Valorization of Agri-Food Wastes as Sustainable Eco-Materials for Wastewater Treatment: Current State and New Perspectives
Abstract
:1. Introduction
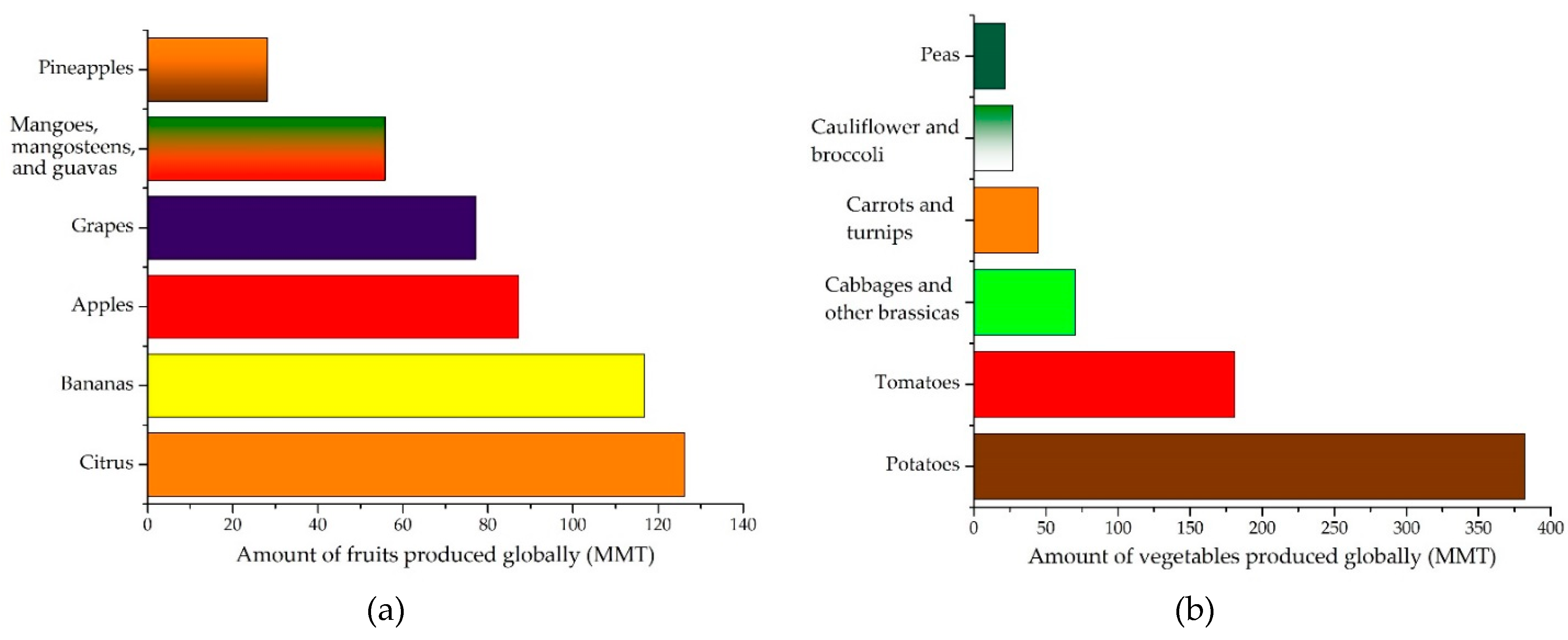
2. Agri-Food Waste Quantification
3. Environmental Impact of Fruit and Vegetable Wastes
4. Valorization of Agri-Food Wastes as Ecological Adsorbents
5. Types of Pollutants
6. Disadvantages-Research Gaps
7. Efficiency and Cost Comparison
8. Conclusions and Future Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef]
- Šeregelj, V.; Vulić, J.; Ćetković, G.; Čanadanovć-Brunet, J.; Šaponjac, V.T.; Stajčić, S. Natural bioactive compounds in carrot waste for food applications and health benefits. Stud. Nat. Prod. Chem. 2021, 67, 307–344. [Google Scholar] [CrossRef]
- Maicas, S.; Mateo, J.J. Sustainability of Wine Production. Sustainability 2020, 12, 559. [Google Scholar] [CrossRef] [Green Version]
- Omo-Okoro, P.N.; Daso, A.P.; Okonkwo, J.O. A review of the application of agricultural wastes as precursor materials for the adsorption of per-and polyfluoroalkyl substances: A focus on current approaches and methodologies. Environ. Technol. Innov. 2018, 9, 100–114. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M.; Witek-Krowiak, A. Agricultural waste peels as versatile biomass for water purification—A review. Chem. Eng. J. 2015, 270, 244–271. [Google Scholar] [CrossRef]
- Yusuf, M. Agro-industrial waste materials and their recycled value-added applications. Handb. Ecomater. 2017, 1–11. [Google Scholar] [CrossRef]
- Oliveira, L.S.; Franca, A.S. Low-cost adsorbents from agri-food wastes. In Food Science and Technology: New Research; Nova Science Publishers: Hauppauge, NY, USA, 2008; Chapter 3; pp. 171–209. ISBN 978-1-60456-715-1. [Google Scholar]
- Yadav, S.; Yadav, P.K.; Yadav, D.; Yadav, K.D.S. Purification and characterization of pectin lyase produced by Aspergillus terricola and its application in retting of natural fibers. Appl. Biochem. Biotechnol. 2009, 159, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Marinari, S.; Masciandaro, G.; Ceccanti, B.; Grego, S. Influence of organic and mineral fertilisers on soil biological and physical properties. Bioresour. Technol. 2000, 72, 9–17. [Google Scholar] [CrossRef]
- Schiewer, S.; Balaria, A. Biosorption of Pb2+ by original and protonated citrus peels: Equilibrium, kinetics, and mechanism. Chem. Eng. J. 2009, 146, 211–219. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Margiotoudis, I.; Massas, I. The use of olive tree pruning waste compost to sequestrate methylene blue dye from aqueous solution. Int. J. Phytoremediat. 2018, 20, 831–838. [Google Scholar] [CrossRef]
- Landin-Sandoval, V.; Mendoza-Castillo, D.; Bonilla-Petriciolet, A.; Aguayo-Villarreal, I.; Reynel-Avila, H.; Gonzalez-Ponce, H. Valorization of agri-food industry wastes to prepare adsorbents for heavy metal removal from water. J. Environ. Chem. Eng. 2020, 8, 104067. [Google Scholar] [CrossRef]
- Maranón, E.; Sastre, H. Ion exchange equilibria of heavy metals onto chemically modified apple residues. Solvent Extr. Ion Exch. 1991, 9, 515–531. [Google Scholar] [CrossRef]
- Orlando, U.; Baes, A.; Nishijima, W.; Okada, M. Preparation of agricultural residue anion exchangers and its nitrate maximum adsorption capacity. Chemosphere 2002, 48, 1041–1046. [Google Scholar] [CrossRef]
- Orlando, U.; Baes, A.; Nishijima, W.; Okada, M. A new procedure to produce lignocellulosic anion exchangers from agricultural waste materials. Bioresour. Technol. 2002, 83, 195–198. [Google Scholar] [CrossRef]
- Marshall, W.E.; Wartelle, L.H. Chromate (CrO42−) and copper (Cu2+) adsorption by dual-functional ion exchange resins made from agricultural by-products. Water Res. 2006, 40, 2541–2548. [Google Scholar] [CrossRef]
- Wartelle, L.H.; Marshall, W.E. Chromate ion adsorption by agricultural by-products modified with dimethyloldihydroxyethylene urea and choline chloride. Water Res. 2005, 39, 2869–2876. [Google Scholar] [CrossRef]
- Wartelle, L.H.; Marshall, W.E. Quaternized agricultural by-products as anion exchange resins. J. Environ. Manag. 2006, 78, 157–162. [Google Scholar] [CrossRef]
- Köse, T.E. Agricultural residue anion exchanger for removal of dyestuff from wastewater using full factorial design. Desalination 2008, 222, 323–330. [Google Scholar] [CrossRef]
- Johnson, T.A.; Jain, N.; Joshi, H.; Prasad, S. Agricultural and agro-processing wastes as low cost adsorbents for metal removal from wastewater: A review. J. Sci. Ind. Res. 2008, 67, 647–658. [Google Scholar]
- Garske, B.; Heyl, K.; Ekardt, F.; Weber, L.M.; Gradzka, W. Challenges of Food Waste Governance An Assessment of European Legislation on Food Waste and Recommendations for Improvement by Economic Instruments. Land 2020, 9, 231. [Google Scholar] [CrossRef]
- Papadaki, M.I.; Mendoza-Castillo, D.I.; Reynel-Avila, H.E.; Bonilla-Petriciolet, A.; Georgopoulos, S. Nut Shells as Adsorbents of Pollutants: Research and Perspectives. Front. Chem. Eng. 2021, 3, 19. [Google Scholar] [CrossRef]
- Ali, I.; Asim, M.; Khan, T.A. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.; Morin-Crini, N. Green adsorbents for pollutant removal. Environ. Chem. Sustain. World 2018, 18, 23–71. [Google Scholar]
- Otero, M.; Rozada, F.; Calvo, L.; Garcıa, A.; Moran, A. Elimination of organic water pollutants using adsorbents obtained from sewage sludge. Dye. Pigment. 2003, 57, 55–65. [Google Scholar] [CrossRef]
- De Laurentiis, V.; Corrado, S.; Sala, S. Quantifying household waste of fresh fruit and vegetables in the EU. Waste Manag. 2018, 77, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Vanham, D.; Bouraoui, F.; Leip, A.; Grizzetti, B.; Bidoglio, G. Lost water and nitrogen resources due to EU consumer food waste. Environ. Res. Lett. 2015, 10, 084008. [Google Scholar] [CrossRef] [Green Version]
- Östergren, K. FUSIONS Definitional Frameword for Food Waste (FP7-Rapport); The Swedish Institute for Food and Biotechnology: Goeteborg, Sweden, 2014; Contract Number: 311972; ISBN 978-91-7290-331-9. [Google Scholar]
- Kummu, M.; De Moel, H.; Porkka, M.; Siebert, S.; Varis, O.; Ward, P.J. Lost food, wasted resources: Global food supply chain losses and their impacts on freshwater, cropland, and fertiliser use. Sci. Total. Environ. 2012, 438, 477–489. [Google Scholar] [CrossRef]
- Pocketbook, F.S. World Food and Agriculture; FAO: Rome Italy, 2020. [Google Scholar] [CrossRef]
- Millati, R.; Cahyono, R.B.; Ariyanto, T.; Azzahrani, I.N.; Putri, R.U.; Taherzadeh, M.J. Agricultural, industrial, municipal, and forest wastes: An Overview. Sustain. Resour. Recovery Zero Waste Approaches 2019, 1–22. [Google Scholar] [CrossRef]
- Velmurugan, R.; Muthukumar, K. Utilization of sugarcane bagasse for bioethanol production: Sono-assisted acid hydrolysis approach. Bioresour. Technol. 2011, 102, 7119–7123. [Google Scholar] [CrossRef] [PubMed]
- Sewsynker-Sukai, Y.; Kana, E.G. Simultaneous saccharification and bioethanol production from corn cobs: Process optimization and kinetic studies. Bioresour. Technol. 2018, 262, 32–41. [Google Scholar] [CrossRef]
- Kim, M.; Kim, B.-C.; Choi, Y.; Nam, K. Minimizing mixing intensity to improve the performance of rice straw anaerobic digestion via enhanced development of microbe-substrate aggregates. Bioresour. Technol. 2017, 245, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Li, X.; Wachemo, A.C.; Yuan, H.; Liu, Y.; Zou, D.; Zuo, X.; Gu, J. Enhancing anaerobic digestion performance and degradation of lignocellulosic components of rice straw by combined biological and chemical pretreatment. Sci. Total Environ. 2018, 637, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Michelin, M.; Ruiz, H.A.; Maria de Lourdes, T.; Teixeira, J.A. Multi-step approach to add value to corncob: Production of biomass-degrading enzymes, lignin and fermentable sugars. Bioresour. Technol. 2018, 247, 582–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatti, H.; Sadaf, S.; Aleem, A. Treatment of textile effluents by low cost agricultural wastes: Batch biosorption study. J. Anim. Plant Sci. 2015, 25, 284–289. [Google Scholar]
- Miura, S.; Arimura, T.; Itoda, N.; Dwiarti, L.; Feng, J.B.; Bin, C.H.; Okabe, M. Production of L-lactic acid from corncob. J. Biosci. Bioeng. 2004, 97, 153–157. [Google Scholar] [CrossRef]
- Ragab, T.I.; Amer, H.; Mossa, A.T.; Emam, M.; Hasaballah, A.; Helmy, W.A. Anticoagulation, fibrinolytic and the cytotoxic activities of sulfated hemicellulose extracted from rice straw and husk. Biocatal. Agric. Biotechnol. 2018, 15, 86–91. [Google Scholar] [CrossRef]
- Besserer, A.; Troilo, S.; Girods, P.; Rogaume, Y.; Brosse, N. Cascading Recycling of Wood Waste: A Review. Polymers 2021, 13, 1752. [Google Scholar] [CrossRef]
- Younas, F.; Mustafa, A.; Farooqi, Z.U.R.; Wang, X.; Younas, S.; Mohy-Ud-Din, W.; Muhammad, A.H.; Muhammad, M.A.; Maitlo, A.A.; Noreen, S.; et al. Current and Emerging Adsorbent Technologies for Wastewater Treatment: Trends, Limitations, and Environmental Implications. Water 2021, 13, 215. [Google Scholar] [CrossRef]
- Pyrzynska, K. Removal of cadmium from wastewaters with low-cost adsorbents. J. Environ. Chem. Eng. 2019, 7, 102795. [Google Scholar] [CrossRef]
- Nor, N.M.; Lau, L.C.; Lee, K.T.; Mohamed, A.R. Synthesis of activated carbon from lignocellulosic biomass and its applications in air pollution control—a review. J. Environ. Chem. Eng. 2013, 1, 658–666. [Google Scholar] [CrossRef]
- Ten, E.; Vermerris, W. Functionalized polymers from lignocellulosic biomass: State of the art. Polymers 2013, 5, 600–642. [Google Scholar] [CrossRef] [Green Version]
- TOKUŞOĞLU, Ö. Agri-Food Chain Wastes and Food By-Products: Importance on NutriFood Chemistry and Anticarcinogenity. Food Health Technol. Innov. 2018, 1, 29–33. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from agri-food wastes: Present insights and future challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. Food Wastage Footprint, Impacts on Natural Resources, Summary Report. 2013. Available online: http://www.fao.org/3/i3347e/i3347e.pdf (accessed on 13 August 2021).
- Jenny Gustavsson, C.C.; Sonesson, U.; van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste—Extent, Causes and Prevention; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2011. [Google Scholar]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halada, K.; Yamamoto, R. The current status of research and development on ecomaterials around the world. MRS Bull. 2001, 26, 871–879. [Google Scholar] [CrossRef]
- Shahbandeh, M. Global Production of Fruit by Variety Selected 2019. Available online: https://www.statista.com/statistics/264001/worldwide-production-of-fruit-by-variety/ (accessed on 13 August 2021).
- Shahbandeh, M. Vegetables: Global Production Volume 2000–2019. Available online: https://www.statista.com/statistics/264059/production-volume-of-vegetables-and-melons-worldwide-since-1990/ (accessed on 13 August 2021).
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; Van Otterdijk, R.; Meybeck, A. Global food losses and waste: Extent, causes and prevention. In Proceedings of the a Study Conducted for International Congress “Save Food” at Interpack, Dusseldorf, Germany, 12–18 May 2011. [Google Scholar]
- Gowe, C. Review on potential use of fruit and vegetables by-products as a valuable source of natural food additives. Food Sci. Qual. Manag. 2015, 45, 47–61. [Google Scholar]
- Panouille, M.; Ralet, M.-C.; Bonnin, E.; Thibault, J.-F. Recovery and reuse of trimmings and pulps from fruit and vegetable processing. In Handbook of Waste Management and Co-Product Recovery in Food Processing; Elsevier: Amsterdam, The Netherlands, 2007; pp. 417–447. [Google Scholar]
- Ayala-Zavala, J.; Vega-Vega, V.; Rosas-Domínguez, C.; Palafox-Carlos, H.; Villa-Rodriguez, J.; Siddiqui, M.W.; Dávila-Aviña, J.; González-Aguilar, G. Agro-industrial potential of exotic fruit byproducts as a source of food additives. Food Res. Int. 2011, 44, 1866–1874. [Google Scholar] [CrossRef]
- Ravani, A.; Joshi, D. Standardization of processing parameters for the production of Ready-To-Serve unripe Mango beverage (Pana). J. Dairy Foods Home Sci. 2011, 30, 94–98. [Google Scholar]
- Schieber, A.; Stintzing, F.C.; Carle, R. By-products of plant food processing as a source of functional compounds—Recent developments. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- Del Borghi, A.; Moreschi, L.; Gallo, M. Circular economy approach to reduce water-energy-food nexus. Curr. Opin. Environ. Sci. Health 2020, 13, 23–28. [Google Scholar] [CrossRef]
- Scholz, K. Carbon Footprint of Retail Food Wastage; Independent Thessis: Uppsala, Sweden, 2013. [Google Scholar]
- Reay, D. Climate-Smart Bananas. In Climate-Smart Food; Springer International Publishing: New York City, NY, USA, 2019; pp. 81–91. [Google Scholar]
- El Barnossi, A.; Moussaid, F.; Housseini, A.I. Tangerine, banana and pomegranate peels valorisation for sustainable environment: A review. Biotechnol. Rep. 2020, 29. [Google Scholar] [CrossRef]
- Bell, E.M.; Horvath, A. Modeling the carbon footprint of fresh produce: Effects of transportation, localness, and seasonality on US orange markets. Environ. Res. Lett. 2020, 15, 034040. [Google Scholar] [CrossRef]
- Litskas, V.D.; Irakleous, T.; Tzortzakis, N.; Stavrinides, M.C. Determining the carbon footprint of indigenous and introduced grape varieties through Life Cycle Assessment using the island of Cyprus as a case study. J. Clean. Prod. 2017, 156, 418–425. [Google Scholar] [CrossRef]
- Dwyer, K.; Hosseinian, F.; Rod, M.R. The market potential of grape waste alternatives. J. Food Res. 2014, 3, 91. [Google Scholar] [CrossRef]
- Page, G.; Kelly, T.; Minor, M.; Cameron, E. Modeling carbon footprints of organic orchard production systems to address carbon trading: An approach based on life cycle assessment. Hortscience 2011, 46, 324–327. [Google Scholar] [CrossRef]
- Galanakis, C.M. Valorization of Fruit Processing By-Products; Academic Press: Chania, Greece, 2019. [Google Scholar]
- Carneiro, J.M.; Dias, A.F.; da Silva Barros, V.; Giongo, V.; Matsuura, M.I.d.S.F.; de Figueirêdo, M.C.B. Carbon and water footprints of Brazilian mango produced in the semiarid region. Int. J. Life Cycle Assess. 2019, 24, 735–752. [Google Scholar] [CrossRef]
- Durán-Aranguren, D.; Barrera, D.; Carreño, L.; Ríos, J.; Saavedra, D.; Morantes, G.; Sierra, R. Mango Fruit Waste: An Amazing Biorefinery Opportunity. Available online: http://www.etaflorence.it/proceedings/?detail=16339 (accessed on 13 August 2021). [CrossRef]
- Ntinas, G.K.; Neumair, M.; Tsadilas, C.D.; Meyer, J. Carbon footprint and cumulative energy demand of greenhouse and open-field tomato cultivation systems under Southern and Central European climatic conditions. J. Clean. Prod. 2017, 142, 3617–3626. [Google Scholar] [CrossRef]
- Løvdal, T.; Van Droogenbroeck, B.; Eroglu, E.C.; Kaniszewski, S.; Agati, G.; Verheul, M.; Skipnes, D. Valorization of tomato surplus and waste fractions: A case study using Norway, Belgium, Poland, and Turkey as examples. Foods 2019, 8, 229. [Google Scholar] [CrossRef] [Green Version]
- Röös, E.; Sundberg, C.; Hansson, P.-A. Uncertainties in the carbon footprint of food products: A case study on table potatoes. Int. J. Life Cycle Assess. 2010, 15, 478–488. [Google Scholar] [CrossRef]
- Jagtap, S.; Bhatt, C.; Thik, J.; Rahimifard, S. Monitoring potato waste in food manufacturing using image processing and internet of things approach. Sustainability 2019, 11, 3173. [Google Scholar] [CrossRef] [Green Version]
- Samotyja, U. Potato Peel as a Sustainable Resource of Natural Antioxidants for the Food Industry. Potato Res. 2019, 62, 435–451. [Google Scholar] [CrossRef] [Green Version]
- Healabel. Available online: https://healabel.com/c-ingredients/cabbage (accessed on 7 February 2021).
- Rogers, G.; Ekman, J.; Titley, M. Identifying New Products, Uses and Markets for Australian Vegetables: A Desktop Study; Horticulture Australia Ltd: Sydney, Australia, 2013; pp. 30–32. ISBN 0 7341 3118 6. [Google Scholar]
- Kreft, C.; Schader, C.; Stolze, M.; Dumondel, M. Lebensmittelverluste in konventionellen und biologischen Gemüsewertschöpfungsketten in der Schweiz am Beispiel von Karotten. In Proceedings of the Grenzen der Qualitätsstrategie im Agrarsektor, Zurich, Switzerland, 1 January 2013; p. 25. [Google Scholar]
- Persiani, A.; Diacono, M.; Monteforte, A.; Montemurro, F. Agronomic performance, energy analysis, and carbon balance comparing different fertilization strategies in horticulture under Mediterranean conditions. Environ. Sci. Pollut. Res. 2019, 26, 19250–19260. [Google Scholar] [CrossRef] [PubMed]
- FAO. Medium-Term Outlook. In Prospects for Global Production and Trade in Bananas and Tropical Fruits 2019–2028; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Ahmad, F.; Zaidi, S. Potential Use of Agro/Food Wastes as Biosorbents in the Removal of Heavy Metals. In Emerging Contaminants; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Alalwan, H.A.; Kadhom, M.A.; Alminshid, A.H. Removal of heavy metals from wastewater using agricultural byproducts. J. Water Supply Res. Technol.AQUA 2020, 69, 99–112. [Google Scholar] [CrossRef]
- Achak, M.; Hafidi, A.; Ouazzani, N.; Sayadi, S.; Mandi, L. Low cost biosorbent “banana peel” for the removal of phenolic compounds from olive mill wastewater: Kinetic and equilibrium studies. J. Hazard. Mater. 2009, 166, 117–125. [Google Scholar] [CrossRef]
- Bilal, M.; Shah, J.A.; Ashfaq, T.; Gardazi, S.M.H.; Tahir, A.A.; Pervez, A.; Haroon, H.; Mahmood, Q. Waste biomass adsorbents for copper removal from industrial wastewater—A review. J. Hazard. Mater. 2013, 263, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Kostoglou, M. Green adsorbents for wastewaters: A critical review. Materials 2014, 7, 333–364. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, J.; Yuan, Q.; Tang, H.; Yu, F.; Lv, X. A green adsorbent derived from banana peel for highly effective removal of heavy metal ions from water. RSC Adv. 2016, 6, 45041–45048. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Utilization of agro-industrial and municipal waste materials as potential adsorbents for water treatment—A review. Chem. Eng. J. 2010, 157, 277–296. [Google Scholar] [CrossRef]
- Feng, N.; Guo, X.; Liang, S. Adsorption study of copper (II) by chemically modified orange peel. J. Hazard. Mater. 2009, 164, 1286–1292. [Google Scholar] [CrossRef]
- Sha, L.; Xueyi, G.; Ningchuan, F.; Qinghua, T. Adsorption of Cu2+ and Cd2+ from aqueous solution by mercapto-acetic acid modified orange peel. Colloids Surf. B Biointerfaces 2009, 73, 10–14. [Google Scholar] [CrossRef]
- Hu, Z.; Srinivasan, M. Mesoporous high-surface-area activated carbon. Microporous Mesoporous Mater. 2001, 43, 267–275. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.; Cazorla-Amorós, D.; Linares-Solano, A. Understanding chemical reactions between carbons and NaOH and KOH: An insight into the chemical activation mechanism. Carbon 2003, 41, 267–275. [Google Scholar] [CrossRef]
- Hayashi, J.i.; Horikawa, T.; Takeda, I.; Muroyama, K.; Ani, F.N. Preparing activated carbon from various nutshells by chemical activation with K2CO3. Carbon 2002, 40, 2381–2386. [Google Scholar] [CrossRef]
- Williams, P.T.; Reed, A.R. Development of activated carbon pore structure via physical and chemical activation of biomass fibre waste. Biomass Bioenergy 2006, 30, 144–152. [Google Scholar] [CrossRef]
- Carrott, P.; Carrott, M.R. Lignin—From natural adsorbent to activated carbon: A review. Bioresour. Technol. 2007, 98, 2301–2312. [Google Scholar]
- Yahya, M.A.; Al-Qodah, Z.; Ngah, C.Z. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renew. Sustain. Energy Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Giraldo, L.; Moreno-Piraján, J.C. Synthesis of activated carbon mesoporous from coffee waste and its application in adsorption zinc and mercury ions from aqueous solution. E-J. Chem. 2012, 9, 938–948. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Chang, C.; Lee, S. A low cost adsorbent from agricultural waste corn cob by zinc chloride activation. Bioresour. Technol. 1998, 64, 211–217. [Google Scholar] [CrossRef]
- Budinova, T.; Ekinci, E.; Yardim, F.; Grimm, A.; Björnbom, E.; Minkova, V.; Goranova, M. Characterization and application of activated carbon produced by H3PO4 and water vapor activation. Fuel Process. Technol. 2006, 87, 899–905. [Google Scholar] [CrossRef]
- Zhu, Z.l.; Li, A.M.; Xia, M.F.; Wan, J.N.; Zhang, Q.X. Preparation and characterization of polymer-based spherical activated carbons. Chin. J. Polym. Sci. 2008, 26, 645–651. [Google Scholar] [CrossRef]
- Lozano-Castello, D.; Lillo-Ródenas, M.; Cazorla-Amorós, D.; Linares-Solano, A. Preparation of activated carbons from Spanish anthracite: I. Activation by KOH. Carbon 2001, 39, 741–749. [Google Scholar] [CrossRef]
- Barakat, M. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Akpor, O.; Otohinoyi, D.; Olaolu, D.; Aderiye, B. Pollutants in wastewater effluents: Impacts and remediation processes. Int. J. Environ. Res. Earth Sci. 2014, 3, 050–059. [Google Scholar]
- Harvey, P.; Handley, H.; Taylor, M. Identification of the sources of metal (lead) contamination in drinking waters in north-eastern Tasmania using lead isotopic compositions. Environ. Sci. Pollut. Res. 2015, 22, 12276–12288. [Google Scholar] [CrossRef] [PubMed]
- Ali, M. Assessment of some water quality characteristics and determination of some heavy metals in Lake Manzala, Egypt. Egypt. J. Aquat. Biol. Fish. 2008, 12, 133–154. [Google Scholar] [CrossRef] [Green Version]
- Rashed, M.N. Adsorption technique for the removal of organic pollutants from water and wastewater. Org. Pollut-Monit. Risk Treat. 2013, 7, 167–194. [Google Scholar] [CrossRef] [Green Version]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Yadav, M.; Gupta, R.; Arora, G.; Yadav, P.; Srivastava, A.; Sharma, R.K. Current Status of Heavy Metal Contaminants and Their Removal/Recovery Techniques. In Contaminants in Our Water: Identification and Remediation Methods; ACS Publications: Washington, DC, USA, 2020; pp. 41–64. [Google Scholar]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Guechi, E.-K.; Hamdaoui, O. Evaluation of potato peel as a novel adsorbent for the removal of Cu (II) from aqueous solutions: Equilibrium, kinetic, and thermodynamic studies. Desalination Water Treat. 2016, 57, 10677–10688. [Google Scholar] [CrossRef]
- El-Azazy, M.; El-Shafie, A.S.; Issa, A.A.; Al-Sulaiti, M.; Al-Yafie, J.; Shomar, B.; Al-Saad, K. Potato peels as an adsorbent for heavy metals from aqueous solutions: Eco-structuring of a green adsorbent operating Plackett–Burman design. J. Chem. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Feizi, M.; Jalali, M. Removal of heavy metals from aqueous solutions using sunflower, potato, canola and walnut shell residues. J. Taiwan Inst. Chem. Eng. 2015, 54, 125–136. [Google Scholar] [CrossRef]
- Moreno-Piraján, J.; Giraldo, L. Activated carbon obtained by pyrolysis of potato peel for the removal of heavy metal copper (II) from aqueous solutions. J. Anal. Appl. Pyrolysis 2011, 90, 42–47. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Deliyanni, E.A.; Matis, K.A. Activated carbons produced by pyrolysis of waste potato peels: Cobalt ions removal by adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2016, 490, 74–83. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bomis, G.; Kosheleva, R.I.; Efthimiadou, E.K.; Favvas, E.P.; Kostoglou, M.; Mitropoulos, A.C. Nanobubbles effect on heavy metal ions adsorption by activated carbon. Chem. Eng. J. 2019, 356, 91–97. [Google Scholar] [CrossRef]
- Aman, T.; Kazi, A.A.; Sabri, M.U.; Bano, Q. Potato peels as solid waste for the removal of heavy metal copper (II) from waste water/industrial effluent. Colloids Surf. B: Biointerfaces 2008, 63, 116–121. [Google Scholar] [CrossRef]
- Gutha, Y.; Munagapati, V.S.; Naushad, M.; Abburi, K. Removal of Ni (II) from aqueous solution by Lycopersicum esculentum (Tomato) leaf powder as a low-cost biosorbent. Desalination Water Treat. 2015, 54, 200–208. [Google Scholar] [CrossRef]
- Heraldy, E.; Lestari, W.W.; Permatasari, D.; Arimurti, D.D. Biosorbent from tomato waste and apple juice residue for lead removal. J. Environ. Chem. Eng. 2018, 6, 1201–1208. [Google Scholar] [CrossRef]
- Hossain, M.; Ngo, H.; Guo, W.; Nguyen, T.; Vigneswaran, S. Performance of cabbage and cauliflower wastes for heavy metals removal. Desalination Water Treat. 2014, 52, 844–860. [Google Scholar] [CrossRef]
- Nasernejad, B.; Zadeh, T.E.; Pour, B.B.; Bygi, M.E.; Zamani, A. Camparison for biosorption modeling of heavy metals (Cr (III), Cu (II), Zn (II)) adsorption from wastewater by carrot residues. Process. Biochem. 2005, 40, 1319–1322. [Google Scholar] [CrossRef]
- Qin, T.; Wang, Z.; Xie, X.; Xie, C.; Zhu, J.; Li, Y. A novel biochar derived from cauliflower (Brassica oleracea L.) roots could remove norfloxacin and chlortetracycline efficiently. Water Sci. Technol. 2017, 76, 3307–3318. [Google Scholar] [CrossRef]
- Potato News Today. Available online: https://www.potatonewstoday.com/2020/02/25/world-potato-congress-report-revised-fao-figures-show-slowdown-in-the-rise-of-global-potato-production/ (accessed on 16 December 2020).
- Sepelev, I.; Galoburda, R. Industrial potato peel waste application in food production: A review. Res Rural. Dev 2015, 1, 130–136. [Google Scholar]
- Sogi, D.; Bhatia, R.; Garg, S.; Bawa, A. Biological evaluation of tomato waste seed meals and protein concentrate. Food Chem. 2005, 89, 53–56. [Google Scholar] [CrossRef]
- Programme, U.N.E. Food Waste Index Report 2021; United Nations Environment Programme: Nairobi, Kenya, 2021. [Google Scholar]
- Westphal, A.; Bauerfeind, J.; Rohrer, C.; Böhm, V. Analytical characterisation of the seeds of two tomato varieties as a basis for recycling of waste materials in the food industry. Eur. Food Res. Technol. 2014, 239, 613–620. [Google Scholar] [CrossRef]
- Liang, J.L. Functional Properties of Wastes from Cabbage (Brassica Oleracea L. Var. Capitata) and Capsicum (Capsicum Annuum L. Var. Annum); UTAR: Perak, Malaysia, 2016. [Google Scholar]
- Khedkar, M.A.; Nimbalkar, P.R.; Chavan, P.V.; Chendake, Y.J.; Bankar, S.B. Cauliflower waste utilization for sustainable biobutanol production: Revelation of drying kinetics and bioprocess development. Bioprocess Biosyst. Eng. 2017, 40, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.J.; Kumar, D.; Orsat, V.; Singh, A. Assessment of carrot rejects and wastes for food product development and as a biofuel. Biomass Convers. Biorefin. 2020, 1–12. [Google Scholar] [CrossRef]
- Ajmal, M.; Rao, R.A.K.; Ahmad, R.; Ahmad, J. Adsorption studies on Citrus reticulata (fruit peel of orange): Removal and recovery of Ni (II) from electroplating wastewater. J. Hazard. Mater. 2000, 79, 117–131. [Google Scholar] [CrossRef]
- Li, X.; Tang, Y.; Cao, X.; Lu, D.; Luo, F.; Shao, W. Preparation and evaluation of orange peel cellulose adsorbents for effective removal of cadmium, zinc, cobalt and nickel. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 512–521. [Google Scholar] [CrossRef]
- Schiewer, S.; Patil, S.B. Pectin-rich fruit wastes as biosorbents for heavy metal removal: Equilibrium and kinetics. Bioresour. Technol. 2008, 99, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Annadurai, G.; Juang, R.-S.; Lee, D. Adsorption of heavy metals from water using banana and orange peels. Water Sci. Technol. 2003, 47, 185–190. [Google Scholar] [CrossRef]
- Razafsha, A.; Ziarati, P. Removal of heavy metals from Oryza sativa rice by sour lemon peel as bio-sorbent. Biomed. Pharmacol. J. 2016, 9, 543–553. [Google Scholar] [CrossRef]
- Dhakal, R.P.; Ghimire, K.N.; Inoue, K. Adsorptive separation of heavy metals from an aquatic environment using orange waste. Hydrometallurgy 2005, 79, 182–190. [Google Scholar] [CrossRef]
- Li, X.; Tang, Y.; Xuan, Z.; Liu, Y.; Luo, F. Study on the preparation of orange peel cellulose adsorbents and biosorption of Cd2+ from aqueous solution. Sep. Purif. Technol. 2007, 55, 69–75. [Google Scholar] [CrossRef]
- Namasivayam, C.; Muniasamy, N.; Gayatri, K.; Rani, M.; Ranganathan, K. Removal of dyes from aqueous solutions by cellulosic waste orange peel. Bioresour. Technol. 1996, 57, 37–43. [Google Scholar] [CrossRef]
- Fernandez, M.E.; Nunell, G.V.; Bonelli, P.R.; Cukierman, A.L. Activated carbon developed from orange peels: Batch and dynamic competitive adsorption of basic dyes. Ind. Crop. Prod. 2014, 62, 437–445. [Google Scholar] [CrossRef]
- Chen, J.Q.; Hu, Z.J.; Ji, R. Removal of carbofuran from aqueous solution by orange peel. Desalination Water Treat. 2012, 49, 106–114. [Google Scholar] [CrossRef]
- Xu, G.F.; Jing, H.M.; Guo, R.X. The adsorption isotherm studies of orange peel on pesticide furadan. In Proceedings of the Applied Mechanics and Materials, 2014; International Conference on Mechanics and Mechanical Engineering, Wuhan, China, 13–14 September 2014; pp. 1331–1335. [Google Scholar]
- Guo, X.Y.; Liang, S.; Tian, Q.H. Removal of heavy metal ions from aqueous solutions by adsorption using modified orange peel as adsorbent. Available online: https://www.scientific.net/AMR.236-238.237 (accessed on 13 August 2021).
- Nakajima, A.; Sakaguchi, T. Recovery and removal of uranium by using plant wastes. Biomass 1990, 21, 55–63. [Google Scholar] [CrossRef]
- Tasaso, P. Adsorption of copper using pomelo peel and depectinated pomelo peel. J. Clean Energy Technol. 2014, 2, 154–157. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Huang, G.; Deng, J.; Liu, K.; Xie, Y. Adsorbent prepared from waste pomelo peel and its adsorption of Pb2+ in wastewater. J. Ecol. Rural. Environ. 2012, 28, 187–191. [Google Scholar]
- Hou, S.X. Adsorption Properties of Pomelo Peels against Methylene Blue in Dye Wastewater. In Proceedings of the Advanced Materials Research. Available online: https://www.scientific.net/AMR.634-638.178 (accessed on 13 August 2021).
- Bello, O.S.; Ahmad, M.A.; Semire, B. Scavenging malachite green dye from aqueous solutions using pomelo (Citrus grandis) peels: Kinetic, equilibrium and thermodynamic studies. Desalination Water Treat. 2015, 56, 521–535. [Google Scholar] [CrossRef]
- Saeed, A.; Sharif, M.; Iqbal, M. Application potential of grapefruit peel as dye sorbent: Kinetics, equilibrium and mechanism of crystal violet adsorption. J. Hazard. Mater. 2010, 179, 564–572. [Google Scholar] [CrossRef]
- Zou, W.; Zhao, L.; Zhu, L. Efficient uranium (VI) biosorption on grapefruit peel: Kinetic study and thermodynamic parameters. J. Radioanal. Nucl. Chem. 2012, 292, 1303–1315. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Kumar, E.; Minocha, A.; Jeon, B.-H.; Song, H.; Seo, Y.-C. Removal of anionic dyes from water using Citrus limonum (lemon) peel: Equilibrium studies and kinetic modeling. Sep. Sci. Technol. 2009, 44, 316–334. [Google Scholar] [CrossRef]
- Tembhurkar, A.; Deshpande, R.P. Powdered Activated Lemon Peels as Adsorbent for Removal of Cutting Oil from Wastewater. J. Hazard. Toxic Radioact. Waste 2012, 16, 311–315. [Google Scholar] [CrossRef]
- Panadare, D.C.; Lade, V.G.; Rathod, V.K. Adsorptive removal of copper (II) from aqueous solution onto the waste sweet lime peels (SLP): Equilibrium, kinetics and thermodynamics studies. Desalination Water Treat. 2014, 52, 7822–7837. [Google Scholar] [CrossRef]
- Anwar, J.; Shafique, U.; Salman, M.; Dar, A.; Anwar, S. Removal of Pb (II) and Cd (II) from water by adsorption on peels of banana. Bioresour. Technol. 2010, 101, 1752–1755. [Google Scholar] [CrossRef] [PubMed]
- Amela, K.; Hassen, M.A.; Kerroum, D. Isotherm and kinetics study of biosorption of cationic dye onto banana peel. Energy Procedia 2012, 19, 286–295. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.R.; Gomes, T.F.; Andrade, G.C.; Monteiro, S.H.; Dias, A.C.; Zagatto, E.A.; Tornisielo, V.L. Banana peel as an adsorbent for removing atrazine and ametryne from waters. J. Agric. Food Chem. 2013, 61, 2358–2363. [Google Scholar] [CrossRef] [PubMed]
- Chaparadza, A.; Hossenlopp, J.M. Adsorption kinetics, isotherms and thermodynamics of atrazine removal using a banana peel based sorbent. Water Sci. Technol. 2012, 65, 940–947. [Google Scholar] [CrossRef]
- Mohammed, R.R.; Chong, M.F. Treatment and decolorization of biologically treated Palm Oil Mill Effluent (POME) using banana peel as novel biosorbent. J. Environ. Manag. 2014, 132, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Abdulfatai, J.; Saka, A.A.; Afolabi, A.S.; Micheal, O. Development of adsorbent from banana peel for wastewater treatment. In Proceedings of the Applied Mechanics and Materials, International Conference on Mechanics and Mechanical Engineering, Wuhan, China, 13–14 September 2013; pp. 310–315. [Google Scholar]
- Mallampati, R.; Valiyaveettil, S. Apple Peels A Versatile Biomass for Water Purification? ACS Appl. Mater. Interfaces 2013, 5, 4443–4449. [Google Scholar] [CrossRef]
- Iqbal, M.; Saeed, A.; Kalim, I. Characterization of adsorptive capacity and investigation of mechanism of Cu2+, Ni2+ and Zn2+ adsorption on mango peel waste from constituted metal solution and genuine electroplating effluent. Sep. Sci. Technol. 2009, 44, 3770–3791. [Google Scholar] [CrossRef]
- USDA. Citrus: World Markets and Trade. Global Market Analysis; United States Department of Agriculture-Foreign Agricultural Service: Washington, DC, USA, 2021.
- FAO. BANANA Market Review 2019; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Rana, G.K.; Singh, Y.; Mishra, S.; Rahangdale, H.K. Potential use of banana and its by-products: A review. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 1827–1832. [Google Scholar] [CrossRef]
- Shahbandeh, M. Apple Production Worldwide 2010–2019. Available online: https://www.statista.com/statistics/961248/production-of-apples-worldwide/ (accessed on 13 August 2021).
- Dhillon, G.S.; Kaur, S.; Brar, S.K. Perspective of apple processing wastes as low-cost substrates for bioproduction of high value products: A review. Renew. Sustain. Energy Rev. 2013, 27, 789–805. [Google Scholar] [CrossRef]
- Khan, N.; Fahad, S.; Naushad, M.; Faisal, S. Grape Production Critical Review in the World. SSRN 2020, SSRN:3595842. [Google Scholar] [CrossRef]
- Ioannou, L.; Puma, G.L.; Fatta-Kassinos, D. Treatment of winery wastewater by physicochemical, biological and advanced processes: A review. J. Hazard. Mater. 2015, 286, 343–368. [Google Scholar] [CrossRef] [PubMed]
- Chouchouli, V.; Kalogeropoulos, N.; Konteles, S.J.; Karvela, E.; Makris, D.P.; Karathanos, V.T. Fortification of yoghurts with grape (Vitis vinifera) seed extracts. LWT-Food Sci. Technol. 2013, 53, 522–529. [Google Scholar] [CrossRef]
- Shahbandeh, M. Global Mango Production 2000–2019. Available online: https://www.statista.com/statistics/577951/world-mango-production/ (accessed on 13 August 2021).
- Ramasamy, M.; Srirangarayan, R.S.; Shanmugasundaram, R.; Ramesh, P.; Shrirangasami, S.; Prasanthrajan, M.; Saravanakumar, S. Composting of Mango Wastes. Biot. Res. Today 2020, 2, 1034–1035. [Google Scholar]
- Sakaguchi, T.; Nakajima, A. Recovery of uranium from seawater by immobilized tannin. Sep. Sci. Technol. 1987, 22, 1609–1623. [Google Scholar] [CrossRef]
- Azargohar, R.; Dalai, A. Biochar as a precursor of activated carbon. In Proceedings of the Twenty-Seventh Symposium on Biotechnology for Fuels and Chemicals, Twenty-Seventh Symposium on Biotechnology for Fuels and Chemicals, Denver, CO, USA, 1–4 May 2005; pp. 762–773. [Google Scholar]
- Bello, O.S.; Ahmad, M.A.; Ahmad, N. Adsorptive features of banana (Musa paradisiaca) stalk-based activated carbon for malachite green dye removal. Chem. Ecol. 2012, 28, 153–167. [Google Scholar] [CrossRef]
- Dias, J.M.; Alvim-Ferraz, M.C.; Almeida, M.F.; Rivera-Utrilla, J.; Sánchez-Polo, M. Waste materials for activated carbon preparation and its use in aqueous-phase treatment: A review. J. Environ. Manag. 2007, 85, 833–846. [Google Scholar] [CrossRef]
- Getachew, T.; Hussen, A.; Rao, V. Defluoridation of water by activated carbon prepared from banana (Musa paradisiaca) peel and coffee (Coffea arabica) husk. Int. J. Environ. Sci. Technol. 2015, 12, 1857–1866. [Google Scholar] [CrossRef] [Green Version]
- Ioannidou, O.; Zabaniotou, A. Agricultural residues as precursors for activated carbon production—A review. Renew. Sustain. Energy Rev. 2007, 11, 1966–2005. [Google Scholar] [CrossRef]
- Mohammad, S.G.; Ahmed, S.M.; Badawi, A.F.M.; El-Desouki, D. Activated carbon derived from egyptian banana peels for removal of cadmium from water. J. Appl. Life Sci. Int. 2015, 3, 77–88. [Google Scholar] [CrossRef]
- Salman, J.; Hameed, B. Removal of insecticide carbofuran from aqueous solutions by banana stalks activated carbon. J. Hazard. Mater. 2010, 176, 814–819. [Google Scholar] [CrossRef] [PubMed]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef] [Green Version]
- Sulyman, M.; Namiesnik, J.; Gierak, A. Low-cost Adsorbents Derived from Agricultural By-products/Wastes for Enhancing Contaminant Uptakes from Wastewater: A Review. Pol. J. Environ. Stud. 2017, 26, 479–510. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.; Lo, W.-h.; Babel, S. Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci. Total Environ. 2006, 366, 409–426. [Google Scholar] [CrossRef]
- Bharathi, K.; Ramesh, S. Removal of dyes using agricultural waste as low-cost adsorbents: A review. Appl. Water Sci. 2013, 3, 773–790. [Google Scholar] [CrossRef] [Green Version]


| CF of Fruits and Vegetables | CF of Generated Wastes | ||
|---|---|---|---|
| Commodity | CF as CO2 Equiv. | Waste Data | CF as CO2 Equiv./kg [60] |
| Banana | 100–200 g CO2 equivalents (eq) per banana [61] | 30–40% peel (about 34.72–46.29 MMT, 2018) [62] | 5.7 |
| Citrus | 0.07–0.64 kg CO2 eq/kg produced with a median value of 0.29 [63] | About 15–25,000 t waste/year [1] | 2.1 as orange 0.5 as lemon 0.2 as grapefruit 0.25 other citrus |
| Grapes | 0.846 kg CO2 eq/kg [64] | 25% by-product/waste (as pomace, including skins, seeds from wine production) [65] | 0.9 |
| Apples | 2.4 to 5 t of CO2 eq/ha/year (New Zealand) [66] | 25% apple pomace (where: about 3% seeds, 95% skin and 1% stems) [67] | 1.2 |
| Mango | From 0.06 to 0.18 kg of CO2 eq/kg [68] | 25% to 40% of the raw material is left as a residue (from 260.000 t) [69] | 2.1 as exotic fruit |
| Tomatoes | Values varied between 0.1–10.1 CO2 eq/kg/year [70] | 3–7% raw material lost as waste (where about 10% seeds ) [71]. | 8.2 |
| Potatoes | 0.10–0.16 kg CO2 eq/kg with 95% certainty for an arbitrary year and field [72] | 5.8 million per day are thrown away (UK householders) [73] 15–40% peel of the initial potato mass as major waste [74] | 0.3 |
| Cabbage | 0.12 kg CO2 eq/kg [75] | 32.5% (13,406.25 t average annual) [76] | 0.1 |
| Carrots | N.D. | 30% waste resulted from processing step (from 60,214 t/year in Switzerland) [77] | 0.1 |
| Cauliflower | 3.67 kg/unit ha/year [78] | 37.1% or 27,825 tons on-farm and 24% between the farm and the final consumer as average wastes annually [76] | 0.3 |
| Vegetables | Type of Waste/Treatment Conditions | Eco-Materials Parameters | Pollutants and Adsorption Efficiency Data | References |
|---|---|---|---|---|
| Potatoes | Untreated potato peel, dried at 50 °C for 7 days | N.A. | Cu(II) 84.74 mg/g | [113,114] |
| Chemical activated 0.5 M NaOH | N.A. | Cd(II) 90 mg/g; Cu II) 41.7 mg/g; Ni (II) 16.7 mg/g; Zn (II) 52.6 mg/g; Mn(II) 47.6 mg/g; Fe(II) 76.9 mg/g | [114,115] | |
| Pyrolysis and treatment with ZnCl2 (chemical activation) of peels | BET: 1078 m2/g V pores: 0.97cm3/g | Cu (II) 62 mg/g and 74 mg/g for 160 wt.% ZnCl2 | [114,116] | |
| Thermal treatment at 400, 600, 800 °C (P400, P600), chemical activated with solution 75% (w/w) H3PO4 | BET P400: 904.56 cm2/g, V pores: 0.726 cm3/g BET P600: 1041.43 cm2/g, V pores: 2.960 cm3/g | Co (II): 373 mg/g for P400 and 405 mg/g for P600 | [114,117] | |
| Thermal activation at 600 °C (activated carbon peel (ACP)) | BET 498 m2/g V pores 0.987 cm3/g | Pb (II) | [114,118] | |
| Potato peels as charcoal (PPC) | N.A. | Cu (II) 0.3877 mg/g (99.8%) %Recovery: 5 repeated cycles (last cycle: 99.5% ± 0.35) | [114,119] | |
| Tomatoes | Leaf powder | BET 5.0518 m2/g V pores 0.003 cm3/g | NI (II) 58.82 mg/g | [120] |
| Chemical activation with NaOH | BET: 8.83 m2/g V pores 0.0447 cm3/g | Pb (II) 152 mg/g (97%) Desorbtion studies: HCl: 53.473%, Na2-EDTA: 94.247% | [121] | |
| Cabbages | Powder, 102 °C, 24 h | BET: 1.0265 m2/g | Pb(II) 60.57 mg/g ( 98.85%) Cd(II) 20.57 mg/g (54.32%) Recovery: Pb (II) 86.67% and Cd (II) 82.34% | [122] |
| Carrots | N.A. | Cr(III) 45.09 mg/g, Cu(II) 32.74, Zn(II) 29.61 mg/g. | [123] | |
| Cauliflower | Powder 102 °C for 24 h | BET: 0.8905 m2/g | Pb (II): 47.63 mg/g (96.06%); Cd (II) 21.32 mg/g (81.31%). Recovery: Pb(II) 85.67% and Cd (II) 79.74%. | [122] |
| Roots, slow pyrolysis, 500 °C, 6 h | BET 232.15 m2/g V pores 0.15 cm3/g | 31.5 mg/g (92.3%) norfloxacin; 81.3 mg/g (93.2%) chlortetracycline | [124] | |
| Raw cauliflower cores (CC) as comparison with broccoli stalks (BS) and coconut shell (CS) | N.A. | Ni2+: 3.5 to 9.9 mg/g, Zn2+: 2.9 to 14.4 mg/g, Cd2+: 0.4 to 17.9 mg/g and Cu2+: 6.2 to 21.2 mg/g CS > CC > BS | [12] | |
| Pyrolysis or carbonization, temperature between (500 and 800 °C), during (2 and 4 h). | N.A. | Cd2+: 0.81–5.69 mg/g, Ni2+: 0.87–5.57 mg/g, Cu2+: 1.19–7.21 mg/g and Zn2+: 0.79–4.09 mg/g. | [12] | |
| Chemical activation, samples modifed with H2SO4 (1), H3PO4(2), NH4NO3 (3) and NH3 (4) | N.A. | Heavy metals: 4.47–10.13 mg/g(1); 0.80–5.67 mg/g (2); 1.41–2.58 mg/g (3); 2.05–9.86 mg/g (4). | [12] |
| Fruits | Type of Waste/Treatment Conditions, Parameters | Pollutants and Adsorption Efficiency Data | References |
|---|---|---|---|
| Orange | Peels | Ni(II): 80 to 158 mg/g from 30 °C to 50 °C, 96% at 50 °C. Desorption with 0.05 M HCl: 95.83% (column system); 76% (batch process). Recovery studies 89% and 93.33%, respectively | [129] |
| Orange | Peels | As(III) 1.18 mg/g (82.45%) | [5] |
| Orange | Peel cellulose modified with alkali (such as NaOH, NH4OH, Ca(OH)2) and acids (such as C6H6O7·H2O, H2C2O4, H3PO4) | Ni(II): 1.28, Co(II): 1.23, Zn(II): 1.21 and Cd(II): 1.13 mol/kg. Desorption results with 0.05 mol/L HCl: 87.23% Zn(II), and 93.72% Cd(II). Desorption results with 0.1 mol/L HCl: 81.06% Co(II) and 80.11% Ni(II) | [5,130] |
| Orange | Peel modified with mercapto-acetic acid, pretreated with NaOH solution | Cu2+: 70.67 mg/g; Cd2+: 136.05 mg/g | [5,89] |
| Citrus | Peel | Cd(II) between 0.5 and 0.9 meq/g, according to pH values | [131] |
| Orange | Peel | Pb(II): 1.93 mmol/g (400 mg/g Pb) | [10] |
| Orange | Peel | Pb(II) 7.75 mg/g, Ni(II) 6.01 mg/g, Zn(II) 5.25 mg/g, Cu (II) 3.65 mg/g, Co(II) 1.82 mg/g; pH: 4.8–5.0. | [132] |
| Lemon | Peel treated at 400 °C, activated with H3PO4 | Cd 96.4%, Ni 67.9%; Pb 90.11% | [133] |
| Orange | Wastes (as dry-gel), chemical modification with Ca(OH)2 (Ca-form) and washed with 0.1M HCl (H-form) | Ca-form: about 1.1 mol Pb(II), Cd(II) and Zn(II)/kg and 1.55 mol Fe(III)/kg and H-form: 2.64 mol Fe(III)/kg Efficiency removal: 100% Fe, 95% Pb, 80% Cu, 55% Cd, 40% Zn for Ca-form Efficiency removal: 98% Fe and Pb, 80% Cu, 60% Zn, 40% Cd, for H-form | [134] |
| Orange | Modified orange peel with methyl acrylate | Cu(II): 289.0 mg/g, pH 6.0, 94.6% Regeneration: 4 cycles (94.6% to 85.2% at the last cycle) | [88] |
| Orange | Saponified and modified peel with citric acid | Cd(II): 0.90 mol/kg The desorption rate: 94%, 0.15 mol/L HCl | [5,135] |
| Orange | Powdered peels | Congo Red: 22.4 mg/g, pH 5.0, 76.6% Procion orange: 1.3 mg/g, pH 3.0, 49% Rhodamine-B: 3.22 mg/g, pH 3.0, 67.5% Desorbtion studies: pH 12 for congo Red: 37%, pH 11 for procion orange: 78% and pH 11 for rhodamine-B: 27%. | [136] |
| Orange | Peel | Acid violet 17: 19.88 mg/g, pH 6.3 Maximum removal: 87% at pH 2.0 Maximum desorption: 60% at pH 10.0 | [5] |
| Direct Yellow DY 12 Adsorption capacity: 75.76 mg/g Efficiency removal: 96% | [5] | ||
| Orange | Peel | Navy Blue 106 | [5] |
| Orange | Peel activated with H3PO4 BET value: 1090 m2/g | methylene blue and rhodamine B (114 mmol/g MB and 1.23 mmol/g RhB for Langmuir-Freundlich models) | [5,137] |
| Orange | Peel | Toluidine blue (TB): 314.3 mg/g. Removal: 60% at pH 3.5 | [5] |
| Orange | Peel | Direct Red 2: 10.72 mg/g, 92%, Direct Red 80: 21.05 mg/g, 91%, pH 2. Desorption: 97.7% and 93% respectively, pH 2 | [5] |
| Orange | Peel | Carbofuran: 84.49 mg/g at 30 °C, 44.54% for 20 mg/L | [138] |
| Orange | Peel | Furadan 161.29 mg/g | [139] |
| Orange | Peel chemical activated with KCl | Cu2+: 59.77 mg/g, Cd2+: 125.63 mg/g, Pb2+ 141.84 mg/g, Zn2+ 45.29 mg/g and Ni2+ 49.14 mg/g. Efficinecy, after 10 cycles: 97% (Cu2+), 90% (Cd2+) and 99% (Pb2+). | [5,140] |
| Orange | Peel powder (OPP) modified with magnetic nano-adsorbent (MNP–OPP) BET value: OPP 47.03 m2/g and MNP–OPP 65.19 m2/g. | Cd2+: 76.92 mg/g MNP–OPP In case of electroplating effluent: 55.38 mg/g (82%) Cd2+. | [5] |
| Orange | Peel (OP) and by polymerization with formaldehyde (OPF) | Removal U 81.2% (OP) Removal U: 96% (OPF) With other competitive ions: Mn 26.8%, Co 36.2%, Ni 41.5%, Cu 92.9%, Zn 54.9%, Cd 50.7%, U 77.9% for OP and Mn 9.1%, Co 11%, Ni 12.7%, Cu 60.5%, Zn 15.4%, Cd 15.6%, U 64.1% for OPF | [141] |
| Pomelo | Peels chemically acrivated (ZnCl2) | Pb2+, Cu2+ 90% from WWT, 21.1 mg/g from synthetic solution | [142,143] |
| Pomelo | Peel (PP) and depectinated pomelo peel (DPP) | Cu2+ adsorption capacity for PP 19.7 mg/g and DPP 21.1 mg/g at pH 4 | [142] |
| Pomelo | Peel wastes | Methylene blue: 133mg/g (83%). | [5,144] |
| Pomelo | Peel wastes BET value 1357.21 m2/g. V tot pores 1.61 cm3/g | Malachite green: 178.43 mg/g. (95.06%), pH 8.0. Recovery after 4 cycles 96.35% | [5,145] |
| Grapefruit | peels activated ZnCl2 | Pb2+ 12.73 mg/g (90% ) | [5] |
| Grapefruit | Peels, raw and protonated | Cd2+: 1.7 meq/g (raw material) and 2.2 meq/g (protonated peels) | [5] |
| Grapefruit | Peels | crystal violet (CV): 254.16 mg/g. Efficiency 96% Recovery: 98.25% using 1 M NaOH, in repeated cycles | [5,146] |
| Cd(II) Ni(II) 42.09 and 46.13 mg/g | [5] | ||
| U (VI): 140.79 mg/g. Recovery after 3 cycles: 80% | [147] | ||
| Grapefruit | Peel (GP) and by polymerization with formaldehyde (GPF) | Removal U 77.3% (GP) Removal U: 73.4% (GPF) With other competitive ions: Mn 27.6%, Co 38.3%, Ni 43.9%, Cu 94.2%, Zn 56.6%, Cd 54.3%, U 83.3% for GP and Mn 11.5%, Co 14.2%, Ni 16.4%, Cu 65.6%, Zn 21.9%, Cd 20.3%, U 71% for GPF | [141] |
| Lemon | Peels | Methyl orange (MO) 50.3 mg/g, Congo red (CR) 34.5 mg/g | [148] |
| Lemon | Peels chemical activation (1M HCl and 1 M NaOH) | Cutting oil Adsorption capacity 8.896 mg/g, 94% at 5g/L lemon peel | [149] |
| Lemon | peels waste | Co: 22 mg/g | [5] |
| Lemon | Cold alkali peel | Pb2+: 630 mg/g | [5] |
| Lemon | Peels waste | Cd: 11.24 mg/g and efficiency removal 80.8% | [5] |
| Sweet lime | peels | Cu(II): 37.45 mg/g at 293 K | [150] |
| Lemon | Protonated peels | 0.9 meq/g at pH 5 | [131] |
| Bananas | Powder banana peels | 5.71 mg/g Cd(II) and 2.18 mg Pb(II)/g Maximum removal: 89.2% for Cd(II) and 85.3% for Pb(II) | [151] |
| Untreated banana peels (1), alkali-hydrolyzed banana peels (2), acid-hydrolyzed banana peels (3), and bleached Banana peels (4) | 1: Cr(VI): 45% and Mn(II) 51%, 2: Cr(VI) 87% and Mn(II) 90%, 3: Cr (VI) 67% and Mn(II) 74%, 4: Cr (V) 40% and Mn(II) 67%. | [5] | |
| Banana peels wastes | phenolic compounds: 689 mg/g Desorbtion at neutral pH water (pH 7.3) 0.17 g/g, acetic acid (pH 1.2) 0.30 g/g and alkaline water (pH 12) 0.12 g/g phenolic compounds | [5,82] | |
| Banana peels (NBP) and modified with caustic soda (ABP) | methylene blue adsorption capacities: 19.671 mg/g (ABP) and 18.647 mg/g (NBP). ABP: 98.93% for pH 4–8 | [152] | |
| Banana peel waste | atrazine 93.8% and ametryne 95.2%. Desorption of ametryne: 31.5% and 47.5% for atrazine | [153] | |
| Charred banana peels chemical activated with H3PO4 | Atrazine: 14 mg/g. 90–99% atrazine removal | [154] | |
| Banana peel treated with acid, alkali, and water | Adsorption capacities: 7.97 (Pb2+), 6.88 (Ni2+), 5.80 (Zn2+), 4.75 (Cu2+), and 2.55 mg/g (Co2+) at pH of around 5.4–5.8 | [5,132] | |
| Natural banana peel (1), methylated banana peel (2) | Palm oil mill effluent. (1)97 mg/g color, 25 mg/g TSS, and 90.5 mg/g COD. (2); 137.5 mg/g color, 28.5 mg/g TSS and 93 mg/g COD. | [155] | |
| Carbonized banana peels, chemical activation with H2SO4 | Removal for Pb: 33.3%. Removal for Zn: 27.3% Removal for Cr: 77.8% | [156] | |
| Apples | Apple juice residue chemical activated with NaOH | Pb(II) Adsorption capacity: 108 mg/g Removal: Cca 90% Desorbtion studies: HCl: 59,647%, Na2-EDTA: 99,809% BET values before and after activation: 7.04 and 11.13 m2/g. Vpores: 8.34 × 10−3 cc/g | [117] |
| Zr immobilized apple peel | AsO2−: 15.64 mg/g, AsO43−15.68 mg/g, Cr2O72− 25.28 mg/g, and PO43−20.35 mg/g. Desorbtion 90% of pollutants pH 12, after 10 min | [157] | |
| Apple residue (AR) and Apple Phosphate residue (P), and Apple Xanthate residue (CLX) | Cu Zn Ni: 40 30 27 mg/g P-AR Cu Zn Ni: 25 15 12 mg/g CLX-AR Cu Zn NI: 10 6 5 mg/g AR | [13] | |
| Grapes | Grape skins | Cd2+ metal uptake capacity 1.20 meq/g | [131] |
| Mango | Mango peel waste | Cu2+ 46.09 mg/g, Ni2+ 39.75 mg/g, Zn2+ 28.21 mg/g Removal: 89.02%, 76.40%, and 67.27% for Cu(II), Ni(II), and Zn(II) genuine electroplating effluent: Cu(II) cca 90%, Ni(II) cca 80%, Zn(II) cca 80% | [158] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matei, E.; Râpă, M.; Predescu, A.M.; Țurcanu, A.A.; Vidu, R.; Predescu, C.; Bobirica, C.; Bobirica, L.; Orbeci, C. Valorization of Agri-Food Wastes as Sustainable Eco-Materials for Wastewater Treatment: Current State and New Perspectives. Materials 2021, 14, 4581. https://doi.org/10.3390/ma14164581
Matei E, Râpă M, Predescu AM, Țurcanu AA, Vidu R, Predescu C, Bobirica C, Bobirica L, Orbeci C. Valorization of Agri-Food Wastes as Sustainable Eco-Materials for Wastewater Treatment: Current State and New Perspectives. Materials. 2021; 14(16):4581. https://doi.org/10.3390/ma14164581
Chicago/Turabian StyleMatei, Ecaterina, Maria Râpă, Andra Mihaela Predescu, Anca Andreea Țurcanu, Ruxandra Vidu, Cristian Predescu, Constantin Bobirica, Liliana Bobirica, and Cristina Orbeci. 2021. "Valorization of Agri-Food Wastes as Sustainable Eco-Materials for Wastewater Treatment: Current State and New Perspectives" Materials 14, no. 16: 4581. https://doi.org/10.3390/ma14164581










