Alkali Activation of Copper and Nickel Slag Composite Cementitious Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Activity Index of CNS
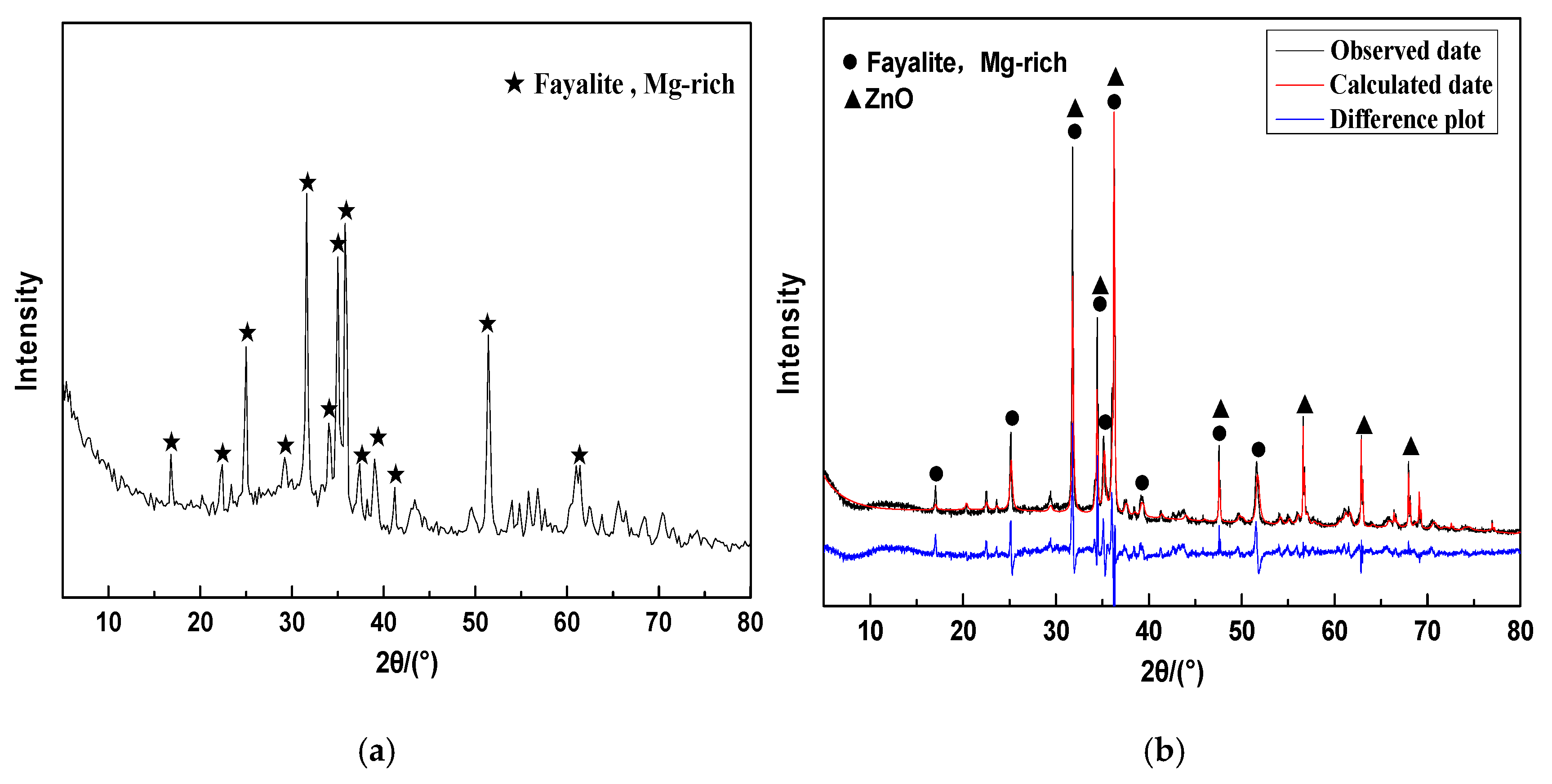
2.2.2. Crystalline Phase and Glass Phase Spatial Structures of CNS
2.2.3. Alkali Dissolution in Glass Phase
2.2.4. Preparation of Samples
2.2.5. Test
3. Results and Discussion
3.1. Activity Index of CNS
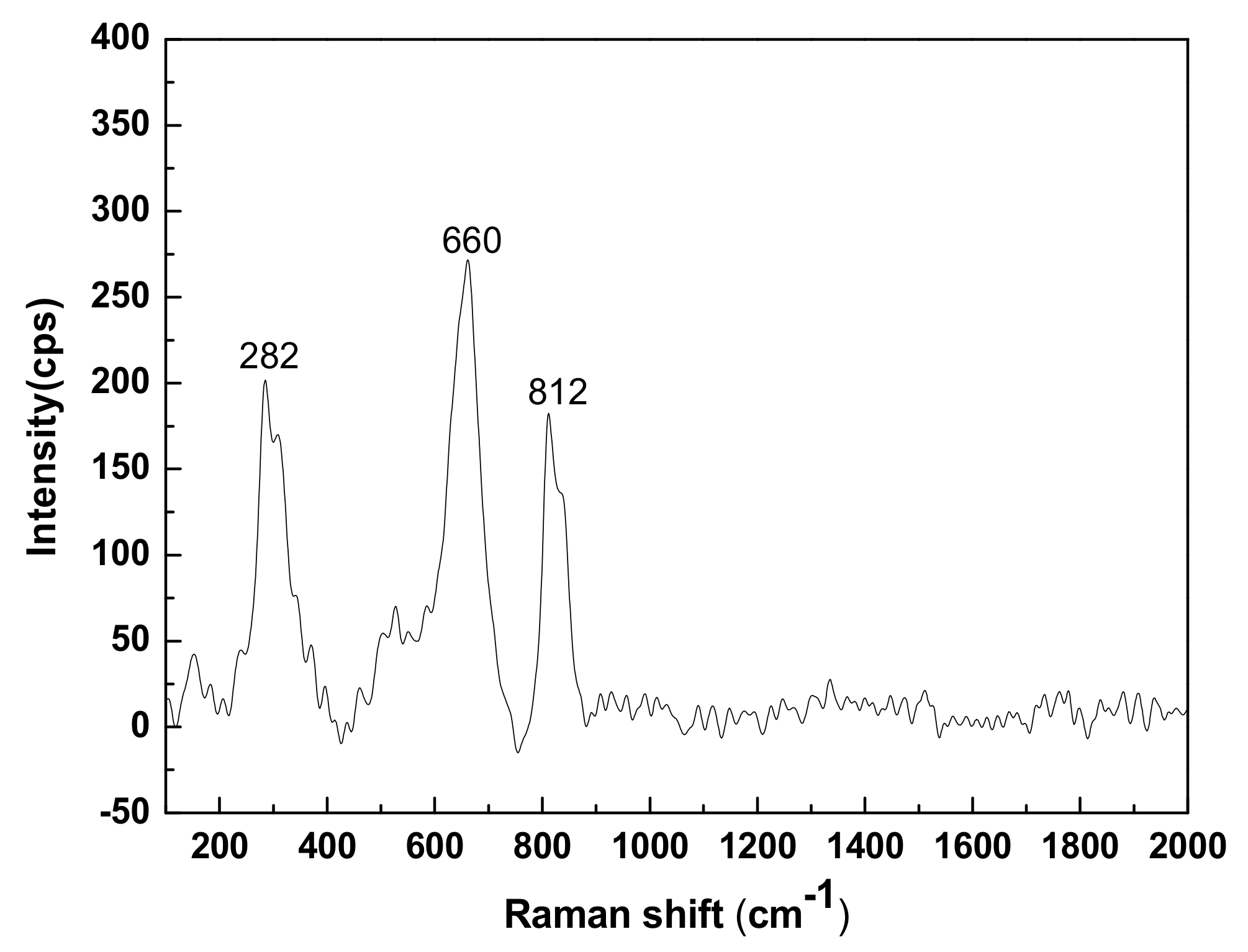
3.2. Raman Analysis
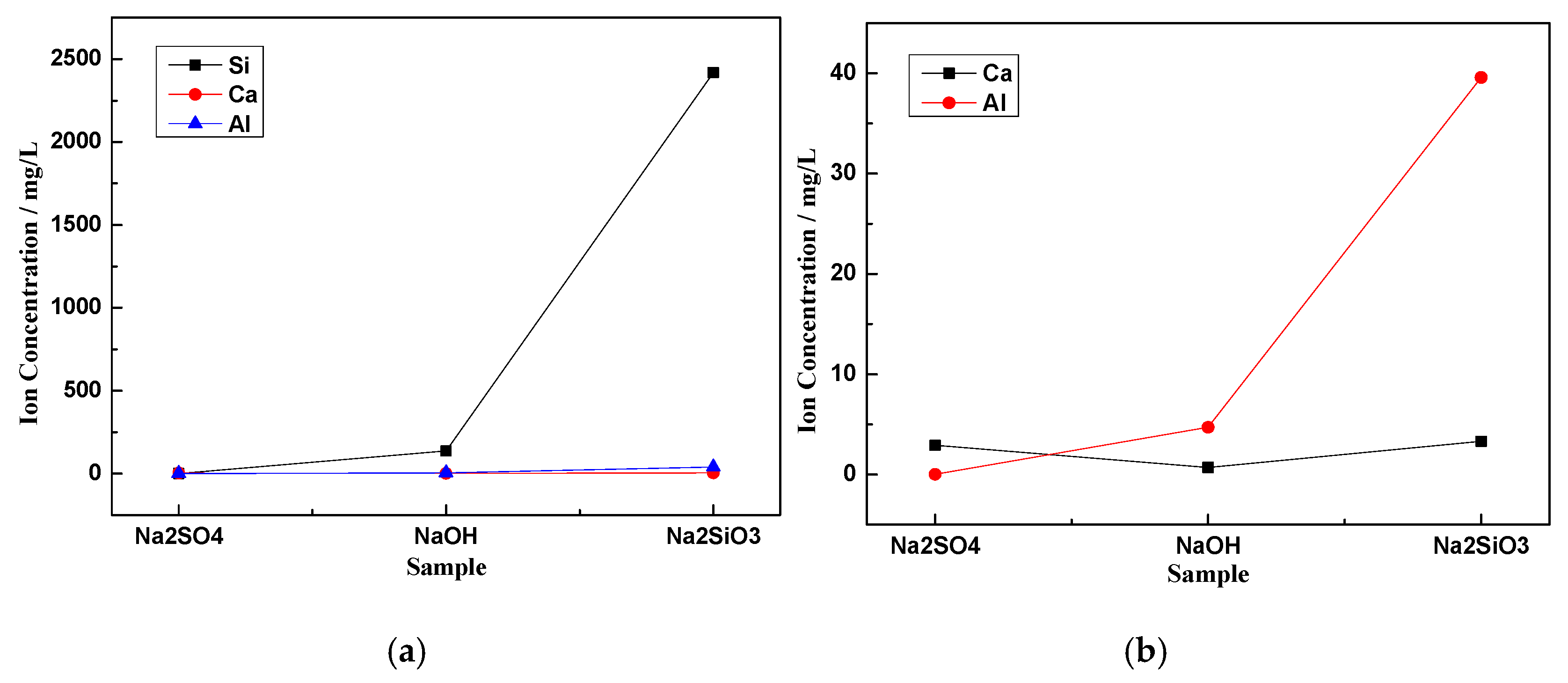
3.3. Ion Dissolution
3.4. Effect of Grinding Time on Hydration of ACNCMs
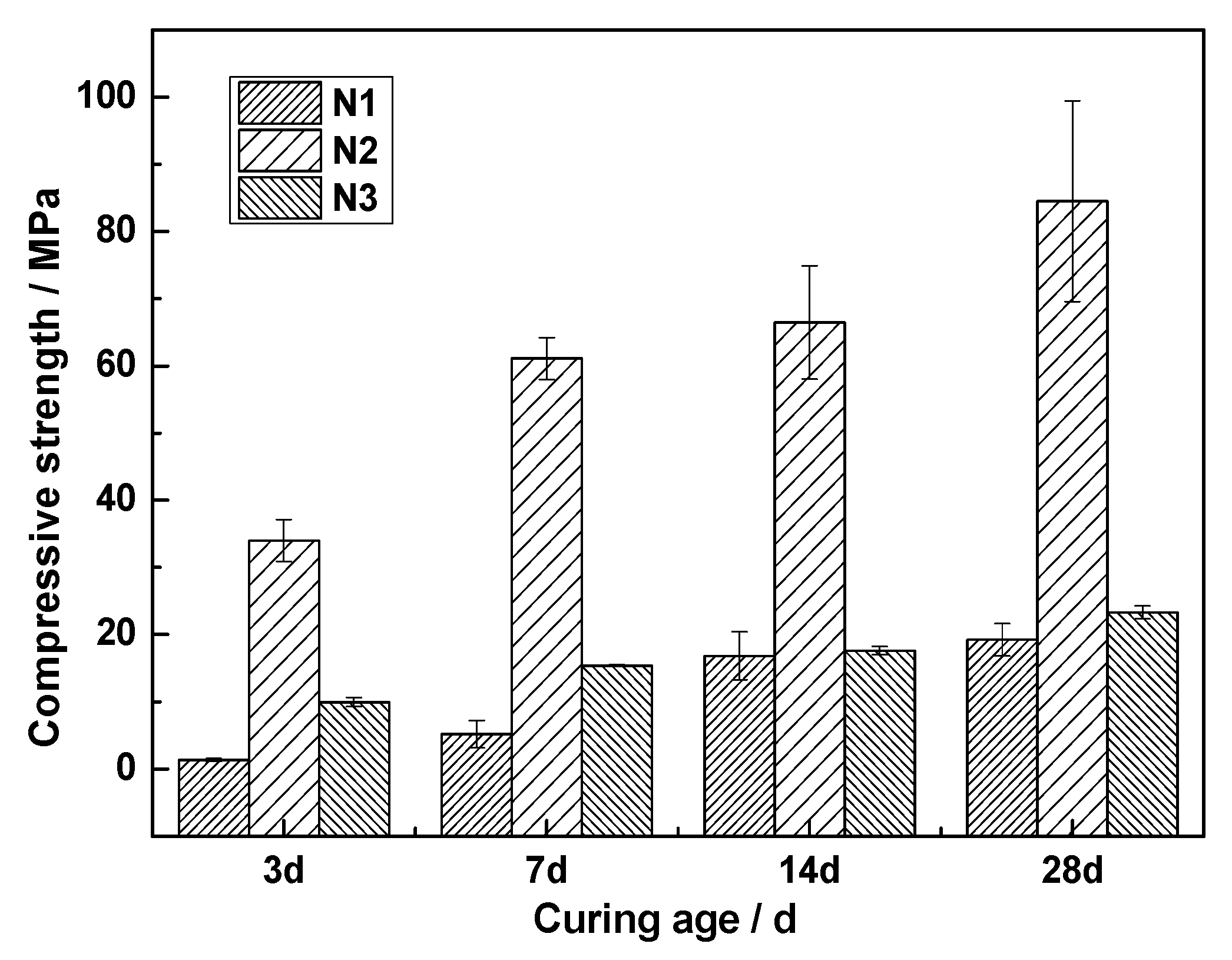
3.5. Effect of Activators on Hydration of ACNCMs
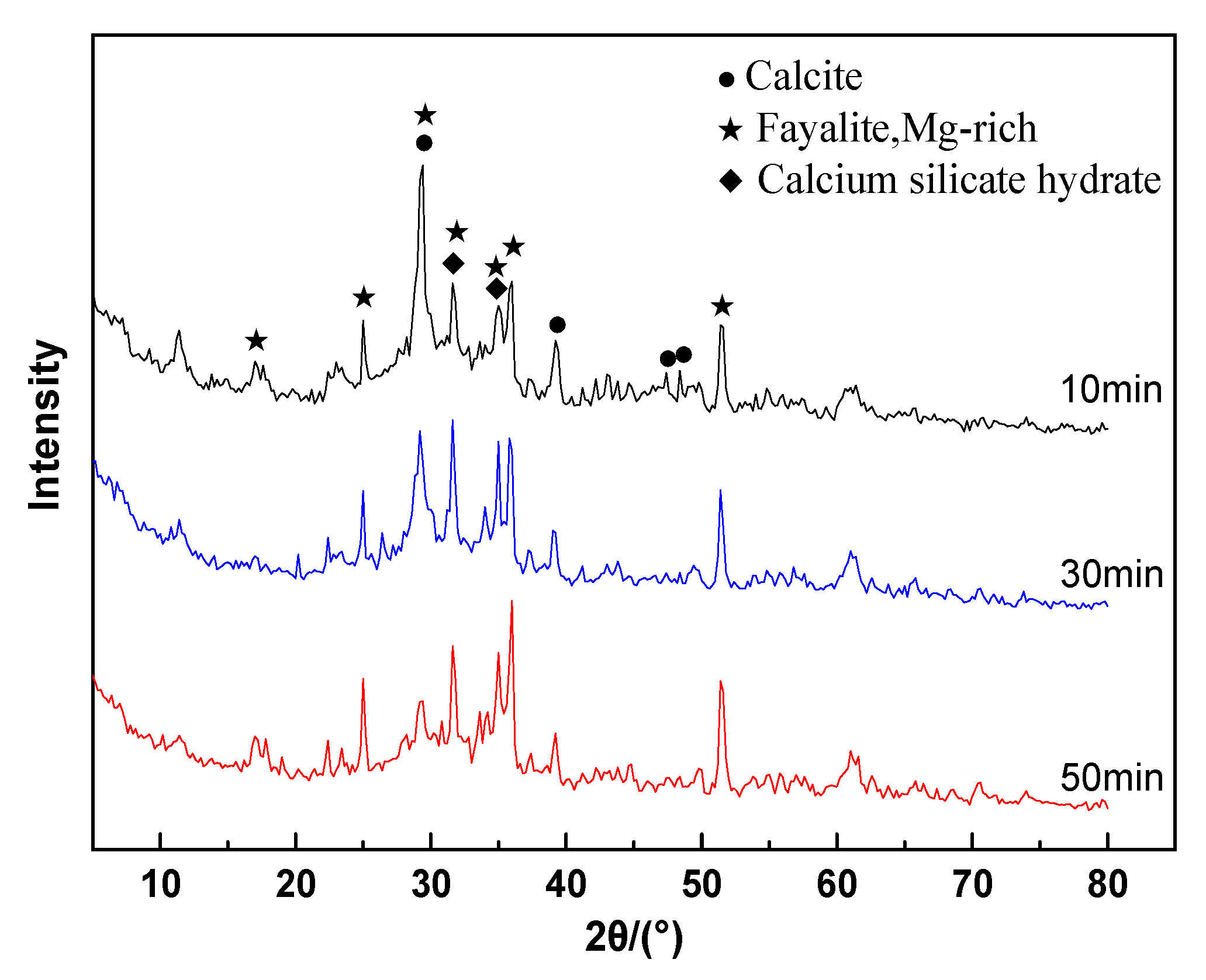
3.6. XRD Analyses
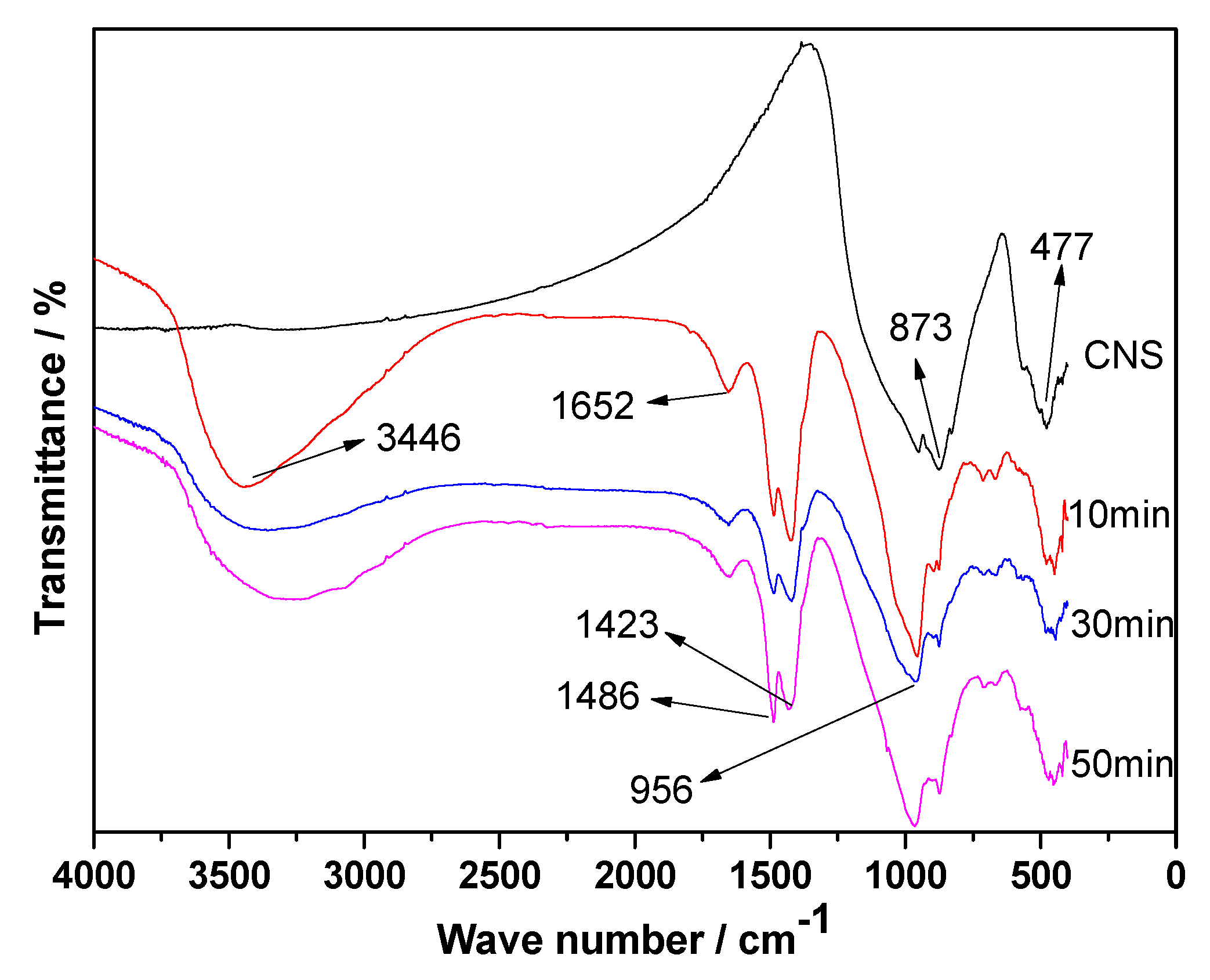
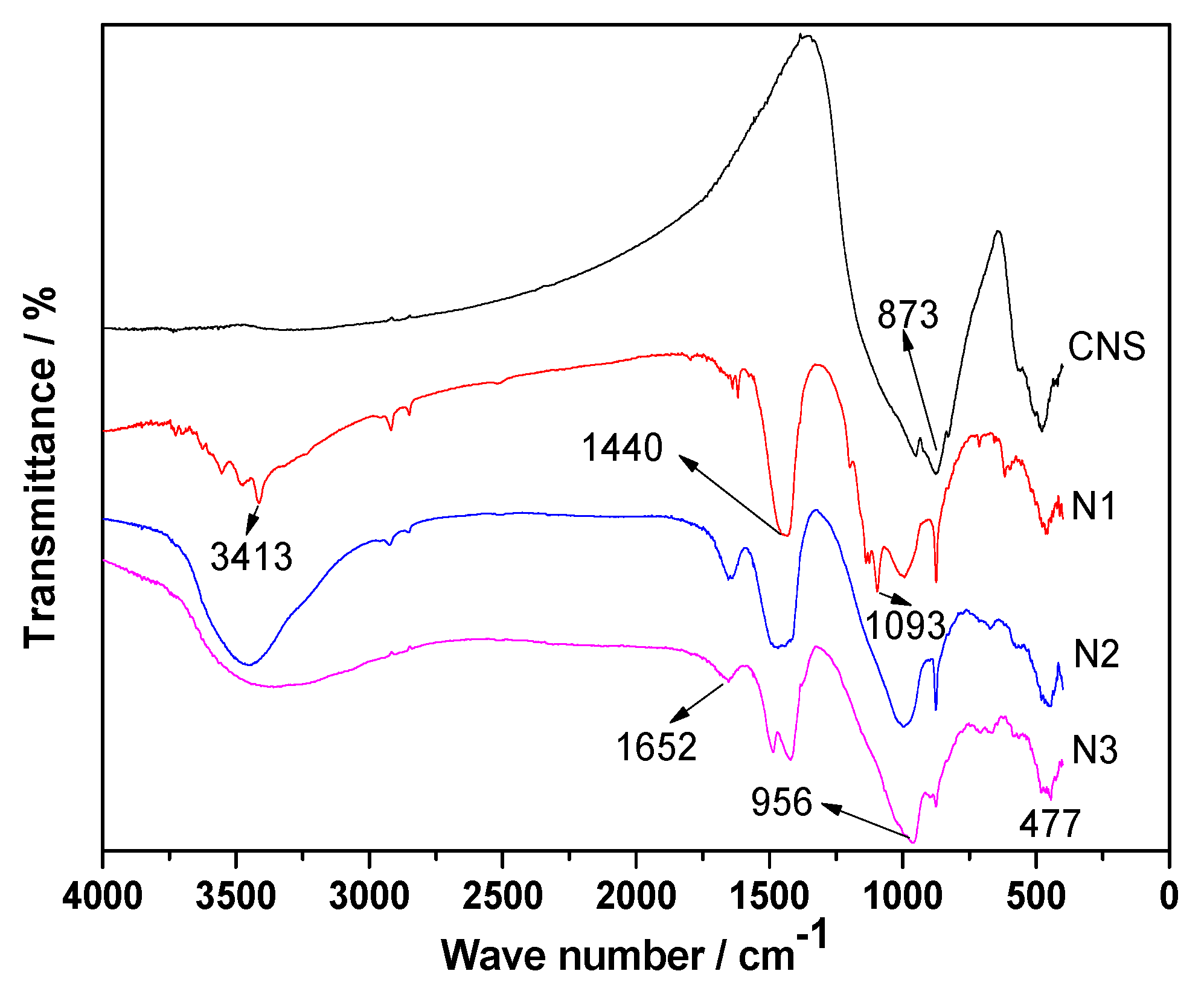
3.7. FT-IR Analyses
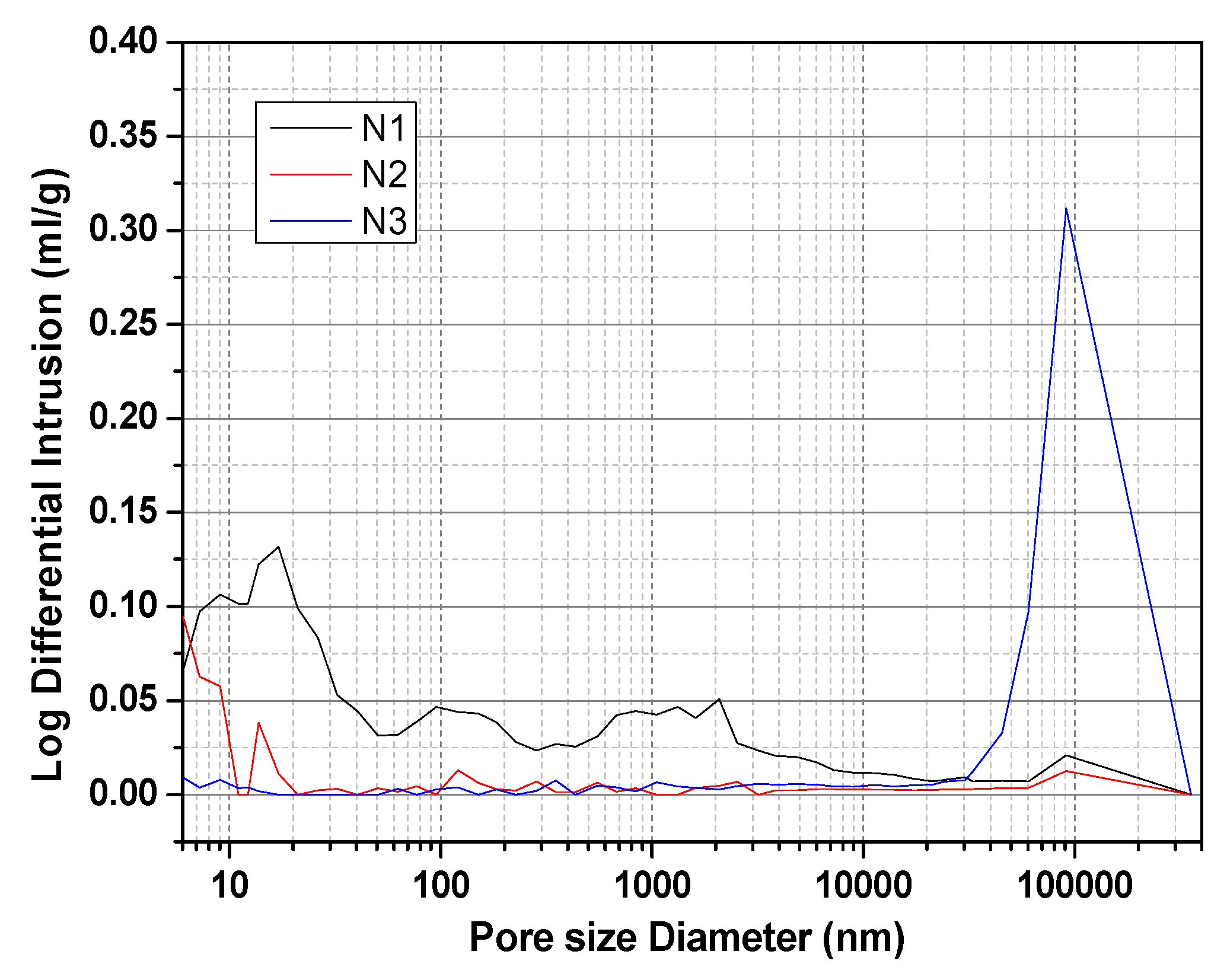
3.8. TG and MIP Analyses
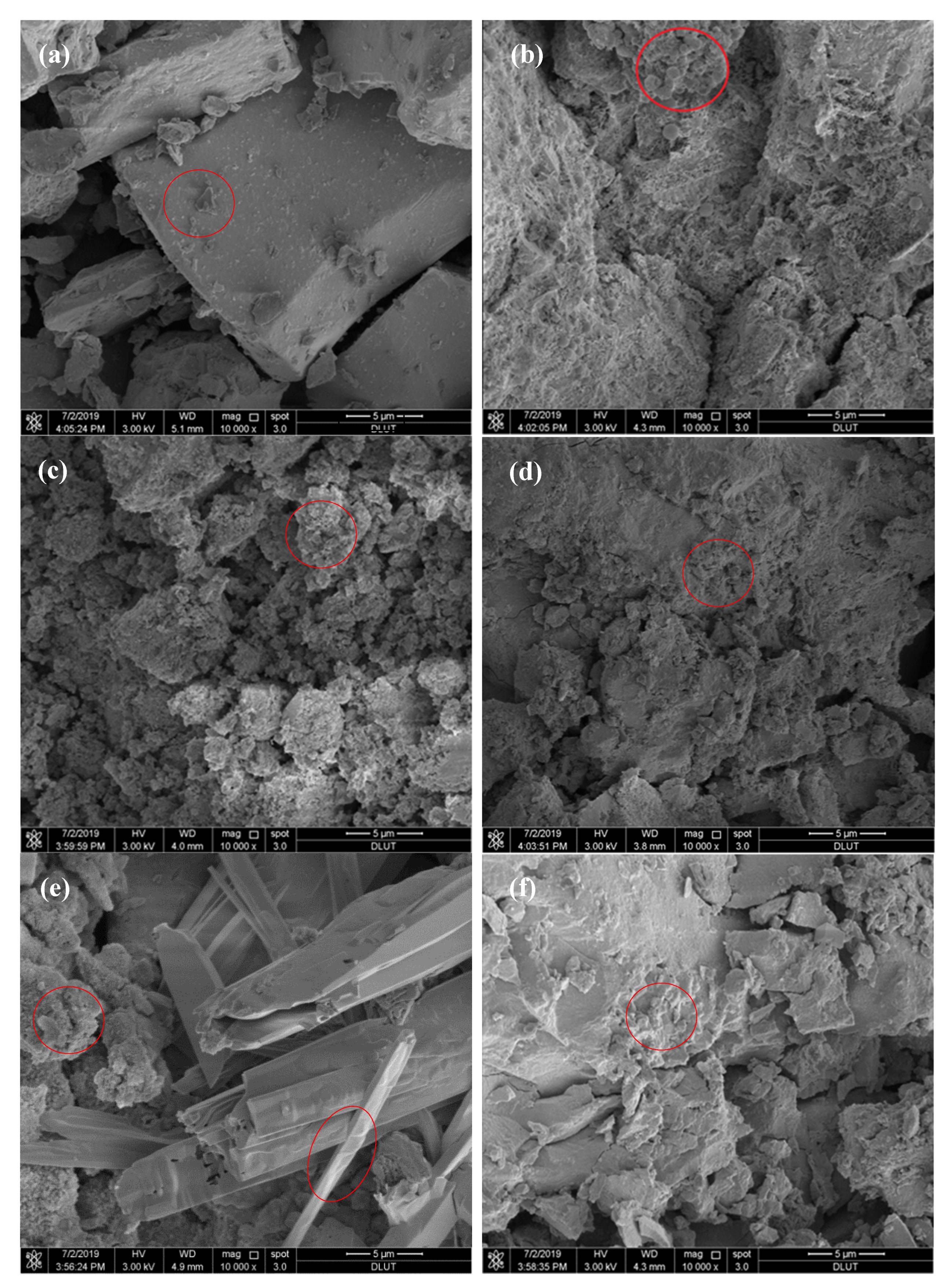
3.9. SEM Analyses
4. Conclusions
- (1)
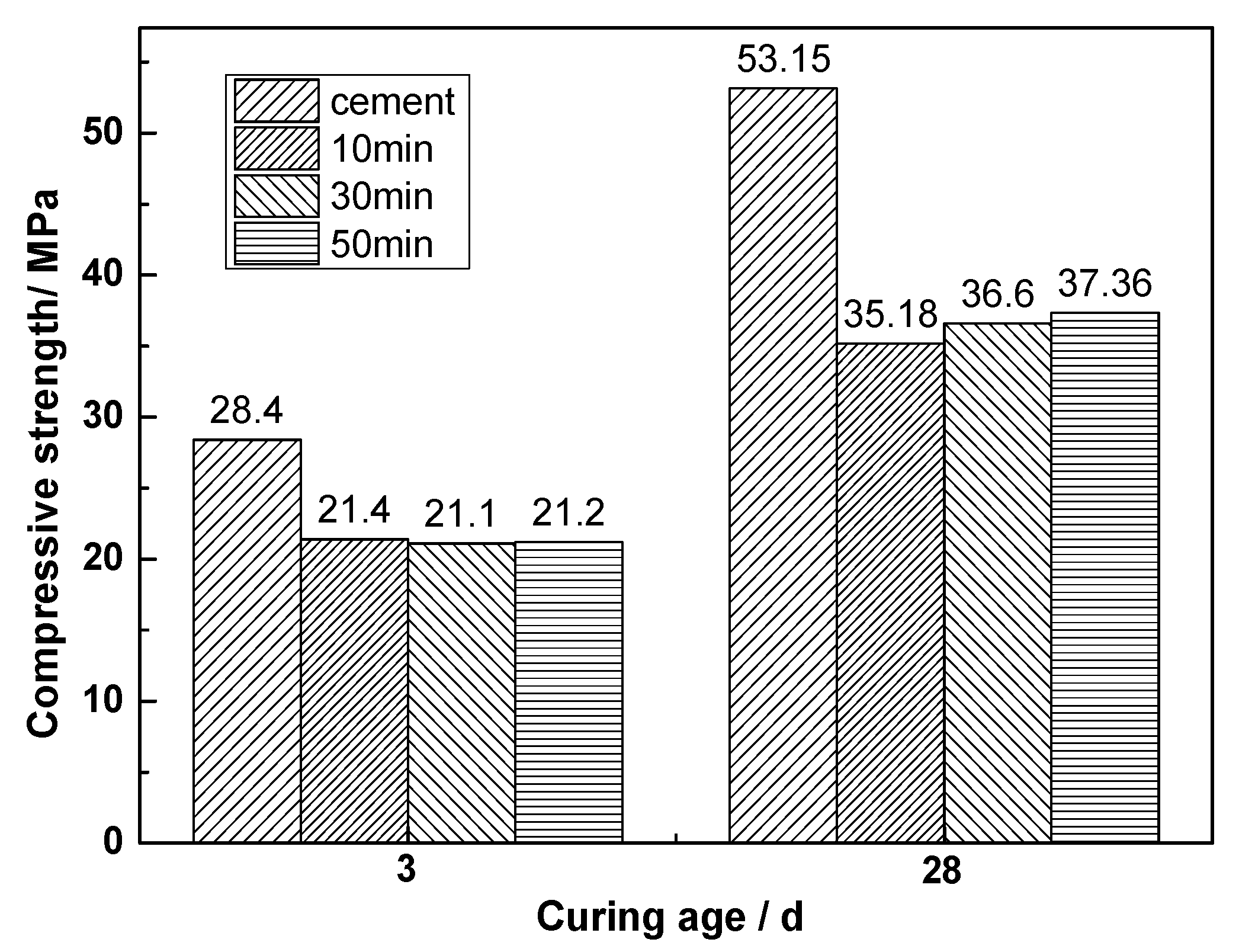
- Mechanical grinding can improve the activity of CNS. The activity indexes of CNS after grinding for 10, 30, and 50 min were found to be 0.662, 0.689, and 0.703, respectively.
- (2)
- Mechanical grinding can promote the hydration reaction of CNS to produce more C-S-H gel, thereby improving the compressive strength of ACNCMs. The 28-day compressive strength of the CNS ground for 50 min was found to be 30.4 MPa, which was 1.3 times that of the CNS ground for 30 min, and 1.9 times that of the CNS ground for 10 min.
- (3)
- CNS is composed of crystalline-phase fayalite and an amorphous glass phase. Fayalite is an island-like [SiO4]4− tetrahedral structure in which the Si-O chemical bond exists as a non-bridged oxygen bond. The glass phase is a bridged oxygen-connected reticular [SiO4]4− tetrahedral structure.
- (4)
- ,, and can break the [SiO4]4− chemical bonds in the glass phase of CNS, but and are better at breaking chemical bonds than . When was used as the activator, the glass phase dissolution was the worst with less Si4+, Al3+, and Ca2+ in the solution. In contrast, when was used as the activator, the dissolution characteristics in the glass phase were the best, and the solution contained the most Si4+, Al3+, and Ca2+ with respective concentrations of 2419, 39.55, and 3.38 mg/L.
- (5)
- When was used as the activator, there was very little C-S-H gel generated by the ACNCMs, the macroscopic mechanical performance was the worst, and the 28-day compressive strength was only 19 MPa. When was used as the activator, there was relatively less C-S-H gel generated by the ACNCMs, and it had a larger pore size and denser structure. The macroscopic mechanical performance was generally good, and the 28-day compressive strength was 23.3 MPa. However, when was used as the activator, CNS dissolved more Si4+ and Ca2+ during the hydration reaction, and the ACNCMs generated more C-S-H gel. The pore size was smaller, the structure was more compact, and the macroscopic mechanical performance was better. The 28-day compressive strength was found to be as high as 84 MPa.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Zhang, Z.; Zhu, Y.; Yang, T.; Li, L.; Zhu, H.; Wang, H. Conversion of local industrial wastes into greener cement through geopolymer technology: A case study of high-magnesium nickel slag. J. Clean. Prod. 2017, 141, 463–471. [Google Scholar] [CrossRef]
- Li, Y.J.; Papangelakis, V.G.; Perederiy, L. High pressure oxidative acid leaching of nickel smelter slag: Characterization of feed and residue. Hydrometallurgy 2009, 97, 185–193. [Google Scholar] [CrossRef]
- Kalinkin, A.; Kumar, S.; Gurevich, B.; Alex, T.; Kalinkina, E.; Tyukavkina, V.; Kalinnikov, V.; Kumar, R. Geopolymerization behavior of Cu–Ni slag mechanically activated in air and in CO2 atmosphere. Int. J. Miner. Process. 2012, 112, 101–106. [Google Scholar] [CrossRef]
- Yang, T.; Wu, Q.Z.; Zhu, H.J.; Zhang, Z.H. Geopolymer with improved thermal stability by incorporating high-magnesium nickel slag. Constr. Build. Mater. 2017, 155, 475–484. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, S.; Yang, T.; Zhu, H.; Li, S. Effect of High-Magnesium Nickel Slag on Hydration Characteristics of Portland Cement. J. Mater. Civ. Eng. 2019, 31, 04019051. [Google Scholar] [CrossRef]
- Li, Y.J.; Perederiy, I.; Papangelakis, V.G. Cleaning of waste smelter slags and recovery of valuable metals by pressure oxidative leaching. J. Hazard. Mater. 2008, 152, 607–615. [Google Scholar] [CrossRef]
- Wang, Z.J.; Ni, W.; Li, K.Q.; Huang, X.Y.; Zhu, L.P. Crystallization characteristics of iron-rich glass ceramics prepared from nickel slag and blast furnace slag. Int. J. Miner. Met. Mater. 2011, 18, 455–459. [Google Scholar] [CrossRef]
- Wang, Z.J.; Ni, W.; Jia, Y.; Zhu, L.P.; Huang, X.Y. Crystallization behavior of glass ceramics prepared from the mixture of nickel slag, blast furnace slag and quartz sand. J. Non-Cryst. Solids 2010, 356, 1554–1558. [Google Scholar] [CrossRef]
- Wu, Q.S.; Wu, Y.; Tong, W.H.; Ma, H. Utilization of nickel slag as raw material in the production of Portland cement for road construction. Constr. Build. Mater. 2018, 193, 426–434. [Google Scholar] [CrossRef]
- Saha, A.K.; Sarker, P.K. Expansion due to alkali-silica reaction of ferronickel slag fine aggregate in OPC and blended cement mortars. Constr. Build. Mater. 2016, 123, 135–142. [Google Scholar] [CrossRef]
- Choi, Y.C.; Choi, S. Alkali-silica reactivity of cementitious materials using ferro-nickel slag fine aggregates produced in different cooling conditions. Constr. Build. Mater. 2015, 99, 279–287. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, Z.H.; Zhu, H.J.; Gao, X.; Dai, C.D.; Wu, Q. Re-examining the suitability of high magnesium nickel slag as precursors for alkali-activated materials. Constr. Build. Mater. 2019, 213, 109–120. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Reddy, M.S.; Dinakar, P.; Rao, B.H. A review of the influence of source material’s oxide composition on the compressive strength of geopolymer concrete. Microporous Mesoporous Mater. 2016, 234, 12–23. [Google Scholar] [CrossRef]
- Rakhimova, N.R.; Rakhimov, R.Z. Alkali-activated cements and mortars based on blast furnace slag and red clay brick waste. Mater. Des. 2015, 85, 324–331. [Google Scholar] [CrossRef]
- Hanzlicek, T.; Steinerova-Vondrakova, M. Immobilization of Toxic Metals in Solidified Systems of Siloxo-Sial Networks. J. Am. Ceram. Soc. 2006, 89, 968–970. [Google Scholar] [CrossRef]
- Nazer, A.; Payá, J.; Borrachero, M.V.; Monzó, J. Use of ancient copper slags in Portland cement and alkali activated cement matrices. J. Environ. Manag. 2016, 167, 115–123. [Google Scholar] [CrossRef]
- Song, W.; Zhu, Z.; Peng, Y.; Wan, Y.; Xu, X.; Pu, S.; Song, S.; Wei, Y. Effect of steel slag on fresh, hardened and microstructural properties of high-calcium fly ash based geopolymers at standard curing condition. Constr. Build. Mater. 2019, 229, 116933. [Google Scholar] [CrossRef]
- Nan, S.Q.; Gao, Q.; Zhang, J.X.; Guo, X.Z. Analysis of Hydration Mechanism of New Type Filling Cementitious Materials. Adv. Mater. Res. 2013, 753–755, 814–818. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Gao, Q.; Wang, Y.Q.; Ni, W.; Chen, D. Experimental study on new filling cementing material using water-hardening nickel slag tailings of Jinchuan Mine. Chin. J. Geotech. Eng. 2014, 36, 1498–1506. [Google Scholar]
- Ding, G.Y.; Xu, J.; Wei, Y.; Chen, R.; Li, X. Engineered reclamation fill material created from excavated soft material and granulated blast furnace slag. Resour. Conserv. Recycl. 2019, 150, 104428. [Google Scholar] [CrossRef]
- Van, D.J.; Provis, J.L.; Duxson, P.; Brice, D.G. Chemical Research and Climate Change as Drivers in the Commercial Adoption of Alkali Activated Materials. Waste Biomass Valorization 2010, 1, 145–155. [Google Scholar]
- Yang, T.; Yao, X.; Zhang, Z.H. Gepolymer prepared with high-magnesium nickel slag: Characterization of properties and microstructure. Constr. Build. Mater. 2014, 59, 188–194. [Google Scholar] [CrossRef]
- Lan, W.T.; Wu, A.X.; Yu, P. Development of a new controlled low strength filling material from the activation of copper slag: Influencing factors and mechanism analysis. J. Clean. Prod. 2019, 246, 119060. [Google Scholar] [CrossRef]
- Singh, J.; Singh, S.P. Development of Alkali-activated Cementitious Material using Copper Slag. Constr. Build. Mater. 2019, 211, 73–79. [Google Scholar] [CrossRef]
- Chao, L. Research on the Glass Phase of Slag, High Calcium Fly Ash and Low Calcium Fly Ash and Their Hydration Mechanism. Ph.D. Thesis, Tsinghua University, Beijing, China, 2011. [Google Scholar]
- Fu, X.H.; Wang, A.; Krawczynski, M.J. Characterizing amorphous silicates in extraterrestrial materials: Polymerization effects on Raman and mid-IR spectral features of alkali and alkali earth silicate glasses. J. Geophys. Res. Planets 2017, 122, 839–855. [Google Scholar] [CrossRef]
- Jiang, F. A Study on the Composition, Structure and Performance of Slag-Fines Cementing Materials Alkall-Activated. Ph.D. Thesis, Xi’an University of Architecture and Technology, Xi’an, China, 2008. [Google Scholar]
- Yuan, R.; Zhu, J.A.; Ouyang, S.X.; Gao, Q.Y. Study on the structure of slag glass and its properties. J. Wuhan Univ. Technol. 1981, 3, 33–43. [Google Scholar]
- Wu, Q.; Guan, J. Mechanochemical effect of nickel slag and its impact on reactive activity. Mater. Sci. Technol. 2016, 24, 22–27. [Google Scholar]
- Bernal, S.A.; Gutierrez, R.M.; Provis, J.L.; Rose, V. Effect of silicate modulus and metakaolin incorporation on the carbonation of alkali silicate-activated slags. Cem. Concr. Res. 2010, 40, 898–907. [Google Scholar] [CrossRef]
- Allahverdi, A.; Mahinroosta, M. Mechanical activation of chemically activated high phosphorous slag content cement. Powder Technol. 2013, 245, 182–188. [Google Scholar] [CrossRef]
- Feng, Y.; Kero, J.; Yang, Q.X.; Chen, Q.S.; Engström, F.; Samuelsson, C.; Qi, C.C. Mechanical Activation of Granulated Copper Slag and Its Influence on Hydration Heat and Compressive Strength of Blended Cement. Materials 2019, 12, 772. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Ayuso, E.; Nugteren, H.W. Synthesis of ettringite: A way to deal with the acid wastewaters of aluminium anodising industry. Water Res. 2005, 39, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Mollah, M.Y.; Yu, W.H.; Schennach, R.; Cocke, D.L. A Fourier transform infrared spectroscopic investigation of the early hydration of Portland cement and the influence of sodium lignosulfonate. Cem. Concr. Res. 2000, 30, 267–273. [Google Scholar] [CrossRef]
- Lin, C.Q.; Wei, W.; Hu, Y.H. Catalytic behavior of graphene oxide for cement hydration process. J. Phys. Chem. Solids 2016, 89, 128–133. [Google Scholar] [CrossRef]
- Cadore, D.E.; Angulski Da Luz, C.; Farias De Medeiros, M.H. An investigation of the carbonation of alkaline activated cement made from blast furnace slag generated by charcoal. Constr. Build. Mater. 2019, 226, 117–125. [Google Scholar] [CrossRef]
- Peng, G.F.; Huang, Z.S. Change in microstructure of hardened cement paste subjected to elevated temperatures. Constr. Build. Mater. 2008, 22, 593–599. [Google Scholar] [CrossRef]
- Ma, Q.M.; Guo, R.X.; Zhao, Z.M.; Lin, Z.W.; He, K.C. Mechanical properties of concrete at high temperature—A review. Constr. Build. Mater. 2015, 93, 371–383. [Google Scholar] [CrossRef]
- Li, N.; Farzadnia, N.; Shi, C.J. Microstructural changes in alkali-activated slag mortars induced by accelerated carbonation. Cem. Concr. Res. 2017, 100, 214–226. [Google Scholar] [CrossRef]
- Song, K.; Song, J.K.; Lee, B.Y.; Yang, K.H. Carbonation Characteristics of Alkali-Activated Blast-Furnace Slag Mortar. Adv. Mater. Sci. Eng. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L. Designing Precursors for Geopolymer Cements. J. Am. Ceram. Soc. 2008, 91, 3864–3869. [Google Scholar] [CrossRef]



| Composition | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CNS | 32.37 | 53.87 | 1.18 | 1.66 | 6.53 | 1.00 | 0.56 | 0.54 | 0.49 | 0.43 |
| GGFBS | 15.43 | 0.73 | 19.20 | 46.27 | 14.74 | --- | 0.62 | --- | --- | --- |
| Rietveld | Spiked | Original | |
|---|---|---|---|
| Amorphous | 0 | 24.82 | 27.58 |
| Fayalite, Mg-rich | 86.70 | 65.18 | 72.42 |
| Zincite | 13.30 | 10.00 | 0.000 |
| Element | Type | Ligancy | M-O Bond Energy (KJ) |
|---|---|---|---|
| Si | Network-forming | 4 | 106 |
| Al | Network-forming | 4 | 101–79 |
| Mg | Intermediate | 4 | 55.5 |
| Ca | Network-modifying | 8 | 32 |
| Na | Network-modifying | 6 | 20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Zhi, S.; Li, T.; Zhou, Z.; Li, M.; Han, J.; Li, W.; Zhang, D.; Guo, L.; Wu, Z. Alkali Activation of Copper and Nickel Slag Composite Cementitious Materials. Materials 2020, 13, 1155. https://doi.org/10.3390/ma13051155
Zhang T, Zhi S, Li T, Zhou Z, Li M, Han J, Li W, Zhang D, Guo L, Wu Z. Alkali Activation of Copper and Nickel Slag Composite Cementitious Materials. Materials. 2020; 13(5):1155. https://doi.org/10.3390/ma13051155
Chicago/Turabian StyleZhang, Tingting, Shiwei Zhi, Tong Li, Ziyu Zhou, Min Li, Junnan Han, Wenchen Li, Dan Zhang, Lijie Guo, and Zhenlin Wu. 2020. "Alkali Activation of Copper and Nickel Slag Composite Cementitious Materials" Materials 13, no. 5: 1155. https://doi.org/10.3390/ma13051155
APA StyleZhang, T., Zhi, S., Li, T., Zhou, Z., Li, M., Han, J., Li, W., Zhang, D., Guo, L., & Wu, Z. (2020). Alkali Activation of Copper and Nickel Slag Composite Cementitious Materials. Materials, 13(5), 1155. https://doi.org/10.3390/ma13051155






