Simultaneous Removal of Pb2+ and Zn2+ Heavy Metals Using Fly Ash Na-X Zeolite and Its Carbon Na-X(C) Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbent Preparation
2.2. Solids Characteristics
2.3. Adsorption/Desorption Measurements
2.4. Electrokinetic Parameters Determination
3. Results and Discussion
3.1. Physicochemical Properties of Adsorbents
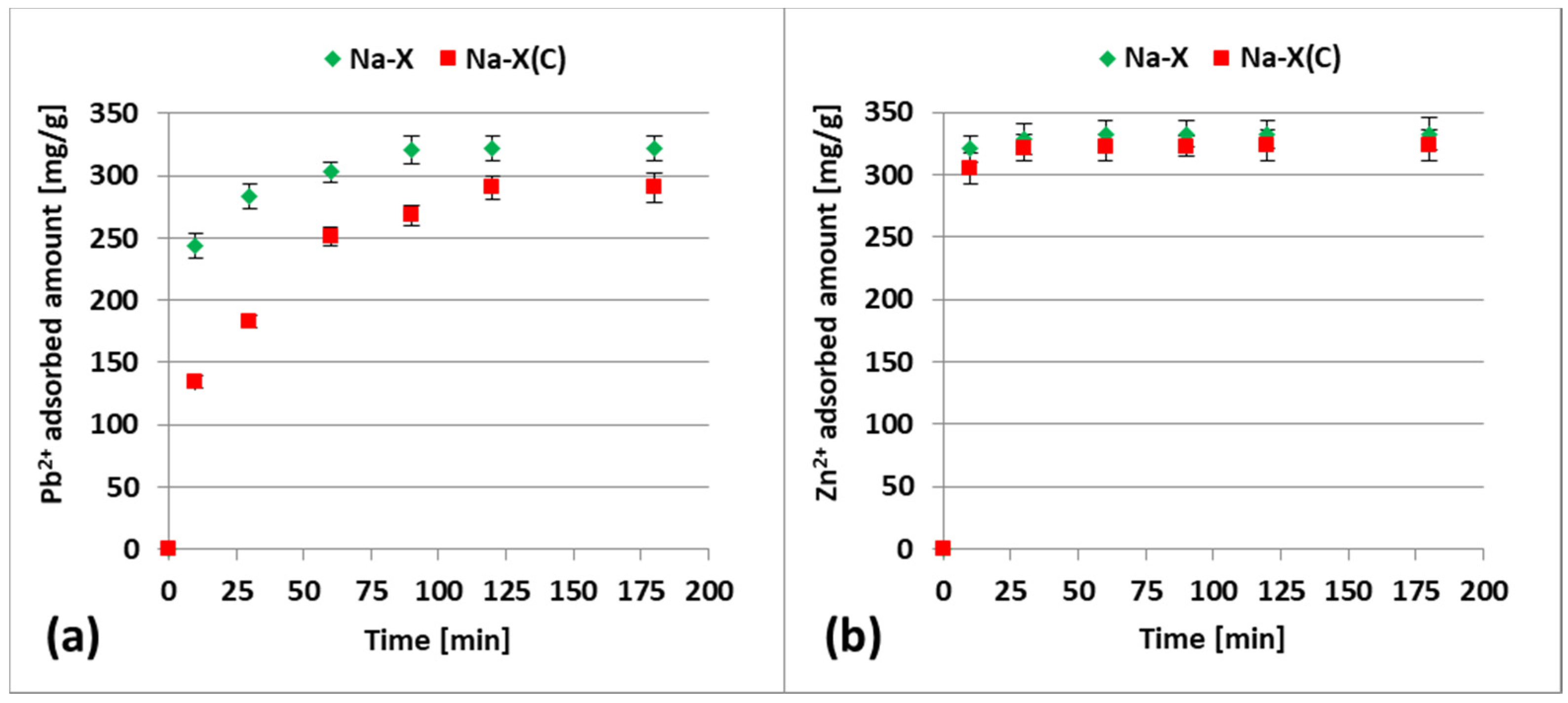
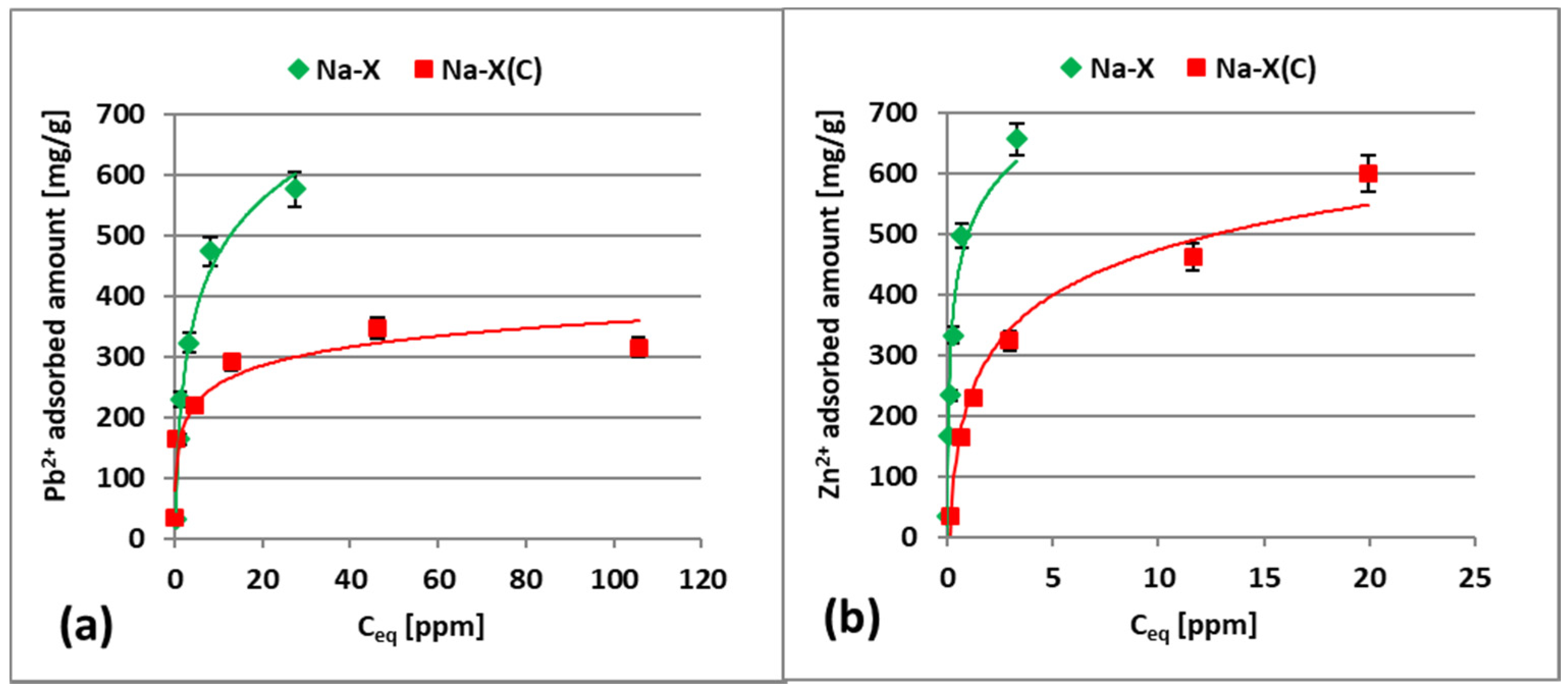
3.2. Zn2+ and Pb2+ Adsorption on Zeolite and Zeolite–Carbon Composite in the Single Systems
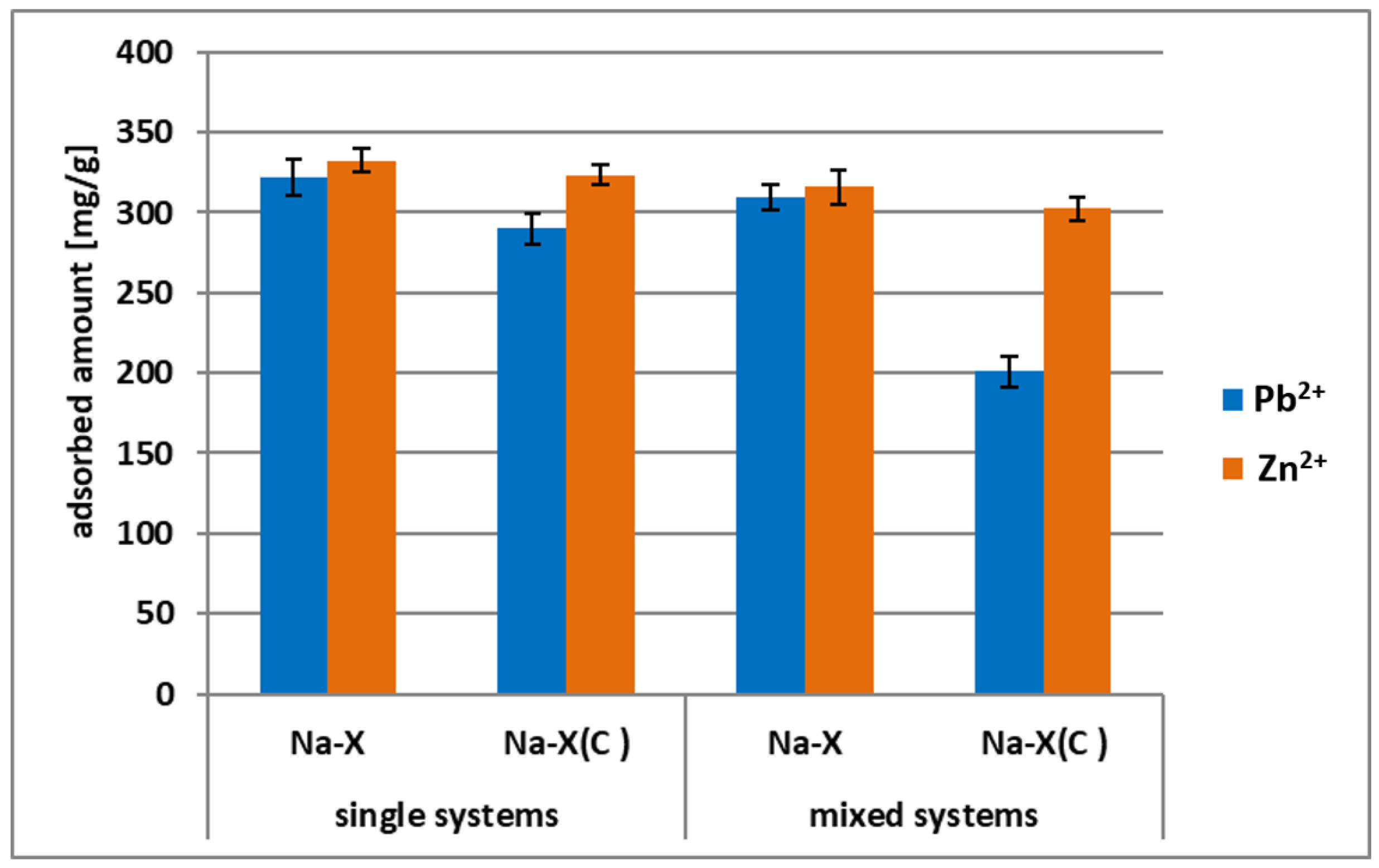
3.3. Zn2+ and Pb2+ Adsorption on Zeolite and its Composite in the Mixed Systems
3.4. Impact of Zn2+/Pb2+ Adsorption on Surface Charge Density of Na-X and Na-X(C) Particles
3.5. Impact of Zn2+/Pb2+ Adsorption on Zeta Potential of Na-X and Na-X(C) Particles
3.6. Desorption of Zn2+ and Pb2+ from Zeolite and Its Composite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gottardi, G.; Galli, E. Natural Zeolites; Springer: Berlin/Heidelberg, Germany, 1985; pp. 1–34. [Google Scholar]
- Pleśniak, J.; Trzop, W. Everyday life with zeolite. Analit 2016, 2, 146–151. (In Polish) [Google Scholar]
- Szala, B.; Turek, P.; Jeleń, A.; Bajda, T. Synthesis and sorption properties of organo-zeolites. ZN UZ IŚ 2013, 30, 5–12. (In Polish) [Google Scholar]
- Biegańska, M. Use of the Bark of the Braided Willow Salix americana for Adsorption of Heavy Metals. Ph.D. Thesis, Poznań University of Economics and Business, Poznań, Poland, 2011; pp. 64–66. (In Polish). [Google Scholar]
- Inglezakis, V.J.; Zorpas, A.A. Handbook of Natural Zeolites; Bentham Science: Sharjah, United Arab Emirates, 2012; pp. 3–133. [Google Scholar]
- Muir, B. Production and Utilization of Organo-Zeolites as Petroleum Sorbents. Ph.D. Thesis, AGH University of Science and Technology, Kraków, Poland, 2016; pp. 12–32. (In Polish). [Google Scholar]
- Shahrokhi-Shahraki, R.; Benally, C.; El-Din, M.G.; Park, J. High efficiency removal of heavy metals using tire derived activated carbon vs. commercial activated carbon: Insights into the adsorption mechanisms. Chemosphere 2021, 264, 128455. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ogata, F.; Nakamura, T.; Kawasaki, N. Synthesis of novel zeolites produced from fly ash by hydrothermal treatment in alkaline solution and its evaluation as an adsorbent for heavy metal removal. J. Environ. Chem. Eng. 2020, 8, 103687. [Google Scholar] [CrossRef]
- Visa, M. Synthesis and characterization of new zeolite materials obtained from fly ash for heavy metals removal in advanced wastewater treatment. Powder Technol. 2016, 294, 338. [Google Scholar] [CrossRef]
- Cheng, T.; Chen, C.; Tang, R.; Han, C.H.; Tian, Y. Competitive adsorption of Cu, Ni, Pb, and Cd from aqueous solution onto fly ash-based linde F(K) Zeolite. Iran. J. Chem. Chem. Eng. 2018, 37, 61. [Google Scholar]
- Joseph, I.V.; Tosheva, L.; Doyle, A.M. Simultaneous removal of Cd(II), Co(II), Cu(II), Pb(II), and Zn(II) ions from aqueous solutions via adsorption on FAU-type zeolites prepared from coal fly ash. J. Environ. Chem. Eng. 2020, 8, 103895. [Google Scholar] [CrossRef]
- Kragović, M.; Pašalić, S.; Marković, M.; Petrović, M.; Nedeljković, B.; Momčilović, M.; Stojmenović, M. Natural and Modified Zeolite—Alginate Composites. Application for Removal of Heavy Metal Cations from Contaminated Water Solutions. Minerals 2018, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.A.; Kamala-Kannan, S.; Lee, K.-J.; Park, Y.-J.; Shea, P.J.; Lee, W.-H.; Kim, H.-M.; Oh, B.-T. Removal of Pb(II) from aqueous solution by a zeolite–nanoscale zero-valent iron composite. Chem. Eng. J. 2013, 217, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wang, L.; Meng, J.; Liu, X.; Xu, J.; Wang, F.; Brookes, P. Zeolite-supported nanoscale zero-valent iron: New findings on simultaneous adsorption of Cd(II), Pb(II), and As(III) in aqueous solution and soil. J. Hazard. Mater. 2018, 344, 1–11. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Lv, Z.; Xie, Y.; Shu, J.; Alsaedi, A.; Hayat, T.; Chen, C. Exploration of the adsorption performance and mechanism of zeolitic imidazolate framework-8@graphene oxide for Pb(II) and 1-naphthylamine from aqueous solution. J. Colloid Interf. Sci. 2019, 542, 410–420. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Abou El-Reash, Y.G.; Youssef, H.M.; Kotp, Y.H.; Hegazey, R.M. Utilization of rice husk and waste aluminum cans for the synthesis of some nanosized zeolite, zeolite/zeolite, and geopolymer/zeolite products for the efficient removal of Co(II), Cu(II), and Zn(II) ions from aqueous media. J. Hazard. Mater. 2020, 401, 123813. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, K.; Cai, J.; Hou, S.; Wang, Y.; Jiang, P.; Liang, M. Silica Oxide Encapsulated Natural Zeolite for High Efficiency Removal of Low Concentration Heavy Metals in Water. Colloids Surf. A 2019, 561, 388–394. [Google Scholar] [CrossRef]
- Panek, R.; Madej, J.; Bandura, L.; Słowik, G. Recycling of Waste Solution after Hydrothermal Conversion of Fly Ash on a Semi-Technical Scale for Zeolite Synthesis. Materials 2021, 14, 1413. [Google Scholar] [CrossRef]
- Bandura, L.; Panek, R.; Madej, J.; Franus, W. Synthesis of zeolite-carbon composites using high-carbon fly ash and their adsorption abilities towards petroleum substances. Fuel 2021, 283, 119173. [Google Scholar] [CrossRef]
- Bandura, L.; Franus, M.; Panek, R.; Woszuk, A.; Franus, W. Characteristics of zeolites and their application as adsorbents of petroleum substances. Przem. Chem. 2015, 93–94, 323–327. (In Polish) [Google Scholar]
- Lu, C.A.; Zhang, J.F.; Jiang, H.M.; Yang, J.C.; Zhang, J.T.; Wang, J.Z.; Shan, H.X. Assessment of soil contamination with Cd, Pb and Zn and source identification in the area around the Huludao Zinc Plant. J. Hazard. Mater. 2010, 182, 743–748. [Google Scholar] [CrossRef]
- Fajardo, C.; Costa, G.; Nande, M.; Botías, P.; García-Cantalejo, J.; Martín, M. Pb, Cd, and Zn soil contamination: Monitoring functional and structural impacts on the microbiome. Appl. Soil Ecol. 2019, 135, 56–64. [Google Scholar] [CrossRef]
- Rengel, Z.; Graham, R.D. Importance of seed Zn content for wheat growth on Zn-deficient soil. I. Vegetative growth. Plant Soil 1995, 173, 259–266. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data For Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Jean Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 9–10. [Google Scholar] [CrossRef] [Green Version]
- Ościk, J. Adsorption; UMCS: Lublin, Poland, 1969; pp. 91–124. (In Polish) [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Application of kinetics models to the sorption of copper(II) onto peat. Adsorpt. Sci. Technol. 2002, 20, 797–815. [Google Scholar] [CrossRef]
- Janusz, W. Electrical double layer at the metal oxide–electrolyte interface. In Interfacial Forces and Fields: Theory and Applications; Marcel Dekker Inc.: New York, NY, USA, 1999; Volume 85, Chapter 4. [Google Scholar]
- Oshima, H. A simple expansion for Henry’s function for the retardation effect in electrophoresis of spherical colloidal particles. J. Colloid Interface Sci. 1994, 168, 269–271. [Google Scholar] [CrossRef]
- Ansari, M.; Aroujalian, A.; Raisi, A.; Dabir, B.; Fathizadeh, M. Preparation and Characterization of Nano-NaX Zeolite by Microwave Assisted Hydrothermal Method. Adv. Powder Technol. 2014, 25, 722–727. [Google Scholar] [CrossRef]
- Bandura, L.; Franus, M.; Józefaciuk, G.; Franus, W. Synthetic Zeolites from Fly Ash as Effective Mineral Sorbents for Land-Based Petroleum Spills Cleanup. Fuel 2015, 147, 100–107. [Google Scholar] [CrossRef]
- Muriithi, G.N.; Petrik, L.F.; Doucet, F.J. Synthesis, Characterisation and CO2 Adsorption Potential of NaA and NaX Zeolites and Hydrotalcite Obtained from the Same Coal Fly Ash. J. CO2 Util. 2020, 36, 220–230. [Google Scholar] [CrossRef]
- Boycheva, S.; Marinov, I.; Miteva, S.; Zgureva, D. Conversion of coal fly ash into nanozeolite Na-X by applying ultrasound assisted hydrothermal and fusion-hydrothermal alkaline activation. Sustain. Chem. Pharm. 2020, 15, 100217. [Google Scholar] [CrossRef]
- Babajide, O.; Musyoka, N.; Petrik, L.; Ameer, F. Novel Zeolite Na-X Synthesized from Fly Ash as a Heterogeneous Catalyst in Biodiesel Production. Catal. Today 2012, 190, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Kunecki, P.; Panek, R.; Wdowin, M.; Bień, T.; Franus, W. Influence of the Fly Ash Fraction after Grinding Process on the Hydrothermal Synthesis Efficiency of Na-A, Na-P1, Na-X and Sodalite Zeolite Types. Int. J. Coal Sci. Technol. 2021, 8, 291–311. [Google Scholar] [CrossRef]
- Zinal Abidin, A.; Abu Bakar, N.H.H.; Ng, E.P.; Tan, W.L. Rapid degragation of methyl orange by Ag doped zeolite X in the presence of borohydrite. J. Taibah Univ. Sci. 2017, 11, 1070–1079. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second-order model for sorption process. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Jovanovic, M.; Rajic, N.; Obradovic, B. Novel kinetic model of the removal of divalent heavy metal ions from aqueous solutions by natural clinoptilolite. J. Hazard. Mater. 2012, 233–234, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Fijałkowska, G.; Szewczuk-Karpisz, K.; Wiśniewska, M. Anionic polyacrylamide as a substance strengthening the Pb(II) immobilization on the kaolinite surface. Int. J. Environ. Sci. Technol. 2020, 17, 1101–1112. [Google Scholar] [CrossRef] [Green Version]
- Szewczuk-Karpisz, K.; Fijałkowska, G.; Wiśniewska, M.; Wójcik, G. Chromium(VI) reduction and accumulation on the kaolinite surface in the presence of cationic soil flocculant. J. Soil Sediment. 2020, 20, 3688–3697. [Google Scholar] [CrossRef]
- Szewczuk-Karpisz, K.; Tomczyk, A.; Komaniecka, I.; Choma, A.; Adamczuk, A.; Sofińska-Chmiel, W. Impact of Sinorhizobium meliloti exopolysaccharide on adsorption and aggregation in the copper(II) ions/supporting electrolyte/kaolinite system. Materials 2021, 14, 1950. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P.; Szewczuk-Karpisz, K. Comparison of monovalent and divalent removal from aqueous solutions using agricultural waste biochars prepared at different temperatures—Experimental and model study. Int. J. Mol. Sci. 2020, 21, 5851. [Google Scholar] [CrossRef]
- Szewczuk-Karpisz, K.; Nowicki, P.; Sokołowska, Z.; Pietrzak, R. Hay-based activated biochars obtained using two different heating methods as effective low-cost sorbents: Solid surface characteristics, adsorptive properties and aggregation in the mixed Cu(II)/PAM system. Chemosphere 2020, 250, 126312. [Google Scholar] [CrossRef]
- Płaziński, W.; Rudziński, W. Adsorption kinetics at solid/solution interfaces. The meaning of the pseudo-first- and pseudo-second-order equations. Wiadomości Chem. 2011, 65, 1055–1067. [Google Scholar]
- Qiu, H.; Pan, B.-C.; Zhang, Q.-J.; Zhang, W.-M.; Zhang, Q.-X. Critical review in adsorption kinetic models. J. Zhejiang Univ. Sci. 2009, 10, 716–724. [Google Scholar] [CrossRef]
- Ho, Y. The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Breviglieri, S.T.; Cavalheiro, É.T.G.; Chierice, G.O. Correlation between ionic radius and thermal decomposition of Fe(II), Co(II), Ni(II), Cu(II) and Zn(II) diethanoldithiocarbamates. Thermochim. Acta 2000, 356, 79–84. [Google Scholar] [CrossRef]
- Lee, J.K.W.; Williams, I.S.; Ellis, D.J. Pb, U and Th diffusion in natural zircon. Nature 1997, 390, 159–162. [Google Scholar] [CrossRef]
- Nibou, D.; Mekatel, H.; Amokrane, S.; Barkat, M.; Trari, M. Adsorption of Zn2+ ions onto NaA and Na-X zeolites: Kinetic, equilibrium and thermodynamic studies. J. Hazard. Mater. 2010, 173, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, M.; Fijałkowska, G.; Szewczuk-Karpisz, K.; Teresa, U.; Nosal-Wiercińska, A.; Wójcik, G. Comparison of adsorption affinity of anionic and cationic polyacrylamides for montmorillonite surface in the presence of chromium(VI) ions. Adsorption 2019, 25, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Fijałkowska, G.; Szewczuk-Karpisz, K.; Wiśniewska, M. Chromium(VI) and lead(II) accumulation at the montmorillonite/aqueous solution interface in the presence of polyacrylamide containing quaternary amine groups. J. Mol. Liq. 2019, 293, 1–8. [Google Scholar] [CrossRef]
- Fijałkowska, G.; Wiśniewska, M.; Szewczuk-Karpisz, K. The structure of electrical double layer formed on the kaolinite surface in the mixed system of cationic polyacrylamide and lead(II) ions. Physicochem. Prob. Min. Process. 2019, 55, 1339–1349. [Google Scholar]
- Fijałkowska, G.; Szewczuk-Karpisz, K.; Wiśniewska, M. Anionic polyacrylamide influence on the lead(II) ion accumulation in soil—the study on montmorillonite. J. Environ. Health Sci. Eng. 2020, 18, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Szewczuk-Karpisz, K.; Wiśniewska, M.; Medykowska, M.; Galaburda, M.V.; Bogatyrov, V.M.; Oranska, O.I.; Błachnio, M.; Oleszczuk, P. Simultaneous adsorption of Cu(II) ions and poly(acrylic acid) on the hybrid carbon-mineral nanocomposites with metallic elements. J. Hazard. Mat. 2021, 412, 125138. [Google Scholar] [CrossRef]
- Szewczuk-Karpisz, K.; Wiśniewska, M.; Medykowska, M.; Bogatyrov, V.; Sokołowska, Z. Adsorption layer structure on the surface of carbon-silica composite in the presence of proteins of different internal stability and Cu(II) ions—the effect on solid aggregation. J. Mol. Liq. 2020, 309, 113072. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Fijałkowska, G.; Nosal-Wiercińska, A.; Franus, M.; Panek, R. Adsorption mechanism of poly(vinyl alcohol) on the surfaces of synthetic zeolites: Sodalite, Na-P1 and Na-A. Adsorption 2019, 25, 567–574. [Google Scholar] [CrossRef] [Green Version]
- Wiśniewska, M.; Urban, T.; Chibowski, S.; Fijałkowska, G.; Medykowska, M.; Nosal-Wiercińska, A.; Franus, W.; Panek, R.; Szewczuk-Karpisz, K. Investigation of adsorption mechanism of phosphate(V) ions on the nanostructured Na-A zeolite surface modified with ionic polyacrylamide with regard to their removal from aqueous solution. Appl. Nanosci. 2020, 10, 4475–4485. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Franus, W.; Fijałkowska, G.; Ostolska, I.; Wójcik, G.; Nosal-Wiercińska, A.; Gościańska, J. Adsorption and electrokinetic studies of sodalite/lithium/poly(acrylic acid) aqueous system. Physicochem. Probl. Miner. Process. 2020, 56, 158–166. [Google Scholar] [CrossRef]
- Lament, K. Study on the Mechanism of Adsorption of Ca2+ and Fe2+ Cations on Metal Oxides Using an Ion-Selective Electrode and a Combined Platinum Electrode. Ph.D. Thesis, Maria Curie-Skłodowska University, Lublin, Poland, 2021; pp. 97–102. (In Polish). [Google Scholar]
- Riddick, T.M. Control of Colloid Stability through Zeta Potentia; Livingston Pub. Co. Edition: Wynnewood, PA, USA, 1968. [Google Scholar]
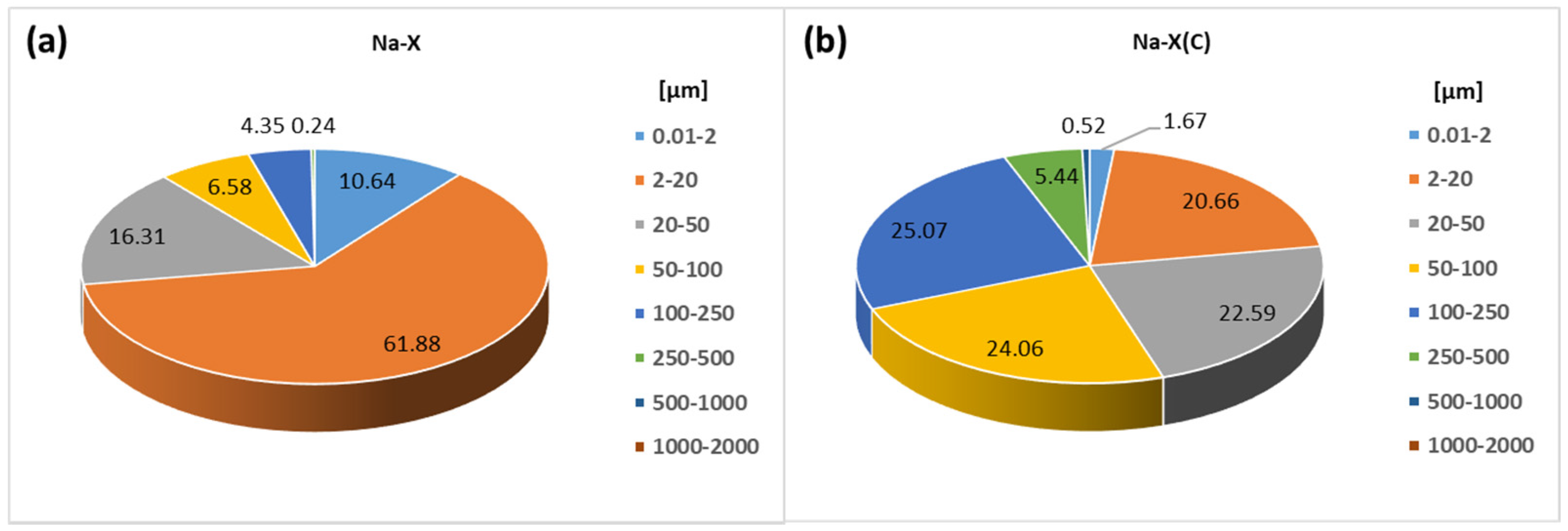
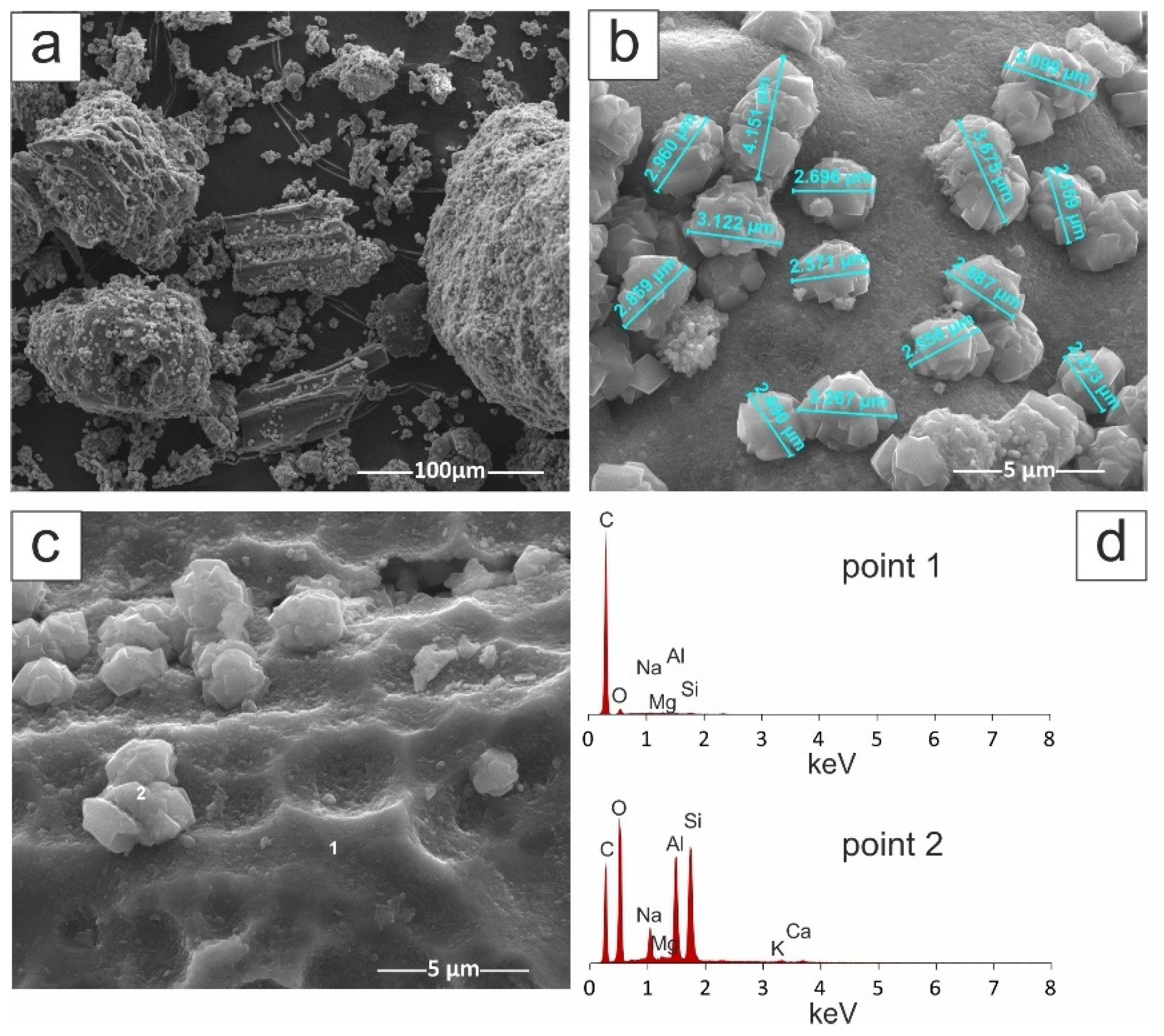

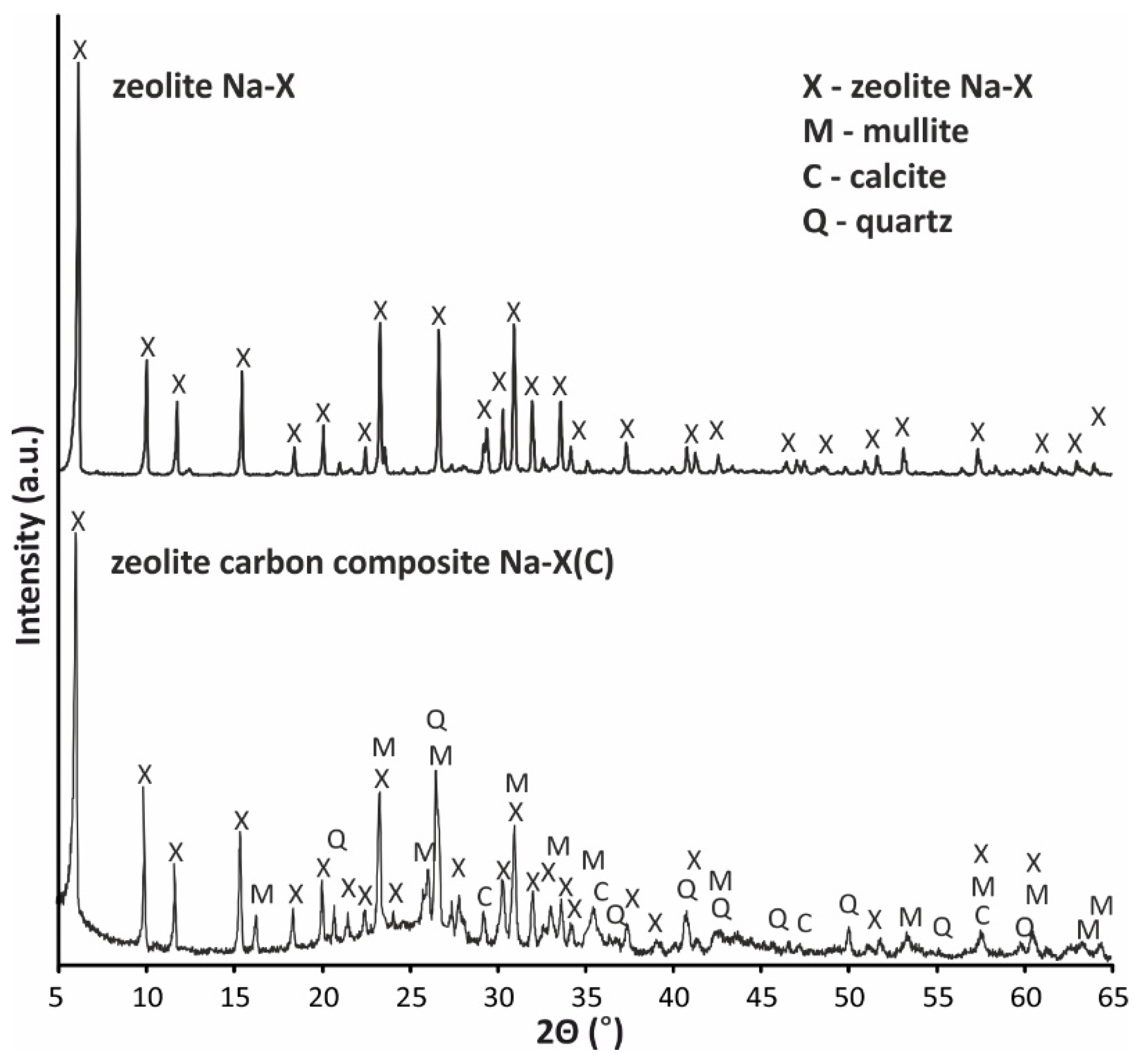
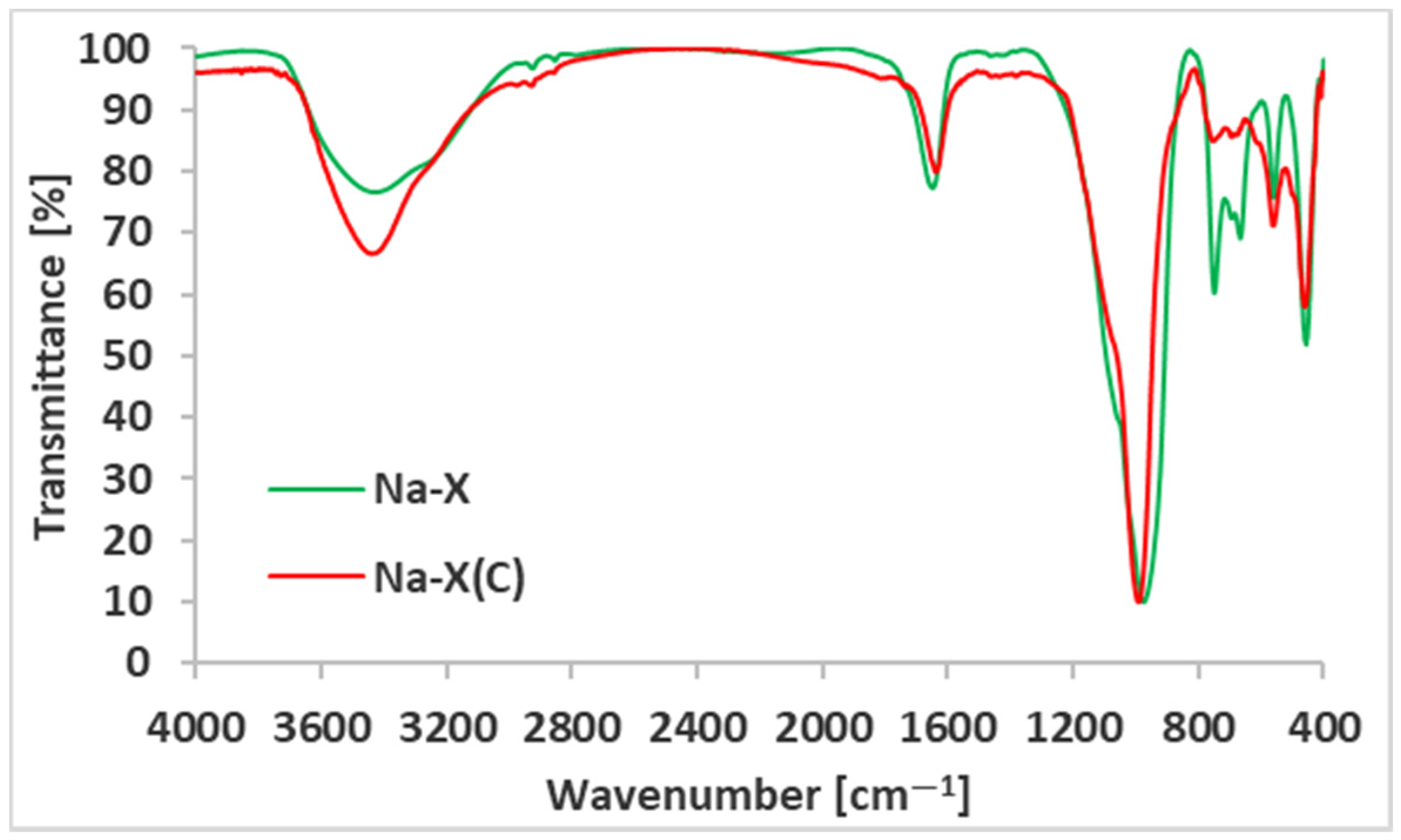



| Material | SBET (m2/g) | Smicro (m2/g) | Vt (cm3/g) | Vmicro (cm3/g) | D (4V/A) (nm) |
|---|---|---|---|---|---|
| Na-X | 728 | 694 | 0.31 | 0.27 | 1.73 |
| Na-X(C) | 272 | 189 | 0.17 | 0.07 | 2.56 |
| Adsorbent | Compounds | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na2O | MgO | Al2O3 | SiO2 | P2O5 | SO3 | K2O | CaO | TiO2 | Fe2O3 | LOI | C | |
| Na-X | 7.32 | 1.69 | 23.41 | 41.12 | 0.02 | - | 0.71 | 7.48 | 0.05 | 1.31 | 16.88 | - |
| Na-X(C) | 3.53 | 1.21 | 14.37 | 28.19 | 0.05 | 0.48 | 1.34 | 2.21 | 1.05 | 9.33 | 38.23 | 31.46 |
| Pseudo-II-Order Model | |||||
|---|---|---|---|---|---|
| System | qe (mg/g) | k2 (g/(mg·min)) | R2 | Linear Form | |
| Pb2+ | Na-X | 333.333 | 0.0007 | 0.999 | y = 0.003x + 0.0129 |
| Na-X(C) | 322.581 | 0.0002 | 0.997 | y = 0.0031x + 0.0533 | |
| Zn2+ | Na-X | 333.333 | 0.0090 | 1.000 | y = 0.003x + 0.001 |
| Na-X(C) | 322.581 | 0.0069 | 1.000 | y = 0.0031x + 0.0014 | |
| Langmuir Model | Freundlich Model | ||||||
|---|---|---|---|---|---|---|---|
| System | qm (mg/g) | KL (dm3/mg) | R2 | n | KF (mg/g (mg/dm3)−1/nF) | R2 | |
| Pb2+ | Na-X | 676.594 | 0.227 | 0.966 | 1.625 | 0.0005 | 0.794 |
| Na-X(C) | 321.909 | 1.178 | 0.997 | 2.501 | 0.0014 | 0.719 | |
| Zn2+ | Na-X | 693.289 | 4.878 | 0.997 | 2.148 | 0.0002 | 0.943 |
| Na-X(C) | 640.938 | 0.395 | 0.979 | 1.899 | 0.0006 | 0.916 | |
| Desorbing Agent | Desorbed Ion | Na-X | Na-X(C) |
|---|---|---|---|
| Single Systems | |||
| HCl | Pb2+ | 61.00 ± 1.5 | 39.55 ± 1.6 |
| NaOH | 17.13 ± 0.6 | 12.29 ± 0.4 | |
| HCl | Zn2+ | 6.11 ± 0.1 | 3.82 ± 0.2 |
| NaOH | 2.48 ± 0.1 | 1.32 ± 0.1 | |
| Mixed Systems | |||
| HCl | Pb2+ | 77.94 ± 2.1 | 64.31 ± 1.8 |
| NaOH | 24.82 ± 1.2 | 22.33 ± 1.5 | |
| HCl | Zn2+ | 6.43 ± 0.2 | 4.08 ± 0.1 |
| NaOH | 2.61 ± 0.1 | 1.41 ± 0.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panek, R.; Medykowska, M.; Wiśniewska, M.; Szewczuk-Karpisz, K.; Jędruchniewicz, K.; Franus, M. Simultaneous Removal of Pb2+ and Zn2+ Heavy Metals Using Fly Ash Na-X Zeolite and Its Carbon Na-X(C) Composite. Materials 2021, 14, 2832. https://doi.org/10.3390/ma14112832
Panek R, Medykowska M, Wiśniewska M, Szewczuk-Karpisz K, Jędruchniewicz K, Franus M. Simultaneous Removal of Pb2+ and Zn2+ Heavy Metals Using Fly Ash Na-X Zeolite and Its Carbon Na-X(C) Composite. Materials. 2021; 14(11):2832. https://doi.org/10.3390/ma14112832
Chicago/Turabian StylePanek, Rafał, Magdalena Medykowska, Małgorzata Wiśniewska, Katarzyna Szewczuk-Karpisz, Katarzyna Jędruchniewicz, and Małgorzata Franus. 2021. "Simultaneous Removal of Pb2+ and Zn2+ Heavy Metals Using Fly Ash Na-X Zeolite and Its Carbon Na-X(C) Composite" Materials 14, no. 11: 2832. https://doi.org/10.3390/ma14112832
APA StylePanek, R., Medykowska, M., Wiśniewska, M., Szewczuk-Karpisz, K., Jędruchniewicz, K., & Franus, M. (2021). Simultaneous Removal of Pb2+ and Zn2+ Heavy Metals Using Fly Ash Na-X Zeolite and Its Carbon Na-X(C) Composite. Materials, 14(11), 2832. https://doi.org/10.3390/ma14112832









