRef]
- Andrews, B.; Almahdali, S.; James, K.; Ly, S.; Crowder, K.N. Copper oxide surfaces modified by alkylphosphonic acids with terminal pyridyl-based ligands as a platform for supported catalysis. Polyhedron 2016, 114, 360–369. [Google Scholar] [CrossRef]
- Yan, Y.; Chibowski, E.; Szcześ, A. Surface properties of Ti-6Al-4V alloy part I: Surface roughness and apparent surface free energy. Mater. Sci. Eng. C 2017, 70, 207–215. [Google Scholar] [CrossRef] [PubMed]
- van Oss, C.J.; Chaudhury, M.K.; Good, R.J. Interfacial Lifschitz-van der Waals and Polar Interactions in Microscopic Systems. J. Chem. Rev. 1988, 88, 927–941. [Google Scholar] [CrossRef]
- Chen, Z.; He, X.; Xiao, C.; Kim, S.H. Effect of humidity on friction and wear-a critical review. Lubricants 2018, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Ebbens, S.; Chen, X.; Jin, Z.; Luk, S.; Madden, C.; Patel, N.; Roberts, C.J. Determination of the Surface Free Energy of Crystalline and Amorphous Lactose by Atomic Force Microscopy Adhesion Measurement. Pharm. Res. 2006, 23, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.N. Intermolecular and Surface Forces, 2nd ed.; Academic Press: London, UK, 1991. [Google Scholar]
- Choe, H.; Hong, M.H.; Seo, Y.; Lee, K.; Kim, G.; Cho, Y.; Ihm, J.; Jhe, W. Formation, Manipulation, and Elasticity Measurement of a Nanometric Column of Water Molecules. Phys. Rev. Lett. 2005, 95, 187801. [Google Scholar] [CrossRef] [PubMed] [Green Version]




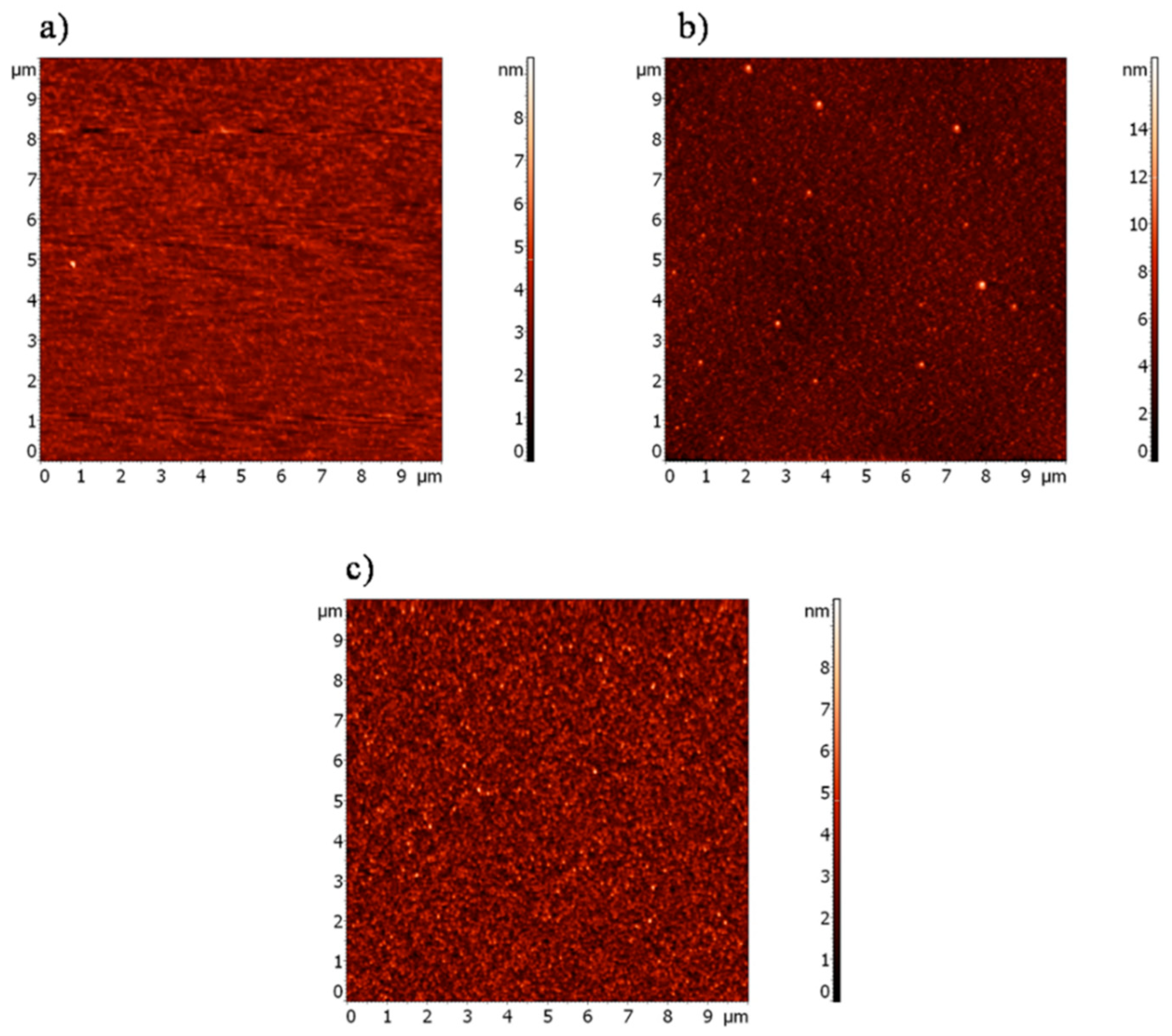



| Materials | SFE (mJ/m2) | LW (mJ/m2) | AB (mJ/m2) |
|---|---|---|---|
| Ti-6Al-4V | 49.9 ± 0.6 | 34.8 ± 0.2 | 12.1 ± 0.6 |
| Ti-6Al-4V/PFDA | 21.4 ± 0.3 | 19.9 ± 0.9 | 1.5 ± 0.1 |
| Ti-6Al-4V/PFDPA | 9.1 ± 0.3 | 8.1 ± 0.9 | 1.0 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cichomski, M.; Prowizor, M.; Kowalczyk, D.A.; Sikora, A.; Batory, D.; Dudek, M. Comparison of the Physicochemical Properties of Carboxylic and Phosphonic Acid Self-Assembled Monolayers Created on a Ti-6Al-4V Substrate. Materials 2020, 13, 5137. https://doi.org/10.3390/ma13225137
Cichomski M, Prowizor M, Kowalczyk DA, Sikora A, Batory D, Dudek M. Comparison of the Physicochemical Properties of Carboxylic and Phosphonic Acid Self-Assembled Monolayers Created on a Ti-6Al-4V Substrate. Materials. 2020; 13(22):5137. https://doi.org/10.3390/ma13225137
Chicago/Turabian StyleCichomski, Michal, Milena Prowizor, Dorota Anna Kowalczyk, Andrzej Sikora, Damian Batory, and Mariusz Dudek. 2020. "Comparison of the Physicochemical Properties of Carboxylic and Phosphonic Acid Self-Assembled Monolayers Created on a Ti-6Al-4V Substrate" Materials 13, no. 22: 5137. https://doi.org/10.3390/ma13225137