Effect of Nitrided and Nitrocarburised Austenite
Abstract
:1. Introduction
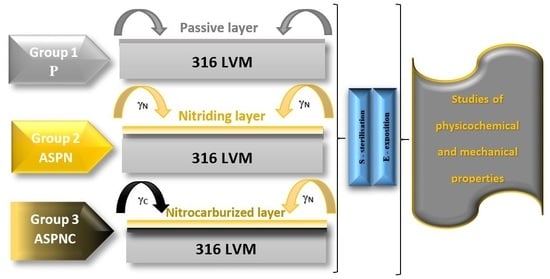
2. Materials and Methods
2.1. Layer Microstructure Test
2.2. Surface Roughness Test
2.3. Wettability and Surface Energy Tests
2.4. Pitting Corrosion Resistance Tests
2.5. Crevice Corrosion Resistance Tests
2.6. Macroscopic Assessment of the Surface
2.7. Nanohardness Measurement
2.8. Statistical Analysis
3. Results
3.1. Layer Microstructure Test
3.2. Surface Roughness
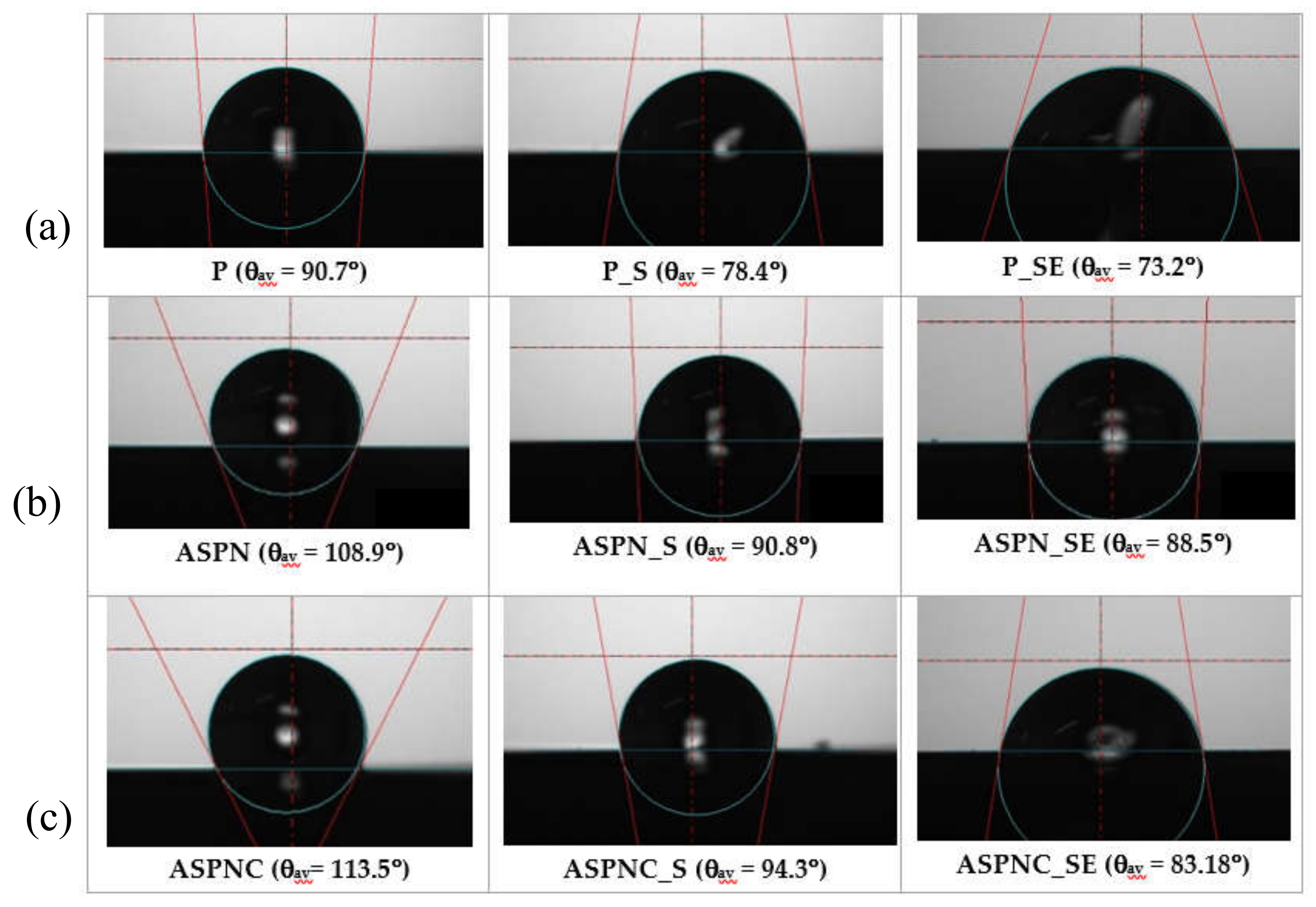
3.3. Wettability and Surface Energy Results
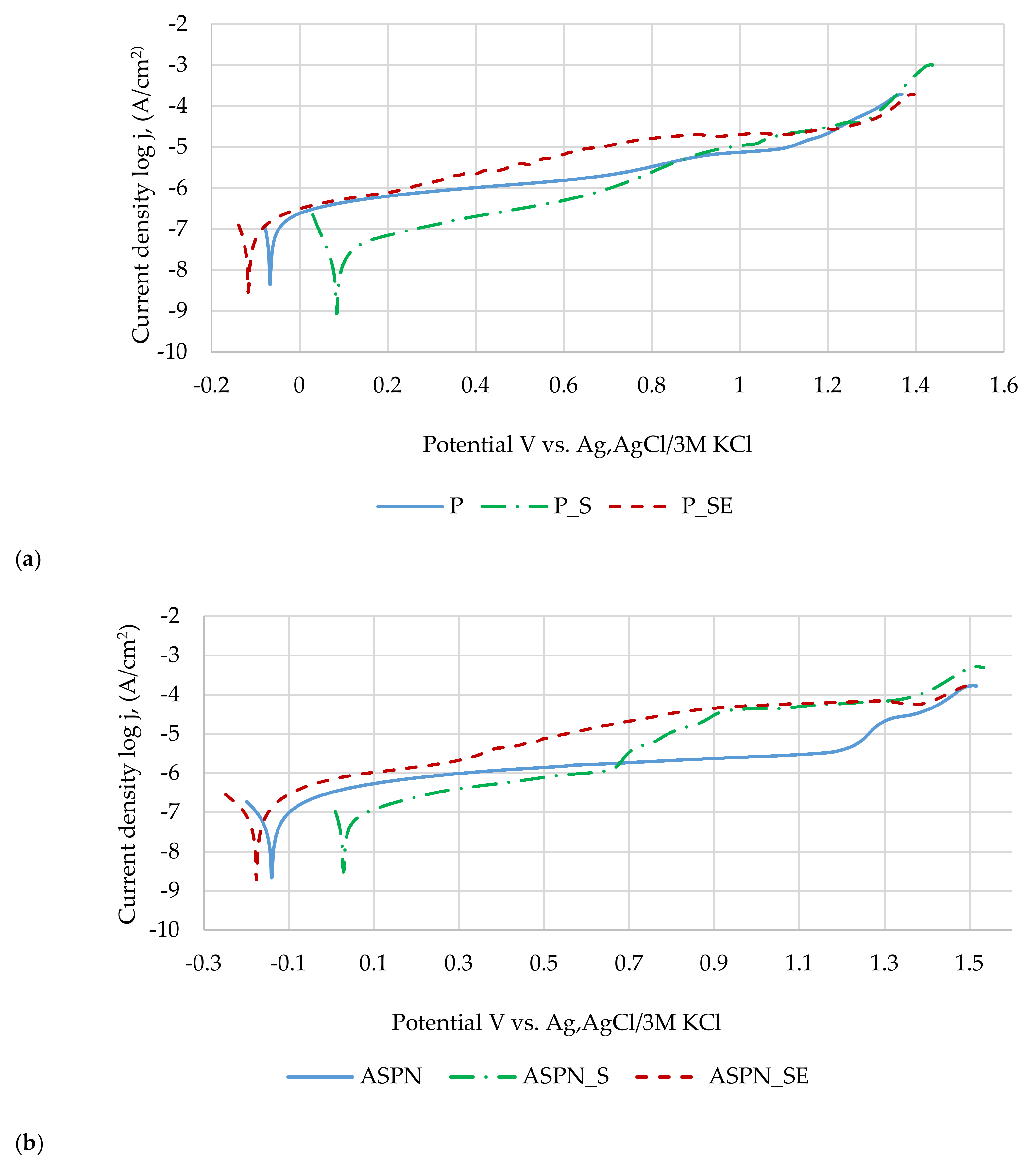
3.4. Pitting Corrosion Resistance Results
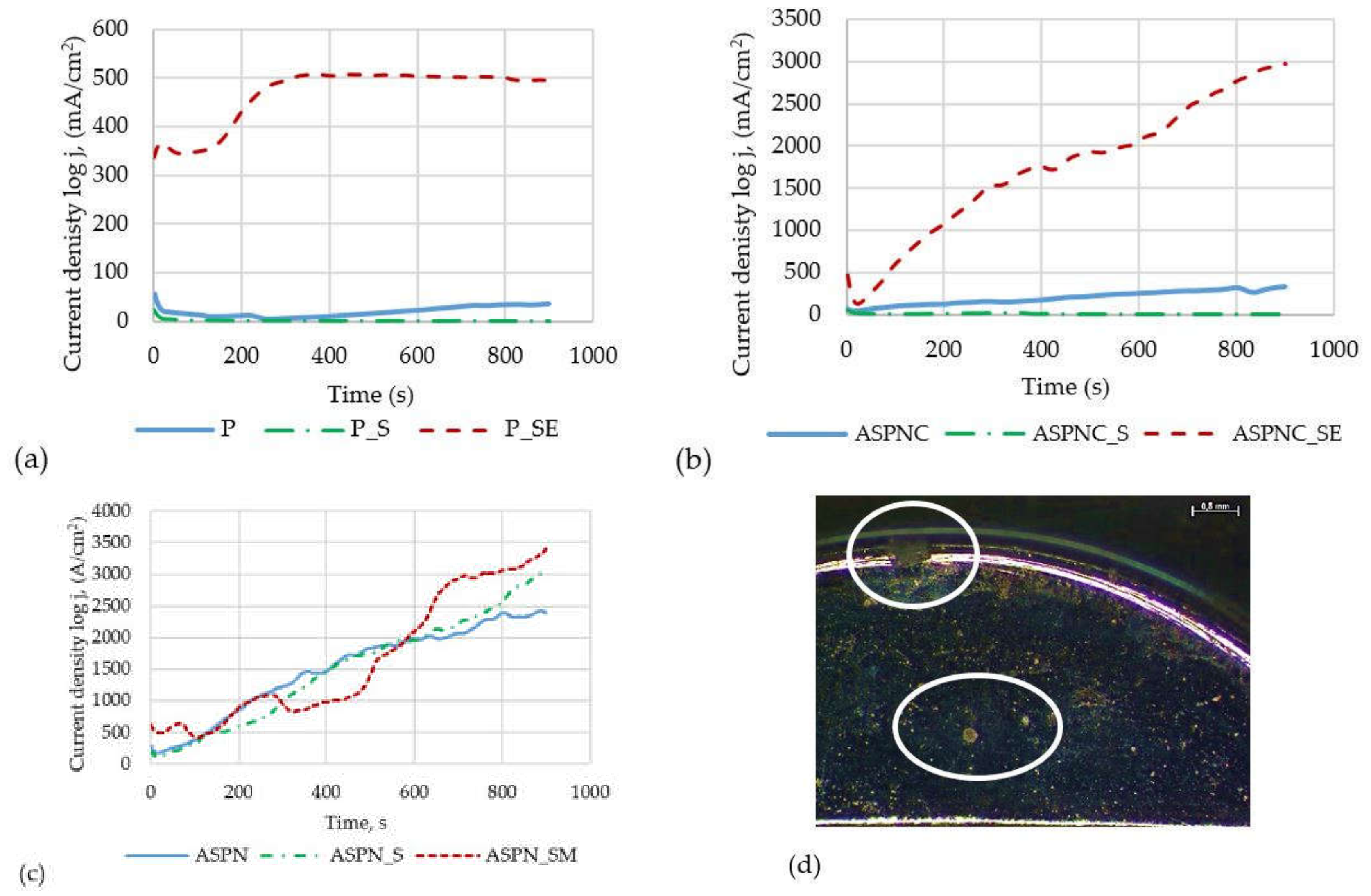
3.5. Crevice Corrosion Resistance Results
3.6. Results of Nanohardness Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ratner, B.D.; Hoffman, A.S.; Lemons, F.J.J.E. Biomaterials Science. An Introduction to Materials in Medicine; Elsevier Academic Press: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Marciniak, J. Biomaterials; Silesian University of Technology: Gliwice, Poland, 2013. [Google Scholar]
- Marciniak, J. Modification of surface films on chromium-nickel-molybdenum steel implants used in orthopaedics and traumatology. J. Ach. Mater. Manufac. Eng. 2010, 43, 108–116. [Google Scholar]
- Szewczenko, J.; Jaglarz, J.; Basiaga, M.; Kurzyk, J.; Skoczek, E.; Paszenda, Z. Topography and thickness of passive layers on anodically oxidized Ti6Al4V alloys. Przegląd Elektrotechniczny 2012, 88, 228–231. [Google Scholar]
- Szewczenko, J.; Jaglarz, J.; Basiaga, M.; Kurzyk, J.; Paszenda, Z. Optical methods applied in thickness and topography testing of passive layers on implantable titanium alloys. Opt. Appl. 2013, 43, 173–180. [Google Scholar]
- Romańczuk, E.; Oksiuta, Z. Comparison of corrosion resistance in physiological saline solution of two austenitic stainless steels—316LV and REX734. Acta Mech. Autom. 2017, 11, 91–95. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhu, D.; Pierre, D.; Gilbert, J.L. Fretting Initiated Crevice Corrosion of 316LVM Stainless Steel in Physiological Phosphate Buffered Saline: Potential and Cycles to Initiation. Acta Biomater. 2019, 97, 565–577. [Google Scholar] [CrossRef]
- Wielowiejska-Giertuga, A.; Wiśniewski, T.; Rubach, R. Fretting Corrosion Studies of Materials Used for Elements of Hip Joint Endoprostheses. Tribologia 2018, 5, 143–151. [Google Scholar] [CrossRef]
- Sesia, S.B.; Haecker, F.M.; Shah, B.; Goretsky, M.J.; Kelly, R.E., Jr.; Obermeyer, R.J. Development of metal allergy after Nuss procedure for repair of pectus excavatum despite preoperative negative skin test. J. Pediatric Surg. Case Rep. 2013, 1, 152–155. [Google Scholar] [CrossRef] [Green Version]
- Lau, T.; Leung, F.; Chan, C.; Chow, S. Wound complication of minimally invasive plate osteosynthesis in distal tibia fractures. Int. Orthop. 2008, 32, 697–703. [Google Scholar] [CrossRef] [Green Version]
- Pokrowiecki, R.; Tyski, S.; Zaleska, M. Problematyka zakażeń okołowszczepowych. Post. Mikrobiol. 2014, 53, 123–134. [Google Scholar]
- Basiaga, M.; Staszuk, M.; Walke, W.; Opilski, Z. Mechanical properties of atomic layer deposition (ALD) TiO2 layers on stainless steel substrates. Materialwissenschaft und Werkstofftechnik 2016, 47, 512–520. [Google Scholar] [CrossRef]
- Basiaga, M.; Walke, W.; Staszuk, M.; Kajzer, W.; Kajzer, A.; Nowinska, K. Influence of ALD process parameters on the physical and chemical properties of the surface of vascular stents. Arch. Civ. Mech. Eng. 2017, 17, 32–42. [Google Scholar] [CrossRef]
- Skolek-Stefaniszyn, E.; Kamiński, J.; Sobczak, J.; Wierzchon, T. Modifying the properties of AISI 316L steel by glow discharge assisted low-temperature nitriding and oxynitriding. Vacuum 2010, 85, 164–169. [Google Scholar] [CrossRef]
- Lu, S.; Zhao, X.; Wang, S.; Li, J.; Wei, W.; Hu, J. Performance enhancement by plasma nitriding at low gas pressure for304 austenitic stainless steel. Vacuum 2017, 145, 334–339. [Google Scholar] [CrossRef]
- Ura-Bińczyk, E.; Krawczyńska, A.; Sitek, R.; Lewandowska, M. Mechanical properties and corrosion resistance of hydrostatically extruded 316 LVM stainless steel after low-temperature plasma nitriding. Surf. Coat. Technol. 2019, 375, 565–572. [Google Scholar] [CrossRef]
- Rajendran, P.; Devaraju, A. Experimental Evaluation of Mechanical and tribological behaviours of gas nitride treated AISI 316LN austenitic stainless steel. Mater. Today Proc. 2018, 5, 14333–14338. [Google Scholar] [CrossRef]
- Manova, D.; Gerlach, J.W.; Scholze, F.; Mändl, S.; Neumann, H. Nitriding of austenitic stainless steel by pulsed low energy ion implantation. Surf. Coat. Technol. 2010, 204, 2919–2922. [Google Scholar] [CrossRef]
- Sun, Y. Corrosion behaviour of low temperature plasma carburised 316L stainless steel in chloride containing solutions. Corros. Sci. 2010, 52, 2661–2670. [Google Scholar] [CrossRef]
- Hummelshøj, T.S.; Christiansen, T.L.; Somers, M.A.J. Lattice expansion of carbon-stabilized expanded austenite. Scr. Mater. 2010, 63, 761–763. [Google Scholar] [CrossRef]
- Christiansen, T.L.; Somers, M.A.J. Low-temperature gaseous surface hardening of stainless steel: The current status. Int. J. Mater. Res. 2009, 100, 1361–1377. [Google Scholar] [CrossRef]
- Christiansen, T.; Somers, M.A.J. On the crystallographic structure of S-phase. Scr. Mater. 2004, 50, 35–37. [Google Scholar] [CrossRef]
- Blawert, C.; Kalvelage, H.; Mordike, B.L.; Collins, G.A.; Short, K.T.; Jirásková, Y.; Schneeweiss, O. Nitrogen and carbon expanded austenite produced by PI3. Surf. Coat. Technol. 2001, 136, 181–187. [Google Scholar] [CrossRef]
- Fewell, M.F.; Mitchell, D.R.G.; Priest, J.M.; Short, K.T.; Collins, G.A. The nature of expanded austenite. Surf. Coat. Technol. 2000, 131, 300–306. [Google Scholar] [CrossRef]
- Fernandes, F.A.P.; Casteletti, L.C.; Gallego, J. Microstructure of nitrided and nitrocarburized layers produced on a superaustenitic stainless steel. J. Mater. Res. Technol. 2013, 2, 158–164. [Google Scholar] [CrossRef] [Green Version]
- Biehler, J.; Hoche, H.; Oechsner, M. Corrosion properties of polished and shot-peened austenitic stainlesssteel 304L and 316L with and without plasma nitriding. Surf. Coat. Technol. 2017, 313, 40–46. [Google Scholar] [CrossRef]
- Fernandes, F.A.P.; Heck, S.C.; Pereira, R.G.; Picon, C.A.; Nascente, P.A.P.; Casteletti, L.C. Ion nitriding of a superaustenitic stainless steel: Wear and corrosion characterization. Surf. Coat. Technol. 2010, 204, 3087–3090. [Google Scholar] [CrossRef]
- Borowski, T.; Adamczyk-Cieślak, B.; Brojanowska, A.; Kulikowski, K.; Wierzchoń, T. Surface modification of austenitic steel by various glow-discharge nitriding methods. Mater. Sci. 2015, 21, 376–381. [Google Scholar] [CrossRef]
- De Sousa, R.R.M.; de Araújo, F.O.; Gontijo, L.C.; da Costa, J.A.P.; Alves, C., Jr. Cathodic cage plasma nitriding (CCPN) of austenitic stainless steel (AISI 316): Influence of the different ratios of the (N2/H2) on the nitrided layers properties. Vacuum 2012, 86, 2048–2053. [Google Scholar] [CrossRef]
- PN–EN ISO 4287/A1:2010. Specyfikacje Geometrii Wyrobów. Struktura Geometryczna Powierzchni.; Polish Committee for Standardization: Warsaw, Poland, 2010. [Google Scholar]
- Standard PN EN ISO 10993-15:2009. Biological Evaluation of Medical Devices—Part 15: Identification and Quantification of Degradation Products from Metals and Alloys; Polish Committee for Standardization: Warsaw, Poland, 2009. [Google Scholar]
- ASTM F746–04. Standard Test Method for Pitting or Crevice Corrosion of Metallic Surgical Implant Materials; ASTM International: Conshohocken, PA, USA, 2014. [Google Scholar]
- Borowski, T.; Kulikowski, K.; Adamczyk-Cieślak, B.; Rożniatowski, K.; Spychalski, M.; Tarnowski, M. Influence of nitrided and nitrocarburised layers on the functional properties of nitrogen-doped soft carbon-based coatings deposited on 316L steel under DC glow-discharge conditions. Surf. Coat. Technol. 2020, 392, 125705. [Google Scholar] [CrossRef]
- Celik, O.; Baydogan, M.; Atar, E.; SabriKayali, E.; Cimenoglu, H. Fatigue performance of low temperature nitrided AISI 321 grade austenitic stainless steel. Mater. Sci. Eng. A 2013, 565, 38–43. [Google Scholar] [CrossRef]
- Borgioli, F.; Galvanetto, E.; Bacci, T. Corrosion behaviour of low temperature nitrided nickel-free, AISI 200 and AISI 300 series austenitic stainless steels in NaCl solution. Corros. Sci. 2018, 136, 352–365. [Google Scholar] [CrossRef]
- Borgioli, F.; Galvanetto, E.; Bacci, T. Influence of surface morphology and roughness on water wetting properties of low temperature nitrided austenitic stainless steels. Mater. Charact. 2014, 95, 278–284. [Google Scholar] [CrossRef]
- Borgioli, F.; Galvanetto, E.; Bacci, T. Low temperature nitriding of AISI 300 and 200 series austenitic stainless steels. Vacuum 2016, 127, 51–60. [Google Scholar] [CrossRef]
- Lei, M.K.; Zhu, X.M. In vitro corrosion resistance of plasma source ion nitrided austenitic stainless steels. Biomaterials 2001, 22, 641–647. [Google Scholar] [CrossRef]
- Zhou, Y.N.; Breyen, M.D. Breyen: Joining and Assembly of Medical Materials and Divices; Woodhead Publishing: Woodhead, UK, 2013. [Google Scholar]
- Shih, C.C.; Su, Y.Y.; Chen, L.C.; Shih, C.M.; Lin, S.J. Degradation of 316L stainless steel sternal wire by steam sterilization. Acta Biomater. 2010, 6, 2322–2328. [Google Scholar] [CrossRef] [PubMed]
- Pegueroles, M.; Gil, F.J.; Planell, J.A.; Aparicio, C. The influence of blasting and sterilization on static and time-related wettability and Surface-energy properties of titanium Surface. Surf. Coat. Technol. 2008, 202, 3470–3479. [Google Scholar] [CrossRef]
- Xu, L.C. Effect of surface wettability and contact time on protein adhesion to biomaterial surfaces. Biomaterials 2007, 28, 3273–3283. [Google Scholar] [CrossRef] [Green Version]
- Kajzer, A.; Głąb, E.; Kajzer, W.; Wróbel, T.; Antonowicz, M. Corrosion Resistance of Stabilizers for Funnel Chest Treatment. Innov. Biomed. Eng. 2017, 526, 25–32. [Google Scholar]
- Bociąga, D.; Jastrzębski, K.; Olejnik, A.; Świątek, L.; Marchwicka, M. Influence of the multiple sterilization proces on the biomateriał properties. Eng. Biomater. 2016, 136, 11–20. [Google Scholar]
- Kajzer, A.; Rabij, K.; Basiaga, M.; Nowińska, K.; Kaczmarek, M.; Borowski, T.; Wierzchoń, T. Influence of sterilization and exposure to the Ringer’s solution on mechanical and physicochemical properties of nitrocarburized 316 LVM steel. Arch. Metall. Mater. 2018, 63, 1257–1266. [Google Scholar]
- Sobczyk-Guzenda, A.; Pietrzyk, B.; Jakubowski, W.; Szymanowski, H.; Szymanski, W.; Kowalski, J.; Olesko, K.; Gazicki-Lipman, M. Mechanical, photocatalytic and microbiological properties of titanium dioxide thin films synthesized with the sol–gel and low temperature plasma deposition techniques. Mater. Res. Bull. 2013, 48, 4022–4031. [Google Scholar] [CrossRef]
- Lee, I.; Barua, A. Behavior of the S-phase of plasma nitrocarburized 316L austenitic stainless steel on changing pulse frequency and discharge voltage at fixed pulse-off time. Surf. Coat. Technol. 2016, 307, 1045–1052. [Google Scholar] [CrossRef]







| Layer | Temperature, °C | Time, min | Pressure, hPa | N2 | H2 | CH4 |
|---|---|---|---|---|---|---|
| ASPN | 420 | 60 | 2 | 50 | 150 | - |
| ASPNC | 420 | 60 | 2 | 47 | 143 | 10 |
| Sample | Initial State | After Sterilisation | After Sterilisation and Exposure |
|---|---|---|---|
| Passivated—group 1 | P | P_S | P_SE |
| Nitrided—group 2 | ASPN | ASPN_S | ASPN_SE |
| Nitrocarburised—group 3 | ASPNC | ASPNC_S | ASPNC_SE |
| Sample | Ecorr, mV | SD 1 | Eb, mV | SD | Rp, kΩ⋅cm2 | SD |
|---|---|---|---|---|---|---|
| P | −44 | 21 | 1263 | 62 | 187 | 66 |
| P_S | 112 | 38 | 1364 | 8 | 528 | 75 |
| P_SE | −85 | 55 | 1357 | 28 | 203 | 68 |
| ASPN | −38 | 35 | 1385 | 33 | 524 | 85 |
| ASPN_S | −3 | 44 | 1398 | 5 | 398 | 71 |
| ASPN_SE | −155 | 20 | 1354 | 34 | 370 | 97 |
| ASPNC | 232 | 27 | 1393 | 26 | 274 | 89 |
| ASPNC_S | 88 | 46 | 1357 | 27 | 279 | 95 |
| ASPNC_SE | −97 | 24 | 1125 | 97 | 94 | 22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kajzer, A.; Ceglarska, M.; Sura, N.; Kajzer, W.; Borowski, T.; Tarnowski, M.; Pilecki, Z.
Effect of Nitrided and Nitrocarburised Austenite
Kajzer A, Ceglarska M, Sura N, Kajzer W, Borowski T, Tarnowski M, Pilecki Z.
Effect of Nitrided and Nitrocarburised Austenite
Kajzer, Anita, Magdalena Ceglarska, Nika Sura, Wojciech Kajzer, Tomasz Borowski, Michał Tarnowski, and Zbigniew Pilecki.
2020. "Effect of Nitrided and Nitrocarburised Austenite
Kajzer, A., Ceglarska, M., Sura, N., Kajzer, W., Borowski, T., Tarnowski, M., & Pilecki, Z.
(2020). Effect of Nitrided and Nitrocarburised Austenite