Silver-Nanoparticles Embedded Pyridine-Cholesterol Xerogels as Highly Efficient Catalysts for 4-nitrophenol Reduction
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of 4-pyridyl Cholesterol (PC) Compound
2.2. Preparation of 4-pyridyl Cholesterol (PC) Xerogels
2.3. Preparation of Silver-Nanocomposites of 4-pyridyl Cholesterol (SNC-PC) Xerogels
3. Characterization Techniques
4. Results and Discussion
4.1. UV-Vis Spectroscopic Analysis
4.2. Functional Group Analysis
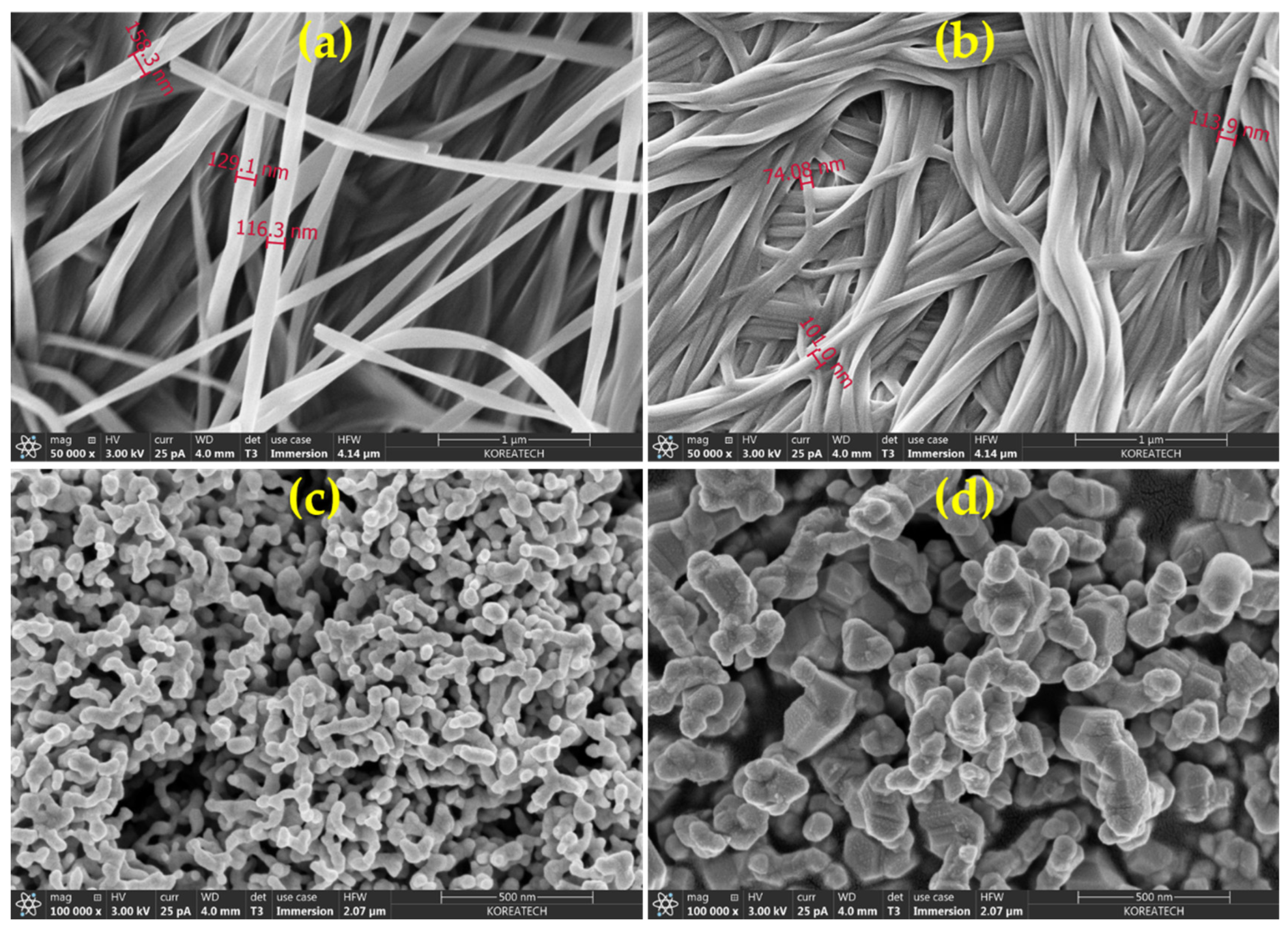
4.3. FESEM and XRD Analysis
4.4. Thermal Properties
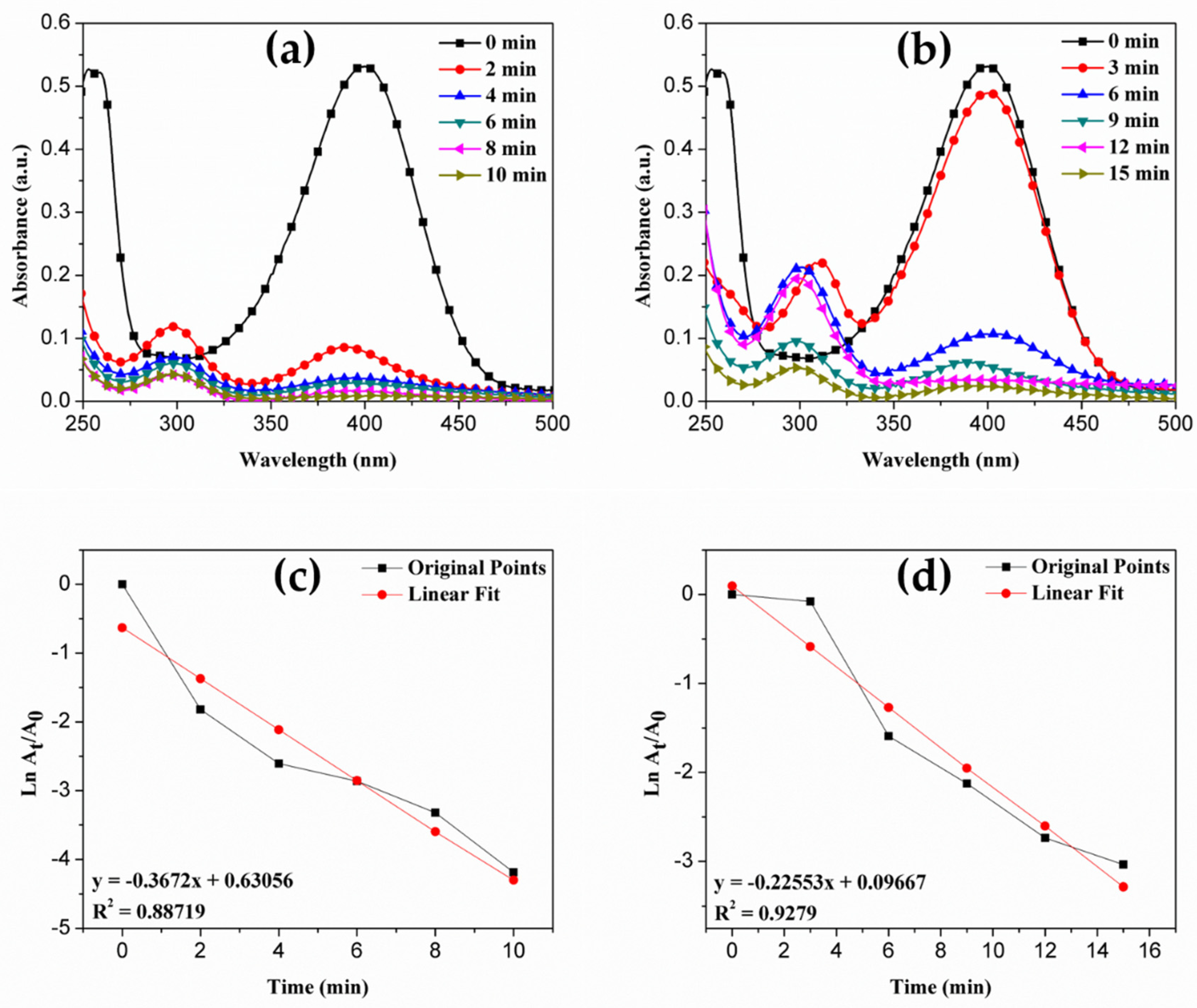
4.5. Catalytic Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dao, A.T.N.; Mott, D.M.; Higashimine, K.; Maenosono, S. Enhanced Electronic Properties of Pt@Ag Heterostructured Nanoparticles. Sensors 2013, 13, 7813–7826. [Google Scholar] [CrossRef]
- Guil-López, R.; Mota, N.; Llorente, J.; Millán, E.; Pawelec, B.; Fierro, J.L.G.; Navarro, R.M. Methanol Synthesis from CO2: A Review of the Latest Developments in Heterogeneous Catalysis. Materials 2019, 12, 3902. [Google Scholar] [CrossRef]
- Shanmugaraj, K.; Bustamante, T.M.; Campos, C.H.; Torres, C.C. Liquid Phase Hydrogenation of Pharmaceutical Interest Nitroarenes over Gold-Supported Alumina Nanowires Catalysts. Materials 2020, 13, 925. [Google Scholar] [CrossRef]
- El-Ansary, A.; Faddah, L.M.; Navarro, R.M. Nanoparticles as biochemical sensors. Nanotechnol. Sci. Appl. 2010, 3, 65–76. [Google Scholar] [CrossRef]
- Wang, L.; Ma, W.; Xu, L.; Chen, W.; Zhu, Y.; Xu, C.; Kotov, N.A. Nanoparticle-based environmental sensors. Mater. Sci. Eng. R 2010, 70, 265–274. [Google Scholar] [CrossRef]
- Navalón, S.; García, H. Nanoparticles for Catalysis. Nanomaterials 2016, 6, 123. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; da Silva, A.G.M.; Camargo, P.H.C. Nanocatalysis by noble metal nanoparticles: Controlled synthesis for the optimization and understanding of activities. J. Mater. Chem. A 2019, 7, 5857. [Google Scholar] [CrossRef]
- Sambur, J.B.; Chen, P. Approaches to Single-Nanoparticle Catalysis. Ann. Rev. Phys. Chem. 2014, 65, 395–422. [Google Scholar] [CrossRef]
- Lim, T.-C.; Ramakrishna, S. A Conceptual Review of Nanosensors. Z. für Nat. A 2006, 61, 402–412. [Google Scholar] [CrossRef]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H. Nanotube Molecular Wires as Chemical Sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Goebi, J.; Yin, Y. Templated synthesis of nanostructured materials. Chem. Soc. Rev. 2013, 42, 2610–2653. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Angelatos, A.S.; Caruso, F. Template Synthesis of Nanostructured Materials via Layer-by-Layer Assembly. Chem. Mater. 2008, 20, 848–858. [Google Scholar] [CrossRef]
- Bera, D.; Kuiry, S.C.; Seal, S. Synthesis of nanostructured materials using template-assisted electrodeposition. JOM 2004, 56, 49–53. [Google Scholar] [CrossRef]
- Xie, Y.; Kocaefe, D.; Chen, C.; Kocaefe, Y. Review of Research on Template Methods in Preparation of Nanomaterials. J. Nanomater. 2016, 2016, 2302595. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Skilling, K.J.; Citossi, F.; Bradshaw, T.D.; Ashford, M.; Kellam, B.; Marlow, M. Insights into low molecular mass organic gelators: A focus on drug delivery and tissue engineering applications. Soft Matter 2014, 10, 237–256. [Google Scholar] [CrossRef]
- Abdallah, D.J.; Weiss, R.G. Organogels and Low Molecular Mass Organic Gelators. Adv. Mater. 2000, 12, 1237–1247. [Google Scholar] [CrossRef]
- George, M.; Weiss, R.G. Molecular Organogels. Soft Matter Comprised of Low-Molecular-Mass Organic Gelators and Organic Liquids. Acc. Chem. Res. 2006, 39, 489–497. [Google Scholar] [CrossRef]
- Draper, E.R.; Adams, D.J. Low-Molecular-Weight Gels: The State of the Art. Chem 2017, 3, 390–410. [Google Scholar] [CrossRef]
- Hanabusa, K.; Suzuki, M. Development of low-molecular-weight gelators and polymer-based gelators. Polym. J. 2014, 46, 776–782. [Google Scholar] [CrossRef]
- Dastidar, P. Designing Supramolecular Gelators: Challenges, Frustrations, and Hopes. Gels 2019, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Charlesby, A. Gel formation and molecular weight distribution in long-chain polymers. Proc. R. Soc. A 1954, 222, 542–557. [Google Scholar] [CrossRef]
- Garrec, D.A.; Norton, I.T. Understanding fluid gel formation and properties. J. Food Eng. 2011, 112, 175–182. [Google Scholar] [CrossRef]
- Hirst, A.R.; Coates, I.A.; Boucheteau, T.R.; Miravet, J.F.; Escuder, B.; Castelletto, V.; Hamley, I.W.; Smith, D.K. Low-Molecular-Weight Gelators: Elucidating the Principles of Gelation Based on Gelator Solubility and a Cooperative Self-Assembly Model. J. Am. Chem. Soc. 2008, 130, 9113–9121. [Google Scholar] [CrossRef] [PubMed]
- Mangunuru, H.P.R.; Yang, H.; Wang, G. Synthesis of peptoid based small molecular gelators by a multiple component reaction. Chem. Commun. 2013, 49, 4489–4491. [Google Scholar] [CrossRef]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef]
- Wang, G.; Yang, H.; Cheuk, S.; Coleman, S. Synthesis and self-assembly of 1-deoxyglucose derivatives as low molecular weight organogelators. Beilstein J. Org. Chem. 2011, 7, 234–242. [Google Scholar] [CrossRef]
- Panja, A.; Ghosh, K. Cholesterol-based simple supramolecular gelators: An approach to selective sensing of CN− ion with application in dye adsorption. Supramol. Chem. 2019, 31, 239–250. [Google Scholar] [CrossRef]
- Zinic, M.; Vögtle, F.; Fages, F. Cholesterol-based gelators. Top. Curr. Chem. 2005, 256, 39–76. [Google Scholar] [CrossRef]
- Jeong, S.W.; Murata, K.; Shinkai, S. Dual-component cholesterol-based gelators bearing complementary hydrogen-bonding sites. Supramol. Sci. 1996, 3, 83–86. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, D.; Zhang, G.; Zhu, D. New Cholesterol-Based Gelators with Maleimide Unit and the Relevant Michael Adducts: Chemoresponsive Organogels. Langmuir 2009, 25, 11436–11441. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Liu, J.; Jing, P.; Xu, C.; Wu, J.; Gao, D.; Fang, Y. Cholesterol-based low-molecular mass gelators towards smart ionogels. Soft Matter 2012, 8, 11697–11703. [Google Scholar] [CrossRef]
- Kavano, S.-I.; Fujita, N.; van Bommel, K.J.C.; Shinkai, S. Pyridine-containing Cholesterols as Versatile Gelators of Organic Solvents and the Subtle Influence of Ag(I) on the Gel Stability. Chem Lett. 2003, 32, 12–13. [Google Scholar] [CrossRef]
- Malik, S.; Kavano, S.-I.; Fujita, N.; Shinkai, S. Pyridine-containing versatile gelators for post-modification of gel tissues toward construction of novel porphyrin nanotubes. Tetrahedron 2007, 63, 7326–7333. [Google Scholar] [CrossRef]
- Liu, M.; Ouyang, G.; Niu, D.; Sang, Y. Supramolecular gelatons: Towards the design of molecular gels. Org. Chem. Front. 2018, 5, 2885–2900. [Google Scholar] [CrossRef]
- Ai, L.; Jiang, J. Catalytic reduction of 4-nitrophenol by silver nanoparticles stabilized on environmentally benign macroscopic biopolymer hydrogel. Bioresour. Technol. 2013, 132, 374–377. [Google Scholar] [CrossRef]
- Gong, W.; Wu, Q.; Jiang, G.; Li, G. Ultrafine silver nanoparticles supported on a covalent carbazole framework as high-efficiency nanocatalysts for nitrophenol reduction. J. Mater. Chem. A 2019, 7, 13449–13454. [Google Scholar] [CrossRef]
- Geng, Q.; Du, J. Reduction of 4-nitrophenol catalyzed by silver nanoparticles supported on polymer micelles and vesicles. RSC Adv. 2014, 4, 16425. [Google Scholar] [CrossRef]
- Palem, R.R.; Ganesh, S.D.; Saha, N.; Kronek, J.; Sáha, P. ‘Green’ synthesis of silver polymer Nanocomposites of poly(2-isopropenyl-2-oxazoline-co-N vinylpyrrolidone) and its catalytic activity. J. Polym. Res. 2018, 25, 152. [Google Scholar] [CrossRef]
- Shimoga, G.; Shin, E.-J.; Kim, S.-Y. Thermal, dielectric and catalytic behavior of palladium doped PVC films. Polímeros 2019, 29, e2019028. [Google Scholar] [CrossRef]
- Shimoga, G.; Shin, E.-J.; Kim, S.-Y. Silver nanoparticles incorporated PVC films: Evaluation of structural, thermal, dielectric and catalytic properties. Polímeros 2019, 29, e2019032. [Google Scholar] [CrossRef]
- Vinogradov, A.P.; Dorofeenko, A.V.; Pukhov, A.A.; Lisyansky, A.A. Exciting surface plasmon polaritons in the Kretschmann configuration by a light beam. Phys. Rev. B 2018, 97, 235407. [Google Scholar] [CrossRef]
- Oliveira, M.B.; Calixto, G.; Graminha, M.; Cerecetto, H.; González, M.; Chorilli, M. Development, Characterization, and In Vitro Biological Performance of Fluconazole-Loaded Microemulsions for the Topical Treatment of Cutaneous Leishmaniasis. BioMed Res. Int. 2015, 2015, 396894. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.V.; Cabau, L.; Koukaras, E.N.; Sharma, A.; Sharma, G.D.; Palomares, E. A–π–D–π–A based porphyrin for solution processed small molecule bulk heterojunction solar cells. J. Mater. Chem. A 2015, 3, 16287–16301. [Google Scholar] [CrossRef]
- Broido, A. A simple, sensitive graphical method of treating thermogravimetric analysis data. J. Polym. Sci. Part A-2 Polym. Phys. 1969, 7, 1761–1773. [Google Scholar] [CrossRef]
- Zhao, P.; Feng, X.; Huang, D.; Yang, G.; Astruc, D. Basic concepts and recent advances in nitrophenol reduction by gold- and other transition metal nanoparticles. Coord. Chem. Rev. 2015, 287, 114–136. [Google Scholar] [CrossRef]
- Verma, S.; Baig, R.B.N.; Nadagouda, M.N.; Varma, R.S. Hydroxylation of Benzene via C–H Activation Using Bimetallic CuAg@g-C3N4. ACS Sustain. Chem. Eng. 2017, 5, 3637–3640. [Google Scholar] [CrossRef]
- Din, M.I.; Khalid, R.; Hussain, Z.; Hussain, T.; Mujahid, A.; Najeeb, J.; Izhar, F. Nanocatalytic Assemblies for Catalytic Reduction of Nitrophenols: A Critical Review. Crit. Rev. Anal. Chem. 2019, 1–17. [Google Scholar] [CrossRef]
- Esumi, K.; Isono, R.; Yoshimura, T. Preparation of PAMAM− and PPI−Metal (Silver, Platinum, and Palladium) Nanocomposites and Their Catalytic Activities for Reduction of 4-Nitrophenol. Langmuir 2004, 20, 237–243. [Google Scholar] [CrossRef]
- Chang, M.; Kim, T.; Park, H.-W.; Kang, M.; Reichmanis, E.; Yoon, H. Imparting chemical stability in nanoparticulate silver via a conjugated polymer casing approach. ACS Appl. Mater. Interfaces 2012, 4, 4357–4365. [Google Scholar] [CrossRef] [PubMed]
- Murugan, E.; Jebaranjitham, J.N. Synthesis and characterization of silver nanoparticles supported on surface-modified poly(N-vinylimidazale) as catalysts for the reduction of 4-nitrophenol. J. Mol. Catal. A Chem. 2012, 365, 128–135. [Google Scholar] [CrossRef]
- Safari, J.; Najafabadi, A.E.; Zarnegar, Z.; Masoule, S.F. Catalytic performance in 4-nitrophenol reduction by Ag nanoparticles stabilized on biodegradable amphiphilic copolymers. Green Chem. Lett. Rev. 2016, 9, 20–26. [Google Scholar] [CrossRef]






| Compound | Ea (kJ/mol) × 10-3 | lnA | ∆H (kJ/mol) | ∆S (kJ/K) | ∆G (kJ/mol) |
|---|---|---|---|---|---|
| PC | 26.045 | −5.542 | −4.780 | −162.464 | 93.900 |
| PC-X1 | 24.533 | −5.599 | −4.781 | −162.479 | 97.172 |
| PC-X2 | 25.389 | −4.269 | −4.768 | −162.118 | 93.699 |
| Compound | Ea (kJ/mol) × 10-3 | lnA | ∆H (kJ/mol) | ∆S (kJ/K) | ∆G (kJ/mol) |
|---|---|---|---|---|---|
| SNC-PC-X1 | 3.157 | −9.205 | −4.222 | −164.186 | 83.444 |
| SNC-PC-X2 | 3.913 | −8.943 | −4.221 | −164.166 | 83.435 |
| Compound | Ea (kJ/mol) × 10-3 | lnA | ∆H (kJ/mol) | ∆S (kJ/K) | ∆G (kJ/mol) |
|---|---|---|---|---|---|
| SNC-PC-X1 | 23.260 | −5.717 | −4.782 | −161.894 | 93.570 |
| SNC-PC-X2 | 23.160 | −5.714 | −4.782 | −161.527 | 93.358 |
| Time (min) | Percentage Conversion of 4-NP to 4-AP |
|---|---|
| 0 | 0 |
| 2 | 83.80 |
| 4 | 92.61 |
| 6 | 94.29 |
| 8 | 96.38 |
| 10 | 98.48 |
| Time (min) | Percentage Conversion of 4-NP to 4-AP |
|---|---|
| 0 | 0 |
| 3 | 7.54 |
| 6 | 79.67 |
| 9 | 88.06 |
| 12 | 93.51 |
| 15 | 95.19 |
| Recycle Attempts | Catalyst SNC-PC-X1 | Catalyst SNC-PC-X2 |
|---|---|---|
| 1 | 95.21 | 91.62 |
| 2 | 93.15 | 87.49 |
| 3 | 86.13 | 85.69 |
| 4 | 82.48 | 81.93 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimoga, G.; Shin, E.-J.; Kim, S.-Y. Silver-Nanoparticles Embedded Pyridine-Cholesterol Xerogels as Highly Efficient Catalysts for 4-nitrophenol Reduction. Materials 2020, 13, 1486. https://doi.org/10.3390/ma13071486
Shimoga G, Shin E-J, Kim S-Y. Silver-Nanoparticles Embedded Pyridine-Cholesterol Xerogels as Highly Efficient Catalysts for 4-nitrophenol Reduction. Materials. 2020; 13(7):1486. https://doi.org/10.3390/ma13071486
Chicago/Turabian StyleShimoga, Ganesh, Eun-Jae Shin, and Sang-Youn Kim. 2020. "Silver-Nanoparticles Embedded Pyridine-Cholesterol Xerogels as Highly Efficient Catalysts for 4-nitrophenol Reduction" Materials 13, no. 7: 1486. https://doi.org/10.3390/ma13071486
APA StyleShimoga, G., Shin, E.-J., & Kim, S.-Y. (2020). Silver-Nanoparticles Embedded Pyridine-Cholesterol Xerogels as Highly Efficient Catalysts for 4-nitrophenol Reduction. Materials, 13(7), 1486. https://doi.org/10.3390/ma13071486








