Development of Bacterial Cellulose Biocomposites Combined with Starch and Collagen and Evaluation of Their Properties
Abstract
1. Introduction
2. Materials and Methods
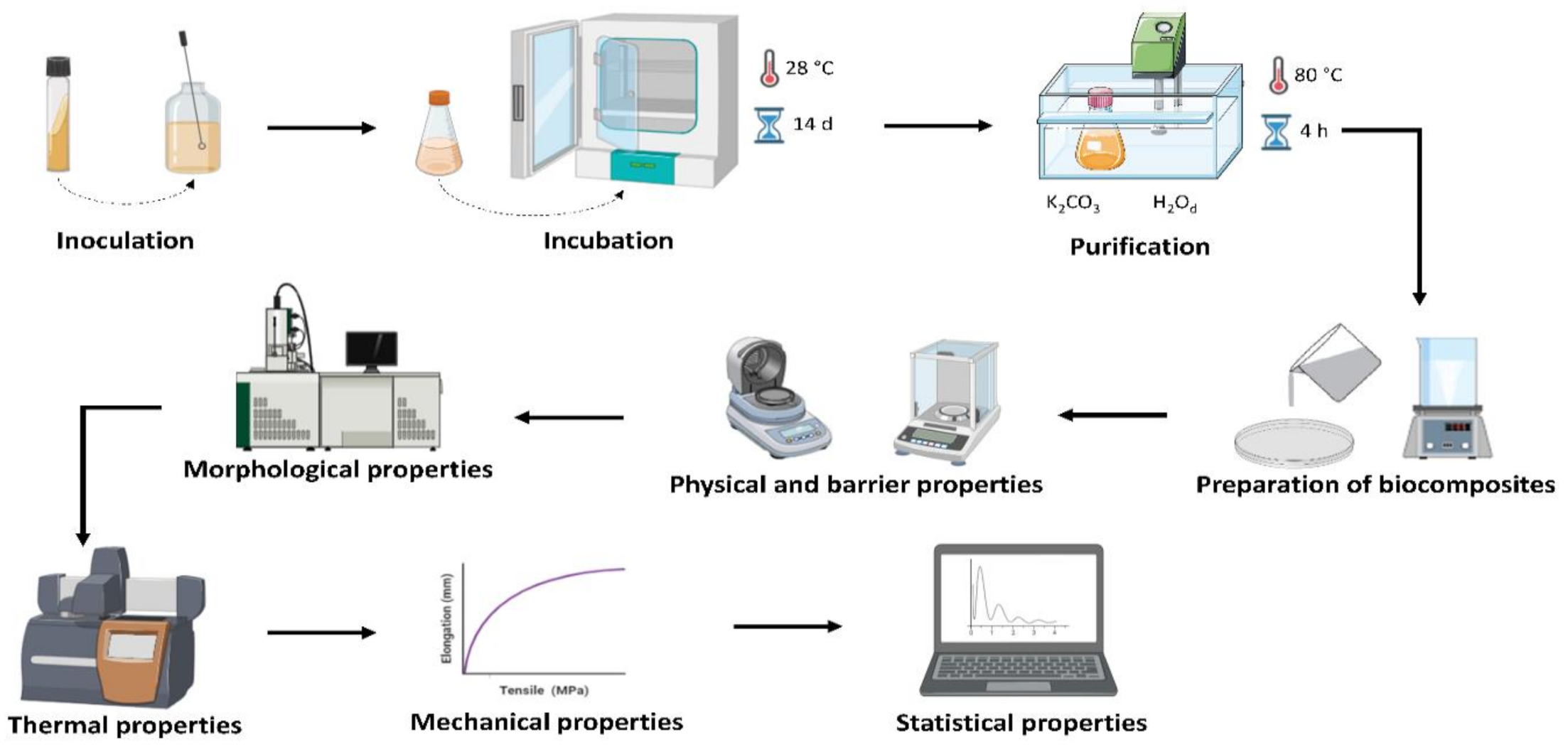
2.1. Bacterial Cellulose (BC) Production and Purification
2.2. Preparation of the Biocomposites
2.3. Biocomposite Characterization
2.3.1. Moisture, Total Solids (TS), and Water Activity (aw)
2.3.2. Water Solubility (WS) and Water Vapor Permeability (WVP)
2.3.3. Opacity and Grammage
2.3.4. Thickness and Mechanical Properties
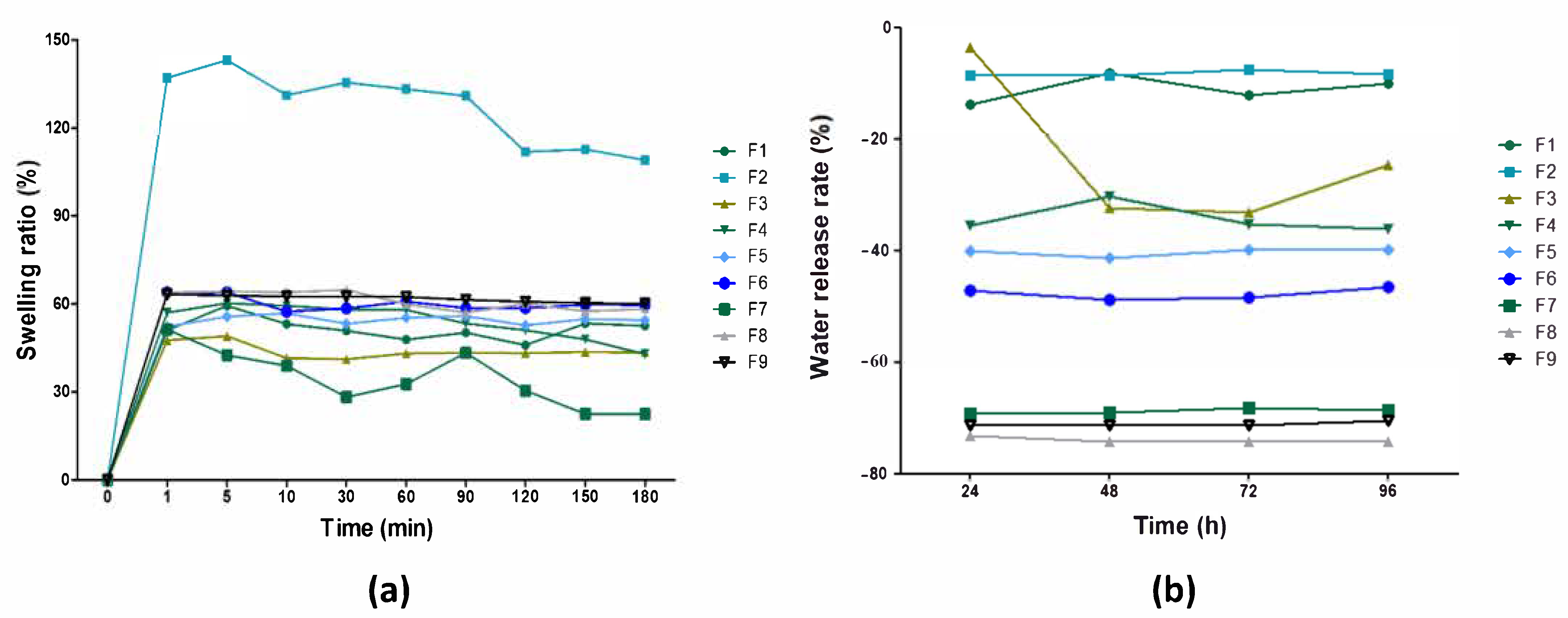
2.3.5. Swelling Rate (SR) and Water Release Rate (WRR)
2.3.6. Scanning Electron Microscopy (SEM)
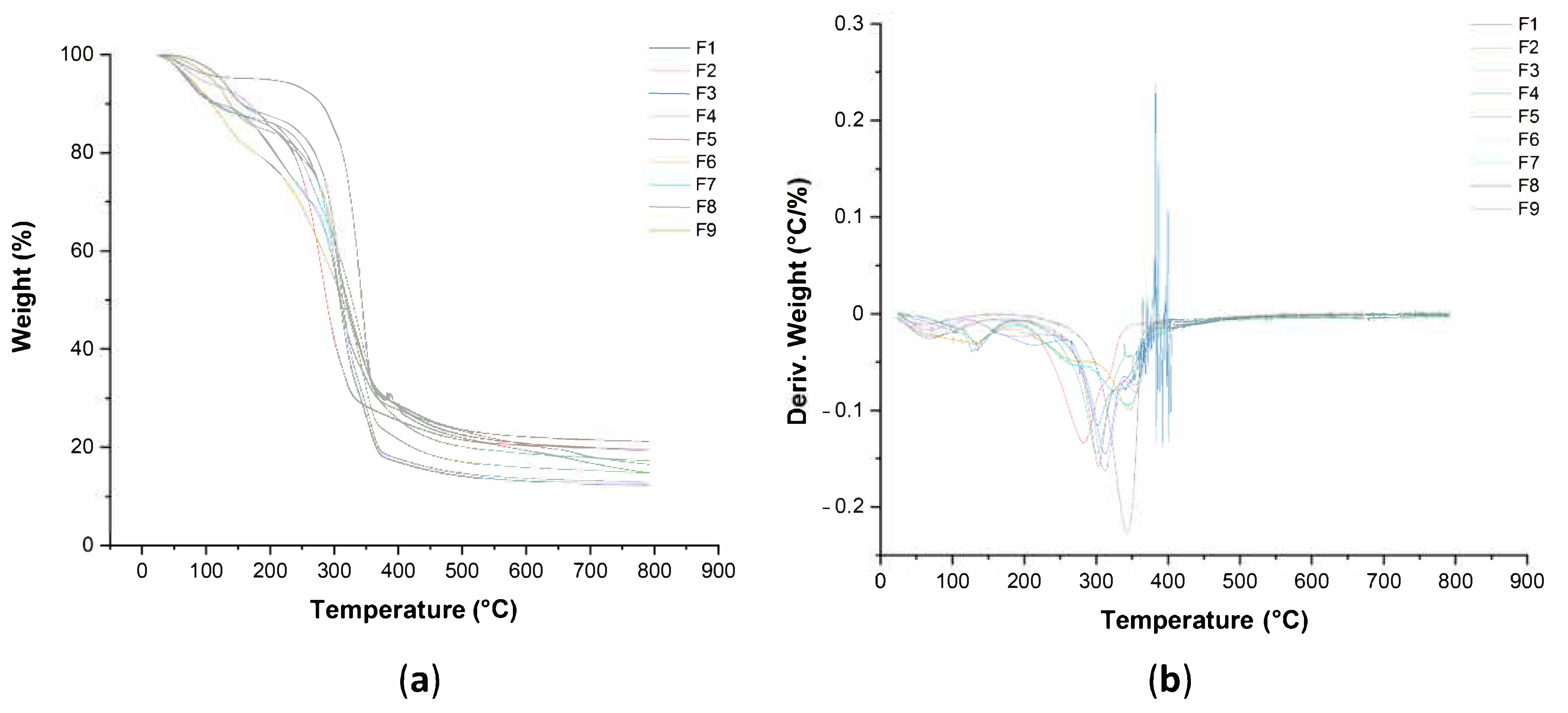
2.3.7. Thermogravimetric Analysis (TGA)
2.4. Statistical Analysis
3. Results
3.1. Visual Appearance of Pure BC and BC–Collagen–Starch Biocomposites
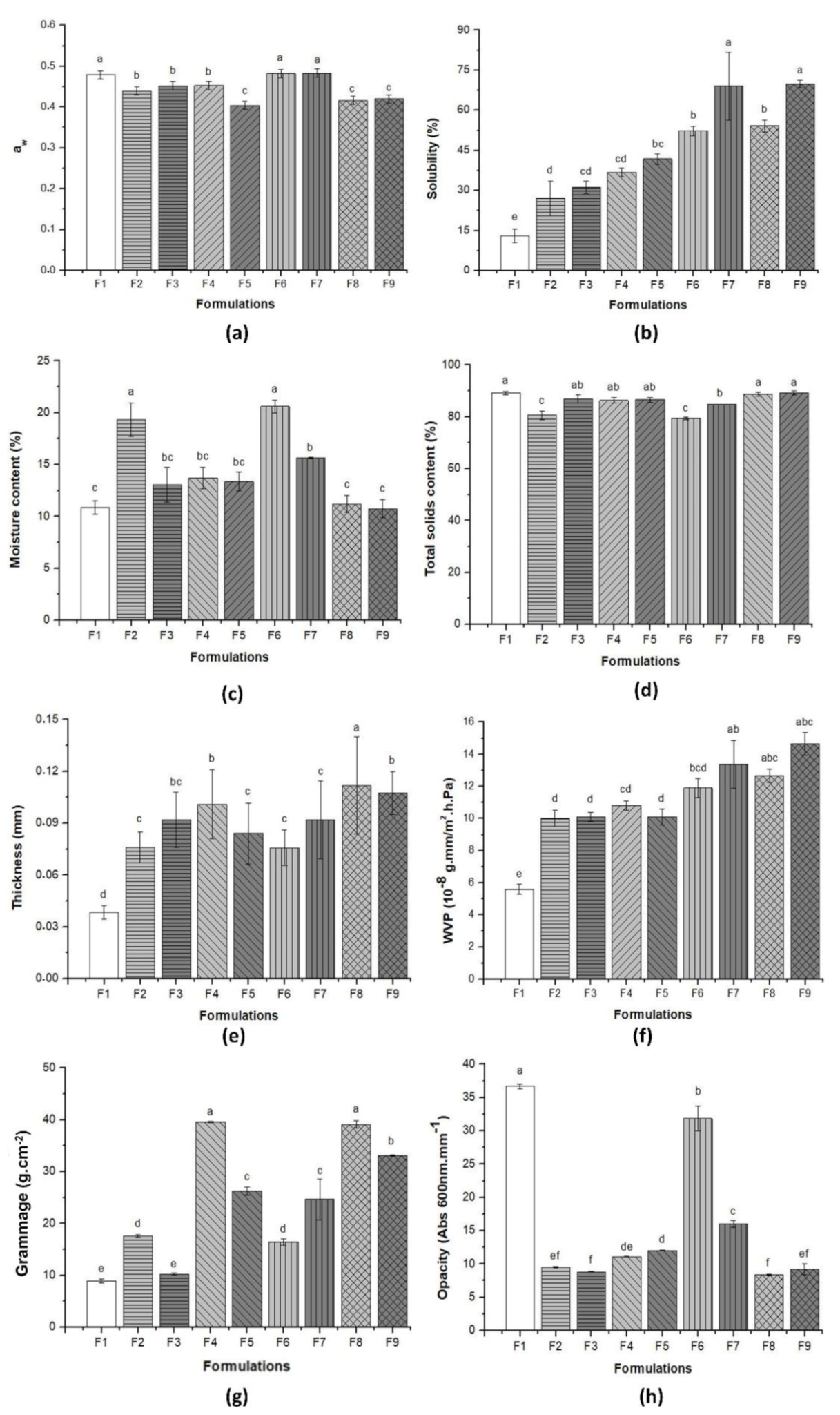
3.2. Physical and Barrier Properties of Pure BC and BC–Collagen–Starch Biocomposites
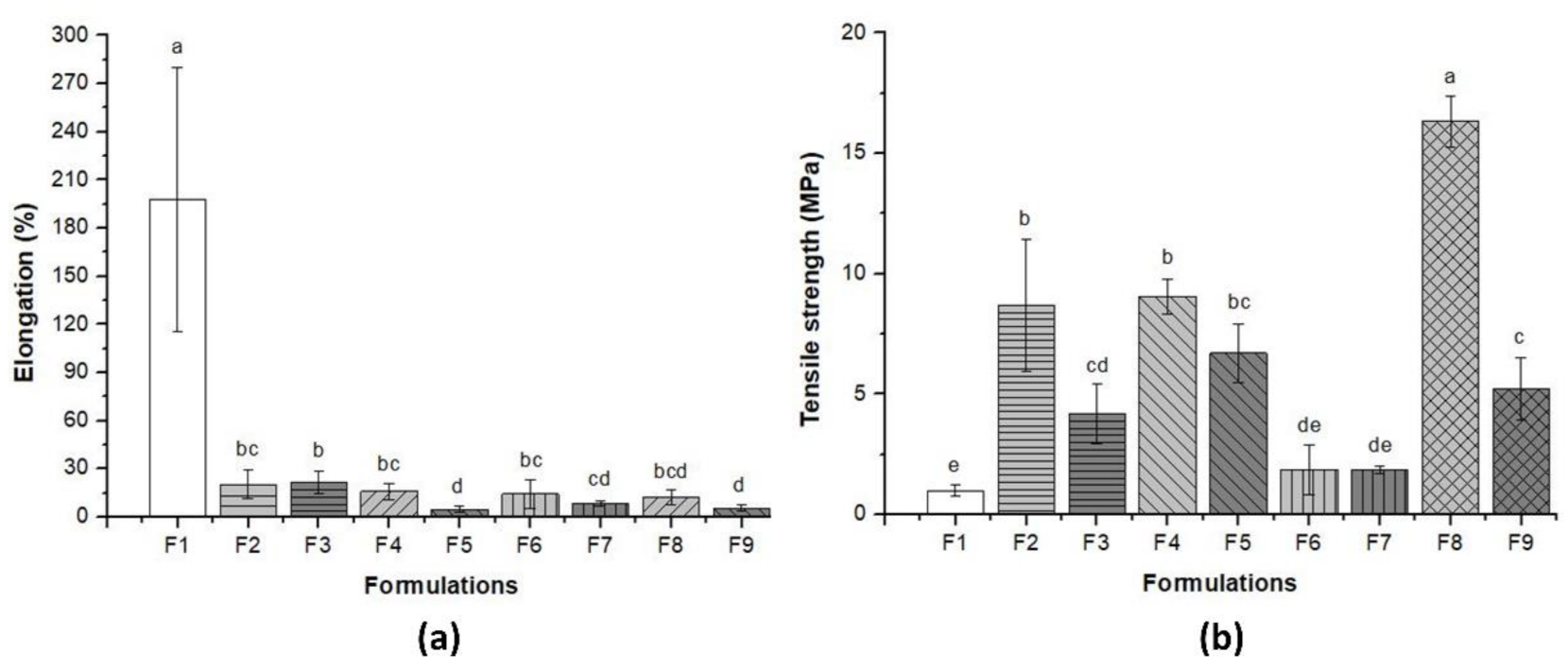
3.3. Mechanical Properties of Pure BC and BC–Collagen–Starch Biocomposites
3.4. Morphological Property of Pure BC and BC–Collagen–Starch Biocomposites
3.5. Thermal Property of Pure BC and BC–Collagen–Starch Biocomposites
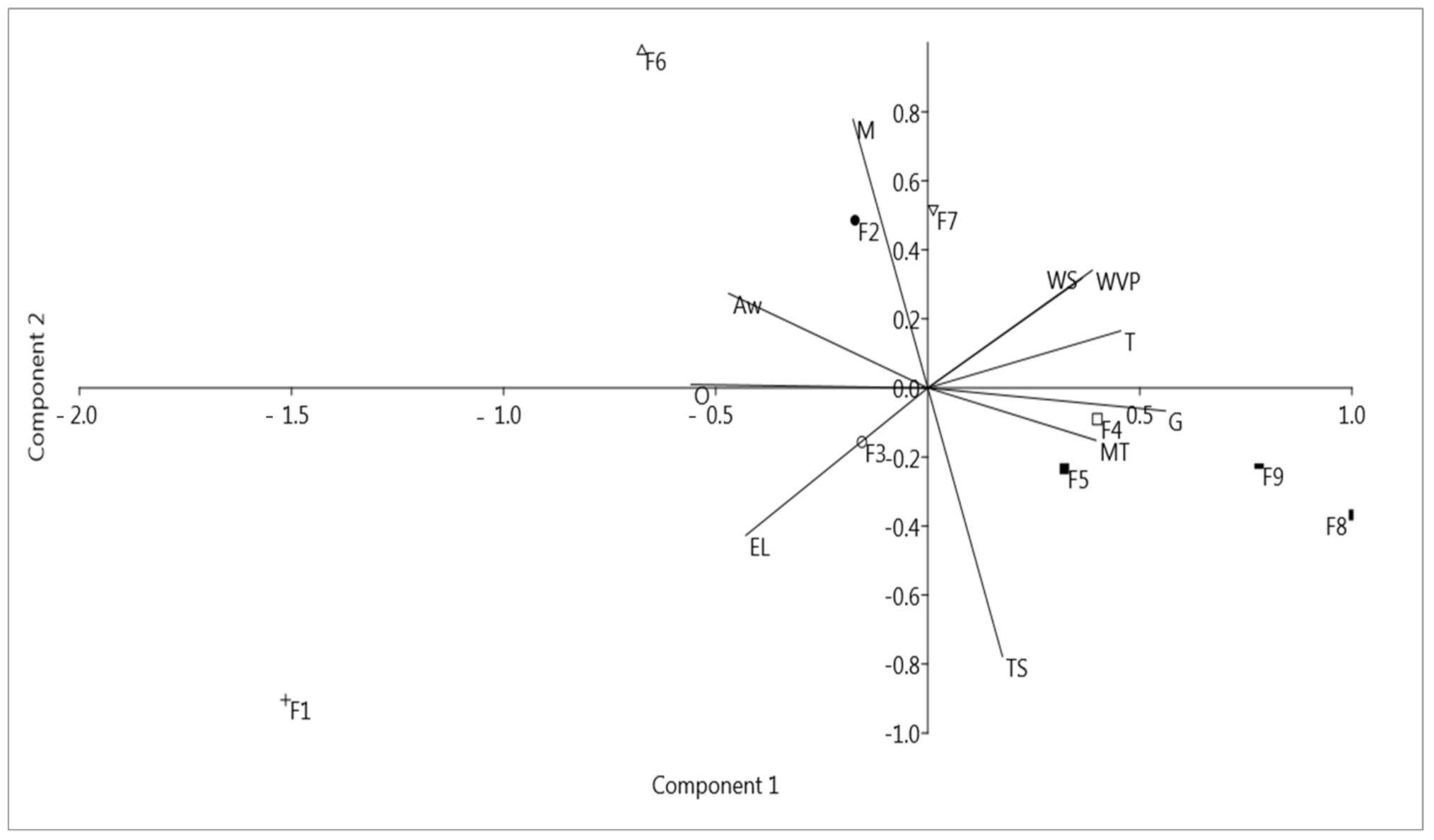
3.6. Effect of the Presence of Components on the Properties of BC–Collagen–Starch Biocomposites: Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Y.; Chen, C.; Hellwarth, P.B.; Bao, X. Biomaterials for stem cell engineering and biomanufacturing. Bioact. Mater. 2019, 4, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Wong, S.; Mohamed, I.; Mohamed, N.; Chin, K.-Y.; Ima-Nirwana, S.; Shuid, A. Wound Healing Properties of Selected Natural Products. Int. J. Environ. Res. Public Health 2018, 15, 2360. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Aljghami, M.E.; Saboor, S.; Amini-Nik, S. Emerging Innovative Wound Dressings. Ann. Biomed. Eng. 2019, 47, 659–675. [Google Scholar] [CrossRef]
- Tricco, A.C.; Cogo, E.; Isaranuwatchai, W.; Khan, P.A.; Sanmugalingham, G.; Antony, J.; Hoch, J.S.; Straus, S.E. A systematic review of cost-effectiveness analyses of complex wound interventions reveals optimal treatments for specific wound types. BMC Med. 2015, 13, 90. [Google Scholar] [CrossRef]
- Cheng, Q.; Gibb, M.; Graves, N.; Finlayson, K.; Pacella, R.E. Cost-effectiveness analysis of guideline-based optimal care for venous leg ulcers in Australia. BMC Health Serv. Res. 2018, 18, 421. [Google Scholar] [CrossRef]
- Hajialyani, M.; Tewari, D.; Sobarzo-Sánchez, E.; Nabavi, S.M.; Farzaei, M.H.; Abdollahi, M. Natural product-based nanomedicines for wound healing purposes: Therapeutic targets and drug delivery systems. Int. J. Nanomed. 2018, 13, 5023–5043. [Google Scholar] [CrossRef]
- Naseri-Nosar, M.; Ziora, Z.M. Wound dressings from naturally-occurring polymers: A review on homopolysaccharide-based composites. Carbohydr. Polym. 2018, 189, 379–398. [Google Scholar] [CrossRef]
- Rezaie, F.; Momeni-Moghaddam, M.; Naderi-Meshkin, H. Regeneration and Repair of Skin Wounds: Various Strategies for Treatment. Int. J. Low. Extrem. Wounds 2019, 18, 247–261. [Google Scholar] [CrossRef]
- Mir, M.; Murtaza, N.A.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic polymeric biomaterials for wound healing: A review. Prog. Biomater. 2018, 7, 1–21. [Google Scholar] [CrossRef]
- Bacakova, M.; Pajorova, J.; Sopuch, T.; Bacakova, L. Fibrin-Modified Cellulose as a Promising Dressing for Accelerated Wound Healing. Materials 2018, 11, 2314. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Huang, Y.; Hu, Q.; He, W.; Duan, B.; Yan, X.; Yang, Z.; Liang, W.; Liu, Z.; Peng, Z.; et al. Elucidation of molecular pathways responsible for the accelerated wound healing induced by a novel fibrous chitin dressing. Biomater. Sci. 2019, 7, 5247–5257. [Google Scholar] [CrossRef] [PubMed]
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic acid—Based wound dressings: A review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef] [PubMed]
- Eulálio, H.Y.C.; Vieira, M.; Fideles, T.B.; Tomás, H.; Silva, S.M.L.; Peniche, C.A.; Fook, M.V.L. Physicochemical Properties and Cell Viability of Shrimp Chitosan Films as Affected by Film Casting Solvents. I-Potential Use as Wound Dressing. Materials 2020, 13, 5005. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, L.; Chen, J.; Wang, L.; Gui, X.; Ran, J.; Xu, G.; Zhao, H.; Zeng, M.; Ji, J.; et al. Silk Fibroin Biomaterial Shows Safe and Effective Wound Healing in Animal Models and a Randomized Controlled Clinical Trial. Adv. Healthc. Mater. 2017, 6, 1–16. [Google Scholar] [CrossRef]
- İnal, M.; Mülazımoğlu, G. Production and characterization of bactericidal wound dressing material based on gelatin nanofiber. Int. J. Biol. Macromol. 2019, 137, 392–404. [Google Scholar] [CrossRef]
- Park, S.B.; Lih, E.; Park, K.S.; Joung, Y.K.; Han, D.K. Biopolymer-based functional composites for medical applications. Prog. Polym. Sci. 2017, 68, 77–105. [Google Scholar] [CrossRef]
- Carvalho, T.; Guedes, G.; Sousa, F.L.; Freire, C.S.R.; Santos, H.A. Latest Advances on Bacterial Cellulose-Based Materials for Wound Healing, Delivery Systems, and Tissue Engineering. Biotechnol. J. 2019, 14, 1–19. [Google Scholar] [CrossRef]
- Islam, M.U.; Ullah, M.W.; Khan, S.; Shah, N.; Park, J.K. Strategies for cost-effective and enhanced production of bacterial cellulose. Int. J. Biol. Macromol. 2017, 102, 1166–1173. [Google Scholar] [CrossRef]
- Fang, L.; Catchmark, J.M. Characterization of cellulose and other exopolysaccharides produced from Gluconacetobacter strains. Carbohydr. Polym. 2015, 115, 663–669. [Google Scholar] [CrossRef]
- Gorgieva, S.; Trček, J. Bacterial Cellulose: Production, Modification and Perspectives in Biomedical Applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef] [PubMed]
- Shoda, M.; Sugano, Y. Recent advances in bacterial cellulose production. Biotechnol. Bioprocess Eng. 2005, 10, 1–8. [Google Scholar] [CrossRef]
- Sharma, C.; Bhardwaj, N.K. Bacterial nanocellulose: Present status, biomedical applications and future perspectives. Mater. Sci. Eng. C 2019, 104, 109963. [Google Scholar] [CrossRef] [PubMed]
- de Fernandes, I.A.A.; Pedro, A.C.; Ribeiro, V.R.; Bortolini, D.G.; Ozaki, M.S.C.; Maciel, G.M.; Haminiuk, C.W.I. Bacterial cellulose: From production optimization to new applications. Int. J. Biol. Macromol. 2020, 164, 2598–2611. [Google Scholar] [CrossRef]
- Naomi, R.; Bt Hj Idrus, R.; Fauzi, M.B. Plant- vs. Bacterial-Derived Cellulose for Wound Healing: A Review. Int. J. Environ. Res. Public Health 2020, 17, 6803. [Google Scholar] [CrossRef]
- Gao, X.; Shi, Z.; Kus̈mierczyk, P.; Liu, C.; Yang, G.; Sevostianov, I.; Silberschmidt, V.V. Time-dependent rheological behaviour of bacterial cellulose hydrogel. Mater. Sci. Eng. C 2016, 58, 153–159. [Google Scholar] [CrossRef]
- Aboelnaga, A.; Elmasry, M.; Adly, O.A.; Elbadawy, M.A.; Abbas, A.H.; Abdelrahman, I.; Salah, O.; Steinvall, I. Microbial cellulose dressing compared with silver sulphadiazine for the treatment of partial thickness burns: A prospective, randomised, clinical trial. Burns 2018, 44, 1982–1988. [Google Scholar] [CrossRef]
- Liang, J.; Wang, R.; Chen, R. The Impact of Cross-linking Mode on the Physical and Antimicrobial Properties of a Chitosan/Bacterial Cellulose Composite. Polymer 2019, 11, 491. [Google Scholar] [CrossRef]
- Khalid, A.; Khan, R.; Ul-Islam, M.; Khan, T.; Wahid, F. Bacterial cellulose-zinc oxide nanocomposites as a novel dressing system for burn wounds. Carbohydr. Polym. 2017, 164, 214–221. [Google Scholar] [CrossRef]
- Malmir, S.; Karbalaei, A.; Pourmadadi, M.; Hamedi, J.; Yazdian, F.; Navaee, M. Antibacterial properties of a bacterial cellulose CQD-TiO2 nanocomposite. Carbohydr. Polym. 2020, 234, 115835. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khan, S.; Ullah, M.W.; Park, J.K. Bacterial cellulose composites: Synthetic strategies and multiple applications in bio-medical and electro-conductive fields. Biotechnol. J. 2015, 10, 1847–1861. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lv, X.; Chen, S.; Li, Z.; Feng, C.; Wang, H.; Xu, Y. In situ fabrication of a microporous bacterial cellulose/potato starch composite scaffold with enhanced cell compatibility. Cellulose 2014, 21, 1823–1835. [Google Scholar] [CrossRef]
- Orlando, I.; Basnett, P.; Nigmatullin, R.; Wang, W.; Knowles, J.C.; Roy, I. Chemical Modification of Bacterial Cellulose for the Development of an Antibacterial Wound Dressing. Front. Bioeng. Biotechnol. 2020, 8, 557885. [Google Scholar] [CrossRef] [PubMed]
- Ciecholewska-Juśko, D.; Żywicka, A.; Junka, A.; Drozd, R.; Sobolewski, P.; Migdał, P.; Kowalska, U.; Toporkiewicz, M.; Fijałkowski, K. Superabsorbent crosslinked bacterial cellulose biomaterials for chronic wound dressings. Carbohydr. Polym. 2021, 253. [Google Scholar] [CrossRef]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 2007, 59, 207–233. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- de Barud, H.G.O.; da Silva, R.R.; da Silva Barud, H.; Tercjak, A.; Gutierrez, J.; Lustri, W.R.; de Oliveira, O.B.; Ribeiro, S.J.L. A multipurpose natural and renewable polymer in medical applications: Bacterial cellulose. Carbohydr. Polym. 2016, 153, 406–420. [Google Scholar] [CrossRef]
- Zhijiang, C.; Guang, Y. Bacterial cellulose/collagen composite: Characterization and first evaluation of cytocompatibility. J. Appl. Polym. Sci. 2011, 120, 2938–2944. [Google Scholar] [CrossRef]
- Albu, M.G.; Vuluga, Z.; Panaitescu, D.M.; Vuluga, D.M.; Cǎşǎricǎ, A.; Ghiurea, M. Morphology and thermal stability of bacterial cellulose/collagen composites. Cent. Eur. J. Chem. 2014, 12, 968–975. [Google Scholar] [CrossRef]
- Combrzyński, M.; Matwijczuk, A.; Wójtowicz, A.; Oniszczuk, T.; Karcz, D.; Szponar, J.; Niemczynowicz, A.; Bober, D.; Mitrus, M.; Kupryaniuk, K.; et al. Potato Starch Utilization in Ecological Loose-Fill Packaging Materials—Sustainability and Characterization. Materials 2020, 13, 1390. [Google Scholar] [CrossRef]
- Liu, G.; Gu, Z.; Hong, Y.; Cheng, L.; Li, C. Electrospun starch nanofibers: Recent advances, challenges, and strategies for potential pharmaceutical applications. J. Control. Release 2017, 252, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Moura, L.I.F.; Dias, A.M.A.; Carvalho, E.; De Sousa, H.C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—A review. Acta Biomater. 2013, 9, 7093–7114. [Google Scholar] [CrossRef] [PubMed]
- Wound Dressings Market—Global Forecast to 2025 | MarketsandMarkets. Available online: https://www.marketsandmarkets.com/Market-Reports/wound-dressings-market-123903496.html (accessed on 28 November 2020).
- Pruim, L.; Wind, A.; van Harten, W.H. Assessing and comparing the quality of wound centres: A literature review and benchmarking pilot. Int. Wound J. 2017, 14, 1120–1136. [Google Scholar] [CrossRef] [PubMed]
- Weller, C.D.; Team, V.; Sussman, G. First-Line Interactive Wound Dressing Update: A Comprehensive Review of the Evidence. Front. Pharmacol. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of Appropriate Wound Dressing for Various Wounds. Front. Bioeng. Biotechnol. 2020, 8, 1–17. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, C.; Yang, J.; Nie, Y.; Chen, C.; Sun, D. Recent advances in bacterial cellulose. Cellulose 2014, 21, 1–30. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef]
- Hodel, K.V.S.; dos Fonseca, L.M.S.; da Santos, I.M.S.; Cerqueira, J.C.; dos Santos-Júnior, R.E.; Nunes, S.B.; Barbosa, J.D.V.; Machado, B.A.S. Evaluation of Different Methods for Cultivating Gluconacetobacter hansenii for Bacterial Cellulose and Montmorillonite Biocomposite Production: Wound-Dressing Applications. Polymer 2020, 12, 267. [Google Scholar] [CrossRef]
- Yang, L.; Paulson, A.T. Mechanical and water vapour barrier properties of edible gellan films. Food Res. Int. 2000, 33, 563–570. [Google Scholar] [CrossRef]
- Cerqueira, J.C.; da Penha, J.S.; Oliveira, R.S.; Guarieiro, L.L.N.; da Melo, P.S.; Viana, J.D.; Machado, B.A.S. Production of biodegradable starch nanocomposites using cellulose nanocrystals extracted from coconut fibers. Polímeros 2017, 27, 320–329. [Google Scholar] [CrossRef]
- Leal, I.L.; da Rosa, Y.C.S.; da Penha, J.S.; Correia, P.R.C.; da Melo, P.S.; Guimarães, D.H.; Barbosa, J.D.V.; Druzian, J.I.; Machado, B.A.S. Development and application starch films:PBAT with additives for evaluating the shelf life of Tommy Atkins mango in the fresh-cut state. J. Appl. Polym. Sci. 2019, 48150–48169. [Google Scholar] [CrossRef]
- De Moura, M.R.; Aouada, F.A.; Souza, J.R.; Mattoso, L.H.C. Preparação de novos nanobiocompósitos comestíveis ativos contendo nanoemulsão de canela e pectina. Polímeros 2014, 24, 486–490. [Google Scholar] [CrossRef][Green Version]
- ASTM. Standard Test Methods for Water Vapor Transmission of Materials ASTM 69-00x; ASTM: West Conshohocken, PA, USA, 2000. [Google Scholar]
- Cazón, P.; Velázquez, G.; Vázquez, M. Characterization of bacterial cellulose films combined with chitosan and polyvinyl alcohol: Evaluation of mechanical and barrier properties. Carbohydr. Polym. 2019, 216, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.M.; Prestes, R.A.; Pinheiro, L.A.; Woiciechowski, A.L.; Wosiacki, G. Propriedades Físicas, Químicas e de Barreira em Filme Formados por Blenda de Celulose Bacteriana e Fécula de Batata. Polímeros Ciência e Tecnol. 2013, 23, 538–546. [Google Scholar] [CrossRef]
- ASTM Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM Int. 2018, 14, 1–12. [CrossRef]
- Rahmi, D.; Paramadina, S.; Anjelika, M.; Widjajanti, R. Optimized swelling properties of hydrogels based on poly(vinyl alcohol)-carrageenan. AIP Conf. Proc. 2020, 2243, 0300191–0300196. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khan, T.; Park, J.K. Water holding and release properties of bacterial cellulose obtained by in situ and ex situ modification. Carbohydr. Polym. 2012, 88, 596–603. [Google Scholar] [CrossRef]
- Machado, B.A.S.; Silva, R.P.D.; de Barreto, G.A.; Costa, S.S.; da Silva, D.F.; Brandão, H.N.; da Rocha, J.L.C.; Dellagostin, O.A.; Henriques, J.A.P.; Umsza-Guez, M.A.; et al. Chemical Composition and Biological Activity of Extracts Obtained by Supercritical Extraction and Ethanolic Extraction of Brown, Green and Red Propolis Derived from Different Geographic Regions in Brazil. PLoS ONE 2016, 11, e0145954. [Google Scholar] [CrossRef]
- Machado, B.A.S.; Reis, J.H.O.; Da Silva, J.B.; Cruz, L.S.; Nunes, I.L.; Pereira, F.V.; Druzian, J.I. Obtenção de nanocelulose da fibra de coco verde e incorporação em filmes biodegradáveis de amido plastificados com glicerol. Quim. Nov. 2014, 37, 1275–1282. [Google Scholar] [CrossRef]
- de Francisco, A.S.e.S.; de Carlos, A.V.A. The Assistat Software Version 7.7 and its use in the analysis of experimental data. Afr. J. Agric. Res. 2016, 11, 3733–3740. [Google Scholar] [CrossRef]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Nanomaterials for Wound Dressings: An Up-to-Date Overview. Molecules 2020, 25, 2699. [Google Scholar] [CrossRef] [PubMed]
- Girard, D.; Laverdet, B.; Buhé, V.; Trouillas, M.; Ghazi, K.; Alexaline, M.M.; Egles, C.; Misery, L.; Coulomb, B.; Lataillade, J.J.; et al. Biotechnological Management of Skin Burn Injuries: Challenges and Perspectives in Wound Healing and Sensory Recovery. Tissue Eng. Part B Rev. 2017, 23, 59–82. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Sun, X.; Lee, J.H.; Kim, H.W.; Fu, X.; Leong, K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev. 2019, 146, 209–239. [Google Scholar] [CrossRef] [PubMed]
- Khang, G.; Martino, M.M.; Balmayor, E.R.; Pandit, A.; Browne, S. Biomaterial-mediated modification of the local inflammatory environment. Front. Bioeng. Biotechnol 2015, 3, 67. [Google Scholar] [CrossRef]
- Singla, R.; Soni, S.; Patial, V.; Markand Kulurkar, P.; Kumari, A.; Padwad, Y.S.; Kumar Yadav, S.; SPadwad, Y. In vivo diabetic wound healing potential of nanobiocomposites containing bamboo cellulose nanocrystals impregnated with silver nanoparticles. Int. J. Biol. Macromol. Int. J. Biol. 2017, 6, 45–55. [Google Scholar] [CrossRef]
- Wu, C.N.; Fuh, S.C.; Lin, S.P.; Lin, Y.Y.; Chen, H.Y.; Liu, J.M.; Cheng, K.C. TEMPO-Oxidized Bacterial Cellulose Pellicle with Silver Nanoparticles for Wound Dressing. Biomacromolecules 2018, 19, 544–554. [Google Scholar] [CrossRef]
- Cacicedo, M.L.; Pacheco, G.; Islan, G.A.; Alvarez, V.A.; Barud, H.S.; Castro, G.R. Chitosan-bacterial cellulose patch of ciprofloxacin for wound dressing: Preparation and characterization studies. Int. J. Biol. Macromol. 2020, 147, 1136–1145. [Google Scholar] [CrossRef]
- Phomrak, S.; Phisalaphong, M. Reinforcement of Natural Rubber with Bacterial Cellulose via a Latex Aqueous Microdispersion Process. J. Nanomater. 2017, 2017. [Google Scholar] [CrossRef]
- Yamanaka, S.; Watanabe, K.; Kitamura, N.; Iguchi, M.; Mitsuhashi, S.; Nishi, Y.; Uryu, M. The structure and mechanical properties of sheets prepared from bacterial cellulose. J. Mater. Sci. 1989, 24, 3141–3145. [Google Scholar] [CrossRef]
- Cacicedo, M.L.; Castro, M.C.; Servetas, I.; Bosnea, L.; Boura, K.; Tsafrakidou, P.; Dima, A.; Terpou, A.; Koutinas, A.; Castro, G.R. Progress in bacterial cellulose matrices for biotechnological applications. Bioresour. Technol. 2016, 213, 172–180. [Google Scholar] [CrossRef]
- Abral, H.; Hartono, A.; Hafizulhaq, F.; Handayani, D.; Sugiarti, E.; Pradipta, O. Characterization of PVA/cassava starch biocomposites fabricated with and without sonication using bacterial cellulose fiber loadings. Carbohydr. Polym. 2019, 206, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Wilpiszewska, K.; Antosik, A.K.; Schmidt, B.; Janik, J.; Rokicka, J. Hydrophilic Films Based on Carboxymethylated Derivatives of Starch and Cellulose. Polymer 2020, 12, 2447. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.A.; Spinacé, M.A.S. Sandwich panel biocomposite of thermoplastic corn starch and bacterial cellulose. Int. J. Biol. Macromol. 2021, 167, 358–368. [Google Scholar] [CrossRef]
- von Cramon, L.; Markowicz, M.; Nebendahl, J.; Buchinger-Kähler, V.C.; Noah, E.M.; Narwan, M.; Behrendt, A.; Pallua, N.; Steinhoff, A. A clinical evaluation of a transparent, absorbent, adhesive wound dressing. Br. J. Nurs. 2017, 26, S46–S53. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Vázquez, M.; Velazquez, G. Environmentally Friendly Films Combining Bacterial Cellulose, Chitosan, and Polyvinyl Alcohol: Effect of Water Activity on Barrier, Mechanical, and Optical Properties. Biomacromolecules 2020, 21, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Pasaribu, K.M.; Gea, S.; Ilyas, S.; Tamrin, T.; Radecka, I. Characterization of Bacterial Cellulose-Based Wound Dressing in Different Order Impregnation of Chitosan and Collagen. Biomolecules 2020, 10, 1511. [Google Scholar] [CrossRef]
- Saska, S.; Teixeira, L.N.; Tambasco de Oliveira, P.; Minarelli Gaspar, A.M.; Lima Ribeiro, S.J.; Messaddeq, Y.; Marchetto, R. Bacterial cellulose-collagen nanocomposite for bone tissue engineering. J. Mater. Chem. 2012. [Google Scholar] [CrossRef]
- Noh, Y.K.; Dos Santos Da Costa, A.; Park, Y.S.; Du, P.; Kim, I.-H.; Park, K. Fabrication of bacterial cellulose-collagen composite scaffolds and their osteogenic effect on human mesenchymal stem cells. Carbohydr. Polym. 2019, 219, 210–218. [Google Scholar] [CrossRef]
- Priya, B.; Gupta, V.K.; Pathania, D.; Singha, A.S. Synthesis, characterization and antibacterial activity of biodegradable starch/PVA composite films reinforced with cellulosic fibre. Carbohydr. Polym. 2014, 109, 171–179. [Google Scholar] [CrossRef]
- Dutta, S.D.; Patel, D.K.; Lim, K.-T. Functional cellulose-based hydrogels as extracellular matrices for tissue engineering. J. Biol. Eng. 2019, 13, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Burn wound healing: Present concepts, treatment strategies and future directions. J. Wound Care 2017, 26, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Moghaddam, A.S.; Manouchehri, S.; Atoufi, Z.; Amiri, A.; Amirkhani, M.A.; Nilforoushzadeh, M.A.; Saeb, M.R.; Hamblin, M.R.; Mozafari, M. Can regenerative medicine and nanotechnology combine to heal wounds? The search for the ideal wound dressing. Nanomedicine 2017, 12, 2403–2422. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Ruan, S.; Yao, H.; Sun, Y. Physical properties of composite films from tilapia skin collagen with Pachyrhizus starch and rambutan peel phenolics. Mar. Drugs 2019, 17, 662. [Google Scholar] [CrossRef]
- Tibolla, H.; Czaikoski, A.; Pelissari, F.M.; Menegalli, F.C.; Cunha, R.L. Starch-based nanocomposites with cellulose nanofibers obtained from chemical and mechanical treatments. Int. J. Biol. Macromol. 2020, 161, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Samadian, H.; Zamiri, S.; Ehterami, A.; Farzamfar, S.; Vaez, A.; Khastar, H.; Alam, M.; Ai, A.; Derakhshankhah, H.; Allahyari, Z.; et al. Electrospun cellulose acetate/gelatin nanofibrous wound dressing containing berberine for diabetic foot ulcer healing: In Vitro and in vivo studies. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M.; Velazquez, G. Composite Films with UV-Barrier Properties of Bacterial Cellulose with Glycerol and Poly(vinyl alcohol): Puncture Properties, Solubility, and Swelling Degree. Biomacromolecules 2019, 20, 3115–3125. [Google Scholar] [CrossRef]
- Qin, L.; Gao, H.; Xiong, S.; Jia, Y.; Ren, L. Preparation of collagen/cellulose nanocrystals composite films and their potential applications in corneal repair. J. Mater. Sci. Mater. Med. 2020, 31, 1–11. [Google Scholar] [CrossRef]
- Antosik, A.K.; Piątek, A.; Wilpiszewska, K. Carboxymethylated starch and cellulose derivatives-based film as human skin equivalent for adhesive properties testing. Carbohydr. Polym. 2019, 222, 115014. [Google Scholar] [CrossRef]
- Mirza, A.M.J.; Parham, S.Z.; Seyed, M.M.B.; Keyvan, S.Z.; Ameneh, G.; Aref, A.R.; Karimi, M.; Hamblin, M.R. Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv. Drug Deliv. Rev. 2018, 123, 33–64. [Google Scholar] [CrossRef]
- Zaman, H.U.; Islam, J.M.M.; Khan, M.A.; Khan, R.A. Physico-mechanical properties of wound dressing material and its biomedical application. J. Mech. Behav. Biomed. Mater. 2011, 4, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; An, S.-J.; Bae, E.-B.; Gwon, H.-J.; Park, J.-S.; Jeong, S.; Jeon, Y.-C.; Lee, S.-H.; Lim, Y.-M.; Huh, J.-B. The Effect of Thickness of Resorbable Bacterial Cellulose Membrane on Guided Bone Regeneration. Materials 2017, 10, 320. [Google Scholar] [CrossRef] [PubMed]
- Qiu, K.; Netravali, A.N. A review of fabrication and applications of bacterial cellulose based nanocomposites. Polym. Rev. 2014, 54, 598–626. [Google Scholar] [CrossRef]
- Martins, I.M.G.; Magina, S.P.; Oliveira, L.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A. New biocomposites based on thermoplastic starch and bacterial cellulose. Compos. Sci. Technol. 2009, 69, 2163–2168. [Google Scholar] [CrossRef]
- de Moraes, P.R.F.S.; Saska, S.; Barud, H.; de Lima, L.R.; da Martins, V.C.A.; de Plepis, A.M.G.; Ribeiro, S.J.L.; Gaspar, A.M.M. Bacterial Cellulose/Collagen Hydrogel for Wound Healing. Mater. Res. 2016, 19, 106–116. [Google Scholar] [CrossRef]


| Formulation (Sample Name) | Biocomposite Composition (%, m·v−1) | |||
|---|---|---|---|---|
| Bacterial Cellulose | Collagen | Starch | Glycerol | |
| F1 | 50.00 | – | – | – |
| F2 | 50.00 | – | 2.23 | 0.60 |
| F3 | 50.00 | – | 1.12 | 0.60 |
| F4 | 50.00 | 1.00 | 2.23 | 0.60 |
| F5 | 50.00 | 1.00 | 1.12 | 0.60 |
| F6 | 50.00 | 1.00 | – | 0.60 |
| F7 | 50.00 | 2.00 | – | 0.60 |
| F8 | 50.00 | 2.00 | 2.23 | 0.60 |
| F9 | 50.00 | 2.00 | 1.12 | 0.60 |
| Analysis | Sample Name | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | |
| Water activity | 0.48 a ± 0.01 | 0.44 b ± 0.06 | 0.45 b ± 0.01 | 0.45 b ± 0.02 | 0.40 c ± 0.01 | 0.48 a ± 0.01 | 0.48 a ± 0.01 | 0.42 c ± 0.01 | 0.42 c ± 0.01 |
| Moisture (%) | 10.88 c ± 0.64 | 19.34 a ± 1.64 | 13.06 bc ± 1.67 | 13.69 bc ± 1.05 | 13.37 bc ± 0.88 | 20.60 a ± 0.69 | 15.66 b ± 0.04 | 11.19 c ± 0.81 | 10.75 c ± 0.89 |
| Total solids (%) | 89.12 a ± 0.64 | 80.66 c ± 1.64 | 86.94 ab ± 1.67 | 86.31 ab ± 1.05 | 86.63 ab ± 0.88 | 79.4 c ± 0.69 | 84.34b ± 0.04 | 88.81 a ± 0.81 | 89.25 a ± 0.89 |
| Water solubility (%) | 12.94 e ± 2.58 | 27.11 d ± 6.52 | 31.12 cd ± 2.31 | 36.76 cd ± 1.62 | 41.79 bc ± 2.02 | 52.33 b ± 1.74 | 69.67 a ± 12.7 | 54.12 b ± 2.21 | 69.76 a ± 1.43 |
| Water vapor permeability (10−8 g·mm/m2·h·Pa) | 5.59 e ± 0.44 | 10.01 d ± 0.51 | 10.18 d ± 0.38 | 10.89 cd ± 0.33 | 10.19 d ± 0.57 | 11.94 bcd ± 0.61 | 13.37 ab ± 1.52 | 12.66 abc ± 0.45 | 14.06 a ± 0.71 |
| Opacity (Abs 600 nm·mm−1) | 36.67 a ± 0.37 | 9.49 ef ± 0.12 | 8.82 f ± 0.04 | 11.12 d ± 0.05 | 12.03 d ± 0.64 | 31.85 b ± 1.98 | 16.02 c ± 0.52 | 8.37 f ± 0.08 | 9.17 ef ± 0.81 |
| Grammage (g·cm−2) | 8.91 e ± 0.46 | 17.57 d ± 0.21 | 10.25 e ± 0.19 | 39.58 a ± 0.87 | 26.25 c ± 0.84 | 16.41 d ± 0.69 | 24.66 c ± 3.97 | 39.10 a ± 0.73 | 33.11 b ± 0.52 |
| Thickness (mm) | 0.04 d ± 0.10 | 0.08 c ± 0.01 | 0.09 bc ± 0.01 | 0.10 b ± 0.02 | 0.08 c ± 0.01 | 0.08 c ± 0.01 | 0.09 c ± 0.02 | 0.11 a ± 0.03 | 0.10 b ± 0.01 |
| Elongation (%) | 169.54 a ± 6.00 | 20.29 bc ± 6.35 | 21.39 b ± 5.76 | 16.07 bcd ± 5.04 | 4.81 d ± 2.0 | 14.29 bcd ± 3.22 | 8.61 cd ± 1.67 | 10.55 bcd ± 1.75 | 5.57 d ± 1.94 |
| Tensile strength (Mpa) | 0.99 e ± 0.23 | 8.7 b ± 2.74 | 4.17 cd ± 1.23 | 9.05 b ± 0.75 | 6.72bc ± 1.22 | 1.5 de ± 0.76 | 1.8 de ± 0.15 | 16.32a ± 1.04 | 5.21 c ± 1.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, S.B.; Hodel, K.V.S.; Sacramento, G.d.C.; Melo, P.d.S.; Pessoa, F.L.P.; Barbosa, J.D.V.; Badaró, R.; Machado, B.A.S. Development of Bacterial Cellulose Biocomposites Combined with Starch and Collagen and Evaluation of Their Properties. Materials 2021, 14, 458. https://doi.org/10.3390/ma14020458
Nunes SB, Hodel KVS, Sacramento GdC, Melo PdS, Pessoa FLP, Barbosa JDV, Badaró R, Machado BAS. Development of Bacterial Cellulose Biocomposites Combined with Starch and Collagen and Evaluation of Their Properties. Materials. 2021; 14(2):458. https://doi.org/10.3390/ma14020458
Chicago/Turabian StyleNunes, Silmar Baptista, Katharine Valéria Saraiva Hodel, Giulia da Costa Sacramento, Pollyana da Silva Melo, Fernando Luiz Pellegrini Pessoa, Josiane Dantas Viana Barbosa, Roberto Badaró, and Bruna Aparecida Souza Machado. 2021. "Development of Bacterial Cellulose Biocomposites Combined with Starch and Collagen and Evaluation of Their Properties" Materials 14, no. 2: 458. https://doi.org/10.3390/ma14020458
APA StyleNunes, S. B., Hodel, K. V. S., Sacramento, G. d. C., Melo, P. d. S., Pessoa, F. L. P., Barbosa, J. D. V., Badaró, R., & Machado, B. A. S. (2021). Development of Bacterial Cellulose Biocomposites Combined with Starch and Collagen and Evaluation of Their Properties. Materials, 14(2), 458. https://doi.org/10.3390/ma14020458







