Bicontinuous Gyroid Phase of a Water-Swollen Wedge-Shaped Amphiphile: Studies with In-Situ Grazing-Incidence X-ray Scattering and Atomic Force Microscopy
Abstract
:1. Introduction
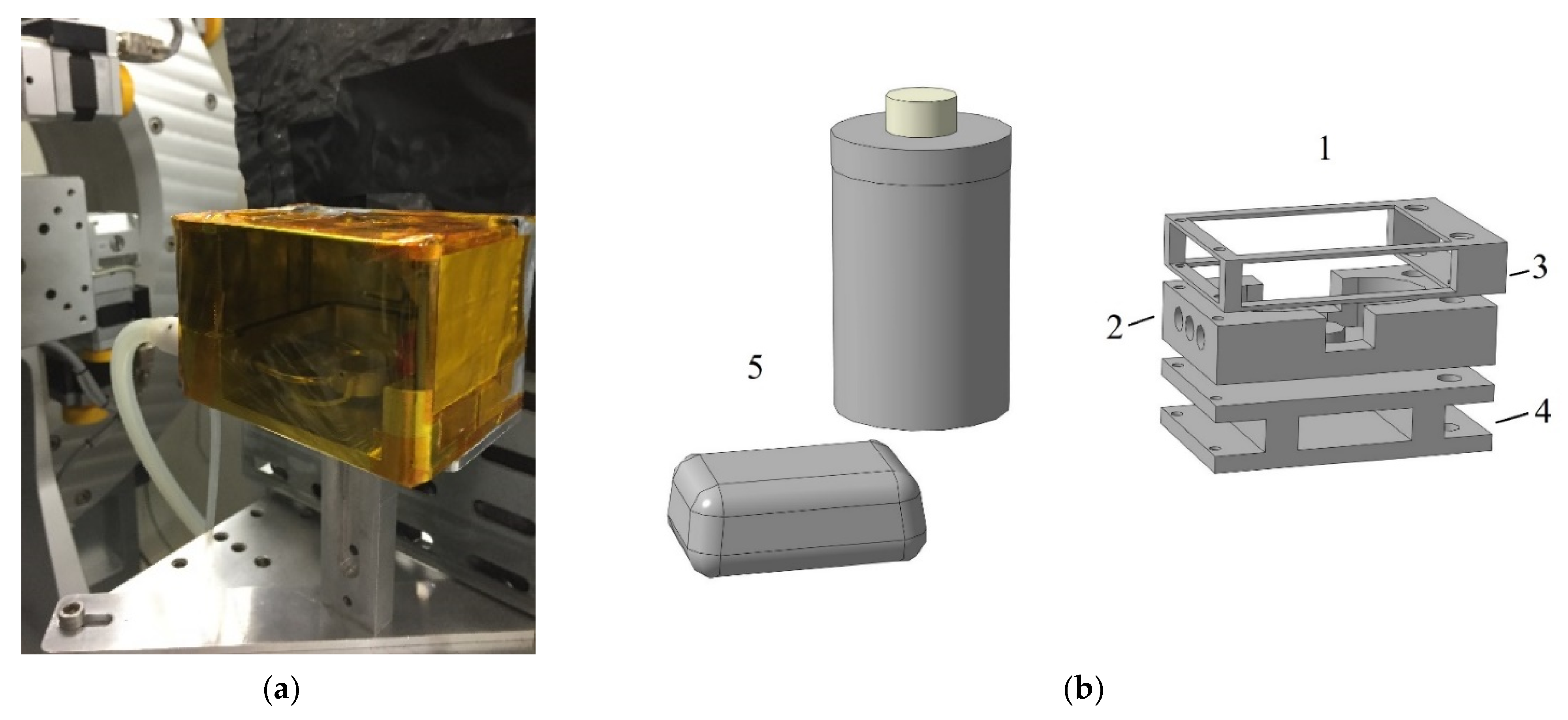
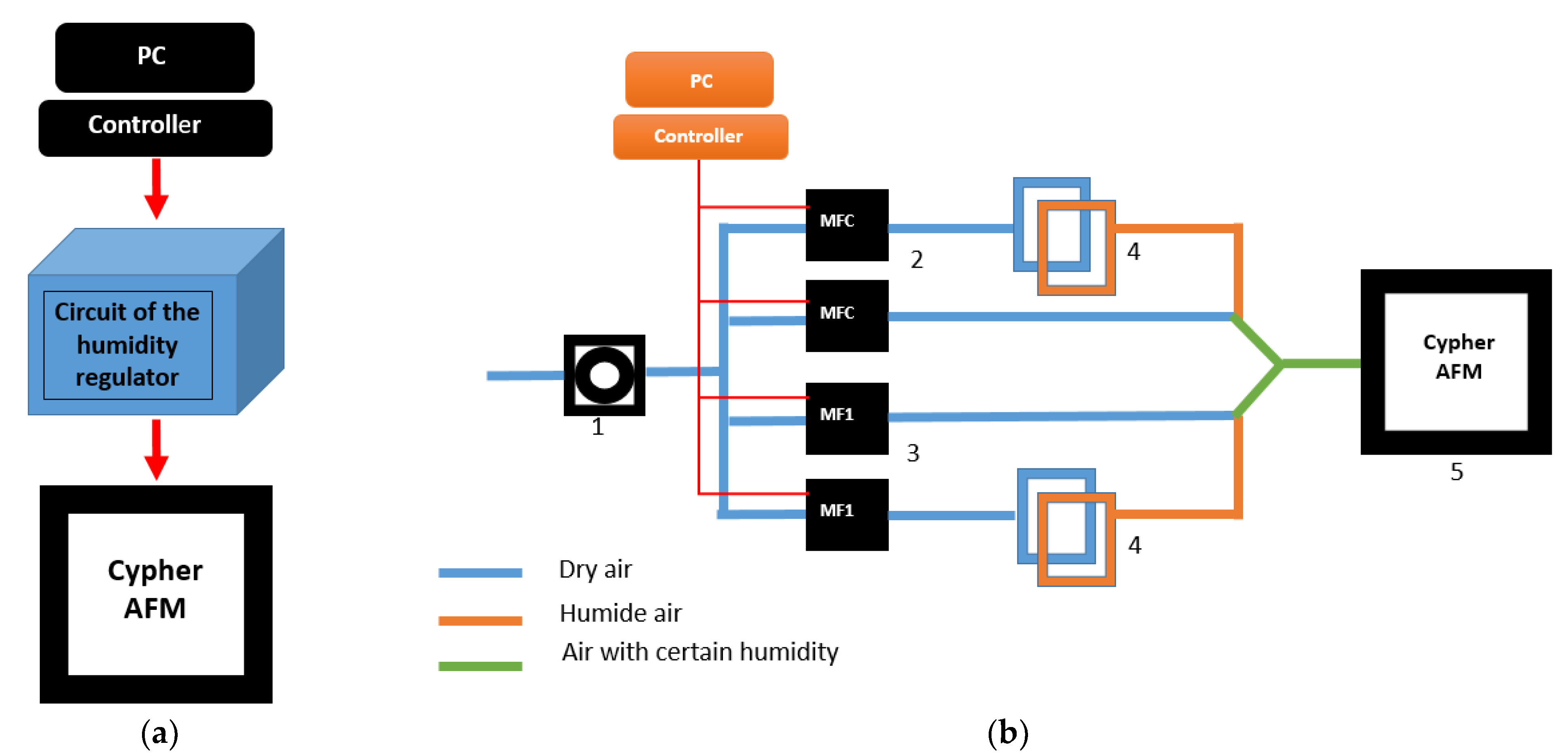
2. Materials and Methods
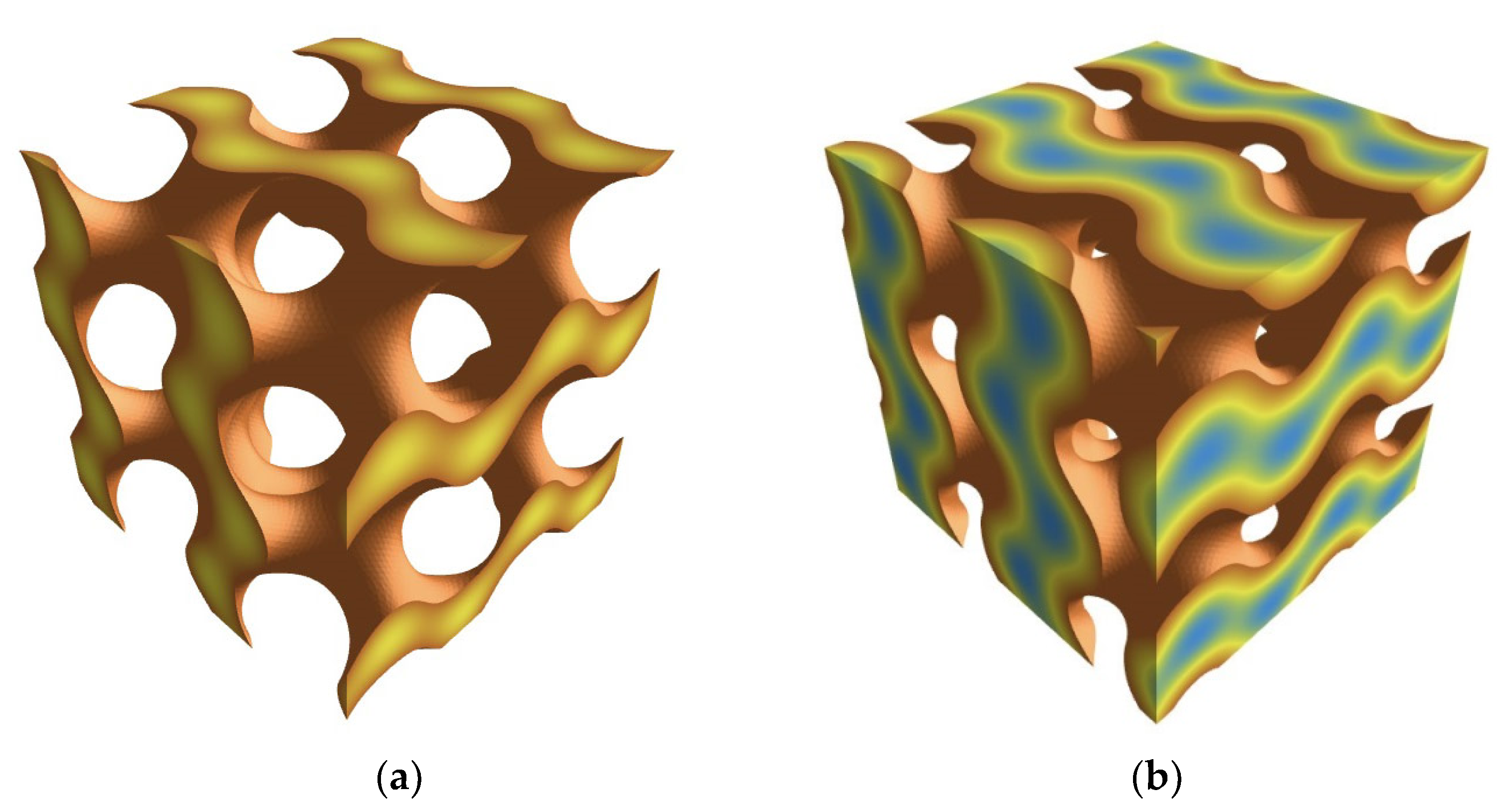
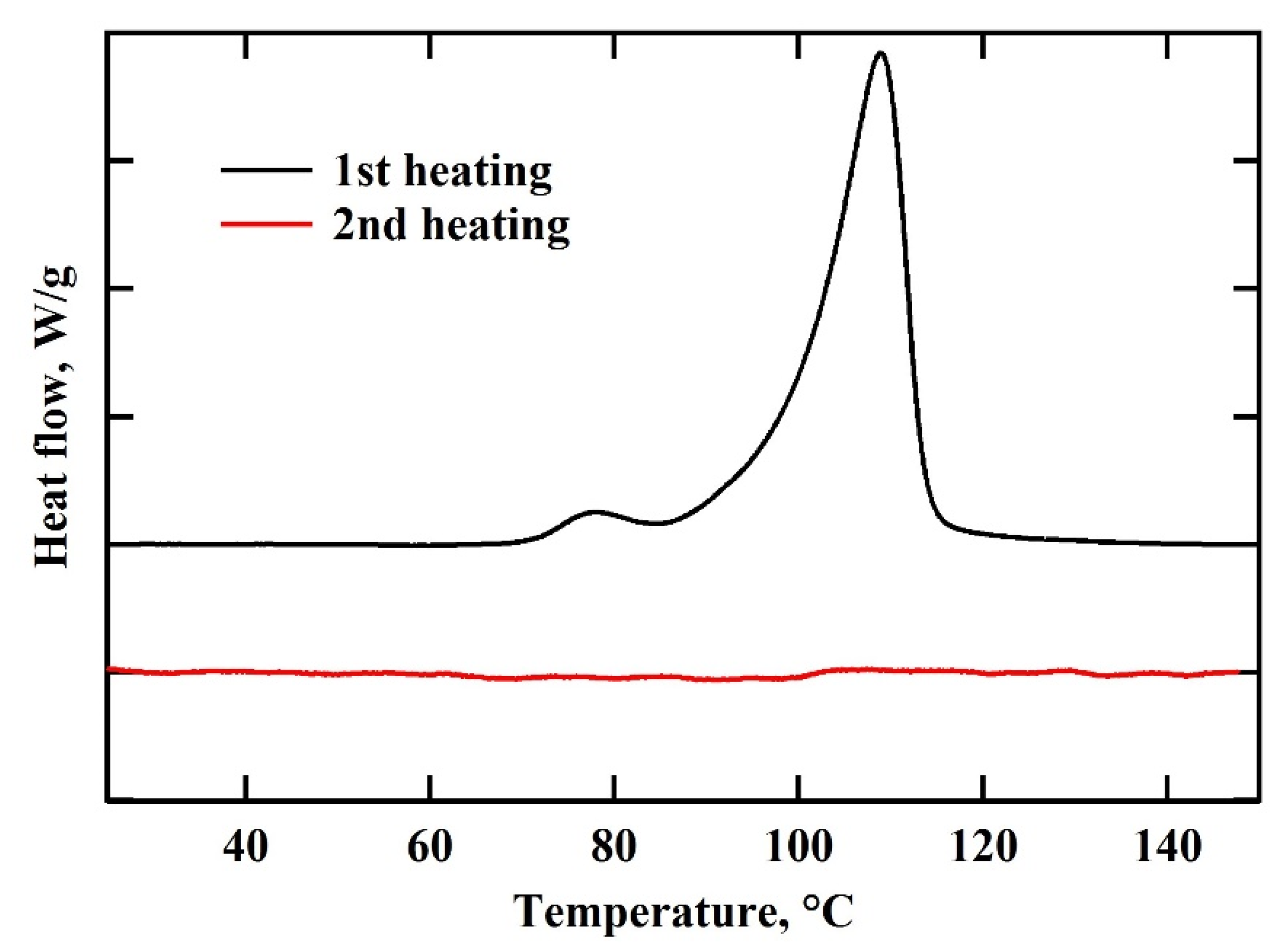
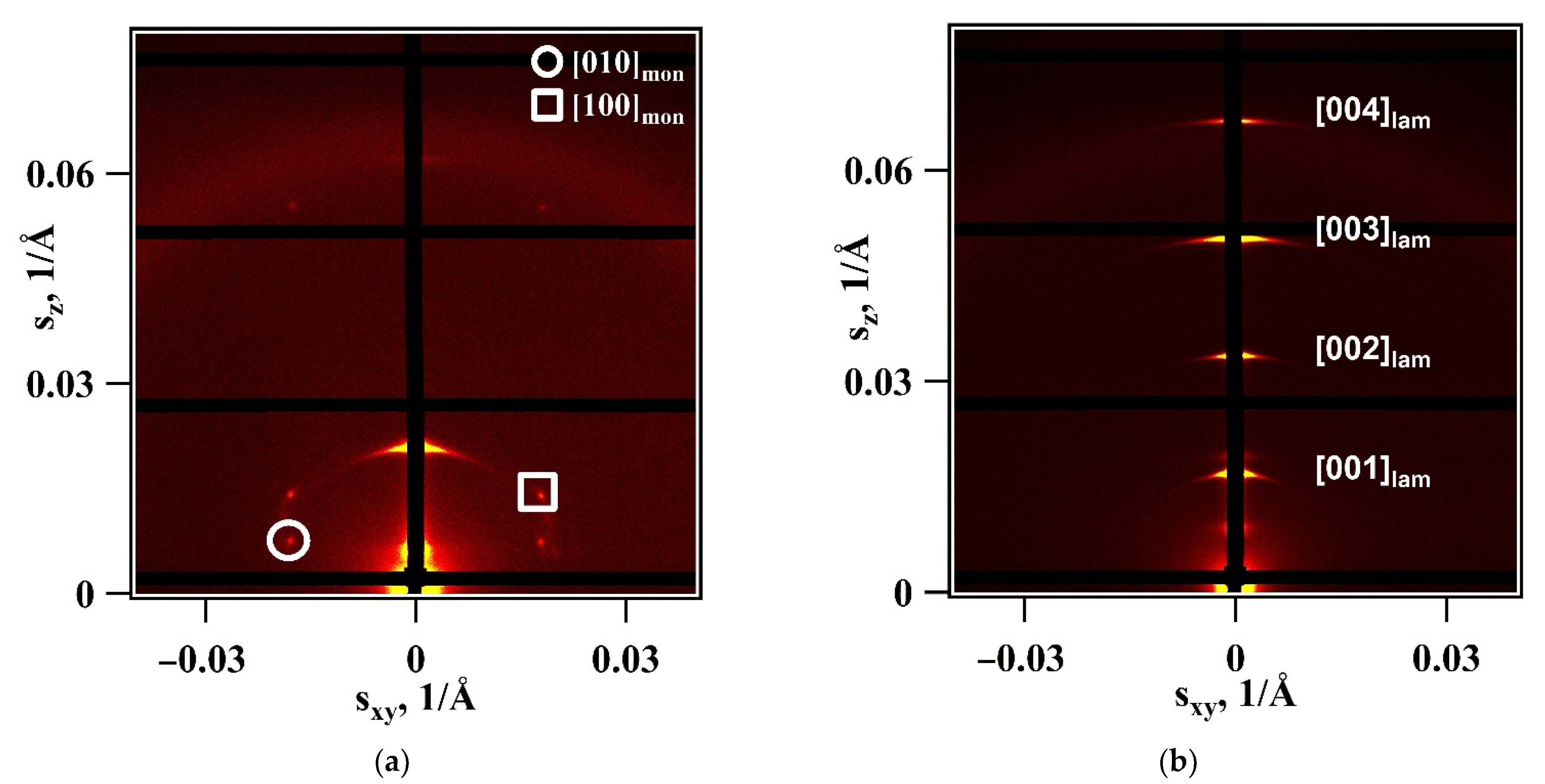
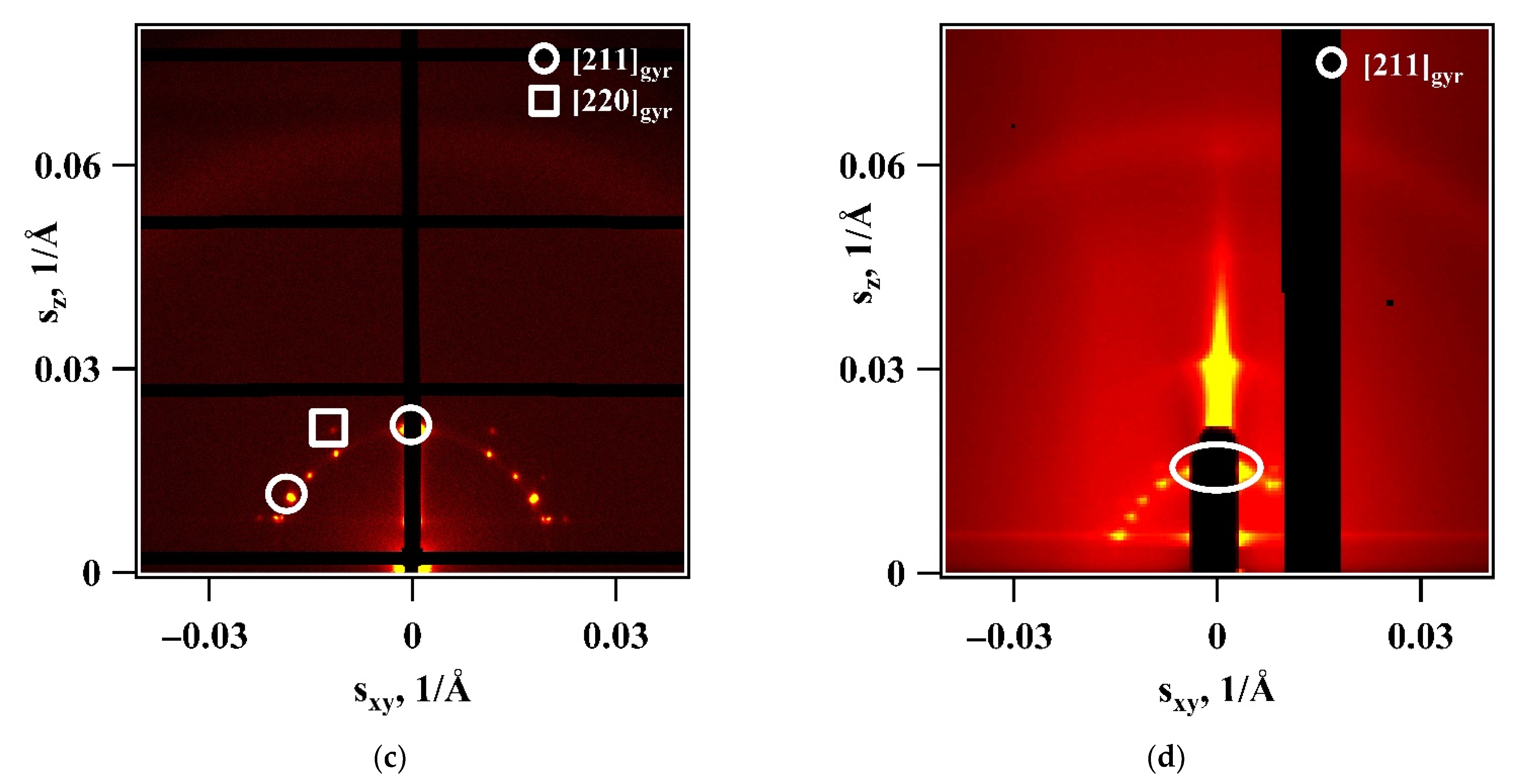
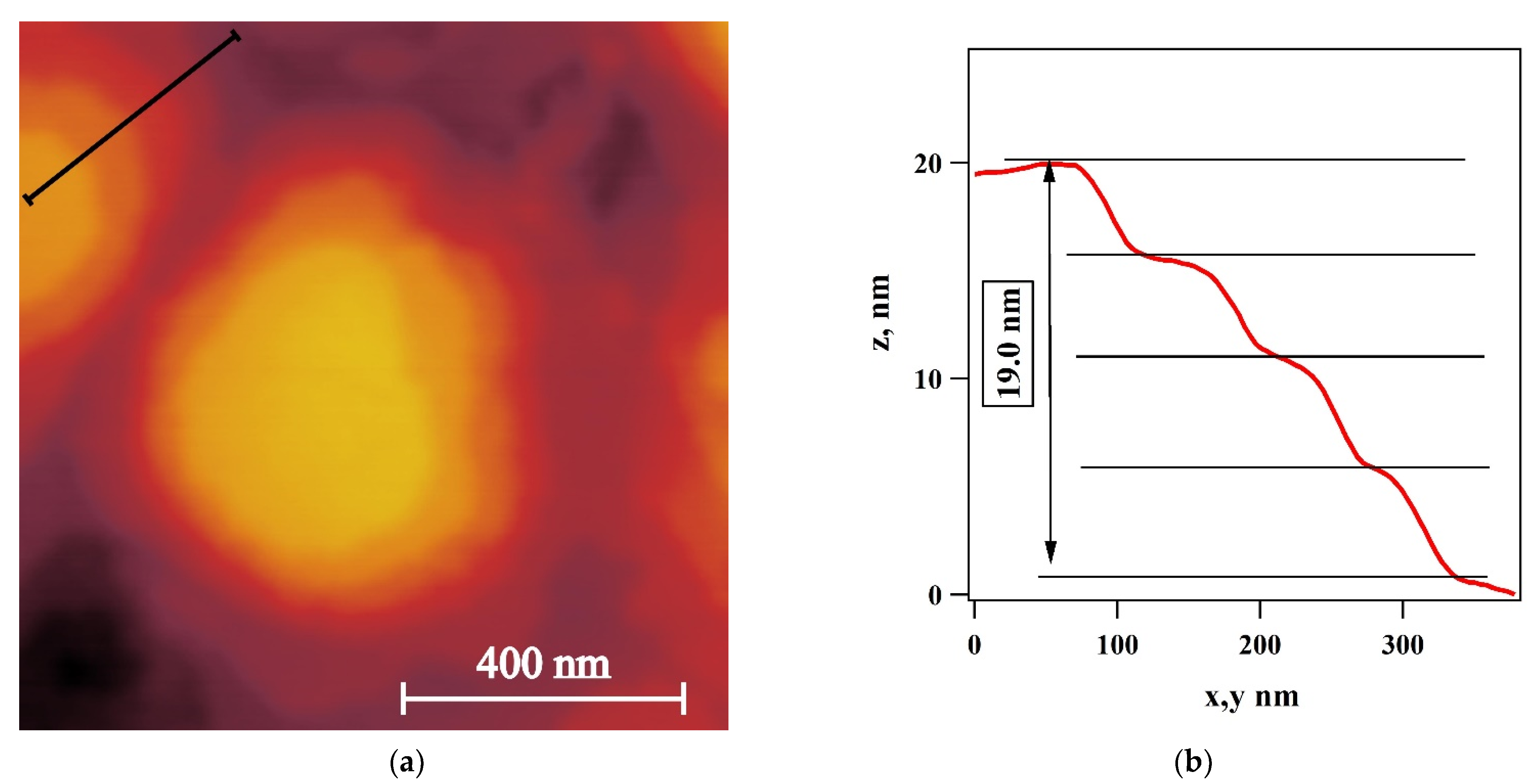
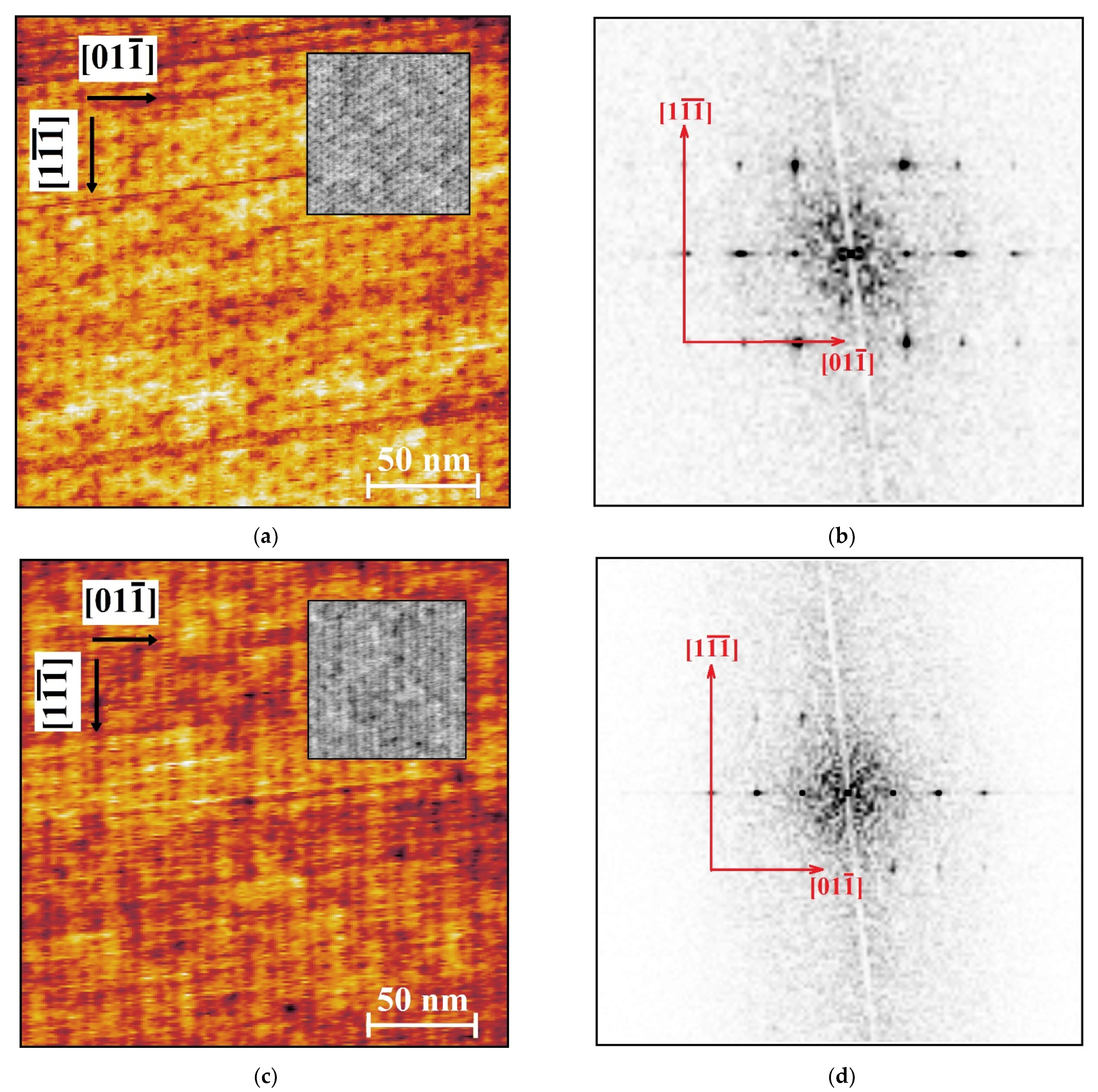
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petsko, G.A.; Ringe, D. Protein Structure and Function; New Science Press, Ltd.: London, UK, 2004. [Google Scholar]
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef]
- Yashima, E.; Ousaka, N.; Taura, D.; Shimomura, K.; Ikai, T.; Maeda, K. Supramolecular helical systems: Helical assemblies of small molecules, foldamers, and polymers with chiral amplification and their functions. Chem. Rev. 2016, 116, 13752–13990. [Google Scholar] [CrossRef] [PubMed]
- Borisch, K.; Diele, S.; Göring, P.; Kresse, H.; Tschierske, C. Tailoring thermotropic cubic mesophases: Amphiphilic polyhydroxy derivatives. J. Mater. Chem. 1998, 8, 529–543. [Google Scholar] [CrossRef]
- Borisch, K.; Tschierske, C.; Göring, P.; Diele, S. Molecular design of thermotropic liquid crystalline polyhydroxy amphiphiles forming type 1 columnar and cubic mesophases. Langmuir 2000, 16, 6701–6708. [Google Scholar] [CrossRef]
- Fong, C.; Le, T.; Drummond, C.J. Lyotropic liquid crystal engineering–ordered nanostructured small molecule amphiphile self-assembly materials by design. Chem. Soc. Rev. 2012, 41, 1297–1322. [Google Scholar] [CrossRef]
- Mezzenga, R.; Seddon, J.M.; Drummond, C.J.; Boyd, B.J.; Schröder-Turk, G.E.; Sagalowicz, L. Nature-Inspired Design and Application of Lipidic Lyotropic Liquid Crystals. Adv. Mater. 2019, 31, 1900818. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Fong, C.; Tran, N.; Drummond, C.J. Non-Lamellar Lyotropic Liquid Crystalline Lipid Nanoparticles for the Next Generation of Nanomedicine. ACS Nano 2019, 13, 6178–6206. [Google Scholar] [CrossRef]
- Barriga, H.M.G.; Holme, M.N.; Stevens, M.M. Cubosomes: The Next Generation of Smart Lipid Nanoparticles? Angew. Chem. Int. Ed. 2019, 58, 2958–2978. [Google Scholar] [CrossRef] [Green Version]
- Caffrey, M. A comprehensive review of the lipid cubic phase or in meso method for crystallizing membrane and soluble proteins and complexes. Struct. Biol. Commun. 2015, 71, 3–18. [Google Scholar]
- Zabara, A.; Meikle, T.G.; Newman, J.; Peat, T.S.; Conn, C.E.; Drummond, C.J. The nanoscience behind the art of in-meso crystallization of membrane proteins. Nanoscale 2017, 9, 754–763. [Google Scholar] [CrossRef] [Green Version]
- Reppe, T.; Poppe, S.; Tschierske, C. Controlling Mirror Symmetry Breaking and Network Formation in Liquid Crystalline Cubic, Isotropic Liquid and Crystalline Phases of Benzil-Based Polycatenars. Chem. Eur. J. 2020, 26, 16066–16079. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jaroniec, M. Strategies for development of nanoporous materials with 2D building units. Chem. Soc. Rev. 2020, 49, 6039–6055. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, J.; Xu, R. Needs and trends in rational synthesis of zeolitic materials. Chem. Soc. Rev. 2012, 41, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Cao, D.; Dai, L. Well-defined two dimensional covalent organic polymers: Rational design, controlled syntheses, and potential applications. Polym. Chem. 2015, 6, 1896–1911. [Google Scholar] [CrossRef]
- Gearba, R.I.; Anokhin, D.V.; Bondar, A.I.; Bras, W.; Lehmann, M.; Ivanov, D.A. Homeotropic Alignment of Columnar Liquid Crystals in Open Films by Means of Surface Nano-Patterning. Adv. Mater. 2007, 19, 815–820. [Google Scholar] [CrossRef]
- Hyde, S.; Blum, Z.; Landh, T.; Lidin, S.; Ninham, B.W.; Andersson, S.; Larsson, K. The Language of Shape: The Role of Curvature in Condensed Matter: Physics, Chemistry and Biology; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Fontell, K. Cubic phases in surfactant and surfactant-like lipid systems. Colloid Polym. Sci. 1990, 268, 264–285. [Google Scholar] [CrossRef]
- Seddon, J.M. An inverse face-centered cubic phase formed by diacylglycerol-phosphatidylcholine mixtures. Biochemistry 1990, 29, 7997–8002. [Google Scholar] [CrossRef]
- Orilall, M.C.; Wiesner, U. Block copolymer based composition and morphology control in nanostructured hybrid materials for energy conversion and storage: Solar cells, batteries, and fuel cells. Chem. Soc. Rev. 2011, 40, 520–535. [Google Scholar] [CrossRef]
- Cochran, E.W.; Garcia-Cervera, C.J.; Fredrickson, G.H. Stability of the gyroid phase in diblock copolymers at strong segregation. Macromolecules 2006, 39, 2449–2451. [Google Scholar] [CrossRef]
- Stefik, M.; Guldin, S.; Vignolini, S.; Wiesner, U.; Steiner, U. Block copolymer self-assembly for nanophotonics. Chem. Soc. Rev. 2015, 44, 5076–5091. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Bonneau, C.; Cantín, A.; Corma, A.; Díaz-Cabañas, M.J.; Moliner, M.; Zhang, D.; Li, M.; Zou, X. The ITQ-37 mesoporous chiral zeolite. Nature 2009, 458, 1154–1157. [Google Scholar] [CrossRef] [PubMed]
- Meuler, A.J.; Hillmyer, M.A.; Bates, F.S. Ordered network mesostructures in block polymer materials. Macromolecules 2009, 42, 7221–7250. [Google Scholar] [CrossRef]
- Beginn, U. Thermotropic columnar mesophases from N-H⋯O, and N⋯H-O hydrogen bond supramolecular mesogenes. Prog. Polym. Sci. 2003, 28, 1049–1105. [Google Scholar] [CrossRef]
- Miskaki, C.; Moutsios, I.; Manesi, G.-M.; Artopoiadis, K.; Chang, C.-Y.; Bersenev, E.A.; Moschovas, D.; Ivanov, D.A.; Ho, R.-M.; Avgeropoulos, A. Self-Assembly of Low-Molecular-Weight Asymmetric Linear Triblock Terpolymers: How Low Can We Go? Molecules 2020, 25, 5527. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Beginn, U.; Möller, M.; Gearba, R.I.; Anokhin, D.V.; Ivanov, D.A. Self-Organization of polybases neutralized with mesogenic wedge-shaped sulfonic acid molecules: An approach toward supramolecular cylinders. Am. Chem. Soc. 2006, 128, 16928–16937. [Google Scholar] [CrossRef]
- Hernandez Rueda, J.J.; Zhang, H.; Rosenthal, M.; Möller, M.; Zhu, X.; Ivanov, D.A. Polymerizable wedge-shaped ionic liquid crystals for fabrication of ion-conducting membranes: Impact of the counterion on the phase structure and conductivity. Eur. Polym. J. 2016, 81, 674–685. [Google Scholar] [CrossRef]
- Chen, Y.; Lingwood, M.D.; Goswami, M.; Kidd, B.E.; Hernandez, J.J.; Rosenthal, M.; Ivanov, D.A.; Perlich, J.; Zhang, H.; Zhu, X.; et al. Humidity-Modulated Phase Control and Nanoscopic Transport in Supramolecular Assemblies. J. Phys. Chem. B 2014, 118, 3207–3217. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.J.; Zhang, H.; Chen, Y.; Rosenthal, M.; Lingwood, M.D.; Goswami, M.; Zhu, X.; Moeller, M.; Madsen, L.A.; Ivanov, D.A. Bottom-Up Fabrication of Nanostructured Bicontinuous and Hexagonal Ion-Conducting Polymer Membranes. Macromolecules 2017, 50, 5392–5401. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Moller, M.; Zhu, X.; Hernandez Rueda, J.J.; Rosenthal, M.; Ivanov, D.A. From channel-forming ionic liquid crystals exhibiting humidity-induced phase transitions to nanostructured ion-conducting polymer membranes. Adv. Mater. 2013, 25, 3543–3548. [Google Scholar] [CrossRef]
- Grafskaia, K.N.; Hernandz Rueda, J.J.; Zhu, X.; Nekipelov, V.M.; Anokhin, D.V.; Moeller, M.; Ivanov, D.A. Designing the topology of ion nano-channels in the mesophases of amphiphilic wedge-shaped molecules. Phys. Chem. Chem. Phys. 2015, 17, 30240–30247. [Google Scholar] [CrossRef]
- Grafskaia, K.N.; Anokhin, D.V.; Hernandez Rueda, J.J.; Ivanov, D.A. In situ studies of molecular self-assembling during the formation of ion-conducting membranes for fuel cells. Appl. Mech. Mater. 2015, 792, 623–628. [Google Scholar] [CrossRef]
- Grafskaia, K.; Zimka, B.; Zhu, X.; Anokhin, D.; Ivanov, D. Engineering of ion channels topology in self-assembled wedge-shaped amphiphiles by combination of temperature and solvent vapor treatment. AIP Conf. Proc. 2016, 1748, 040009. [Google Scholar]
- Grafskaia, K.N.; Anokhin, D.V.; Zimka, B.I.; Izdelieva, I.A.; Zhu, X.; Ivanov, D.A. An “on–off” switchable cubic phase with exceptional thermal stability and water sorption capacity. Chem. Commun. 2017, 53, 13217–13220. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Tartsch, B.; Beginn, U.; Möller, M. Wedge-Shaped Molecules with a Sulfonate Group at the Tip—A New Class of Self-Assembling Amphiphiles. Chem. Eur. J. 2004, 10, 3871–3878. [Google Scholar] [CrossRef]
- Snyder, R.G.; Strauss, H.L.; Elliger, C.A. Carbon-hydrogen Stretching Modes and the Structure of n-Alkyl Chains. 1. Long Disordered Chains. J. Phys. Chem. 1982, 86, 5145–5150. [Google Scholar] [CrossRef]
- Moschovas, D.; Manesi, G.-M.; Karydis-Messinis, A.; Zapsas, G.; Ntetsikas, K.; Zafeiropoulos, N.E.; Piryazev, A.A.; Thomas, E.L.; Hadjichristidis, N.; Ivanov, D.A.; et al. Alternating Gyroid Network Structure in an ABC Miktoarm Terpolymer Comprised of Polystyrene and Two Polydienes. Nanomaterials 2020, 10, 1497. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhuo, M.; Guo, H.; Thomas, E.L. Visualizing the double-gyroid twin. Proc. Natl. Acad. Sci. USA 2021, 118, e2018977118. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grafskaia, K.N.; Akhkiamova, A.F.; Vashurkin, D.V.; Kotlyarskiy, D.S.; Pontoni, D.; Anokhin, D.V.; Zhu, X.; Ivanov, D.A. Bicontinuous Gyroid Phase of a Water-Swollen Wedge-Shaped Amphiphile: Studies with In-Situ Grazing-Incidence X-ray Scattering and Atomic Force Microscopy. Materials 2021, 14, 2892. https://doi.org/10.3390/ma14112892
Grafskaia KN, Akhkiamova AF, Vashurkin DV, Kotlyarskiy DS, Pontoni D, Anokhin DV, Zhu X, Ivanov DA. Bicontinuous Gyroid Phase of a Water-Swollen Wedge-Shaped Amphiphile: Studies with In-Situ Grazing-Incidence X-ray Scattering and Atomic Force Microscopy. Materials. 2021; 14(11):2892. https://doi.org/10.3390/ma14112892
Chicago/Turabian StyleGrafskaia, Kseniia N., Azaliia F. Akhkiamova, Dmitry V. Vashurkin, Denis S. Kotlyarskiy, Diego Pontoni, Denis V. Anokhin, Xiaomin Zhu, and Dimitri A. Ivanov. 2021. "Bicontinuous Gyroid Phase of a Water-Swollen Wedge-Shaped Amphiphile: Studies with In-Situ Grazing-Incidence X-ray Scattering and Atomic Force Microscopy" Materials 14, no. 11: 2892. https://doi.org/10.3390/ma14112892





