Influence of High-Temperature Liquid on Phase Composition and Morphology of Carbothermal Reduction-Nitridation Products from Coal Gasification Slag
Abstract
:1. Introduction
2. Experimental Part
2.1. Experimental Materials
2.2. Experiment Method
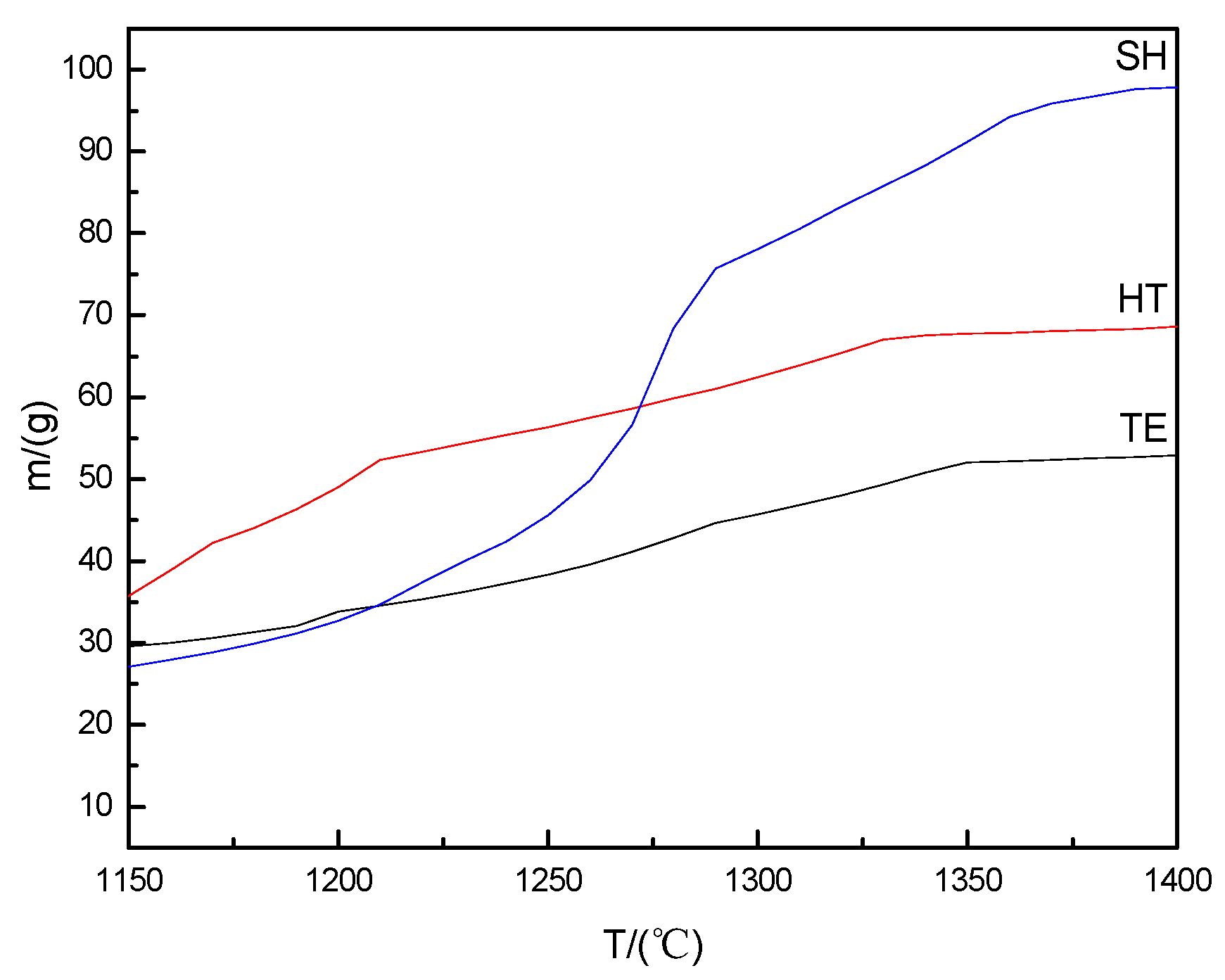
2.2.1. Thermodynamics Calculation of Liquid Phase Quantity and High-Temperature Treatment of Raw Materials
2.2.2. CRN Process
2.2.3. Characterization
3. Results and Discussion
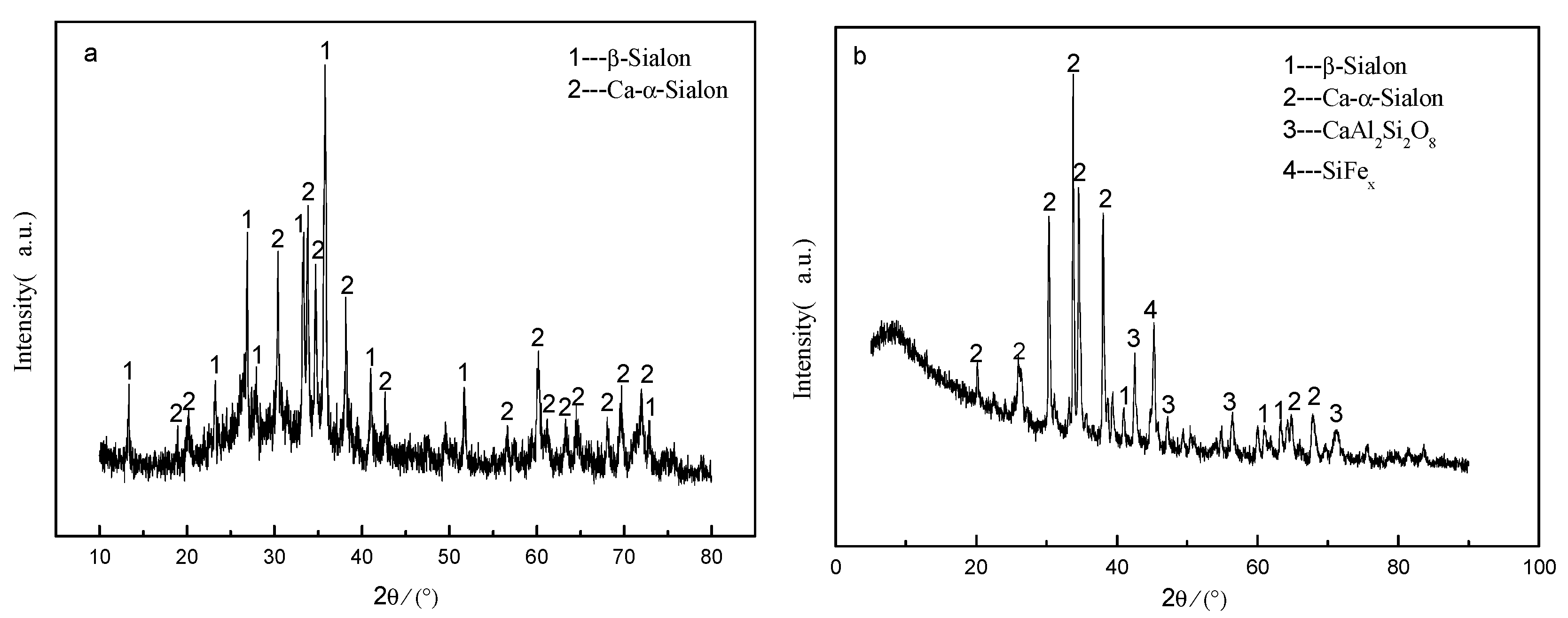
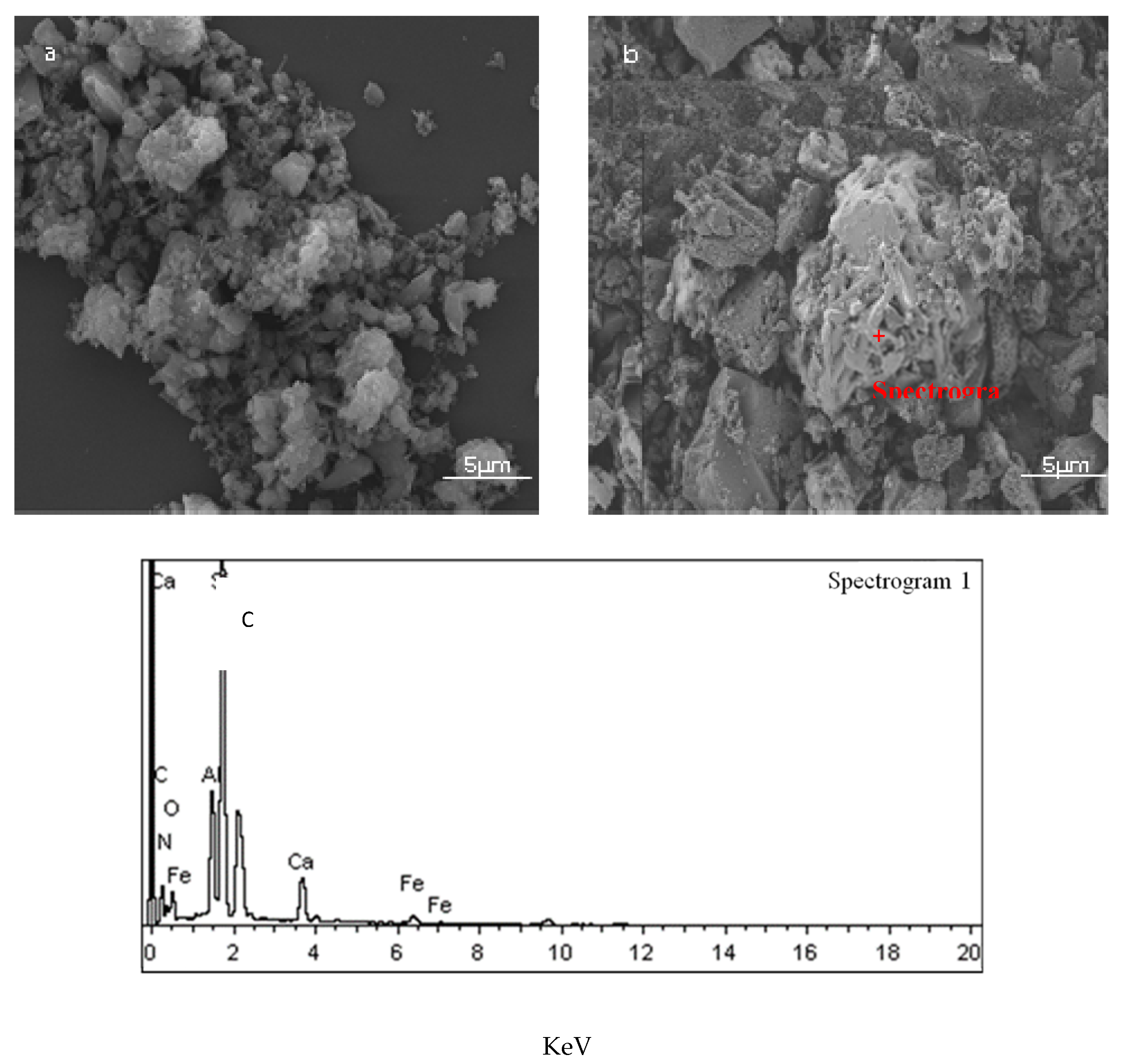
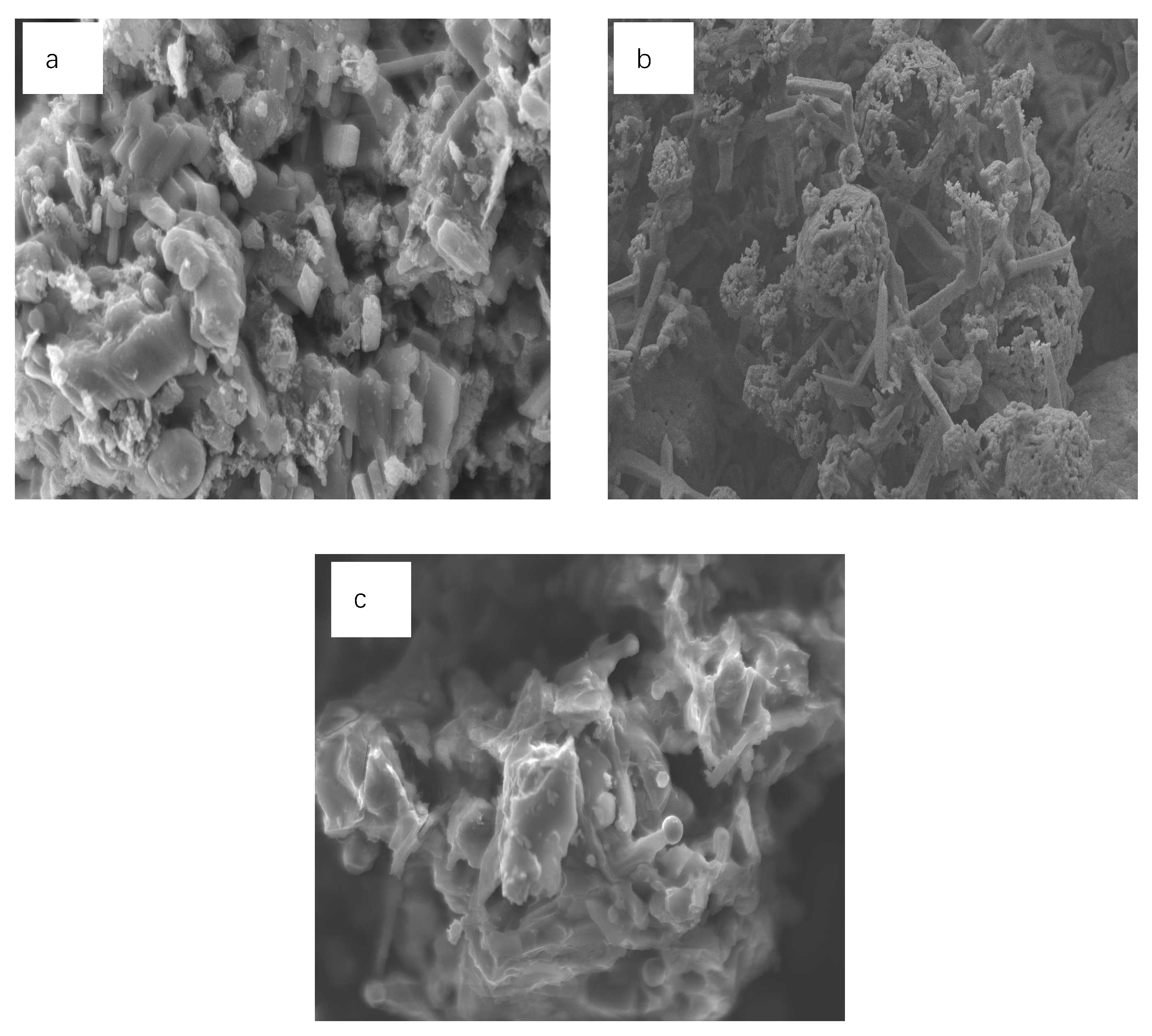
3.1. Influence of the Existence of High-Temperature Liquid Phase on CRN Products
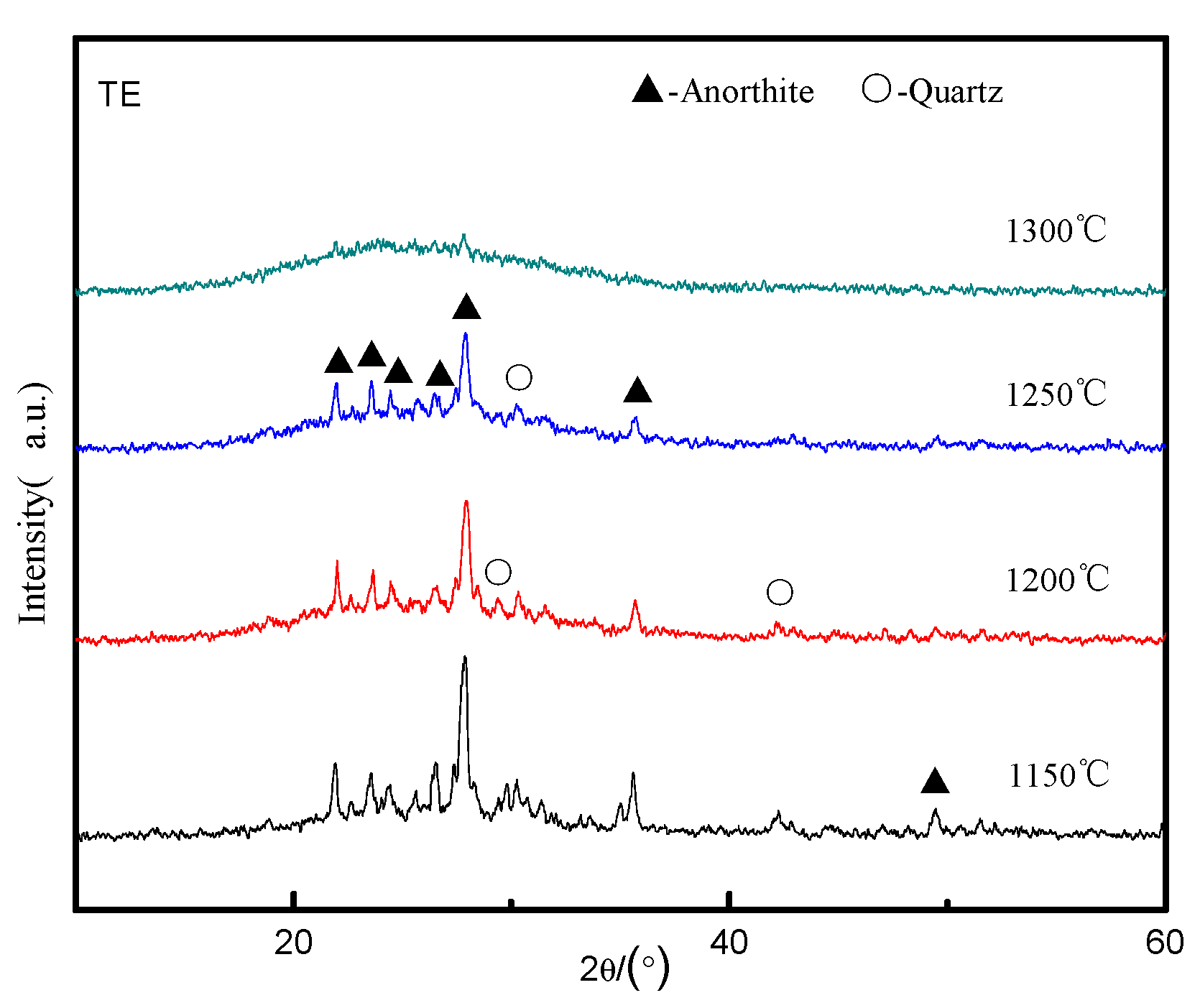
3.2. Effect of the Content of High-Temperature Liquid Phase on CRN Products
4. Conclusions
- (1)
- The presence of a high-temperature liquid phase not only favored the formation of the Ca-α-SiAlON phase but also produced a Ca-α-SiAlON that tended to grow into a columnar shape.
- (2)
- The relationship between the amount of liquid phase and the aspect ratio of Ca-α-SiAlON phase was nonlinear. The morphology of crystal grains was not only related to the amount of liquid phase but also might be affected by the viscosity of the liquid phase.
- (3)
- The HT with many liquid phases (~65 wt. %) and low viscosity (~1.99 Pa·S) was most conducive to the growth of elongated Ca-α-SiAlON; and for the SH with the highest content of liquid-phase (~95 wt. %) and the maximum viscosity (~3.37 Pa·S), the Ca-α-SiAlON showed the largest aspect ratio; while Ca-α-SiAlON grains fabricated by TE slag with the lowest content of the high-temperature liquid (~50 wt. %) and the medium viscosity (~2.81 Pa·S) were of equiaxed shape.
Author Contributions
Funding
Conflicts of Interest
References
- Acosta, A.; Aineto, M.; Iglesias, I.; Romerob, M.; Rincón, J.M. Physico-chemical characterization of slag waste coming from GICC thermal power plant. Mater. Lett. 2001, 50, 246–250. [Google Scholar] [CrossRef] [Green Version]
- Choudhry, V.; Kwan, S.; Hadley, S.R. Utilization of Lightweight Materials Made from Coal Gasification Slags; Technical Report; National Energy Technology Laboratory (NETL): Albany, OR, USA, 1 June 2001.
- Acosta, A.; Iglesias, I.; Aineto, M.; Romero, M.; Rincón, J.M. Thermal and sintering characterization of IGCC slag. J. Therm. Anal. Calorim. 2002, 67, 249–255. [Google Scholar] [CrossRef]
- Aineto, M.; Acosta, A.; Rincón, J.M.; Romero, M. Thermal expansion of slag and fly ash from coal gasification in IGCC power plant. Fuel 2006, 85, 2352–2358. [Google Scholar] [CrossRef]
- Wagner, N.J.; Matjie, R.H.; Slaghuis, J.H.; van Heerden, J.H.P. Characterization of unburned carbon present in coarse gasification ash. Fuel 2008, 87, 683–691. [Google Scholar] [CrossRef]
- Wu, T.; Gong, M.; Lester, E.; Wang, F.C.; Zhou, Z.J.; Yu, Z.H. Characterisation of residual carbon from entrained-bed coal water slurry gasifiers. Fuel 2007, 86, 972–982. [Google Scholar] [CrossRef]
- Xu, S.Q.; Zhou, Z.J.; Gao, X.X.; Yu, G.S.; Gong, X. The gasification reactivity of unburned carbon present in gasification slag from entrained-flow gasifier. Fuel Process. Technol. 2009, 90, 1062–1070. [Google Scholar] [CrossRef]
- Song, W.J.; Tang, L.H.; Zhu, X.D.; Wu, Y.Q.; Zhu, Z.B.; Koyama, S. Flow properties and rheology of slag from coal gasification. Fuel 2010, 89, 1709–1715. [Google Scholar] [CrossRef]
- Song, W.J.; Tang, L.H.; Zhu, X.D.; Wu, Y.Q.; Rong, Y.Q.; Zhu, Z.B.; Koyama, S. Fusibility and flow properties of coal ash and slag. Fuel 2009, 88, 297–304. [Google Scholar] [CrossRef]
- Yu, G.S.; Zhu, Q.R.; Chi, G.Z.; Guo, Q.H.; Zhou, Z.J. Study on slag composition and flow property in a bench-scale OMB gasifier. Fuel Process. Technol. 2012, 104, 136–143. [Google Scholar] [CrossRef]
- Kong, L.X.; Bai, J.; Bai, Z.Q.; Guo, Z.X.; Li, W. Effects of CaCO3 on slag flow properties at high temperatures. Fuel 2013, 109, 76–85. [Google Scholar] [CrossRef]
- Tang, Y.; Yin, H.F.; Yuan, H.D.; Shuai, H.; Xin, Y.L. Phase and morphological transformation stages during carbothermal reduction nitridation process: From coal gasification slag wastes to Ca-α-SiAlON powders. Adv. Powder Technol. 2016, 27, 2232–2237. [Google Scholar] [CrossRef]
- Du, M.J.; Huang, J.J.; Liu, Z.Y.; Zhou, X.; Guo, S.; Wang, Z.Q.; Fang, Y.T. Reaction characteristics and evolution of constituents and structure of a gasification slag during acid treatment. Fuel 2018, 224, 178–185. [Google Scholar] [CrossRef]
- Yin, H.F.; Tang, Y.; Zhang, J.Z. Synthesis of Ca-α-Sialon–SiC Multiphase Ceramics Using Gasification Slag. J. Chin. Ceram. Soc. 2011, 39, 233–238. [Google Scholar]
- Tang, Y.; Yin, H.F.; Ren, Y.; Zhang, J.Z. Preparation of SiAlON powder from coal gasification slag. J. Wuhan Univ. Technol. Mater. 2010, 25, 1044–1046. [Google Scholar] [CrossRef]
- Karakuş, N.; Kurt, A.O.; Duran, C.; Öztürk, C.; Toplan, H.Ö. Sintering behaviour of silicon nitride powders produced by carbothermal reduction and nitridation. Adv. Powder Technol. 2013, 24, 697–702. [Google Scholar] [CrossRef]
- Jiang, T.; Wu, J.; Xue, X.; Duan, P.; Chu, M. Carbothermal formation and microstrutural evolution of α′-Sialon-AlN-BN powders from boron-rich blast furnace slag. Adv. Powder Technol. 2012, 23, 406–413. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, H.; Huang, Z.; Liu, G.; Fang, M.; Liu, Y.; Gauckler, L. Preparation and Formation Mechanism of Elongated (Ca,Dy)-α-Sialon Powder via Carbothermal Reduction and Nitridation. J. Am. Ceram. Soc. 2012, 95, 1871–1877. [Google Scholar] [CrossRef]
- Hao, H.S.; Yang, Y.; Lian, F.; Gao, W.Y.; Liu, G.S.; Hu, Z.Q. Synthesis and performance of Ca-α/β-SiAlON composites from tailings. J. Miner. Metall. Mater. 2014, 21, 515–522. [Google Scholar] [CrossRef]
- Shan, Y.; Wang, G.; Sun, X.; Yi, J.; Guan, C.; Zhang, Z.; Xu, J. Improvement of high-temperature oxidation resistance of Y-α-SiAlON with high nitrogen content by lowering Y/Si ratio. J. Alloys Compd. 2015, 636, 138–144. [Google Scholar] [CrossRef]
- Kurama, S.; Avcioglu, S.; Ayas, E. Optimization of the optical properties of α-SiAlON through a decrease in starting powder size. J. Eur. Ceram. Soc. 2015, 35, 3229–3235. [Google Scholar] [CrossRef]
- Zhao, K.; Huang, Z.H.; Fang, M.H.; Zhang, J. Phases transformation of high-alumina fly ash under carbothermal reduction and nitridation. J. Synth. Cryst. 2009, 38, 387–389. [Google Scholar]
- Yan, M.; Li, Y.; Sun, Y.; Li, L.; Tong, S.; Sun, J. Controllable preparation and synthetic mechanism of mullite from the bauxite with Fe-rich oxide content. Mater. Chem. Phys. 2017, 202, 245–250. [Google Scholar] [CrossRef]
- Liu, W.; Tan, G.; Xue, X.; Dong, G.; Ren, H.; Xia, A. Phase transition and enhanced multiferroic properties of (Sm, Mn and Cr) co-doped BiFeO3 thin films. Ceram. Int. 2014, 40, 12179–12185. [Google Scholar] [CrossRef]
- Tang, Y.; Yin, H.; Yuan, H.; Shuai, H.; Xin, Y. Carbothermal reduction nitridation of slag, glass and minerals: Formation process of SiAlON powders with different morphology. Ceram. Int. 2016, 42, 7499–7505. [Google Scholar] [CrossRef]
- Sang, S.B.; Li, Y.W.; Li, N. Formation mechanism and its influencing factors of the elongated α-Sialon grains. Mater. Rev. 2004, 18, 358–360. [Google Scholar]
- Chen, W.W.; Li, Y.W.; Sun, W.Y.; Yan, D.S. Effect of liquid and AlN-polytypoid on the morphology of α-Sialon. Mater. Sci. Eng. 2000, 18, 221–224. [Google Scholar] [CrossRef]
- Van Rutten, J.W.; Hintzen, H.T.; Metselaar, R. Phase Formation of Ca-a-sialon Reation Sintering. J. Eur. Ceram. Soc. 1996, 16, 995–999. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.; Kim Deug, J. Growth of Elongated Grains in α-SiAlON Ceramics. Mater. Lett. 2001, 47, 329–333. [Google Scholar] [CrossRef]
- Jung, W.S.; Joo, H.U. Catalytic growth of aluminum nitride whiskers by a modified carbothermal reduction and nitridation method. J. Cryst. Growth 2005, 285, 566–571. [Google Scholar] [CrossRef]
- Hotta, M.; Tatami, J.; Komeya, K.; Zhang, C.; Meguro, T.; Terner, M.R.; Cheng, Y.B. Formation Process of Ca-α-SiAlON Hollow Balls Composed of Nanosized Particles by Carbothermal Reduction-Nitridation. J. Am. Ceram. Soc. 2008, 91, 860–864. [Google Scholar] [CrossRef]
- Shuai, H.; Yin, H.F.; Yuan, H.D. Phase composition evolution and viscosity-temperature characteristics of gasification slags at high temperature. Coal Convers. 2015, 38, 44–48. [Google Scholar]
- Shuai, H. Intrinsic Characteristics of Gasification Slags, and the Phase Evolution of Gasification Slags during Carbothermal Reduction-Nitridation Process. Master’s Thesis, Xi’an University of Architecture & Technology, Xi’an, China, 2015. [Google Scholar]
- Jiang, T.; Xue, X.; Duan, P.; Liu, X.; Zhang, S.; Liu, R. Carbothermal reduction–nitridation of titania-bearing blast furnace slag. Ceram. Int. 2008, 34, 1643–1651. [Google Scholar] [CrossRef]
- Deng, C.J. Study on Synthesis Mechanism, Performance and Applications of MgAlON. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 1999. [Google Scholar]


| Material | Gasifier | Trade Names |
|---|---|---|
| HT | HT-L | Luxi Chemical Group Co. Ltd. (Liaocheng, Shandong, China) |
| TE | Texaco | Shaanxi Shenmu Chemical Industry Co.Ltd. (Yulin, Guangxi, China) |
| SH | SHELL | Yiyi Coal Group Kaixiang Chemical Co. Ltd. (Sanmenxia, Henan, China) |
| PRL | - | Zhejiang Qingtian Ye Wax Stone Co.Ltd. (Lishui, Zhejiang, China) |
| Material | Chemical Composition (wt. %) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | Fe2O3 | Na2O | K2O | MgO | TiO2 | C | |
| TE | 5.53 | 31.50 | 11.58 | 3.12 | 0.77 | 1.83 | 0.94 | 0.42 | 37.25 |
| HT | 15.04 | 32.82 | 12.25 | 5.41 | 0.66 | 0.96 | 0.9 | 0.44 | 27.99 |
| SH | 10.35 | 51.53 | 25.05 | 7.49 | 0.58 | 1.03 | 2.51 | 0.88 | 0.71 |
| PRL | - | 67.50 | 21.90 | \0.50 | - | 0.40 | - | 0.30 | - |
| Sample | Quality Percentage/(wt. %) | |||
|---|---|---|---|---|
| Ca-α-SiAlON | β-SiAlON | CaAl2Si2O8 | FeSix | |
| PRL | 61.65 | 38.35 | - | - |
| TE | 99.99 | - | 0.002 | 0.002 |
| Sample | Quality Percentage/(wt. %) | |||||
|---|---|---|---|---|---|---|
| Ca-α-SiAlON | β-SiAlON | CaAl2Si2O8 | Ca2Al2SiO7 | 15R | FeSix | |
| TE | 97.64 | 2.34 | - | - | - | 0.02 |
| HT | 66.48 | - | 33.07 | - | - | 0.45 |
| SH | 78.64 | 15.43 | - | - | 0.70 | 4.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.; Yin, H.; Tang, Y.; Shuai, H.; Xin, Y.; Pu, X. Influence of High-Temperature Liquid on Phase Composition and Morphology of Carbothermal Reduction-Nitridation Products from Coal Gasification Slag. Materials 2020, 13, 1346. https://doi.org/10.3390/ma13061346
Yuan H, Yin H, Tang Y, Shuai H, Xin Y, Pu X. Influence of High-Temperature Liquid on Phase Composition and Morphology of Carbothermal Reduction-Nitridation Products from Coal Gasification Slag. Materials. 2020; 13(6):1346. https://doi.org/10.3390/ma13061346
Chicago/Turabian StyleYuan, Hudie, Hongfeng Yin, Yun Tang, Hang Shuai, Yalou Xin, and Xilou Pu. 2020. "Influence of High-Temperature Liquid on Phase Composition and Morphology of Carbothermal Reduction-Nitridation Products from Coal Gasification Slag" Materials 13, no. 6: 1346. https://doi.org/10.3390/ma13061346




