The Effect of Type-I Photoinitiators on the Kinetics of the UV-Induced Cotelomerization Process of Acrylate Monomers and Properties of Obtained Pressure-Sensitive Adhesives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- (1)
- α-Hydroxyalkylphenones (HPs): 2-hydroxy-1-(4-(4-(2-hydroxy-2-methylpropionyl)benzyl)phenyl)-2-methylpropan-1-one (Omnirad 127; IGM Resin, Waalwijk, The Netherlands) and 1-hydroxycyclohexylphenyl ketone (Omnirad 184, IGM Resins, Waalwijk, The Netherlands);
- (2)
- Acylphosphine oxides (APOs): bis(2,4,6-trimethylbenzoyl)-phenylphosphineoxide (Omnirad 819; IGM Resins, Waalwijk, The Netherlands); 2,4,6-trimethylbenzoyl-diphenyl phosphine oxide (Omnirad TPO; IGM Resins, Waalwijk, The Netherlands) and 2,4,6-trimethylbenzoyldi-phenylphosphinate (Omnirad TPOL; IGM Resins, Waalwijk, The Netherlands);
- (3)
- Mixture of acylphosphine oxides (APOs blend): ethyl phenyl(2,4,6- trimethylbenzoyl)phosphinate (ca. 95 wt%) and phenyl bis(2,4,6-trimethylbenzoyl)-phosphine oxide (ca. 5 wt%) (Omnirad 2100; IGM Resins, Waalwijk, The Netherlands).
2.2. Synthesis and Characterization of BA/AA/ABP Cotelomers
2.3. Preparation and Characterization of Obtained Pressure-Sensitive Adhesives (PSAs)
3. Results
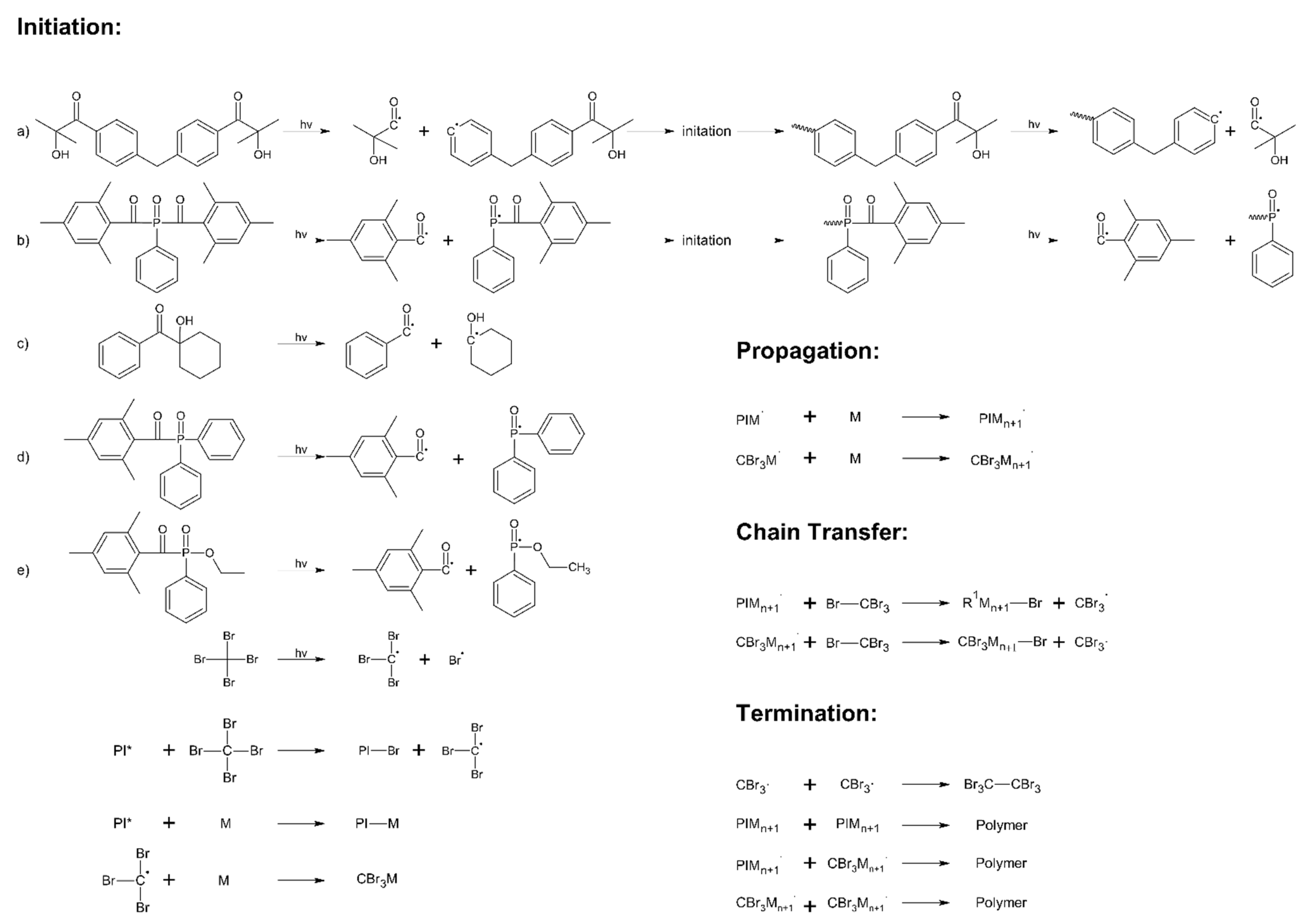
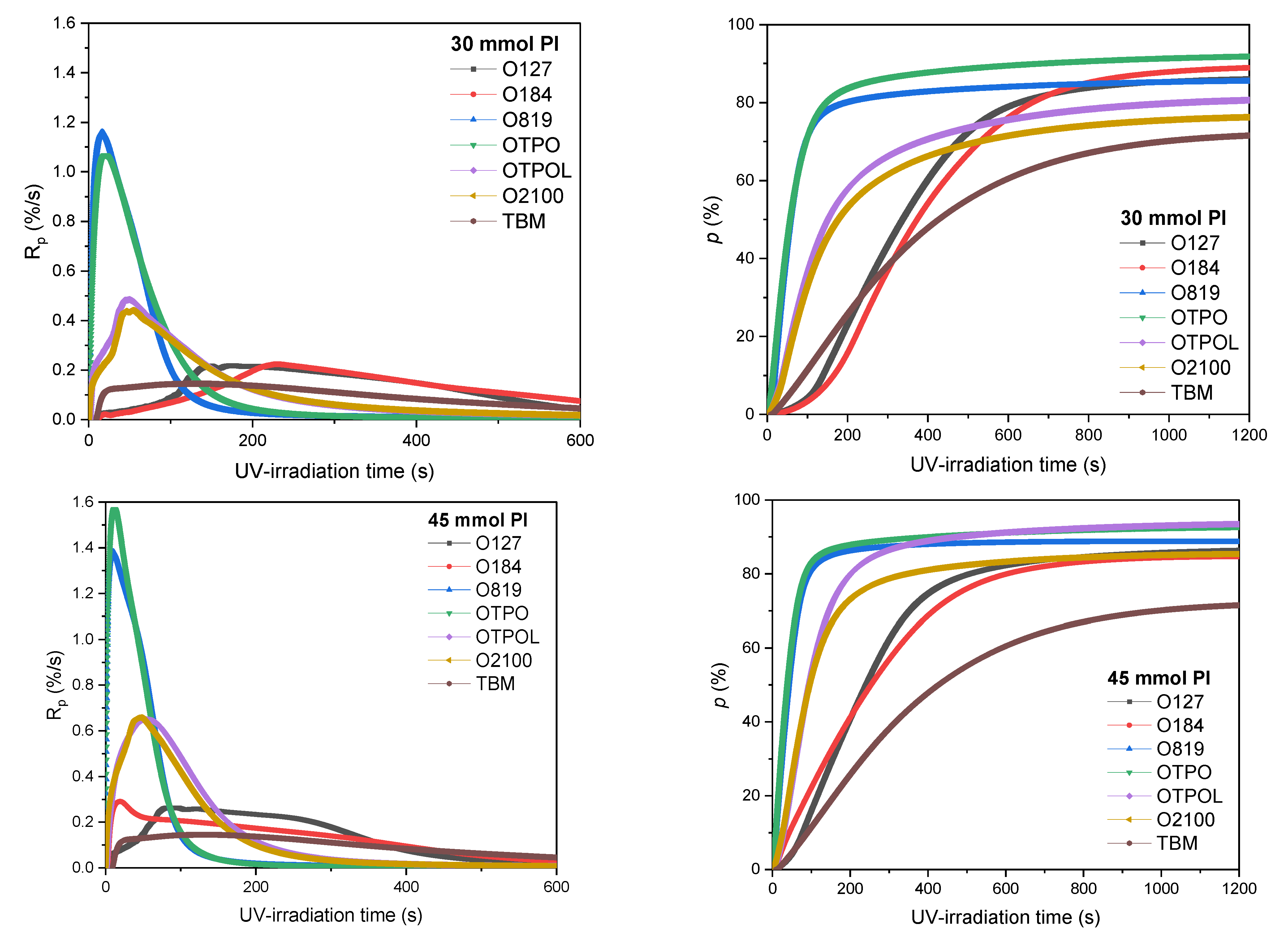
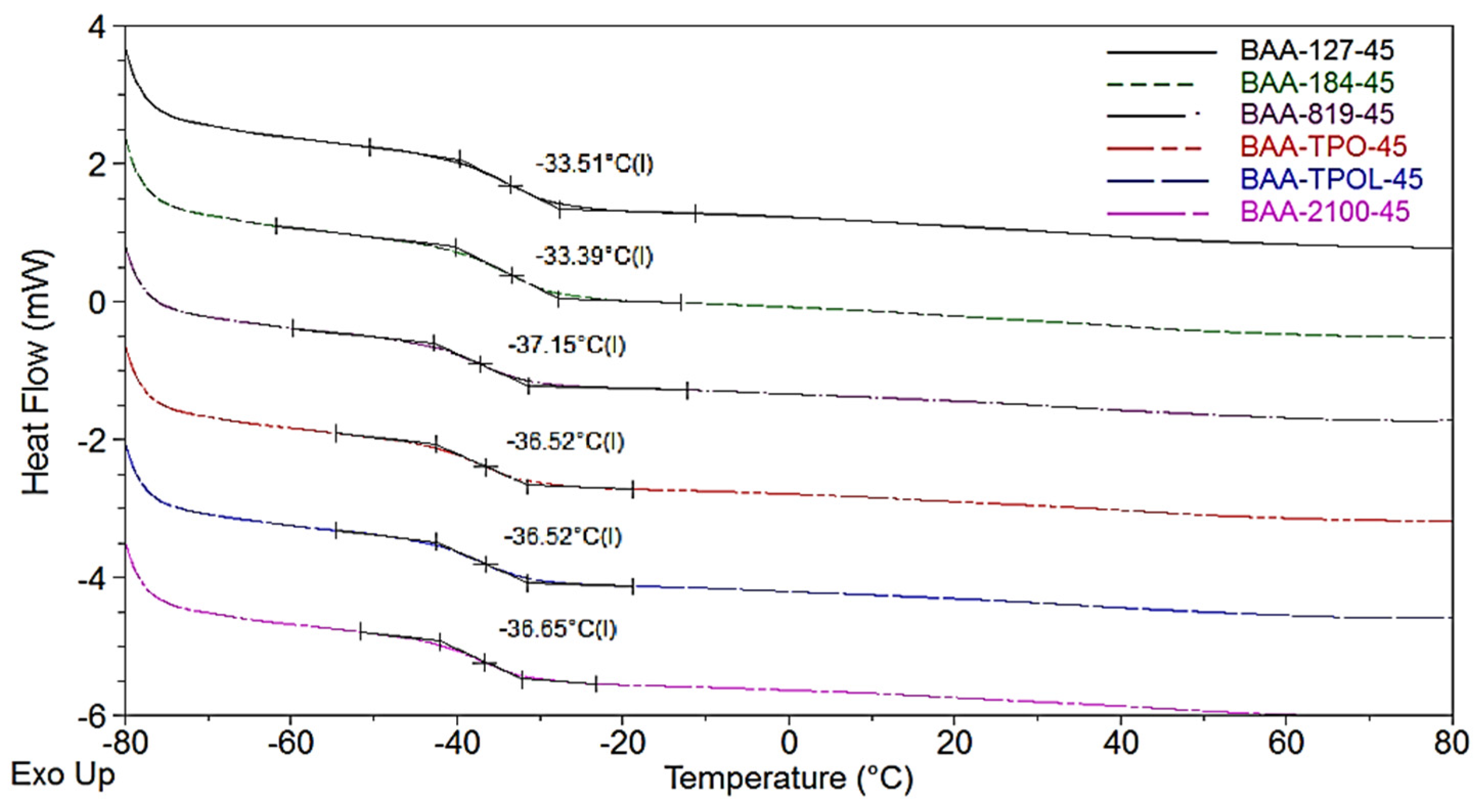
3.1. Kinetics Study of Photo-Cotelomerization Process
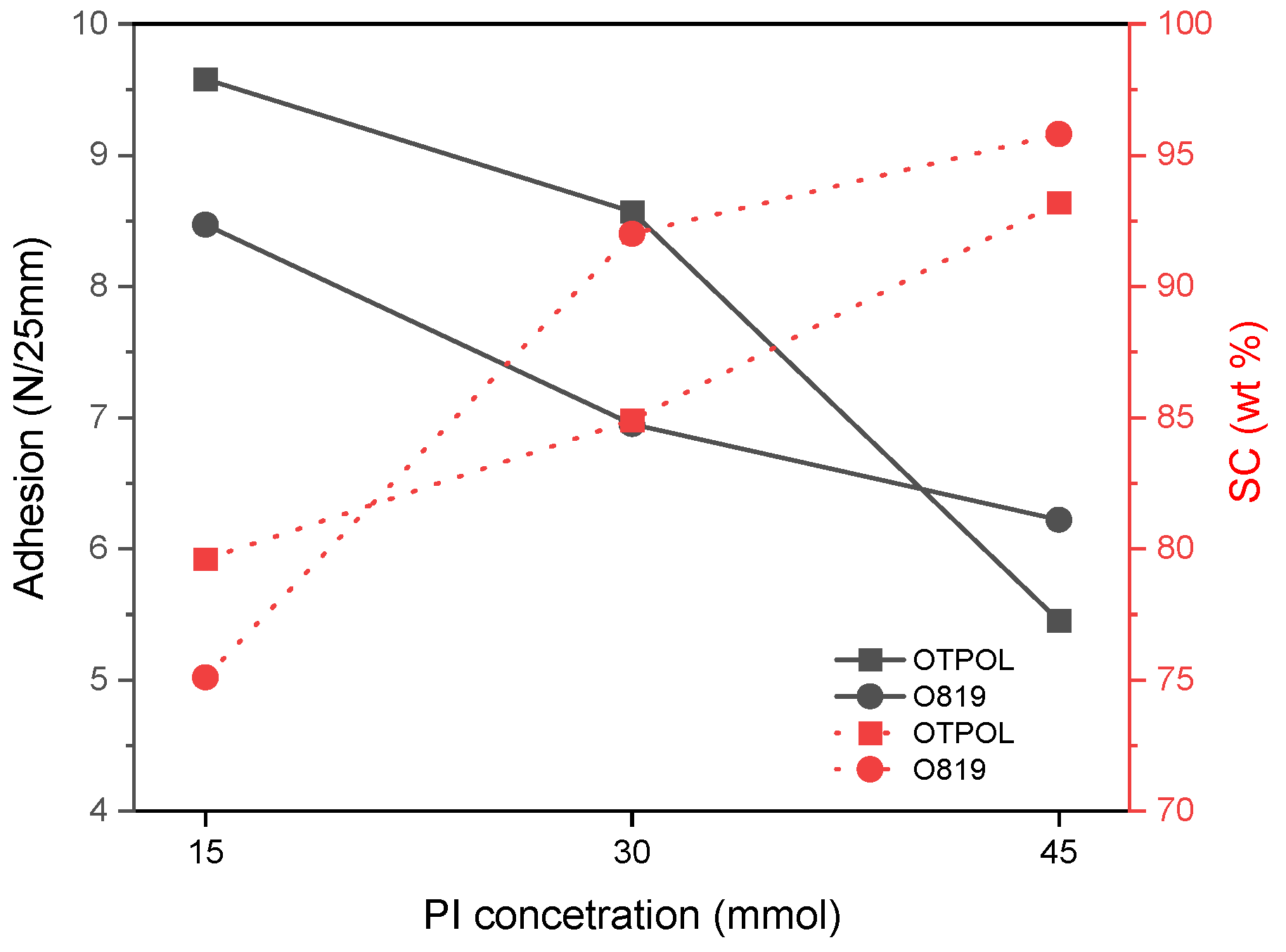
3.2. Properties of BAA Syrups and Cotelomers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slota, J.; Kubit, A.; Trzepieciński, T.; Krasowski, B.; Varga, J. Ultimate Load-Carrying Ability of Rib-Stiffened 2024-T3 and 7075-T6 Aluminium Alloy Panels under Axial Compression. Materials 2021, 14, 1176. [Google Scholar] [CrossRef]
- Satas, D. Handbook of Pressure Sensitive Adhesive Technology; Springer: Warwick, RI, USA, 1999; pp. 346–398. [Google Scholar]
- Creton, C. Pressure-Sensitive Adhesives: An Introductory Course. MRS Bull. 2003, 28, 434–439. [Google Scholar] [CrossRef] [Green Version]
- Brockmann, W.; Geiss, P.; Klingen, J.; Schröder, B. Adhesive Bonding: Materials, Applications and Technology; Wiley-VCH Verlag GmbH&Co. KGaA: Weinheim, Germany, 2009; pp. 39–46. [Google Scholar]
- Doyle, J.; Quinn, R. Adhesives: Types, Mechanics and Applications; Nova Science Publishers: New York, NY, USA, 2011; pp. 47–71. [Google Scholar]
- Adams, R.D. Adhesive Bonding: Science, Technology and Applications; CRP Press: Abington, UK, 2007; p. 34. [Google Scholar]
- Márquez, I.; Alarcia, F.; Velasco, J. Synthesis and Properties of Water-Based Acrylic Adhesives with a Variable Ratio of 2-Ethylhexyl Acrylate and n-Butyl Acrylate for Application in Glass Bottle Labels. Polymers 2020, 12, 428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mapari, S.; Mestry, S.; Mhaske, S.T. Developments in pressure-sensitive adhesives: A review. Polym. Bull. 2021, 78, 4075–4108. [Google Scholar] [CrossRef]
- Arunprasert, K.; Pornpitchanarong, C.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P.; Aumklad, P.; Patrojanasophon, P. Development and Evaluation of Novel Water-Based Drug-in-Adhesive Patches for the Transdermal Delivery of Ketoprofen. Pharmaceutics 2021, 13, 789. [Google Scholar] [CrossRef] [PubMed]
- Back, J.H.; Kwon, Y.; Roldao, J.C.; Yu, Y.; Kim, H.; Gierchner, J.; Lee, W.; Kwon, M.S. Solvent-free acrylic pressure-sensitive adhesives via a visible-light driven photocatalytic radical polymerization without additives. Green Chem. 2020, 22, 8289–8297. [Google Scholar] [CrossRef]
- Back, J.H.; Kwon, Y.; Kim, H.J.; Yu, Y.; Lee, W.; Kwon, M.S. Visible-light-curable solvent-free acrylic pressure-sensitive adhesives via photoredox-mediated radical polymerization. Macromolecules 2021, 26, 385. [Google Scholar] [CrossRef]
- Baek, S.; Jang, S.; Lee, S.; Hwang, S. Effect of Chemical Structure of Acrylate Monomer on the Transparent Acrylic Pressure Sensitive Adhesives for Optical Applications. Polym. Korea 2014, 38, 682. [Google Scholar] [CrossRef] [Green Version]
- Baek, S.; Jang, S.; Hwang, S. Preparation and adhesion performance of transparent acrylic pressure sensitive adhesives: Effects of substituent structure of acrylate monomer. Int. J. Adhes. Adhes. 2016, 64, 72–77. [Google Scholar] [CrossRef]
- Beak, S.; Hwang, S. Preparation and adhesion performance of transparent acrylic pressure-sensitive adhesives containing menthyl acrylate. Polym. Bull. 2016, 73, 687–701. [Google Scholar] [CrossRef]
- Beak, S.; Hwang, S. Eco-friendly UV-curable pressure sensitive adhesives containing acryloyl derivatives of monosaccharides and their adhesive performances. Int. J. Adhes. Adhes. 2016, 70, 110–113. [Google Scholar] [CrossRef]
- Baek, S.; Jang, S.; Hwang, S. Construction and adhesion performance of biomass tetrahydro-geraniol-based sustainable/transparent pressure sensitive adhesives. J. Ind. Eng. Chem. 2017, 53, 429–434. [Google Scholar] [CrossRef]
- Kim, J.; Shim, G.; Baek, D.; Back, J.; Jang, S.; Kim, H.; Choi, J.; Yeom, J. UV/UV step-curing of optically clear acrylate adhesives for mobile devices. Express Polym. Lett. 2019, 13, 794–805. [Google Scholar] [CrossRef]
- Gziut, K.; Kowalczyk, A.; Schmidt, B. Free-radical bulk photopolymerization process as method of obtaining thermally curable structural self-adhesive tapes and effect of used type I photoinitiators. Polymers 2020, 12, 2191. [Google Scholar] [CrossRef]
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated polymerization: Advances, challenges and opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Dietliker, K.A. Compilation of Photoinitiators Commercially Available for UV Today; Sita Technology Ltd.: London, UK, 2002. [Google Scholar]
- Green, W.A. Industrial Photoinitiators. A Technical Guide; CRP Press: New York, NY, USA, 2010; pp. 75–82. [Google Scholar]
- Tehfe, M.A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouasier, J.P.; Lalevée, J. Design of new type I and type II photoinitiators possessing highly coupled pyrene-ketone moieties. Polym. Chem. 2013, 4, 2313. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Garra, P.; Dumur, F.; Bui, T.T.; Goubard, F.; Toufaily, J.; Hamieh, T.; Gra, B.; Gigmes, D.; Fouasier, J.P.; et al. Novel Carbazole Skeleton-Based Photoinitiators for LED Polymerization and LED Projector 3D Printing. Molecules 2017, 22, 2143. [Google Scholar] [CrossRef] [Green Version]
- Nazir, R.; Danilevicius, P.; Gray, D.; Farsani, M.; Gryko, D.T. Push–Pull Acylo-Phosphine Oxides for Two-Photon-Induced Polymerization. Macromolecules 2013, 46, 7239–7244. [Google Scholar] [CrossRef]
- Dietlin, C.; Trinh, T.T.; Schweizer, S.; Graff, B.; Morlet-Savary, F.; Noirot, P.A.; Lelevée, J. New Phosphine Oxides as High Performacne Near-UV Type I Photoinitiators of Radical Polymerization. Molecules 2020, 25, 1671. [Google Scholar] [CrossRef] [Green Version]
- Eren, T.N.; Lalevéel, J.; Avci, D. Water soluble polymeric photoinitiator for dual-curing of acrylates and methacrylates. J. Photochem. Photobiol. A Chem. 2020, 389, 112288. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Weisbrodt, M.; Schmidt, B.; Gziut, K. Influence of Acrylic Acid on Kinetics of UV-Induced Cotelomerization Process and Properties of Obtained Pressure-Sensitive Adhesives. Materials 2020, 13, 5661. [Google Scholar] [CrossRef]
- Boutevin, B. From telomerization to living radical polymerization. J. Polym. Sci. Part. A Polym. Chem. 2000, 38, 3235–3243. [Google Scholar] [CrossRef]
- Clark, S.C.; Hill, D.J.T.; Hoyle, C.E.; Miller, C.W.; Shao, L.Y. N-Substituent effect of maleimides on acrylate polymerization initiated by three-component systems. Polym. Int. 2003, 52, 1701–1710. [Google Scholar] [CrossRef]
- Gziut, K.; Kowalczyk, A.; Schmidt, B.; Kowalczyk, K.; Weisbrodt, M. Epoxy-Based Structural Self-Adhesive Tapes Modified with Acrylic Syrups Prepared via a Free Radical Photopolymerization Process. Polymers 2021, 13, 189. [Google Scholar] [CrossRef]
- Benedek, I. Pressure-Sensitive Adhesives and Applications; Marcel Deker Inc.: New York, NY, USA, 2004; pp. 92–102. [Google Scholar]





| PI Type | PI Names and Chemical Structure | |||
|---|---|---|---|---|
| HPs | Omnirad 127 | Omnirad 184 | ||
 |  | |||
| APOs | Omnirad 819 | Omnirad TPO | Omnirad TPOL | |
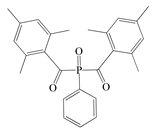 |  | 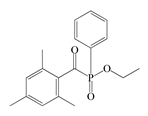 | ||
| APOs Blend | Omnirad 2100 (O.TPO-L + O.819) (95/5) | |||
 | ||||
| Cotelomer Acronym | Monomers (wt%) | PI | |||
|---|---|---|---|---|---|
| BA | AA | APB | Symbol | mmol * | |
| BAA-127-15 | 91.5 | 7.5 | 1 | O127 | 15 |
| BAA-127-30 | 30 | ||||
| BAA-127-45 | 45 | ||||
| BAA-184-15 | O184 | 15 | |||
| BAA-184-30 | 30 | ||||
| BAA-184-45 | 45 | ||||
| BAA-819-15 | O819 | 15 | |||
| BAA-819-30 | 30 | ||||
| BAA-819-45 | 45 | ||||
| BAA-TPO-15 | OTPO | 15 | |||
| BAA-TPO-30 | 30 | ||||
| BAA-TPO-45 | 45 | ||||
| BAA-TPOL-15 | OTPOL | 15 | |||
| BAA-TPOL-30 | 30 | ||||
| BAA-TPOL-45 | 45 | ||||
| BAA-2100-15 | O2100 | 15 | |||
| BAA-2100-30 | 30 | ||||
| BAA-2100-45 | 45 | ||||
| Cotelomer Symbol | Mn (g/mol) | Mw (g/mol) | PDI |
|---|---|---|---|
| BAA-127-15 | 21,530 | 33,770 | 1.57 |
| BAA-127-30 | 20,520 | 32,930 | 1.61 |
| BAA-127-45 | 20,100 | 30,570 | 1.52 |
| BAA-184-15 | 21,010 | 34,400 | 1.64 |
| BAA-184-30 | 21,060 | 32,540 | 1.54 |
| BAA-184-45 | 19,790 | 30,670 | 1.55 |
| BAA-819-15 | 18,150 | 28,440 | 1.57 |
| BAA-819-30 | 17,650 | 27,090 | 1.53 |
| BAA-819-45 | 17,460 | 26,930 | 1.54 |
| BAA-TPO-15 | 19,390 | 31,120 | 1.60 |
| BAA-TPO-30 | 18,210 | 27,190 | 1.49 |
| BAA-TPO-45 | 17,460 | 26,430 | 1.51 |
| BAA-TPOL-15 | 17,500 | 28,670 | 1.64 |
| BAA-TPOL-30 | 18,260 | 27,450 | 1.50 |
| BAA-TPOL-45 | 17,250 | 26,320 | 1.53 |
| BAA-2100-15 | 19,100 | 28,990 | 1.52 |
| BAA-2100-30 | 18,940 | 28,620 | 1.51 |
| BAA-2100-45 | 17,970 | 27,450 | 1.53 |
| PSA Symbol | Cohesion [h] | Tack (N) | |
|---|---|---|---|
| 20 °C | 70 °C | ||
| BAA-127-15 | nd | nd | nd |
| BAA-127-30 | nd | nd | nd |
| BAA-127-45 | 100 ± 3 | 72 ± 2 | 2.1 ± 0.2 |
| BAA-184-15 | nd | nd | nd |
| BAA-184-30 | 100 ± 4 | 72 ± 1 | 3.0± 0.3 |
| BAA-184-45 | 100 ± 5 | 72 ± 2 | 2.5 ± 0.1 |
| BAA-819-15 | 100 ± 3 | 72 ± 2 | 6.5 ± 0.3 |
| BAA-819-30 | 14 ± 0.5 | 3 ± 0.2 | 5.2 ± 0.3 |
| BAA-819-45 | 5 ± 0.5 | 0.1 ± 0.02 | 2.4 ± 0.2 |
| BAA-TPO-15 | nd | nd | nd |
| BAA-TPO-30 | 100 ± 5 | 72 ± 3 | 7.1 ± 0.4 |
| BAA-TPO-45 | 11 ± 0.3 | 2 ± 0.2 | 5.3 ± 0.2 |
| BAA-TPOL-15 | 100 ± 6 | 72 ± 3 | 3.2 ± 0.1 |
| BAA-TPOL-30 | 100 ± 4 | 72 ± 4 | 3.1 ± 0.2 |
| BAA-TPOL-45 | 1 ± 0.2 | 0.3 ± 0.2 | 2.5 ± 0.2 |
| BAA-2100-15 | 100 ± 2 | 72 ± 3 | 5.1 ± 0.4 |
| BAA-2100-30 | 100 ± 5 | 72 ± 3 | 5.0 ± 0.3 |
| BAA-2100-45 | 2 ± 0.2 | 0.6 ± 0.2 | 2.5 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, A.; Weisbrodt, M.; Schmidt, B.; Kraśkiewicz, A. The Effect of Type-I Photoinitiators on the Kinetics of the UV-Induced Cotelomerization Process of Acrylate Monomers and Properties of Obtained Pressure-Sensitive Adhesives. Materials 2021, 14, 4563. https://doi.org/10.3390/ma14164563
Kowalczyk A, Weisbrodt M, Schmidt B, Kraśkiewicz A. The Effect of Type-I Photoinitiators on the Kinetics of the UV-Induced Cotelomerization Process of Acrylate Monomers and Properties of Obtained Pressure-Sensitive Adhesives. Materials. 2021; 14(16):4563. https://doi.org/10.3390/ma14164563
Chicago/Turabian StyleKowalczyk, Agnieszka, Mateusz Weisbrodt, Beata Schmidt, and Agata Kraśkiewicz. 2021. "The Effect of Type-I Photoinitiators on the Kinetics of the UV-Induced Cotelomerization Process of Acrylate Monomers and Properties of Obtained Pressure-Sensitive Adhesives" Materials 14, no. 16: 4563. https://doi.org/10.3390/ma14164563