The Effect of Excessive Sulfate in the Li-Ion Battery Leachate on the Properties of Resynthesized Li[Ni1/3Co1/3Mn1/3]O2
Abstract
:1. Introduction
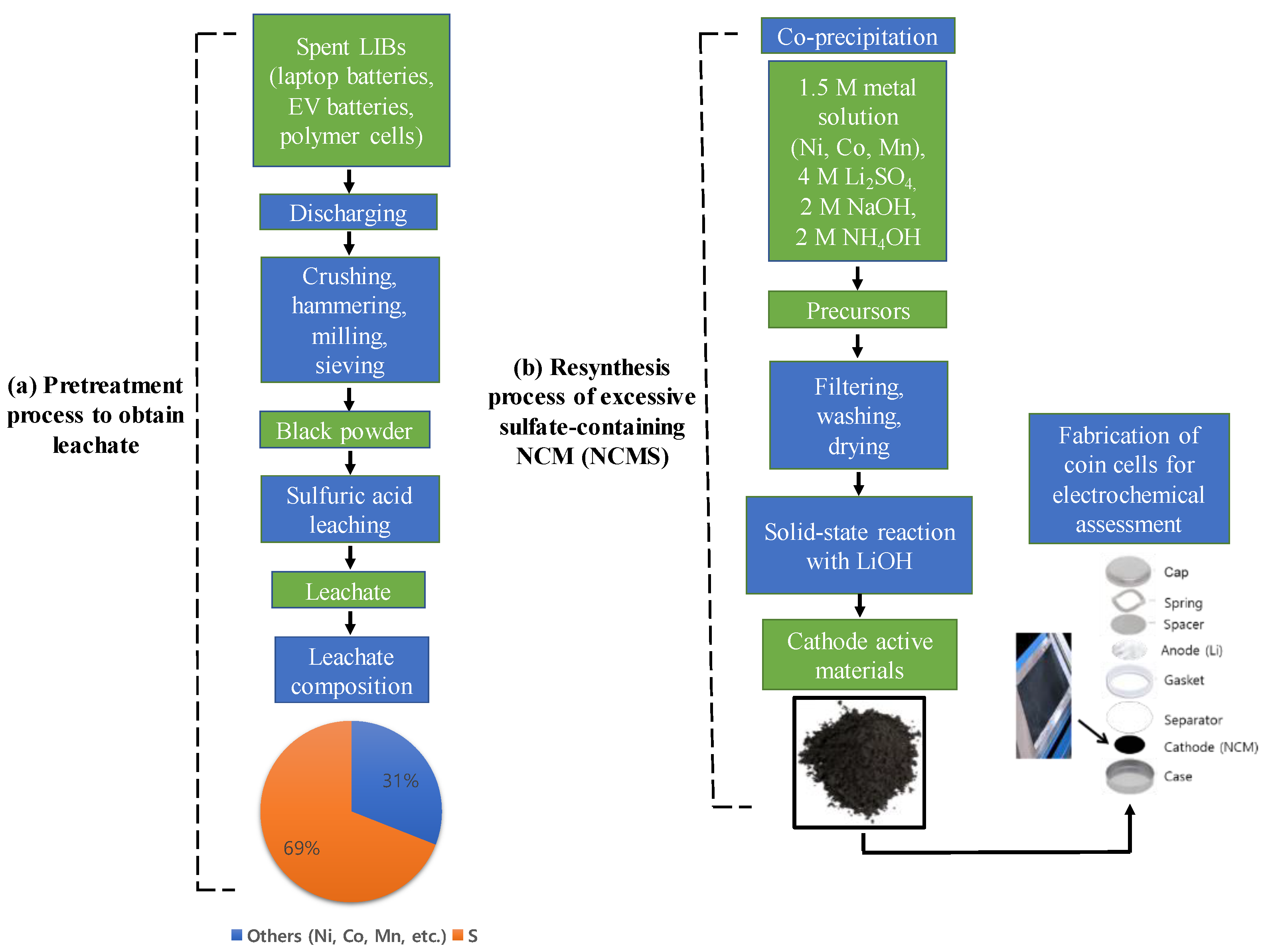
2. Experimental Section
2.1. Synthesis and Characterization of Materials
2.2. Electrochemical Analysis
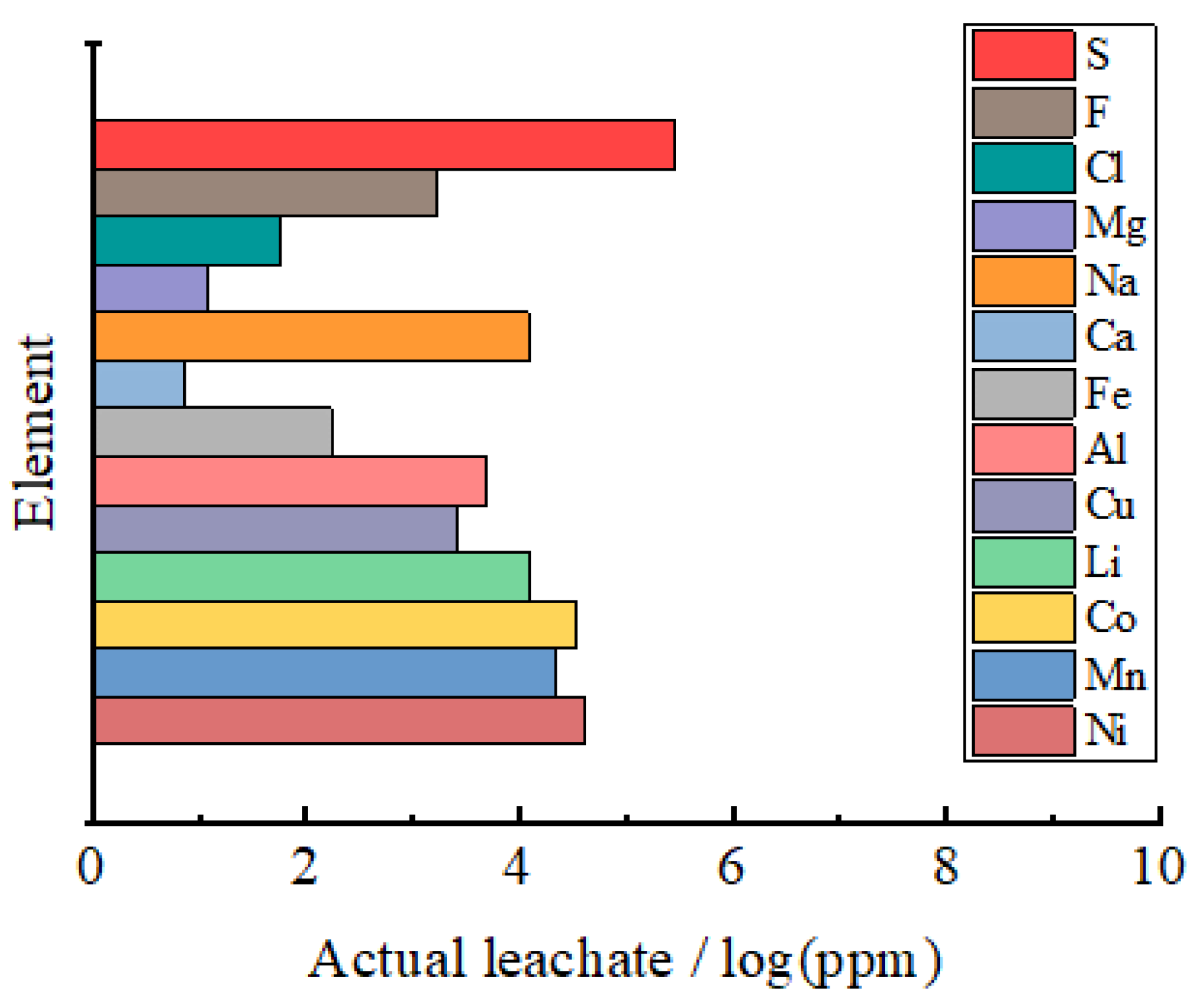
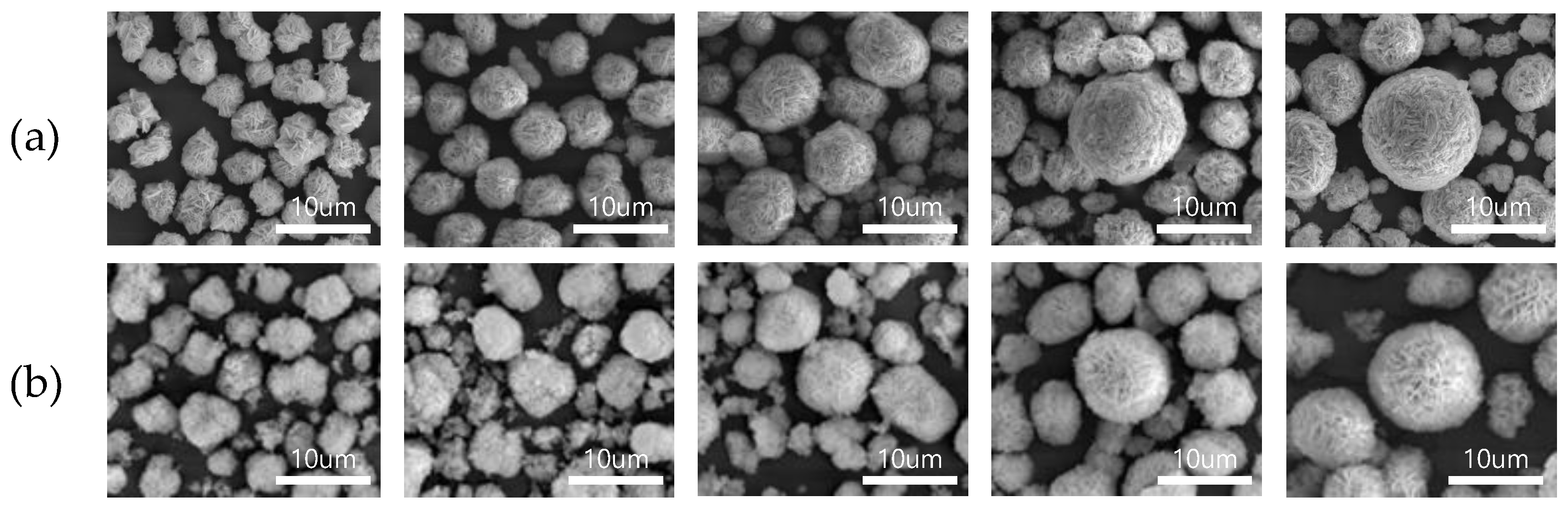
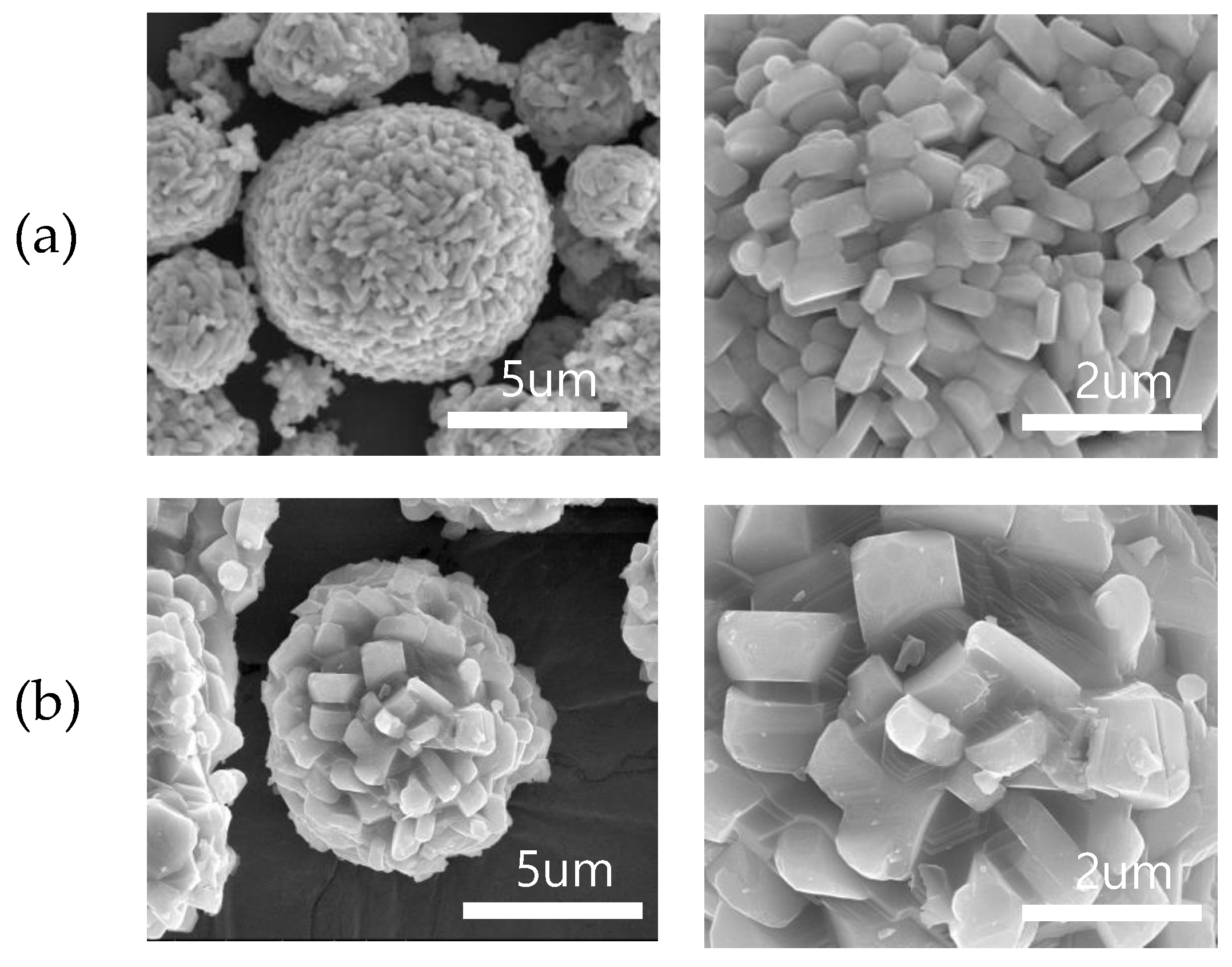
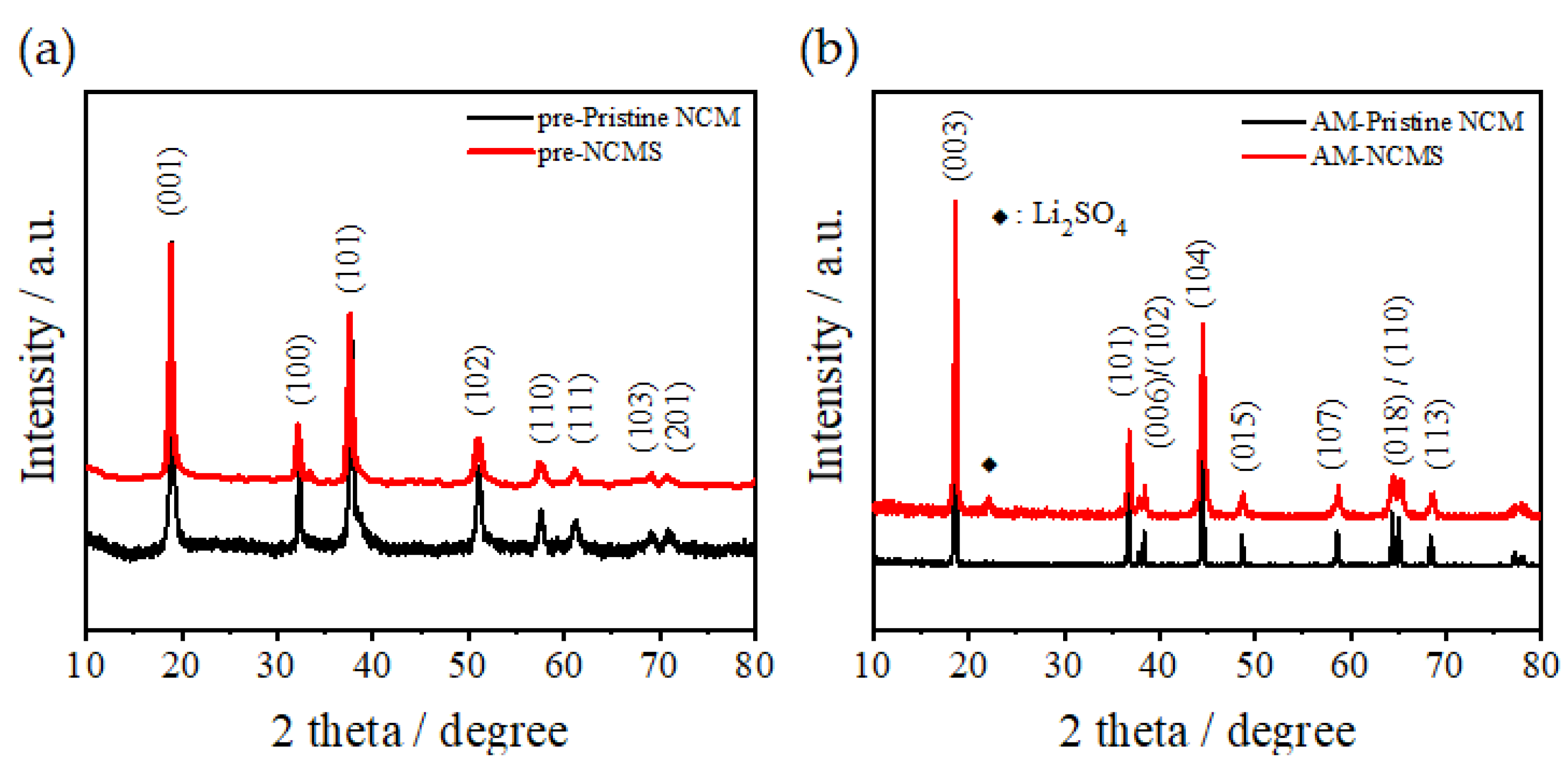
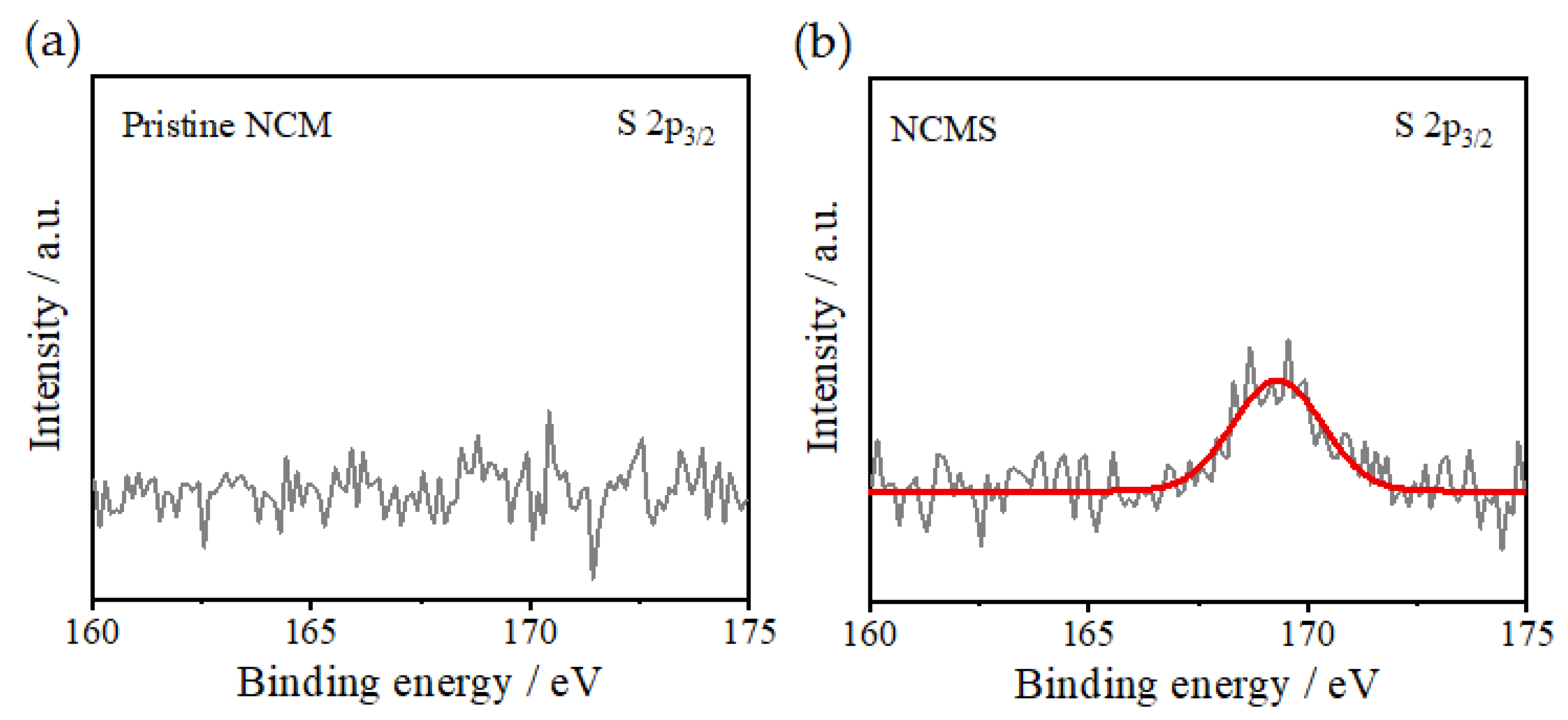
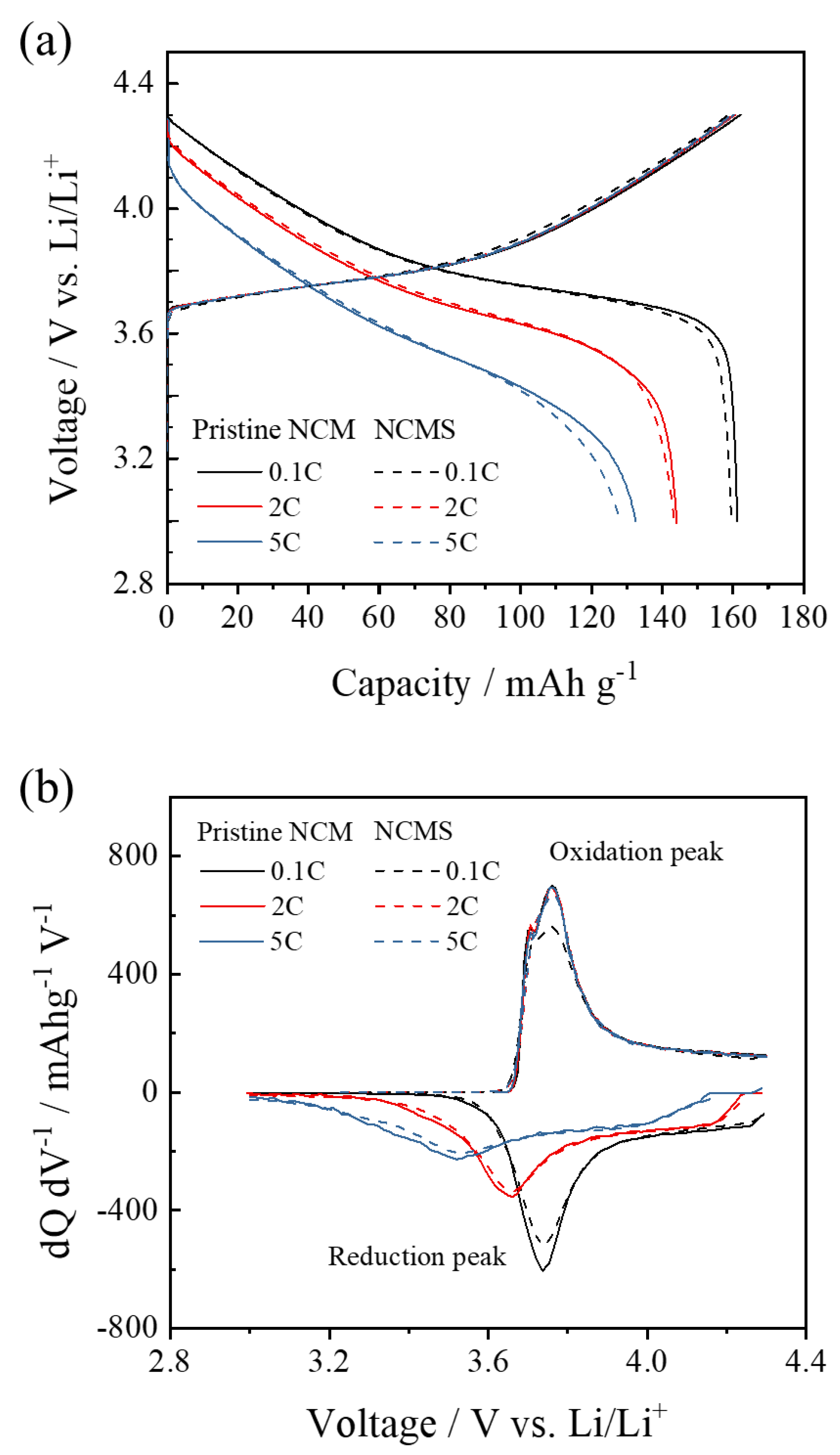
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manthiram, A. An outlook on lithium ion battery technology. ACS Cent. Sci. 2017, 3, 1063–1069. [Google Scholar] [CrossRef]
- Li, W.; Erickson, E.M.; Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 2020, 5, 26–34. [Google Scholar] [CrossRef]
- Xu, J.; Thomas, H.R.; Francis, R.W.; Lum, K.R.; Wang, J.; Liang, B. A review of processes and technologies for the recycling of lithium-ion secondary batteries. J. Power Sources 2008, 177, 512–527. [Google Scholar]
- Zeng, X.; Li, J.; Ren, Y. Prediction of various discarded lithium batteries in China. In Proceedings of the 2012 IEEE International Symposium on Sustainable Systems and Technology (ISSST), Boston, MA, USA, 16–18 May 2012; pp. 1–4. [Google Scholar]
- Contestabile, M.; Panero, S.; Scrosati, B. A laboratory-scale lithium-ion battery recycling process. J. Power Sources 2001, 92, 65–69. [Google Scholar]
- Ku, H.; Jung, Y.; Jo, M.; Park, S.; Kim, S.; Yang, D.; Rhee, K.; An, E.; Sohn, J.; Kwon, K. Recycling of spent lithium-ion battery cathode materials by ammoniacal leaching. J. Hazard. Mater. 2016, 313, 138–146. [Google Scholar] [CrossRef]
- Bruce, R.C. The role of hydrometallurgy in achieving sustainable development. Hydrometallurgy 1992, 30, 1–28. [Google Scholar]
- Yang, Y.; Sun, W.; Bu, Y.; Zhang, C.; Song, S.; Hu, Y. Recovering valuable metals from spent lithium ion battery via a combination of reduction thermal treatment and facile acid leaching. ACS Sustain. Chem. 2018, 6, 10445–10453. [Google Scholar]
- Kim, S.; Bang, J.; Yoo, J.; Shin, Y.; Bae, J.; Jeong, J.; Kim, K.; Dong, P.; Kwon, K. A comprehensive review on the pretreatment process in lithium-ion battery recycling. J. Clean. Prod. 2021, 294, 126329. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef]
- Li, D.F.; Wang, C.Y.; Yin, F.; Chen, Y.Q.; Yang, Y.Q.; Jie, X.W. Carbon reduction of lithium cobalt dioxide and its dissolution in sulfuric acid solution. Nonferrous Met. 2009, 61, 83–86. [Google Scholar]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Hydrometallurgical processing of spent lithium ion batteries (LIBs) in the presence of a reducing agent with emphasis on kinetics of leaching. Chem. Eng. J. 2015, 281, 418–427. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Process optimization and kinetics for leaching of rare earth metals from the spent Ni-metal hydride batteries. Waste Manag. 2016, 51, 196–203. [Google Scholar] [CrossRef]
- Zhang, P.; Yokoyama, T.; Itabashi, O.; Suzuki, T.M.; Inoue, K. Hydrometallurgical process for recovery of metal values from spent lithium-ion secondary batteries. Hydrometallurgy 1998, 47, 259–271. [Google Scholar] [CrossRef]
- Ban, L.; Yin, Y.; Zhuang, W.; Lu, H.; Wang, Z.; Lu, S. Electrochemical performance improvement of Li1.2[Mn0.54Ni0.13Co0.13]O2 cathode material by sulfur incorporation. Electrochim. Acta 2015, 180, 218–226. [Google Scholar] [CrossRef]
- Li, J.; Wu, J.; Li, S.; Liu, G.; Cui, Y.; Dong, Z.; Liu, H.; Sun, X. Stabilizing LiNi0.8Co0.15Mn0.05O2 cathode by doping sulfate for lithium-ion batteries. ChemSusChem 2021, 14, 2721–2730. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.; Ku, H.; Park, S.; Song, J.; Kwon, K. Effects of residual lithium in the precursors of Li[Ni1/3Co1/3Mn1/3]O2 on their lithium-ion battery performance. J. Phys. Chem. Solids 2018, 118, 47–52. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.; Jo, M.; Beak, M.; Park, J.; Jeong, G.; Yu, J.; Kwon, K. Electrochemical effects of residual Al in the resynthesis of Li[Ni1/3Mn1/3Co1/3]O2 cathode materials. J. Alloys Compd. 2021, 857, 157581. [Google Scholar]
- Jo, M.; Park, S.; Song, J.; Kwon, K. Incorporation of Cu into Li[Ni1/3Co1/3Mn1/3]O2 cathode: Elucidating its electrochemical properties and stability. J. Alloys Compd. 2018, 764, 112–121. [Google Scholar] [CrossRef]
- Beak, M.; Park, S.; Kim, S.; Park, J.; Jeong, S.; Thirumalraj, B.; Jeong, G.; Kim, T.; Kwon, K. Effect of Na from the leachate of spent Li-ion batteries on the properties of resynthesized Li-ion battery cathodes. J. Alloys Compd. 2021, 873, 159808. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, L.; Yu, Y.; Sun, J. Progress of enhancing the safety of lithium ion battery from the electrolyte aspect. Nano Energy 2019, 55, 93–114. [Google Scholar]
- Xiao, J.; Guo, J.; Zhan, L.; Xu, Z. A cleaner approach to the discharge process of spent lithium ion batteries in different solu-tions. J. Clean. Prod. 2020, 255, 120064. [Google Scholar] [CrossRef]
- Tang, X.; Tang, W.; Duan, J.; Yang, W.; Wang, R.; Tang, M.; Li, J. Recovery of valuable metals and modification of cathode materials from spent lithium-ion batteries. J. Alloys Compd. 2021, 874, 159853. [Google Scholar]
- Chen, D.; Kramer, D.; Mönig, R. Chemomechanical fatigue of LiMn1.95Al0.05O4 electrodes for lithium-ion batteries. Electrochim. Acta 2018, 259, 939–948. [Google Scholar] [CrossRef]
- Wagner, A.C.; Bohn, N.; Geßwein, H.; Neumann, M.; Osenberg, M.; Hilger, A.; Manke, I.; Schmidt, V.; Binder, J.R. Hierar-chical structuring of NMC111-cathode materials in lithium-ion batteries: An in-depth study on the influence of primary and secondary particle sizes on electrochemical performance. ACS Appl. Energy Mater. 2020, 3, 12565–12574. [Google Scholar] [CrossRef]
- Guan, X.Y.; Deng, J.C. Preparation and electrochemical performance of nano-scale nickel hydroxide with different shapes. Mater. Lett. 2007, 61, 621–625. [Google Scholar]
- Wang, B.; Zhang, F.-L.; Zhou, X.-A.; Wang, P.; Wang, J.; Ding, H.; Dong, H.; Liang, W.-B.; Zhang, N.-S.; Li, S.-Y. Which of the nickel-rich NCM and NCA is structurally superior as a cathode material for lithium-ion batteries? J. Mater. Chem. A 2021, 9, 13540. [Google Scholar] [CrossRef]
- Xu, G.; Li, J.; Xue, Q.; Dai, Y.; Zhou, H.; Xindong, W.; Kang, F. Elevated electrochemical performance of (NH4)3AlF6-coated 0.5Li2MnO3·0.5LiNi1/3Co1/3Mn1/3O2 cathode material via a novel wet coating method. Electrochim. Acta 2014, 117, 41–47. [Google Scholar] [CrossRef]
- Nefedov, V.-I. A comparison of results of an ESCA study of nonconducting solids using spectrometers of different constructions. J. Electron. Spectrosc. Relat. Phenom. 1982, 25, 29–47. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Park, S.; Beak, M.; Park, S.R.; Lee, A.R.; Byun, S.H.; Song, J.; Sohn, J.S.; Kwon, K. The Effect of Excessive Sulfate in the Li-Ion Battery Leachate on the Properties of Resynthesized Li[Ni1/3Co1/3Mn1/3]O2. Materials 2021, 14, 6672. https://doi.org/10.3390/ma14216672
Lee J, Park S, Beak M, Park SR, Lee AR, Byun SH, Song J, Sohn JS, Kwon K. The Effect of Excessive Sulfate in the Li-Ion Battery Leachate on the Properties of Resynthesized Li[Ni1/3Co1/3Mn1/3]O2. Materials. 2021; 14(21):6672. https://doi.org/10.3390/ma14216672
Chicago/Turabian StyleLee, Jimin, Sanghyuk Park, Mincheol Beak, Sang Ryul Park, Ah Reum Lee, Suk Hyun Byun, Junho Song, Jeong Soo Sohn, and Kyungjung Kwon. 2021. "The Effect of Excessive Sulfate in the Li-Ion Battery Leachate on the Properties of Resynthesized Li[Ni1/3Co1/3Mn1/3]O2" Materials 14, no. 21: 6672. https://doi.org/10.3390/ma14216672
APA StyleLee, J., Park, S., Beak, M., Park, S. R., Lee, A. R., Byun, S. H., Song, J., Sohn, J. S., & Kwon, K. (2021). The Effect of Excessive Sulfate in the Li-Ion Battery Leachate on the Properties of Resynthesized Li[Ni1/3Co1/3Mn1/3]O2. Materials, 14(21), 6672. https://doi.org/10.3390/ma14216672






