Utilizing the Intrinsic Thermal Instability of Swedenborgite Structured YBaCo4O7+δ as an Opportunity for Material Engineering in Lithium-Ion Batteries by Er and Ga Co-Doping Processes
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis and Characterization of Materials
2.2. Electrochemical Analysis
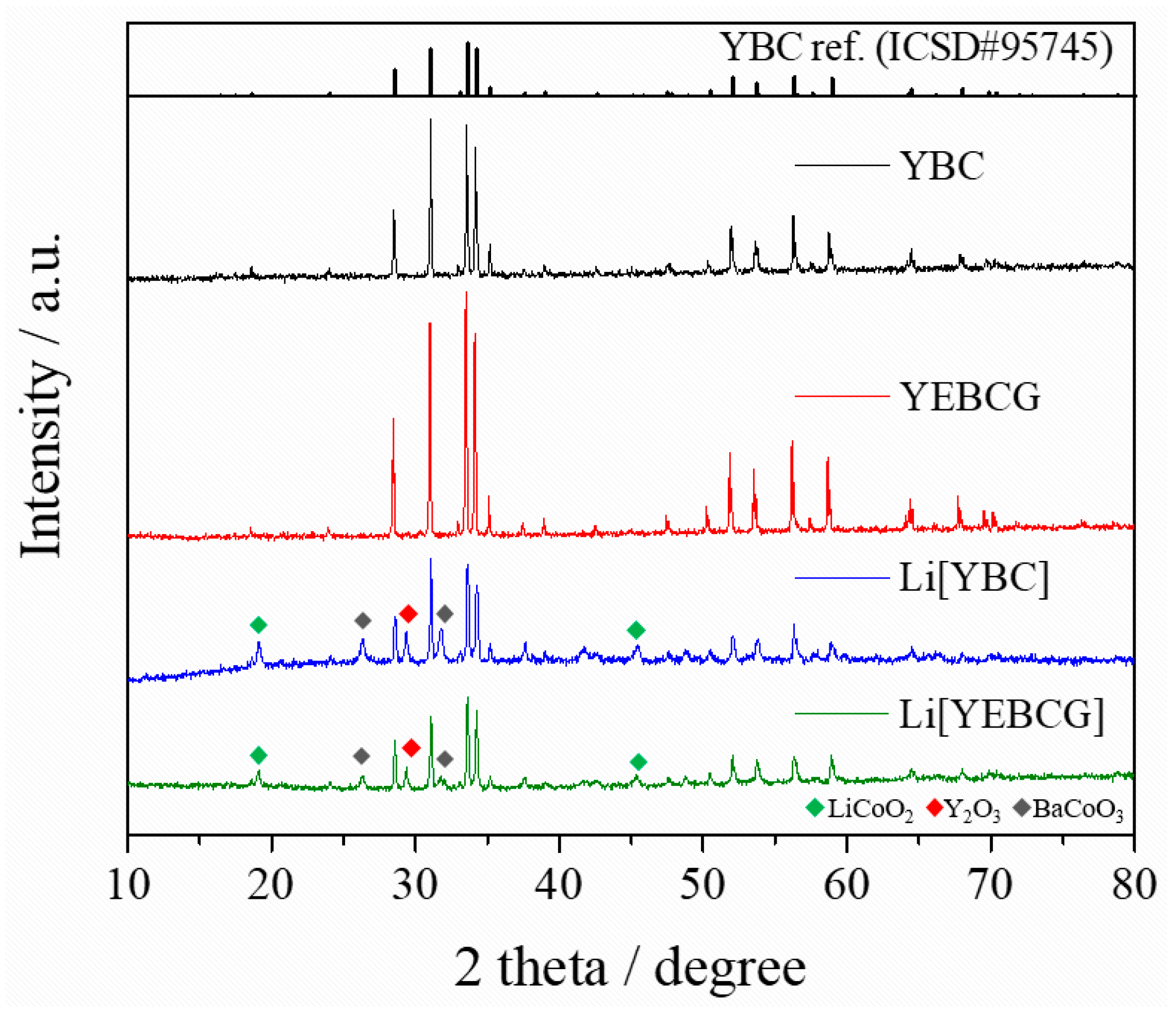
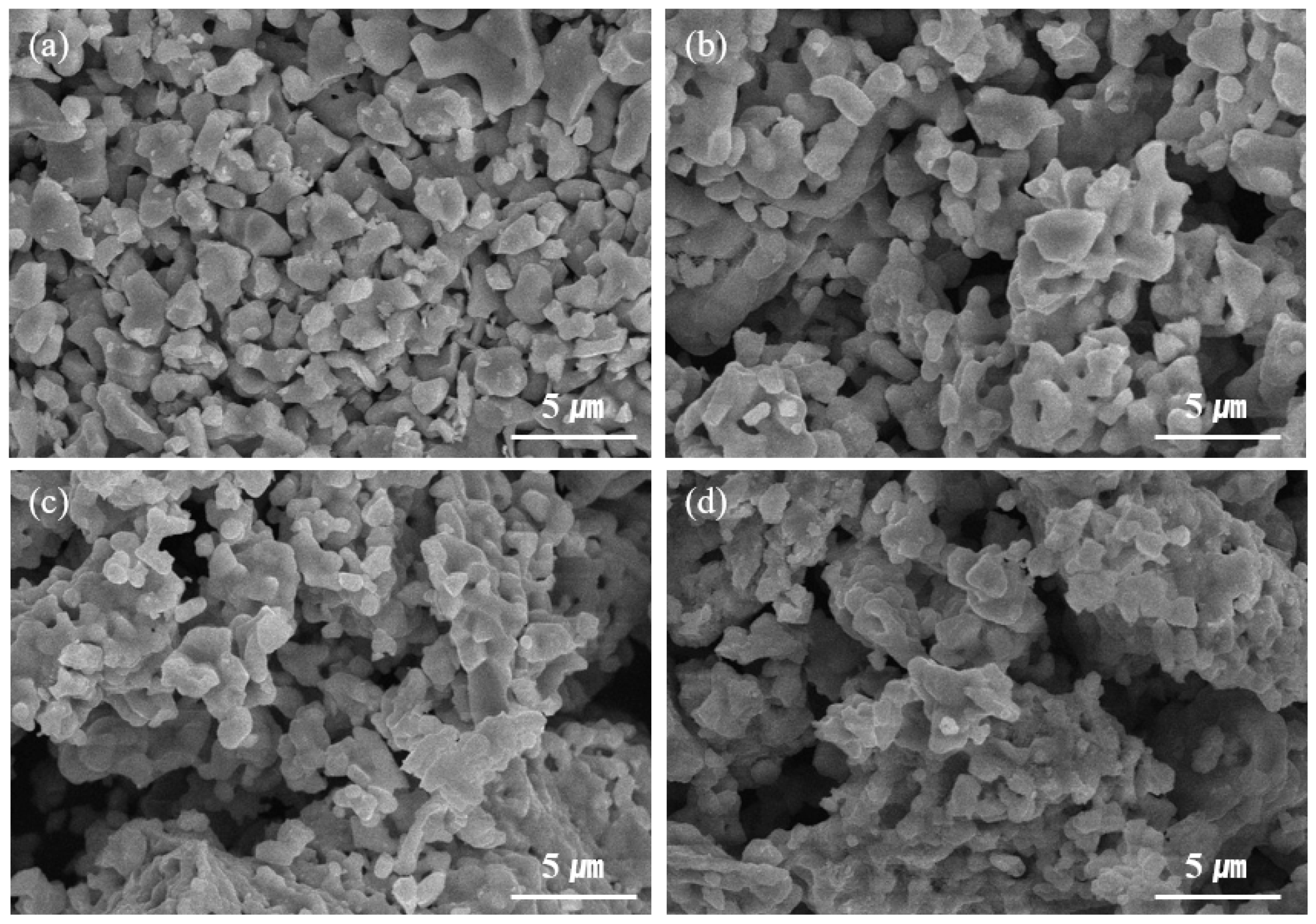
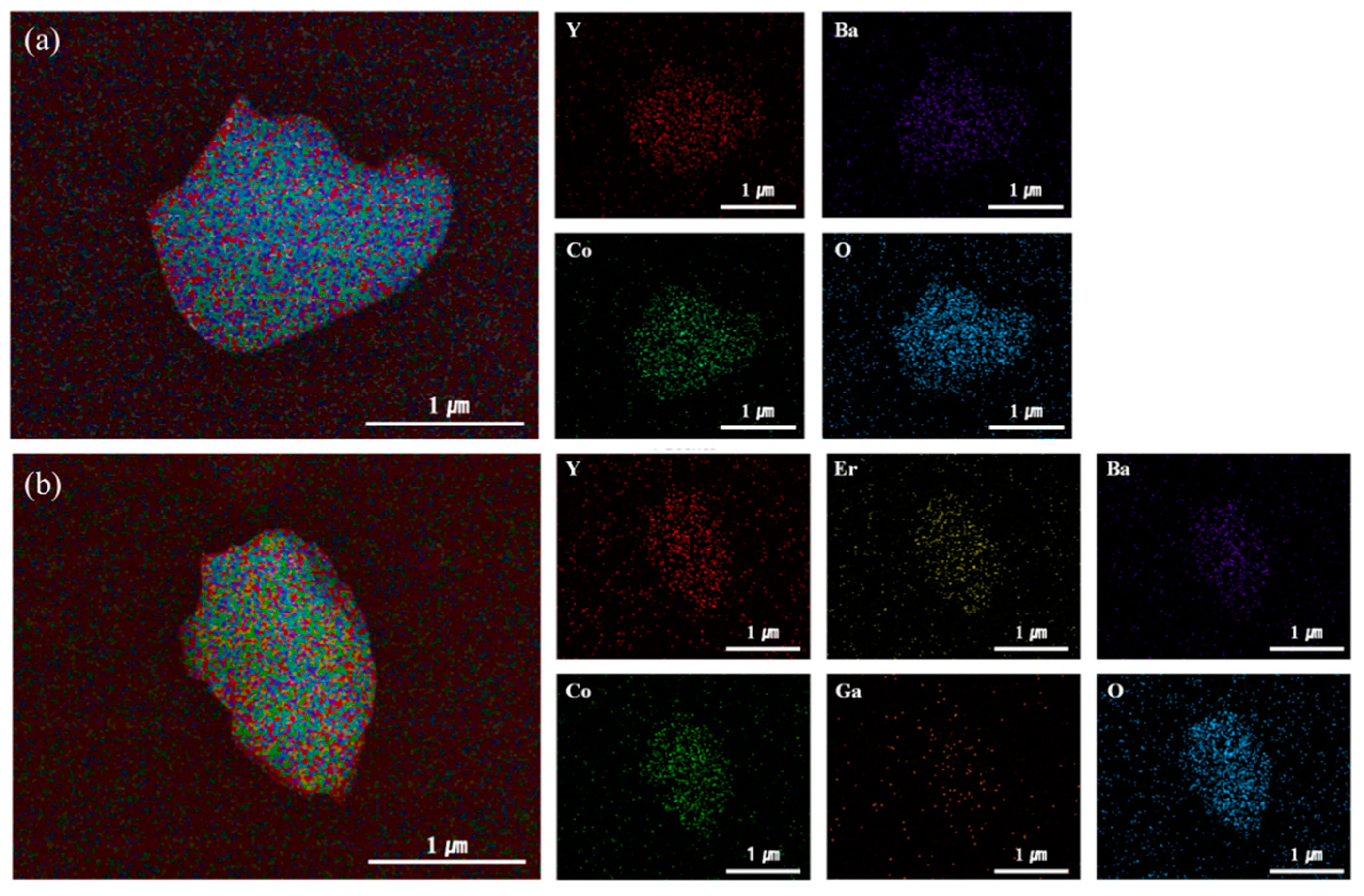
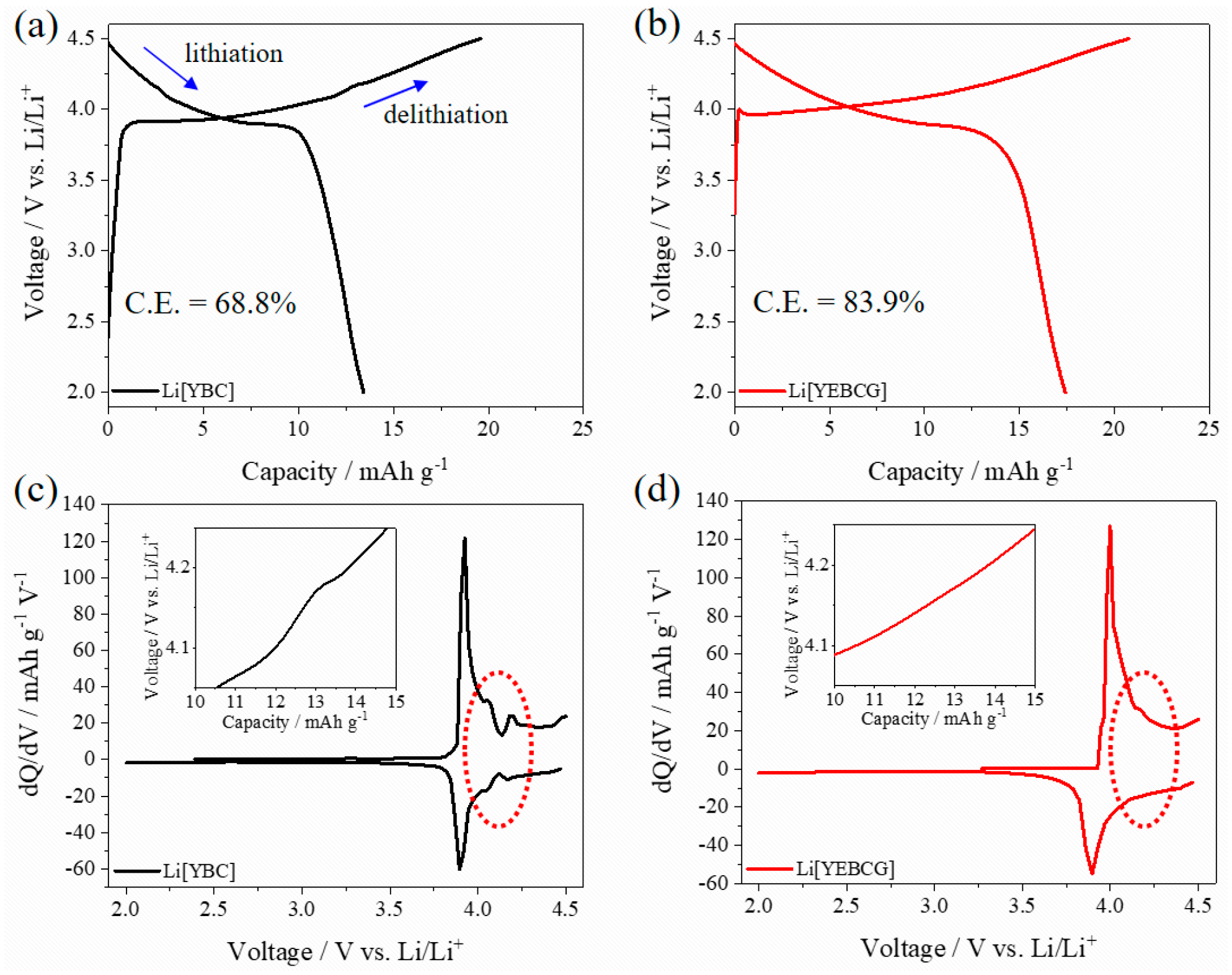
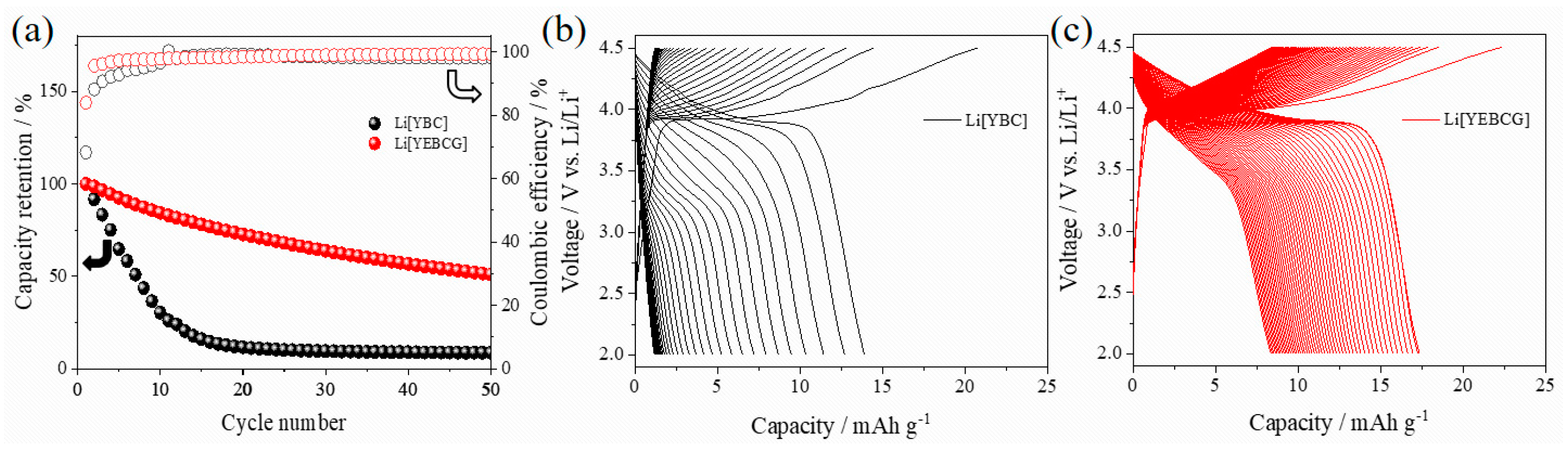
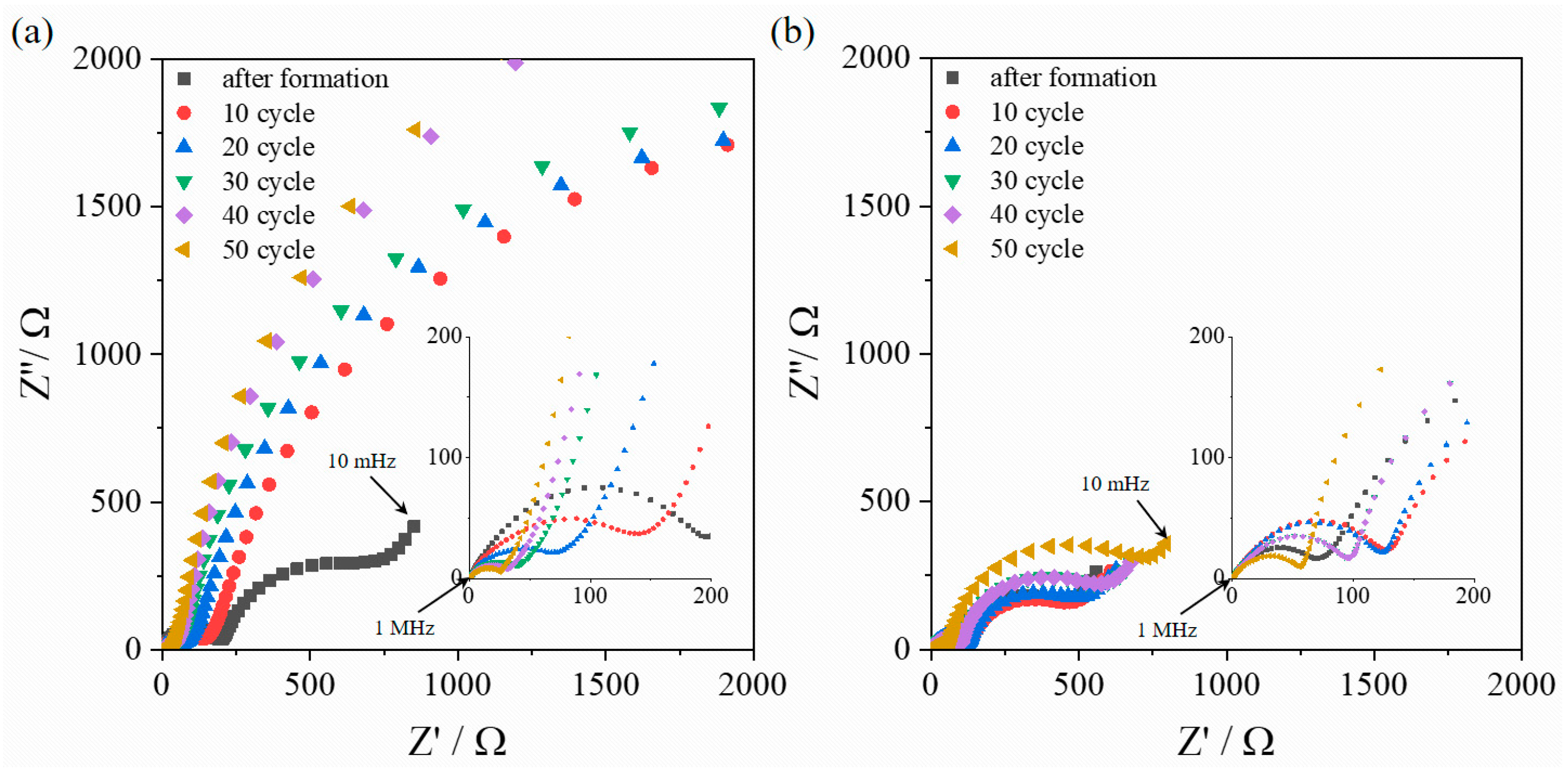
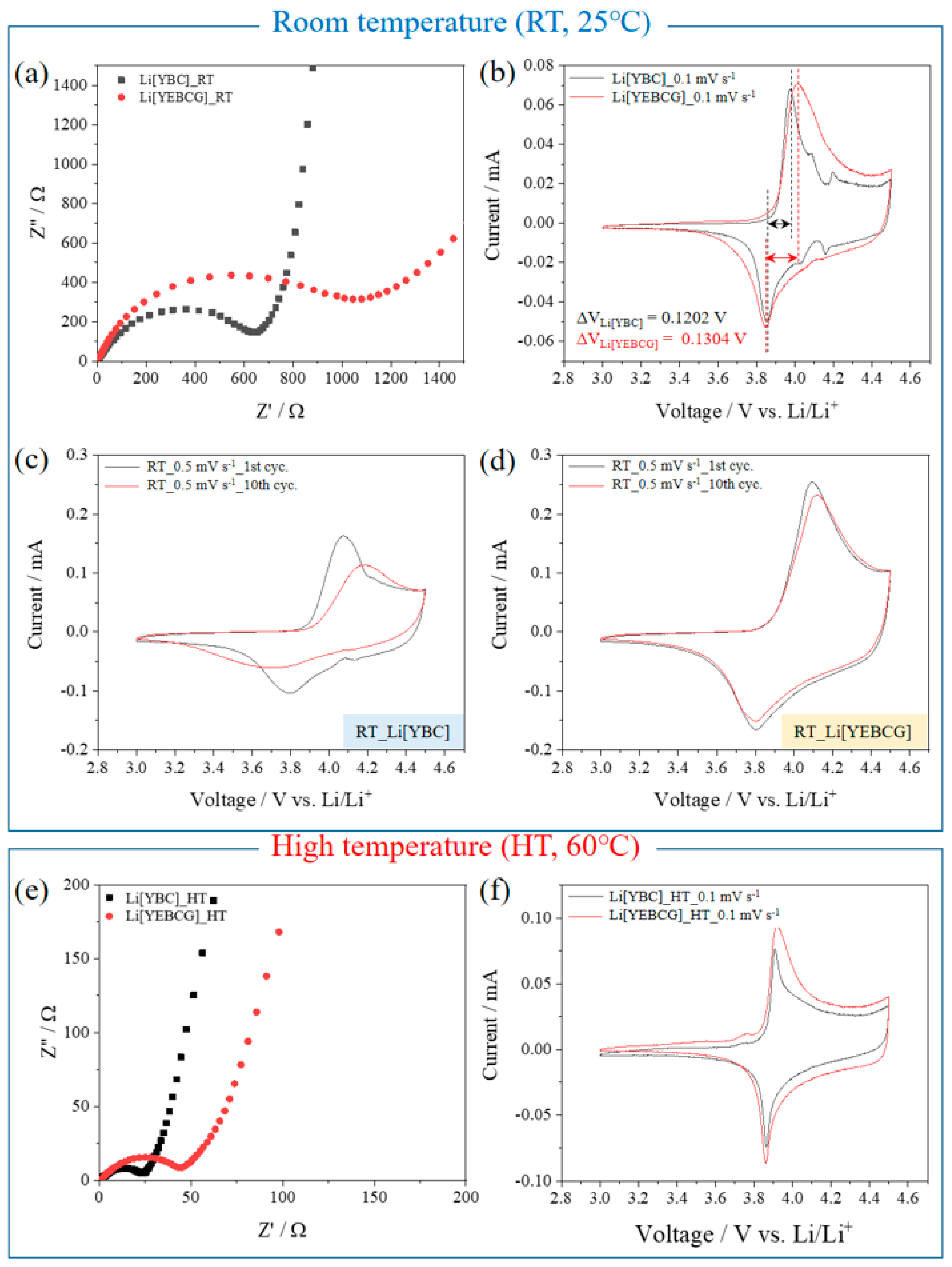
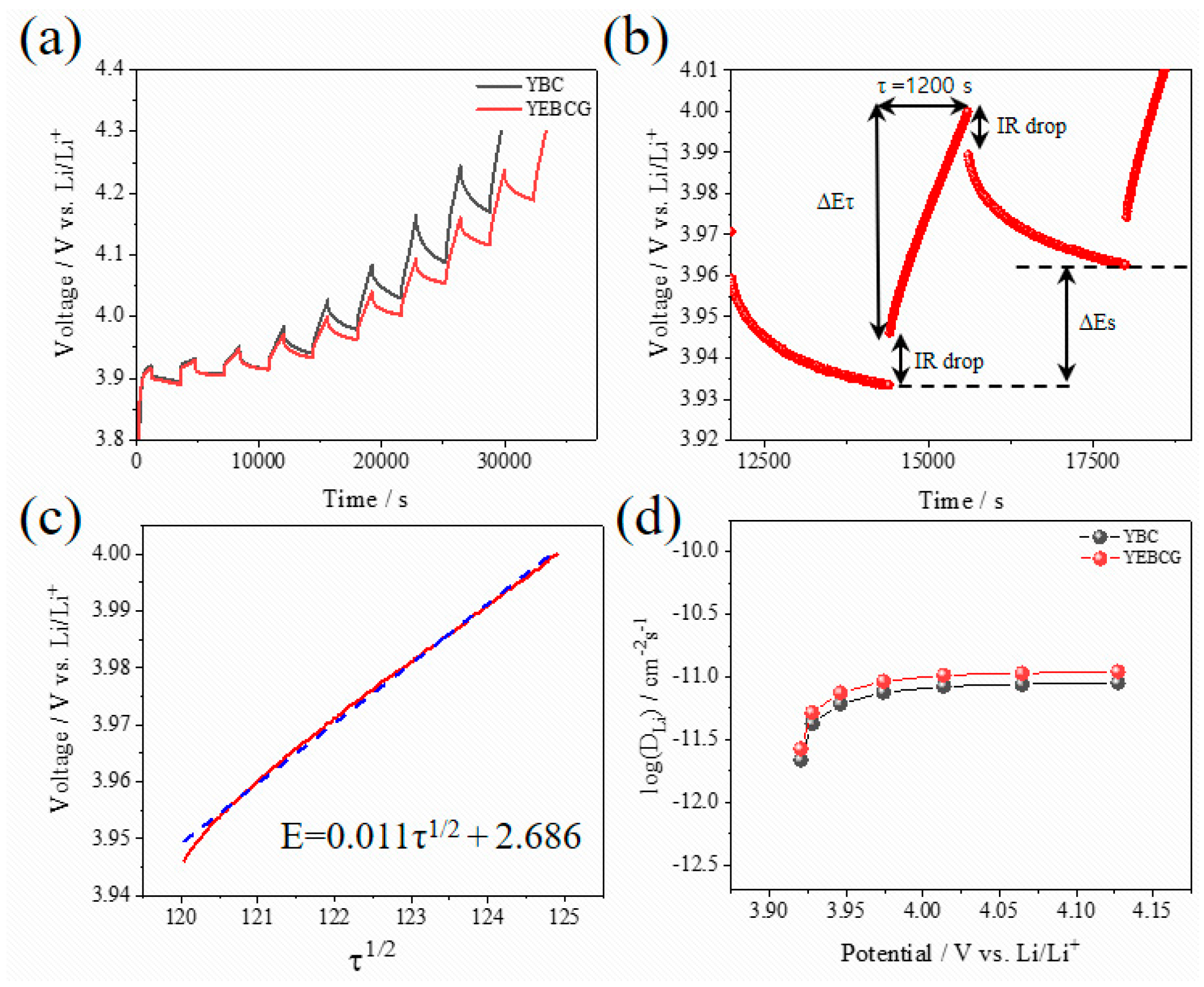
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, W.; Erickson, E.M.; Manthiram, A. High-Nickel Layered Oxide Cathodes for Lithium-Based Automotive Batteries. Nat. Energy 2020, 5, 26–34. [Google Scholar] [CrossRef]
- Xia, H.; Lu, L.; Meng, Y.S.; Ceder, G. Phase Transitions and High-Voltage Electrochemical Behavior of LiCoO2 Thin Films Grown by Pulsed Laser Deposition. J. Electrochem. Soc. 2007, 154, A337–A342. [Google Scholar] [CrossRef]
- Weigel, T.; Schipper, F.; Erickson, E.M.; Susai, F.A.; Markovsky, B.; Aurbach, D. Structural and Electrochemical Aspects of LiNi0.8Co0.1Mn0.1O2 Cathode Materials Doped by Various Cations. ACS Energy Lett. 2019, 4, 508–516. [Google Scholar] [CrossRef]
- Trease, N.M.; Seymour, I.D.; Radin, M.D.; Liu, H.; Hy, S.; Chernova, N.; Parikh, P.; Devaraj, A.; Wiaderek, K.M.; Chupas, P.J.; et al. Identifying the Distribution of Al3+ in LiNi0.8Co0.15Al0.05O2. Chem. Mater. 2016, 28, 8170–8180. [Google Scholar] [CrossRef] [Green Version]
- Sivaprakash, S.; Majumder, S.B. Understanding the Role of Zr4+ Cation in Improving the Cycleability of LiNi0.8Co0.15Zr0.05O2 Cathodes for Li Ion Rechargeable Batteries. J. Alloys Compd. 2009, 479, 561–568. [Google Scholar] [CrossRef]
- Chen, T.; Li, X.; Wang, H.; Yan, X.; Wang, L.; Deng, B.; Qu, M. The Effect of Gradient Boracic Polyanion-Doping on Structure, Morphology, and Cycling Performance of Ni-rich LiNi0.8Co0.15Al0.05O2 Cathode Material. J. Power Sources 2018, 374, 1–11. [Google Scholar] [CrossRef]
- Woo, S.U.; Park, B.C.; Yoon, C.S.; Myung, S.T.; Prakash, J.; Sun, Y.K. Improvement of Electrochemical Performances of LiNi0.8Co0.1Mn0.1O2 Cathode Materials by Fluorine Substitution. J. Electrochem. Soc. 2007, 154, A649–A655. [Google Scholar] [CrossRef]
- Becker, D.; Borner, M.; Nolle, R.; Diehl, M.; Klein, S.; Rodehorst, U.C.; Placke, T. Surface Modification of Ni-rich LiNi0.8Co0.1Mn0.1O2 Cathode Material by Tungsten Oxide Coating for Improved Electrochemical Performance in Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 18404–18414. [Google Scholar] [CrossRef]
- Konishi, H.; Yoshikawa, M.; Hirano, T. The Effect of Thermal Stability for High Ni-Content Layer-Structured Cathode Materials, LiNi0.8Mn0.1−xCo0.1MoxO2 (x = 0, 0.02, 0.04). J. Power Sources 2013, 244, 23–28. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, E.; Chen, D.; Wu, M.; Han, S.; Huang, Q.; Yang, L.; Xiao, X.; Hu, Z. Decreasing Li/Ni Disorder and Improving the Electrochemical Performances of Ni-rich LiNi0.8Co0.1Mn0.1O2 by Ca Doping. Inorg. Chem. 2017, 56, 8355–8362. [Google Scholar] [CrossRef]
- Song, B.; Li, W.; Oh, S.M.; Manthiram, A. Long-Life Nickel-Rich Layered Oxide Cathodes with a Uniform Li2ZrO3 Surface Coating for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 9718–9725. [Google Scholar] [CrossRef]
- Schipper, F.; Bouzaglo, H.; Dixit, M.; Erickson, E.M.; Weigel, T.; Talianker, M.; Grinblat, J.; Erk, C. From Surface ZrO2 Coating to Bulk Zr Doping by High Temperature Annealing of Nickel-Rich Lithiated Oxides and Their Enhanced Electrochemical Performance in Lithium Ion Batteries. Adv. Energy Mater. 2018, 8, 1701682. [Google Scholar] [CrossRef]
- Min, K.; Park, K.; Park, S.Y.; Seo, S.W.; Choi, B.; Cho, E. Improved Electrochemical Properties of LiNi0.91Co0.06Mn0.03O2 Cathode Material via Li-Reactive Coating with Metal Phosphates. Sci. Rep. 2017, 7, 7151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, U.; Myung, S.T.; Yoon, C.S.; Sun, Y.K. Extending the Battery Life Using an Al-Doped Li[Ni0.76Co0.09Mn0.15]O2 Cathode with Concentration Gradients for Lithium Ion Batteries. ACS Energy Lett. 2017, 2, 1848–1854. [Google Scholar] [CrossRef]
- Ryu, H.H.; Park, K.J.; Yoon, C.S.; Sun, Y.K. Capacity Fading of Ni-Rich Li[NixCoyMn1−x−y]O2 (0.6 ≤ x ≤ 0.95) Cathodes for High-Energy-Density Lithium-Ion Batteries: Bulk or Surface Degradation. Chem. Mater. 2018, 30, 1155–1163. [Google Scholar] [CrossRef]
- Lim, J.M.; Hwang, T.; Kim, D.; Park, M.S.; Cho, K.; Cho, M. Intrinsic Origins of Crack Generation in Ni-Rich LiNi0.8Co0.1Mn0.1O2 Layered Oxide Cathode Material. Sci. Rep. 2017, 7, 39669. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.N.; Cho, S.M.; Wang, H.; Manthiram, A. Electrochemical Characterization of YBaCo3ZnO7 + Gd0.2Ce0.8O1.9 Composite Cathodes for Intermediate Temperature Solid Oxide Fuel Cells. Electrochim. Acta 2010, 55, 5312–5317. [Google Scholar] [CrossRef]
- Medvedev, D.; Lyagaeva, J.; Vdovin, G.; Beresnev, S.; Demin, A.; Tsiakaras, P. A Tape Calendering Method as an Effective Way for the Preparation of Proton Ceramic Fuel Cells with Enhanced Performance. Electrochim. Acta 2016, 210, 681–688. [Google Scholar] [CrossRef]
- Bhat, M.A.; Zargar, R.A.; Modi, A.; Arora, M.; Gaur, N.K. Structural, Electrical and Magnetic Features of Kagomé YBaCo4O7 System. Mater. Sci. Pol. 2016, 34, 786–793. [Google Scholar] [CrossRef] [Green Version]
- Tsvetkov, D.S.; Pralong, V.; Tsvetkova, N.S.; Zuev, A.Y. Oxygen Content and Thermodynamic Stability of YBaCo4O7±δ. Solid State Ion. 2015, 278, 1–4. [Google Scholar] [CrossRef]
- Lai, K.Y.; Manthiram, A. Phase Stability, Oxygen-Storage Capability, and Electrocatalytic Activity in Solid Oxide Fuel Cells of (Y, In, Ca) BaCo4–yGayO7+δ. Chem. Mater. 2016, 28, 9077–9087. [Google Scholar] [CrossRef]
- Shin, J.-S.; Park, H.; Park, K.; Saqib, M.; Jo, M.; Kim, J.H.; Lim, H.-T.; Kim, M.; Kim, J.; Park, J.-Y. Activity of Layered Swedenborgite Structured Y0.8Er0.2BaCo3.2Ga0.8O7+δ For Oxygen Electrode Reactions in at Intermediate Temperature Reversible Ceramic Cells. J. Mater. Chem. A 2021, 9, 607–621. [Google Scholar] [CrossRef]
- Goktepe, H.; Sahan, H.; Ulgen, A.; Patat, S. Synthesis and Electrochemical Properties of Carbon-Mixed LiEr0.02Fe0.98PO4 Cathode Material for Lithium-Ion Batteries. J. Mater. Sci. Technol. 2011, 27, 861–864. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, H.; Tan, M.; Hu, Y.; Shu, X.; Zhang, M.; Chen, B.; Liu, X. Er-Doped LiNi0.5Mn1.5O4 Cathode Material with Enhanced Cycling Stability for Lithium-Ion Batteries. Materials 2017, 10, 859. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Li, J.; Zhu, M.; Wang, L.; Kang, Y.; Dang, Z.; Yan, J. Enhanced Structural Stability and Electrochemical Performance of LiNi0.6Co0.2Mn0.2O2 Cathode Materials by Ga Doping. Materials 2021, 14, 1816. [Google Scholar] [CrossRef]
- Parkkima, O.; Karppinen, M. The YBaCo4O7+δ-Based Functional Oxide Material Family: A Review. Eur. J. Inorg. Chem. 2014, 4056–4067. [Google Scholar] [CrossRef]
- Kim, J.-H.; Manthiram, A. Low Thermal Expansion RBa(Co,M)4O7 Cathode Materials Based on Tetrahedral-Site Cobalt Ions for Solid Oxide Fuel Cells. Chem. Mater. 2010, 22, 822–831. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, K.J.; Kim, S.J.; Yoon, C.S.; Sun, Y.K. A Method of Increasing the Energy Density of Layered Ni-Rich Li[Ni1−2xCoxMnx]O2 cathodes (x = 0.05, 0.1, 0.2). J. Mater. Chem. A 2019, 7, 2694–2701. [Google Scholar] [CrossRef]
- Choi, W.; Shin, H.C.; Kim, J.M.; Choi, J.Y.; Yoon, W.S. Modeling and Applications of Electrochemical Impedance Spectroscopy (EIS) for Lithium-Ion Batteries. J. Electrochem. Sci. Technol. 2020, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Beak, M.; Park, S.; Kim, S.; Park, J.; Jeong, S.; Thirumalraj, B.; Jeong, G.; Kim, T.; Kwon, K. Effect of Na from the Leachate of Spent Li-Ion Batteries on the Properties of Resynthesized Li-Ion Battery Cathodes. J. Alloys Compd. 2021, 873, 159808–159816. [Google Scholar] [CrossRef]
- Jeong, S.; Park, S.; Beak, M.; Park, J.; Shon, J.-S.; Kwon, K. Effect of Residual Trace Amounts of Fe and Al in Li[Ni1/3Mn1/3Co1/3]O2 Cathode Active Material for the Sustainable Recycling of Lithium-Ion Batteries. Materials 2021, 14, 2464. [Google Scholar] [CrossRef] [PubMed]
- Shaju, K.M.; Rao, G.V.S.; Chowdari, B.V.R. Electrochemical Kinetic Studies of Li-ion in O2-Structured Li2/3(Ni1/3Mn2/3)O2 and Li(2/3)+x(Ni1/3Mn2/3)O2 by EIS and GITT. J. Electrochem. Soc. 2003, 150, A1–A13. [Google Scholar] [CrossRef]
- Zheng, J.M.; Shi, W.; Gu, M.; Xiao, J.; Zuo, P.J.; Wang, C.M.; Zhang, J.G. Electrochemical Kinetics and Performance of Layered Composite Cathode Material Li[Li0.2Ni0.2Mn0.6]O2. J. Electrochem. Soc. 2013, 160, A2212–A2219. [Google Scholar] [CrossRef]
- Peng, F.W.; Mu, D.Y.; Li, R.H.; Liu, Y.L.; Ji, Y.P.; Dai, C.S.; Ding, F. Impurity Removal with Highly Selective and Efficient Methods and the Recycling of Transition Metals from Spent Lithium-Ion Batteries. RSC Adv. 2019, 9, 21922–21930. [Google Scholar] [CrossRef] [Green Version]
- Shaju, K.M.; Rao, G.V.S.; Chowdari, B.V.R. EIS and GITT Studies on Oxide Cathodes, O2-Li(2/3)+x(Co0.15Mn0.85)O2 (x = 0 and 1/3). Electrochim. Acta 2003, 48, 2691–2703. [Google Scholar] [CrossRef]

| YBC | YEBCG | |
|---|---|---|
| a-axis (Å) | 6.2993 | 6.3097 |
| c-axis (Å) | 10.2534 | 10.2674 |
| Volume (Å3) | 352.3540 | 354.0059 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Park, K.; Shin, J.-S.; Ko, G.; Kim, W.; Park, J.-Y.; Kwon, K. Utilizing the Intrinsic Thermal Instability of Swedenborgite Structured YBaCo4O7+δ as an Opportunity for Material Engineering in Lithium-Ion Batteries by Er and Ga Co-Doping Processes. Materials 2021, 14, 4565. https://doi.org/10.3390/ma14164565
Park S, Park K, Shin J-S, Ko G, Kim W, Park J-Y, Kwon K. Utilizing the Intrinsic Thermal Instability of Swedenborgite Structured YBaCo4O7+δ as an Opportunity for Material Engineering in Lithium-Ion Batteries by Er and Ga Co-Doping Processes. Materials. 2021; 14(16):4565. https://doi.org/10.3390/ma14164565
Chicago/Turabian StylePark, Sanghyuk, Kwangho Park, Ji-Seop Shin, Gyeongbin Ko, Wooseok Kim, Jun-Young Park, and Kyungjung Kwon. 2021. "Utilizing the Intrinsic Thermal Instability of Swedenborgite Structured YBaCo4O7+δ as an Opportunity for Material Engineering in Lithium-Ion Batteries by Er and Ga Co-Doping Processes" Materials 14, no. 16: 4565. https://doi.org/10.3390/ma14164565






