Effect of Co, Ti and Cr Additions on Microstructure, Magnetic Properties and Corrosion Resistance of Magnetocaloric Gd-Ge-Si Alloys
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Structure and Phase Investigations of the Gd58-xGe20Si22Yx (x = 0 or 2; Y = Co, Ti or Cr) Alloys
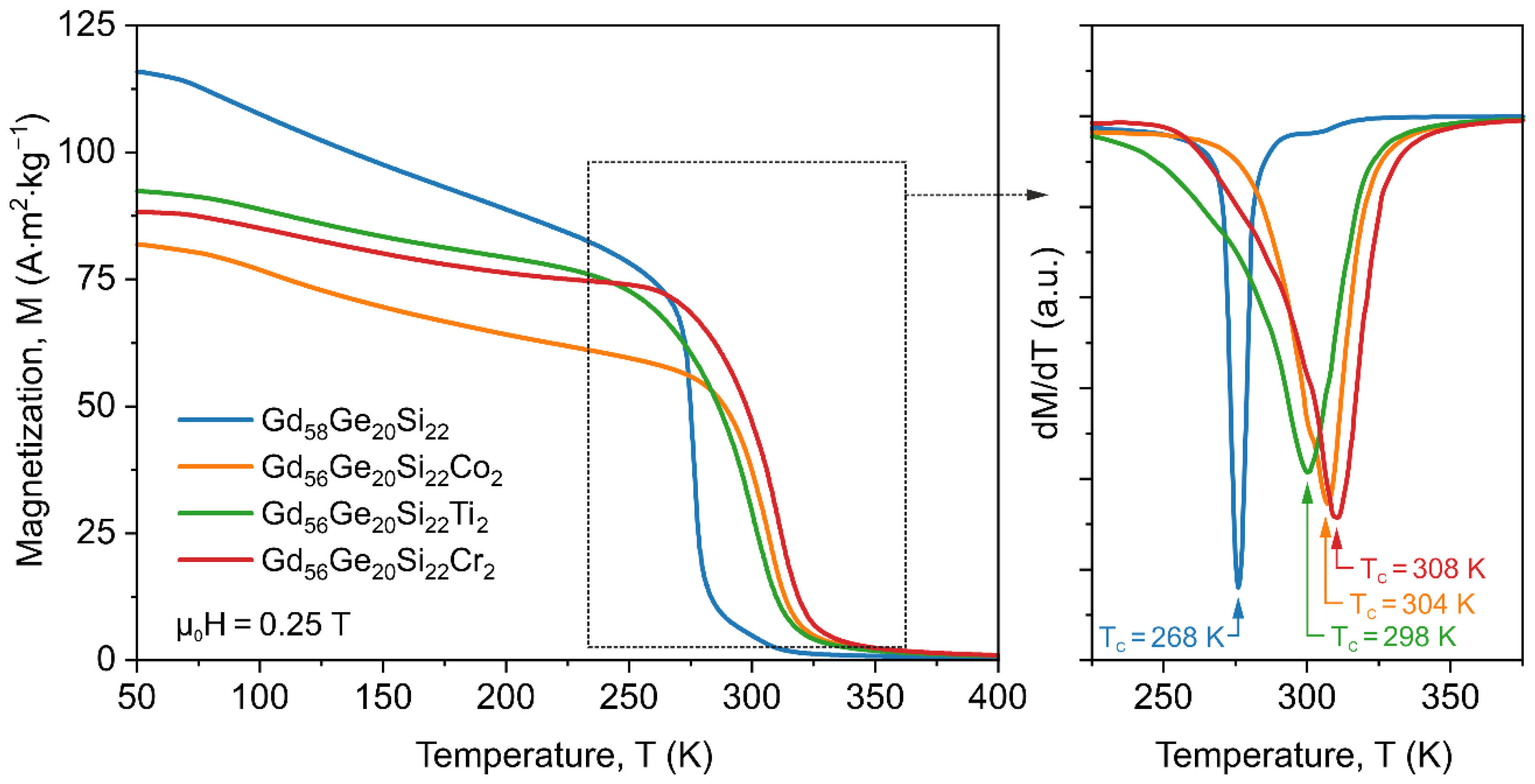
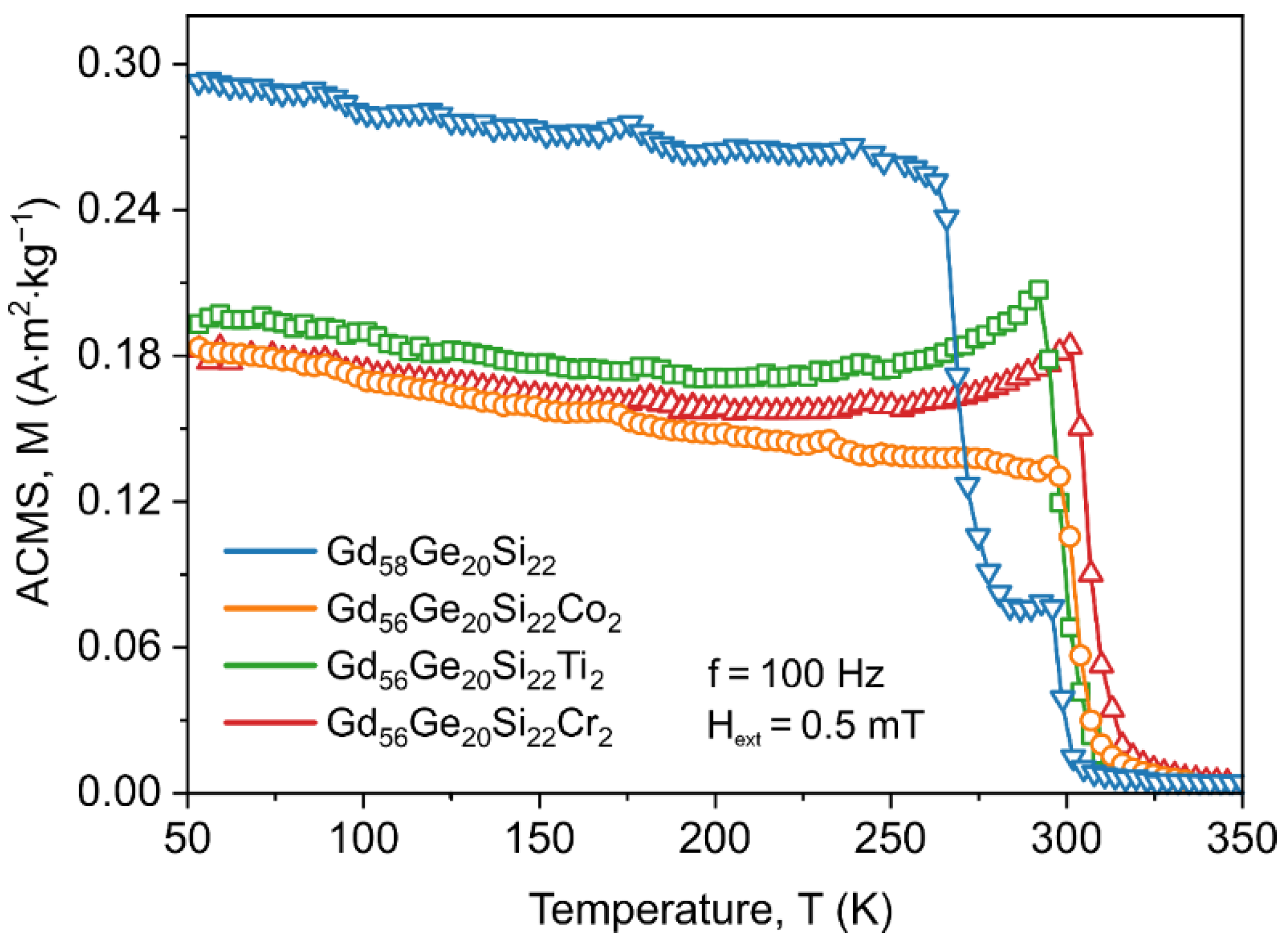
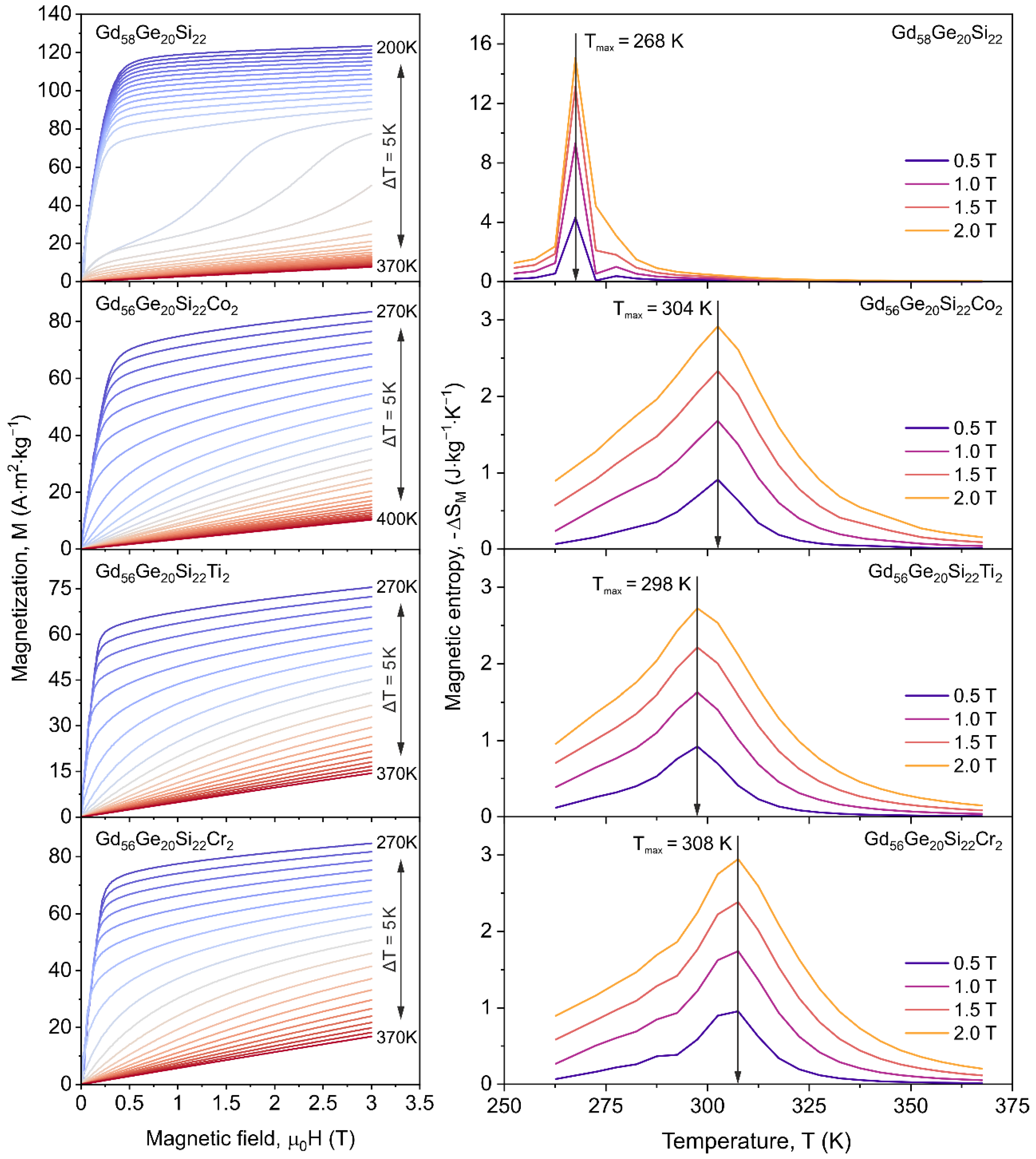
3.2. Magnetic Properties of GdGeSi Alloy without and with the Addition of Co, Ti and Cr
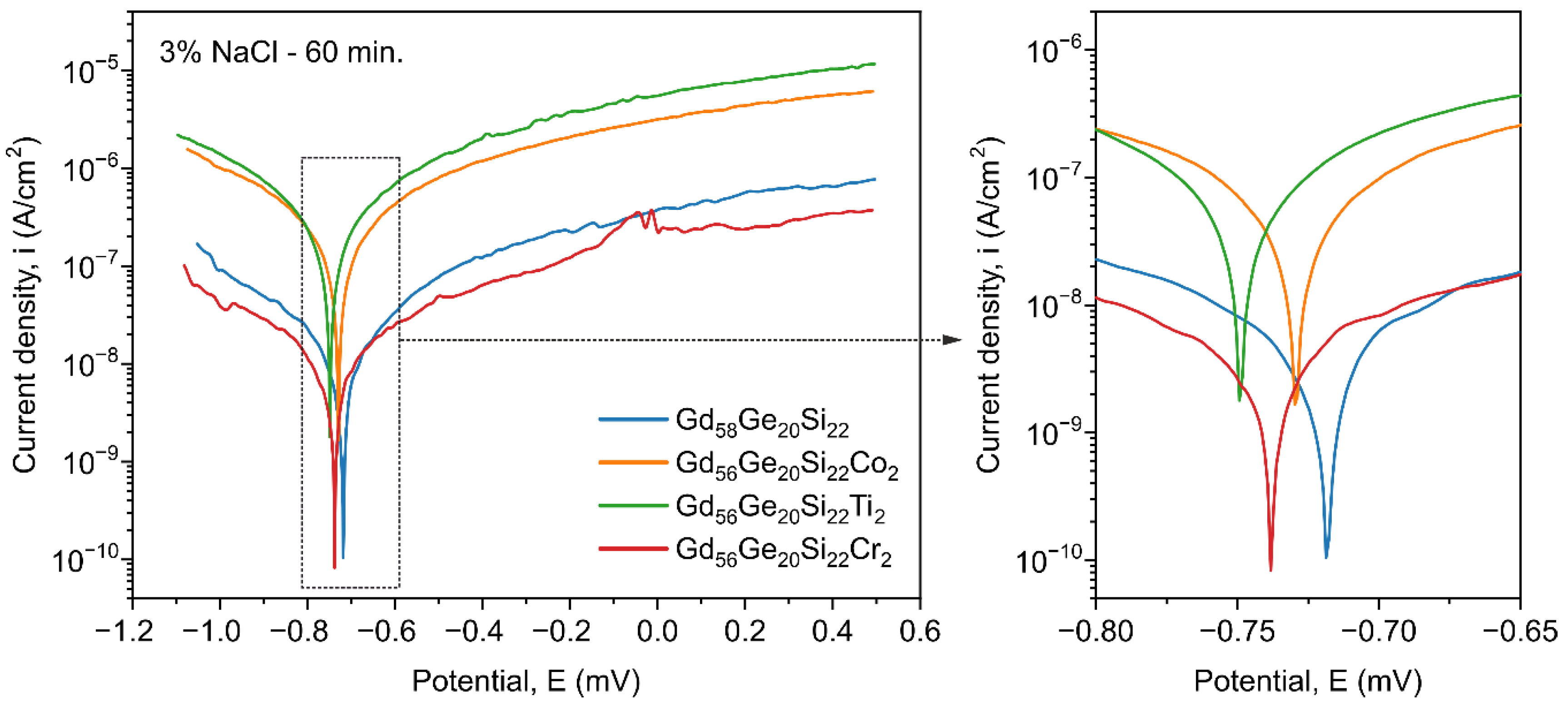
3.3. Corrosion Properties of GdGeSi-Based Alloys
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elliott, R.J. Magnetic Properties of Rare Earth Metals; Elliott, R.J., Ed.; Springer: Boston, MA, USA, 1972; ISBN 978-1-4757-5693-7. [Google Scholar]
- Luo, Q.; Wang, W.H. Magnetocaloric effect in rare earth-based bulk metallic glasses. J. Alloys Compd. 2010, 495, 209–216. [Google Scholar] [CrossRef]
- Gutfleisch, O.; Willard, M.A.; Brück, E.; Chen, C.H.; Sankar, S.G.; Liu, J.P. Magnetic Materials and Devices for the 21st Century: Stronger, Lighter, and More Energy Efficient. Adv. Mater. 2011, 23, 821–842. [Google Scholar] [CrossRef]
- Phan, M.H.; Yu, S.C. Review of the magnetocaloric effect in manganite materials. J. Magn. Magn. Mater. 2007, 308, 325–340. [Google Scholar] [CrossRef]
- Chrobak, A.; Nosenko, V.; Haneczok, G.; Boichyshyn, L.; Kotur, B.; Bajorek, A.; Zivotsky, O.; Hendrych, A. Effect of rare earth additions on magnetic properties of Fe82Nb2B14RE2 (RE = Y, Gd, Tb and Dy) amorphous alloys. Mater. Chem. Phys. 2011, 130, 603–608. [Google Scholar] [CrossRef]
- Xiao, S.; Chen, Y. Magnetic properties and magnetic exchange interactions in Gd1-xREx(RE = Pr, Nd) alloys. J. Rare Earths 2016, 34, 489–494. [Google Scholar] [CrossRef]
- Zhao, L.; Tian, X.; Yao, Z.; Zhao, X.; Wang, R.; Hamt, O.; Jiang, L.; Harris, V.G. Enhanced magnetostrictive properties of lightly Pr-doped Fe83Ga17 alloys. J. Rare Earths 2020, 38, 257–264. [Google Scholar] [CrossRef]
- Aryal, A.; Quetz, A.; Pandey, S.; Dubenko, I.; Stadler, S.; Ali, N. Magnetocaloric effects and transport properties of rare-earth (R = La, Pr, Sm) doped Ni50-xRxMn35Sn15 Heusler alloys. J. Alloys Compd. 2017, 717, 254–259. [Google Scholar] [CrossRef]
- Hasiak, M.; Łaszcz, A. Microstructure to thermomagnetic and mechanical properties relationship in Gd75Ge15Si5Pr5 alloy. J. Rare Earths 2019, 37, 1213–1217. [Google Scholar] [CrossRef]
- Naik, S.R.; Salker, A.V. Change in the magnetostructural properties of rare earth doped cobalt ferrites relative to the magnetic anisotropy. J. Mater. Chem. 2012, 22, 2740–2750. [Google Scholar] [CrossRef]
- Dent, P.C. Rare earth elements and permanent magnets (invited). J. Appl. Phys. 2012, 111, 07A721. [Google Scholar] [CrossRef]
- Yüzüak, E.; Dincer, I.; Elerman, Y. Effects of manganese doping on magnetocaloric effect in Ge-rich Gd5Ge2.05Si1.95 alloy. J. Rare Earths 2012, 30, 217–221. [Google Scholar] [CrossRef]
- Bingham, N.S.; Wang, H.; Qin, F.; Peng, H.X.; Sun, J.F.; Franco, V.; Srikanth, H.; Phan, M.H. Excellent magnetocaloric properties of melt-extracted Gd-based amorphous microwires. Appl. Phys. Lett. 2012, 101, 102407. [Google Scholar] [CrossRef]
- Pecharsky, V.K.; Gschneidner, K.A., Jr. Giant magnetocaloric effect in Gd5(Si2Ge2). Phys. Rev. Lett. 1997, 78, 4494. [Google Scholar] [CrossRef]
- Zhong, X.; Shen, X.; Liu, Z. Magnetocaloric properties, microhardness and corrosion resistance of Gd100-xZrx alloys. J. Rare Earths 2016, 34, 889–894. [Google Scholar] [CrossRef]
- Hasiak, M. Microstructure and magnetocaloric effect in as-quenched GdGeSi alloys with addition of Ni and Ce. Phys. Status Solidi Appl. Mater. Sci. 2016, 213, 1130–1137. [Google Scholar] [CrossRef]
- Belo, J.H.; Pereira, A.M.; Ventura, J.; Oliveira, G.N.P.; Araújo, J.P.; Tavares, P.B.; Fernandes, L.; Algarabel, P.A.; Magen, C.; Morellon, L.; et al. Phase control studies in Gd5Si2Ge2 giant magnetocaloric compound. J. Alloys Compd. 2012, 529, 89–95. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Y.; Tang, Y.; Du, H.; Ren, T.; Tu, M. The phase formation and thermal hysteresis of Gd5Si2Ge2 with the addition of transition elements (Mn, Fe, Co, Ni). J. Alloys Compd. 2007, 433, 18–21. [Google Scholar] [CrossRef]
- Franco, V.; Blázquez, J.S.; Ingale, B.; Conde, A. The magnetocaloric effect and magnetic refrigeration near room temperature: Materials and models. Annu. Rev. Mater. Res. 2012, 42, 305–342. [Google Scholar] [CrossRef]
- Xu, L.; Qian, C.; Ai, Y.; Su, T.; Hou, X. Tunable Magnetocaloric Properties of Gd-Based Alloys by Adding Tb and Doping Fe Elements. Materials 2019, 12, 2877. [Google Scholar] [CrossRef]
- Ferenc, J.; Kowalczyk, M.; Cieålak, G.; Erenc-Sȩdziak, T.; Kulik, T. Improvement of magnetocaloric properties of Gd-Ge-Si alloys by alloying with iron. EPJ Web Conf. 2013, 40, 3–6. [Google Scholar] [CrossRef][Green Version]
- Prabahar, K.; Raj Kumar, D.M.; Manivel Raja, M.; Chandrasekaran, V. Phase analysis and magnetocaloric properties of Zr substituted Gd-Si-Ge alloys. J. Magn. Magn. Mater. 2011, 323, 1755–1759. [Google Scholar] [CrossRef]
- Brück, E. Developments in magnetocaloric refrigeration. J. Phys. D Appl. Phys. 2005, 38. [Google Scholar] [CrossRef]
- Kitanovski, A. Energy Applications of Magnetocaloric Materials. Adv. Energy Mater. 2020, 10. [Google Scholar] [CrossRef]
- Lyubina, J. Magnetocaloric materials for energy efficient cooling. J. Phys. D Appl. Phys. 2017, 50. [Google Scholar] [CrossRef]
- Tishin, A.M.; Spichkin, Y.I. Recent progress in magnetocaloric effect: Mechanisms and potential applications. Int. J. Refrig. 2014, 37, 223–229. [Google Scholar] [CrossRef]
- Waske, A.; Gruner, M.E.; Gottschall, T.; Gutfleisch, O. Magnetocaloric materials for refrigeration near room temperature. MRS Bull. 2018, 43, 269–273. [Google Scholar] [CrossRef]
- Gutfleisch, O.; Gottschall, T.; Fries, M.; Benke, D.; Radulov, I.; Skokov, K.P.; Wende, H.; Gruner, M.; Acet, M.; Entel, P.; et al. Mastering hysteresis in magnetocaloric materials. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374. [Google Scholar] [CrossRef]
- Scheibel, F.; Gottschall, T.; Taubel, A.; Fries, M.; Skokov, K.P.; Terwey, A.; Keune, W.; Ollefs, K.; Wende, H.; Farle, M.; et al. Hysteresis Design of Magnetocaloric Materials—From Basic Mechanisms to Applications. Energy Technol. 2018, 6, 1397–1428. [Google Scholar] [CrossRef]
- Basso, V.; Piazzi, M.; Bennati, C.; Curcio, C. Hysteresis and Phase Transition Kinetics in Magnetocaloric Materials. Phys. Status Solidi Basic Res. 2018, 255, 1700278. [Google Scholar] [CrossRef]
- Cohen, L.F. Contributions to Hysteresis in Magnetocaloric Materials. Phys. Status Solidi Basic Res. 2018, 255, 2–9. [Google Scholar] [CrossRef]
- Guo, L.; Lovell, E.; Wilson, N.; Burdett, P.; Cohen, L.F.; Ryan, M.P. The electrochemical behaviour of magnetocaloric alloys La(Fe,Mn,Si)13Hx under magnetic field conditions. Chem. Commun. 2019, 55, 3642–3645. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Zheng, Z.G.; Huang, B.; Lai, J.W.; Zhou, Q.; Lei, L.; Zeng, D.C. Magnetocaloric effect, corrosion and mechanical properties of Mn1.05Fe0.9P0.5Si0.5Cux alloys. Intermetallics 2019, 113, 106539. [Google Scholar] [CrossRef]
- Hu, J.; Fu, S.; Huo, Y.; Long, Y.; Xue, J. Effect of impurity phase on corrosion resistance and magnetic entropy change for LaFe11.3Co0.4Si1.3C0.15 magnetocaloric compound. J. Rare Earths 2016, 34, 283–287. [Google Scholar] [CrossRef]
- Zhu, F.; Lin, J.C.; Jiang, W.B.; Yang, C.; Li, L.F.; Zhang, X.K.; Song, W.H.; Zhu, X.B.; Tong, P.; Sun, Y.P. Enhanced mechanical properties and large magnetocaloric effect in epoxy-bonded Mn0.98CoGe. Scr. Mater. 2018, 150, 96–100. [Google Scholar] [CrossRef]
- Kavita, S.; Anusha, G.; Bhatt, P.; Suresh, V.; Vijay, R.; Sethupathi, K.; Gopalan, R. On the giant magnetocaloric and mechanical properties of Mn–Fe–P–Si-Ge alloy. J. Alloys Compd. 2020, 817, 153232. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Y.; Niu, E.; Hu, F.; Sun, J.; Shen, B. Enhanced mechanical properties and large magnetocaloric effects in bonded La(Fe, Si)13-based magnetic refrigeration materials. Appl. Phys. Lett. 2014, 104, 062407. [Google Scholar] [CrossRef]
- Salas, F.H.; Mirabal-Garca, M. Quenching of the Hopkinson maximum under contamination in the system Gd(0001)/W(110). Phys. Rev. B 1990, 41, 10859–10861. [Google Scholar] [CrossRef] [PubMed]
- Morrish, A.H. The Physical Principles of Magnetism; Wiley: New York, NY, USA, 2001; ISBN 9780470546581. [Google Scholar]

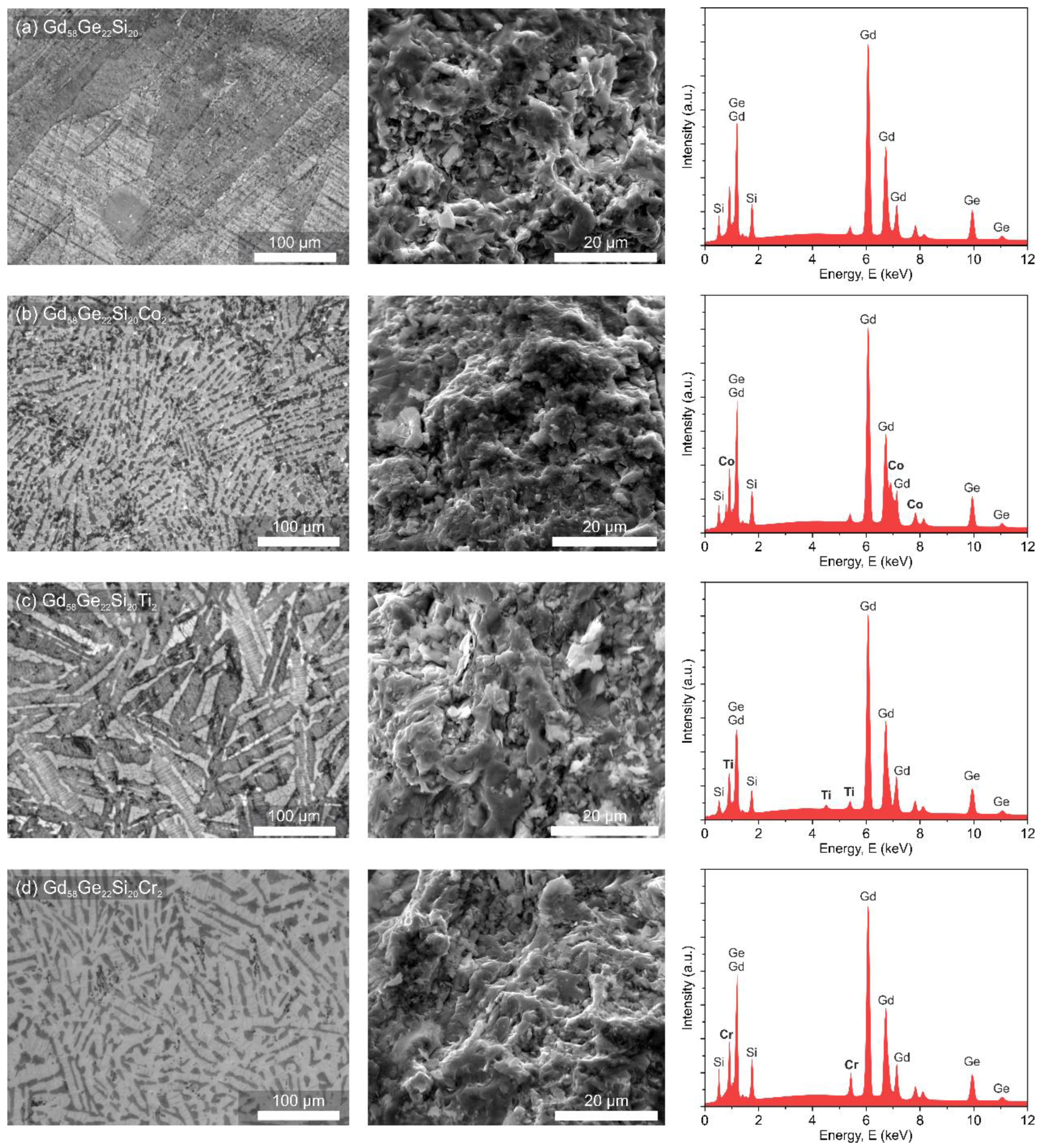

| Time of Exposition | Alloy | Ecorr (mV) | EC-A (mV) | Rp (Ω∙cm2) | icorr (A/cm2) |
|---|---|---|---|---|---|
| 60 min | Gd58Ge20Si22 | −707 | −720 | 3.46 × 106 | 7.54 × 10−9 |
| Gd56Ge20Si22Co2 | −729 | −729 | 2.90 × 105 | 8.98 × 10−8 | |
| Gd56Ge20Si22Ti2 | −737 | −737 | 4.18 × 106 | 6.26 × 10−9 | |
| Gd56Ge20Si22Cr2 | −751 | −748 | 2.27 × 105 | 1.15 × 10−7 | |
| 55 days | Gd58Ge20Si22 | −721 | −713 | 3.56 × 106 | 7.33 × 10−9 |
| Gd56Ge20Si22Co2 | −647 | −766 | 7.49 × 105 | 3.48 × 10−8 | |
| Gd56Ge20Si22Ti2 | −487 | −571 | 3.62 × 106 | 7.21 × 10−9 | |
| Gd56Ge20Si22Cr2 | −704 | −855 | 5.06 × 105 | 5.15 × 10−8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasiak, M.; Chęcmanowski, J.G.; Kucharska, B.; Łaszcz, A.; Kolano-Burian, A.; Kaleta, J. Effect of Co, Ti and Cr Additions on Microstructure, Magnetic Properties and Corrosion Resistance of Magnetocaloric Gd-Ge-Si Alloys. Materials 2020, 13, 5758. https://doi.org/10.3390/ma13245758
Hasiak M, Chęcmanowski JG, Kucharska B, Łaszcz A, Kolano-Burian A, Kaleta J. Effect of Co, Ti and Cr Additions on Microstructure, Magnetic Properties and Corrosion Resistance of Magnetocaloric Gd-Ge-Si Alloys. Materials. 2020; 13(24):5758. https://doi.org/10.3390/ma13245758
Chicago/Turabian StyleHasiak, Mariusz, Jacek G. Chęcmanowski, Barbara Kucharska, Amadeusz Łaszcz, Aleksandra Kolano-Burian, and Jerzy Kaleta. 2020. "Effect of Co, Ti and Cr Additions on Microstructure, Magnetic Properties and Corrosion Resistance of Magnetocaloric Gd-Ge-Si Alloys" Materials 13, no. 24: 5758. https://doi.org/10.3390/ma13245758
APA StyleHasiak, M., Chęcmanowski, J. G., Kucharska, B., Łaszcz, A., Kolano-Burian, A., & Kaleta, J. (2020). Effect of Co, Ti and Cr Additions on Microstructure, Magnetic Properties and Corrosion Resistance of Magnetocaloric Gd-Ge-Si Alloys. Materials, 13(24), 5758. https://doi.org/10.3390/ma13245758






