Adsorption of Copper (II) from Aqueous Solutions with Alginate/Clay Hybrid Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
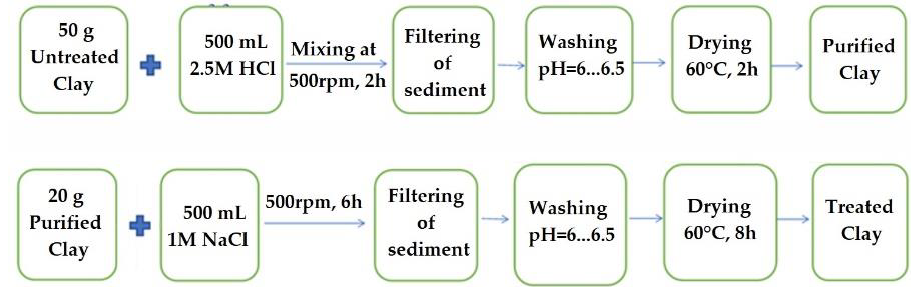
2.2. Preparation of Calcium Alginate/Clay Hybrid Composite Beads
2.3. Structural Characterization
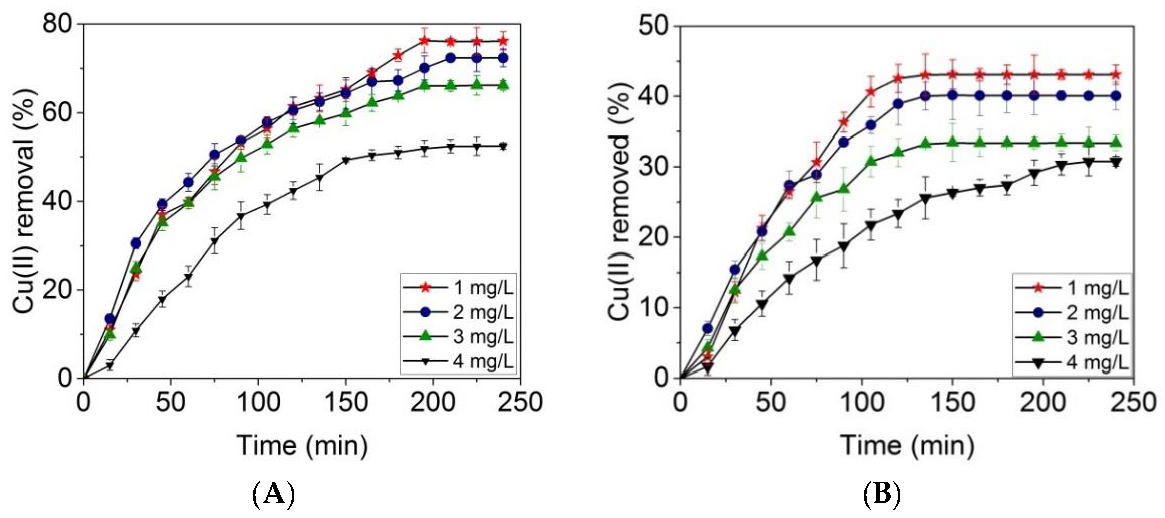
2.4. Batch Adsorption Experiments
3. Results and Discussion
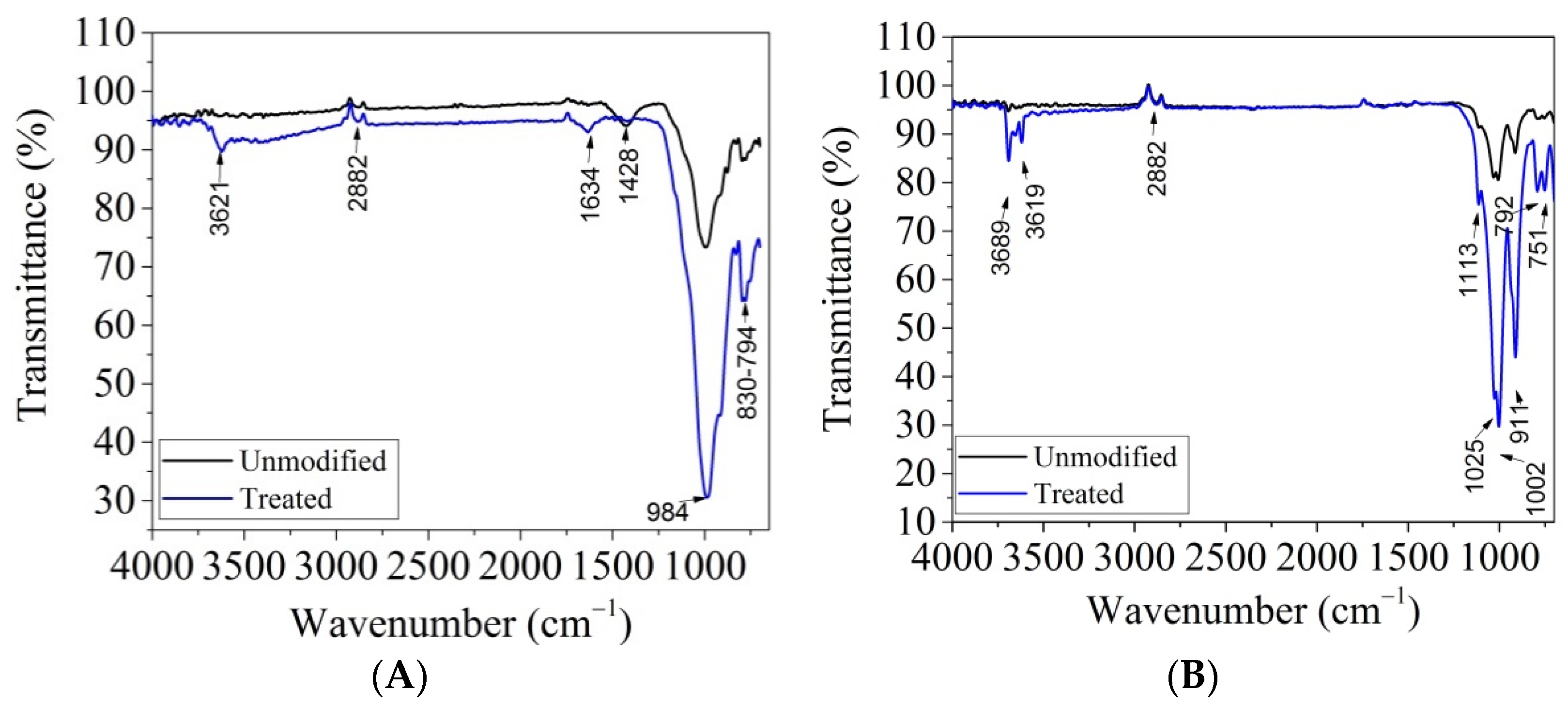
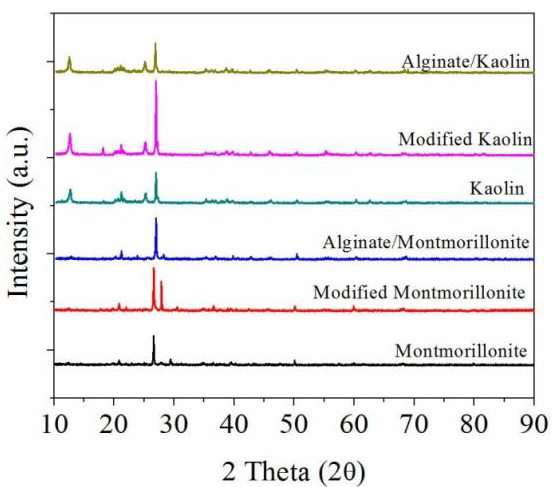
3.1. Characterization of Modified Clays
3.2. Characterization of Alginate/Clay Hybrid Composite Beads
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, H.G.; Chae, M.Y. Novel type of alginate gel-based adsorbents for heavy metal removal. J. Chem. Technol. Biotechnol. 2004, 79, 1080–1083. [Google Scholar] [CrossRef]
- Musarurwa, H.; Tavengwa, N.T. Application of carboxymethyl polysaccharides as bio-sorbents for the sequestration of heavy metals in aquatic environments. Carbohydr. Polym. 2020, 237, 116142. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Chen, C. Chitosan-based biosorbents: Modification and application for biosorption of heavy metals and radionuclides. Bioresour. Technol. 2014, 160, 129–141. [Google Scholar] [CrossRef]
- Matei, E.; Predescu, A.M.; Rapa, M.; Turcanu, A.; Predescu, C.; Vidu, R.; Favier, L.; Covaliu, C.I.; Ignat, D.; Grigore, V. Testing of Alginate/Chitosan/Glass Bubbles Adsorbent for Copper Removal from Wastewater. Mater. Plast. 2021, 58, 19–26. [Google Scholar] [CrossRef]
- Vaid, U.; Mittal, S.; Babu, J.N. Influence of anion induced proton abstraction on Cu(II) adsorption by alginic acid. React. Funct. Polym. 2015, 97, 48–55. [Google Scholar] [CrossRef]
- Predescu, A.M.; Matei, E.; Rapa, M.; Pantilimon, C.; Coman, G.; Savin, S.; Popa, E.E.; Predescu, C. Adsorption of Lead(II) from Aqueous Solution Using Chitosan and Polyvinyl Alcohol Blends. Anal. Lett. 2019, 52, 2365–2392. [Google Scholar] [CrossRef]
- Salehi, E.; Daraei, P.; Shamsabadi, A.A. A review on chitosan-based adsorptive membranes. Carbohydr. Polym. 2016, 152, 419–432. [Google Scholar] [CrossRef]
- Tang, X.Y.; Thankappan, S.K.; Lee, P.; Fard, S.E.; Harmon, M.D.; Tran, K.; Yu, X.J. Polymeric Biomaterials in Tissue Engineering and Regenerative Medicine. Nat. Synth. Biomed. Polym. 2014, 351–371. [Google Scholar] [CrossRef]
- Basumallick, S. Alginates in Drug Delivery. In Alginates: Applications in the Biomedical and Food Industries; Elservier: Amsterdam, The Netherlands, 2019; pp. 141–151. [Google Scholar]
- Ravi, A.; Vijayanand, S.; Rajeshkannan, V.; Aisverya, S.; Sangeetha, K.; Sudha, P.N.; Hemapriya, J. Alginates in Comestibles. In Alginates: Applications in the Biomedical and Food Industries; Elservier: Amsterdam, The Netherlands, 2019; pp. 263–279. [Google Scholar]
- Cordova, B.M.; Venancio, T.; Olivera, M.; Huamani-Palomino, R.G.; Valderrama, A.C. Xanthation of alginate for heavy metal ions removal. Characterization of xanthate-modified alginates and its metal derivatives. Int. J. Biol. Macromol. 2021, 169, 130–142. [Google Scholar] [CrossRef]
- Moral, C.K.; Dogan, O.; Sanin, F.D. Use of laboratory-grown bacterial alginate in copper removal. Water Sci. Technol. 2012, 65, 2003–2009. [Google Scholar] [CrossRef]
- Sotnmez, M.; Ficai, A.; Ficai, D.; Trusca, R.; Andronescu, E. Alginate/cellulose composite beads for environmental applications. Univ. Politeh. Buchar. Sci. Bull. Ser. B Chem. Mater. Sci. 2016, 78, 165–176. [Google Scholar]
- Rapa, M.; Matei, E.; Turcanu, A.; Predescu, A.M.; Pantilimon, M.C.; Predescu, C. Structural, morphological and thermal analysis of some alginate/starch/dellite hps composites for aqueous Cu(II) removal. Cellul. Chem. Technol. 2019, 53, 561–571. [Google Scholar] [CrossRef]
- Ion, R.-M.; Fierăscu, R.-C.; Teodorescu, S.; Fierăscu, I.; Bunghez, I.-R.; Ţurcanu-Caruţiu, D.; Ion, M.-L. (Eds.) Ceramic Materials Based on Clay Minerals. In Cultural Heritage Study, Clays, Clay Minerals and Ceramic Materials Based on Clay Minerals; Intech Open: London, UK, 2016. [Google Scholar]
- Bibi, I.; Icenhower, J.; Niazi, N.K.; Naz, T.; Shahid, M.; Bashir, S. Clay minerals: Structure, chemistry, and significance in contaminated environments and geological CO2 sequestration. In Environmental Materials and Waste Resource Recovery and Pollution Prevention; Academic Press: Cambridge, MA, USA, 2016; pp. 543–567. [Google Scholar] [CrossRef]
- Huggett, J.M. Clay Minerals. In Encyclopedia of Geology; Selley, R.C., Cocks, L.R.M., Plimer, I.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 358–365. [Google Scholar]
- Lezehari, M.; Baudu, M.; Bouras, O.; Basly, J.P. Fixed-bed column studies of pentachlorophenol removal by use of alginate-encapsulated pillared clay microbeads. J. Colloid Interface Sci. 2012, 379, 101–106. [Google Scholar] [CrossRef]
- Ruan, B.; Wu, P.X.; Chen, M.Q.; Lai, X.L.; Chen, L.Y.; Yu, L.F.; Gong, B.N.; Kang, C.X.; Dang, Z.; Shi, Z.Q.; et al. Immobilization of Sphingomonas sp GY2B in polyvinyl alcohol-alginate-kaolin beads for efficient degradation of phenol against unfavorable environmental factors. Ecotoxicol. Environ. Saf. 2018, 162, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Kumar, I.A.; Viswanathan, N. Fabrication of zirconium(IV) cross-linked alginate/kaolin hybrid beads for nitrate and phosphate retention. Arab. J. Chem. 2020, 13, 4111–4125. [Google Scholar] [CrossRef]
- Li, Y.H.; Xia, B.; Zhao, Q.S.; Liu, F.Q.; Zhang, P.; Du, Q.J.; Wang, D.C.; Li, D.; Wang, Z.H.; Xia, Y.Z. Removal of copper ions from aqueous solution by calcium alginate immobilized kaolin. J. Environ. Sci. 2011, 23, 404–411. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Wang, Y.X.; Chen, L.Y. A green composite hydrogel based on cellulose and clay as efficient absorbent of colored organic effluent. Carbohydr. Polym. 2019, 210, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Tu, F.; Wu, Y.; Wang, X.; Lu, G.; Zhao, L. Facile preparation of Kaolin supported silver nanoparticles mediated by Thymbra spicata extract and investigation of the anti-human lung cancer properties. J. Saudi Chem. Soc. 2021, 25, 11. [Google Scholar] [CrossRef]
- Zhou, C.H. An overview on strategies towards clay-based designer catalysts for green and sustainable catalysis. Appl. Clay Sci. 2011, 53, 87–96. [Google Scholar] [CrossRef]
- Zhong, Z.P.; Li, J.F.; Ma, Y.Y.; Yang, Y.X. The adsorption mechanism of heavy metals from coal combustion by modified kaolin: Experimental and theoretical studies. J. Hazard. Mater. 2021, 418, 126256. [Google Scholar] [CrossRef]
- Ely, A.; Baudu, M.; Kankou, M.; Basly, J.P. Copper and nitrophenol removal by low cost alginate/Mauritanian clay composite beads. Chem. Eng. J. 2011, 178, 168–174. [Google Scholar] [CrossRef]
- Edathil, A.A.; Pal, P.; Banat, F. Alginate clay hybrid composite adsorbents for the reclamation of industrial lean methyldiethanolamine solutions. Appl. Clay Sci. 2018, 156, 213–223. [Google Scholar] [CrossRef]
- Uyar, G.; Kaygusuz, H.; Erim, F.B. Methylene blue removal by alginate-clay quasi-cryogel beads. React. Funct. Polym. 2016, 106, 1–7. [Google Scholar] [CrossRef]
- Garmia, D.; Zaghouane-Boudiaf, H.; Ibbora, C.V. Preparation and characterization of new low cost adsorbent beads based on activated bentonite encapsulated with calcium alginate for removal of 2,4-dichlorophenol from aqueous medium. Int. J. Biol. Macromol. 2018, 115, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Komadel, P. Acid activated clays: Materials in continuous demand. Appl. Clay Sci. 2016, 131, 84–99. [Google Scholar] [CrossRef]
- Mobarak, M.; Selim, A.Q.; Mohamed, E.A.; Seliem, M.K. Modification of organic matter-rich clay by a solution of cationic surfactant/H2O2: A new product for fluoride adsorption from solutions. J. Clean. Prod. 2018, 192, 712–721. [Google Scholar] [CrossRef]
- Luna, F.M.T.; Cecilia, J.A.; Saboya, R.M.A.; Barrera, D.; Sapag, K.; Rodriguez-Castellon, E.; Cavalcante, C.L. Natural and Modified Montmorillonite Clays as Catalysts for Synthesis of Biolubricants. Materials 2018, 11, 1764. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Tam, Y.S.; Elefsiniotis, P. Corrosion control in water supply systems: Effect of pH, alkalinity, and orthophosphate on lead and copper leaching from brass plumbing. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2009, 44, 1251–1260. [Google Scholar] [CrossRef]
- Malhotra, N.; Ger, T.R.; Uapipatanakul, B.; Huang, J.C.; Chen, K.H.C.; Hsiao, C.D. Review of Copper and Copper Nanoparticle Toxicity in Fish. Nanomaterials 2020, 10, 1126. [Google Scholar] [CrossRef]
- Bai, D.K.; Ying, Q.H.; Wang, N.; Lin, J.H. Copper Removal from Electroplating Wastewater by Coprecipitation of Copper-Based Supramolecular Materials: Preparation and Application Study. J. Chem. 2016, 2016, 5281561. [Google Scholar] [CrossRef]
- National Primary Drinking Water. Regulations. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations#one (accessed on 5 November 2021).
- Ansari, T.M.; Marr, I.L.; Tariq, N. Heavy Metals in Marine Pollution Perspective–A Mini Review. J. Appl. Sci. 2004, 4, 1–20. [Google Scholar] [CrossRef]
- Wang, Y.H.; Gu, J.C.; Lin, W.L.; Wang, W.Y. Wastewater containing copper precipitation research. Front. Green Build. Mater. Civ. Eng. III Pts 1–3 2013, 368–370, 510–513. [Google Scholar] [CrossRef]
- Sreesai, S.; Sthiannopkao, S. Utilization of zeolite industrial wastewater for removal of copper and zinc from copper-brass pipe industrial wastewater. Can. J. Civ. Eng. 2009, 36, 709–719. [Google Scholar] [CrossRef]
- Yang, C.H.; Lin, W.C.; Liao, W.T. Copper recovery from EDTA—Chelating copper wastewater in a fluidized bed. Can. J. Chem. Eng. 2005, 83, 409–417. [Google Scholar] [CrossRef]
- Kuleyin, A.; Uysal, H.E. Recovery of Copper Ions from Industrial Wastewater by Electrodeposition. Int. J. Electrochem. Sci. 2020, 15, 1474–1485. [Google Scholar] [CrossRef]
- Taleb, K.; Pillin, I.; Grohens, Y.; Saidi-Besbes, S. Gemini surfactant modified clays: Effect of surfactant loading and spacer length. Appl. Clay Sci. 2018, 161, 48–56. [Google Scholar] [CrossRef]
- Baldermann, A.; Fleischhacker, Y.; Schmidthaler, S.; Wester, K.; Nachtnebel, M.; Eichinger, S. Removal of Barium from Solution by Natural and Iron(III) Oxide-Modified Allophane, Beidellite and Zeolite Adsorbents. Materials 2020, 13, 2582. [Google Scholar] [CrossRef]
- Oyekanmi, A.A.; Abd Latiff, A.A.; Daud, Z.; Mohamed, R.M.S.R.; Ismail, N.; Ab Aziz, A.; Rafatullah, M.; Hossain, K.; Ahmad, A.; Abiodun, A.K. Adsorption of cadmium and lead from palm oil mill effluent using bone-composite: Optimisation and isotherm studies. Int. J. Environ. Anal. Chem. 2019, 99, 707–725. [Google Scholar] [CrossRef]
- Ramjhan, Z.; Lokhat, D.; Alshammari, M.B.; Joy, M.N.; Ahmad, A. Trioctylammonium-based Ionic liquids for metal ions Extraction: Synthesis, characterization and application. J. Mol. Liq. 2021, 342, 117534. [Google Scholar] [CrossRef]
- Dong, Y.J.; Sang, D.S.; He, C.D.; Sheng, X.F.; Lei, L.W. Mxene/alginate composites for lead and copper ion removal from aqueous solutions. Rsc Adv. 2019, 9, 29015–29022. [Google Scholar] [CrossRef]
- Bello, O.; Mary, O.; Olatunde, A. Sorption studies of lead ions onto activated carbon produced from Oil-Palm fruit fibre. Adsorpt. Technol. 2010, 1, 14–29. [Google Scholar]
- Wu, H.S.; Zhang, A.Q.; Wang, L.S. Immobilization study of biosorption of heavy metal ions onto activated sludge. J. Environ. Sci. 2004, 16, 640–645. [Google Scholar]
- Deng, P.Y.; Liu, W.; Zeng, B.Q.; Qiu, Y.K.; Li, L.S. Sorption of heavy metals from aqueous solution by dehydrated powders of aquatic plants. Int. J. Environ. Sci. Technol. 2013, 10, 559–566. [Google Scholar] [CrossRef][Green Version]
- Lee, A.Y.W.; Lim, S.F.; Chua, S.N.D.; Sanaullah, K.; Baini, R.; Abdullah, M.O. Adsorption Equilibrium for Heavy Metal Divalent Ions (Cu2+, Zn2+, and Cd2+) into Zirconium-Based Ferromagnetic Sorbent. Adv. Mater. Sci. Eng. 2017, 2017, 1210673. [Google Scholar] [CrossRef]
- Moral, C.K.; Yildiz, M. Alginate Production from Alternative Carbon Sources and Use of Polymer Based Adsorbent in Heavy Metal Removal. Int. J. Polym. Sci. 2016, 2016, 7109825. [Google Scholar] [CrossRef]

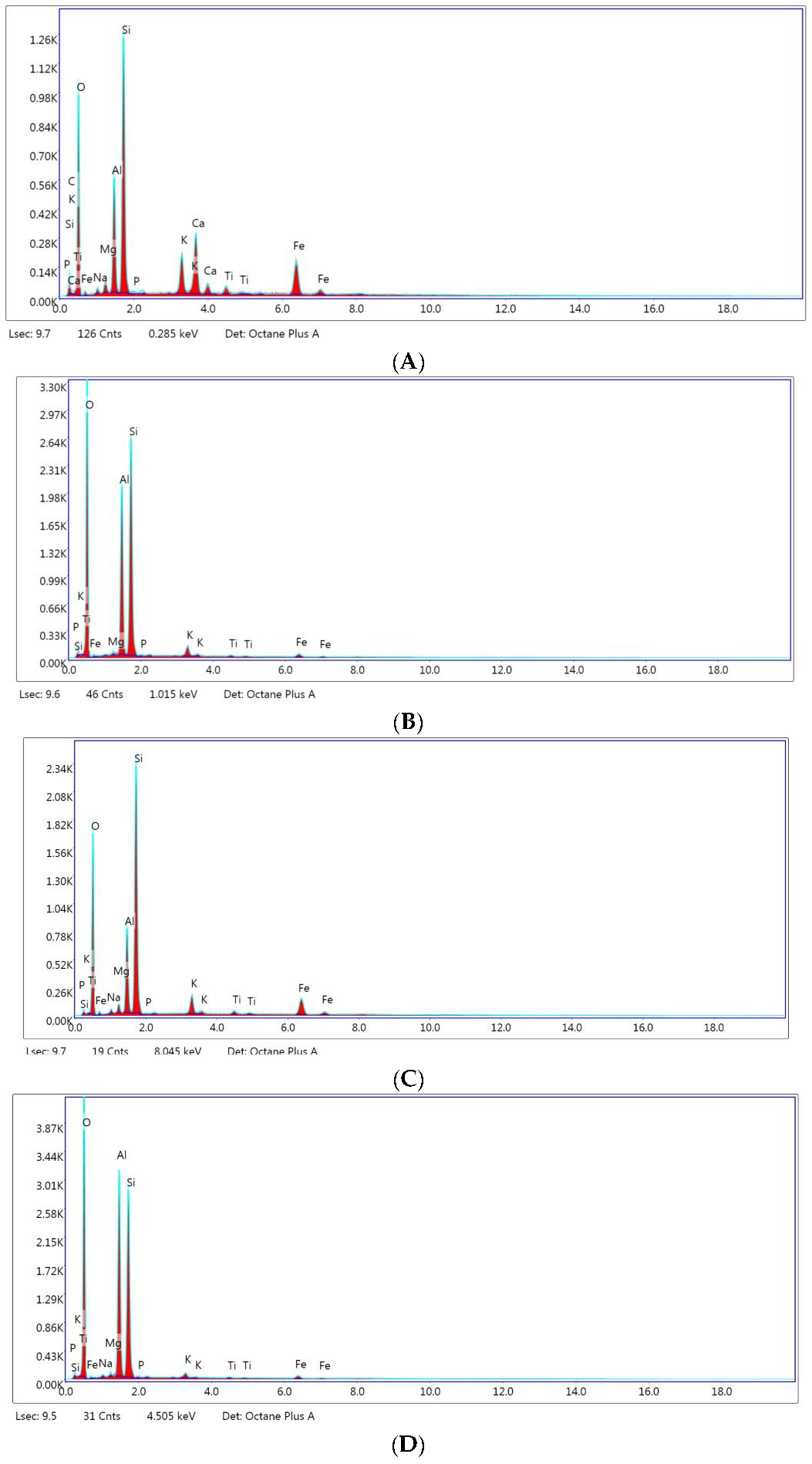




| Element | Untreated Montmorillonite | Untreated Kaolin | Treated Montmorillonite | Treated Kaolin |
|---|---|---|---|---|
| Carbon (C) | 12.45 | N.D. | N.D. | 59.42 |
| Oxygen (O) | 48.84 | 60.15 | 52.62 | N.D. |
| Natrium (Na) | N.D. | N.D. | 1.19 | 0.40 |
| Magnesium (Mg) | 1.53 | 0.44 | 1.60 | 0.53 |
| Aluminum (Al) | 8.08 | 15.83 | 10.20 | 18.57 |
| Silicon (Si) | 16.05 | 21.81 | 27.89 | 19.96 |
| Phosphorus (P) | N.D. | 0.03 | N.D. | 0.04 |
| Potassium (K) | 2.60 | 1.03 | 2.36 | 0.46 |
| Calcium (Ca) | 4.61 | N.D. | N.D. | N.D. |
| Titanium (Ti) | 0.66 | 0.17 | 0.52 | 0.14 |
| Iron (Fe) | 4.29 | 0.55 | 3.64 | 0.48 |
| Clay | Z-Average (nm) | PdI | Size for Peak 1 (nm) | Size for Peak 2 (nm) |
|---|---|---|---|---|
| Treated montmorillonite | 899.5 ± 4.738 | 0.697 ± 0.33 | 515.1 ± 104 | 71.15 ± 20 |
| Treated kaolin | 667 ± 13.08 | 0.614 ± 0.054 | 369.0 ± 33.8 | - |
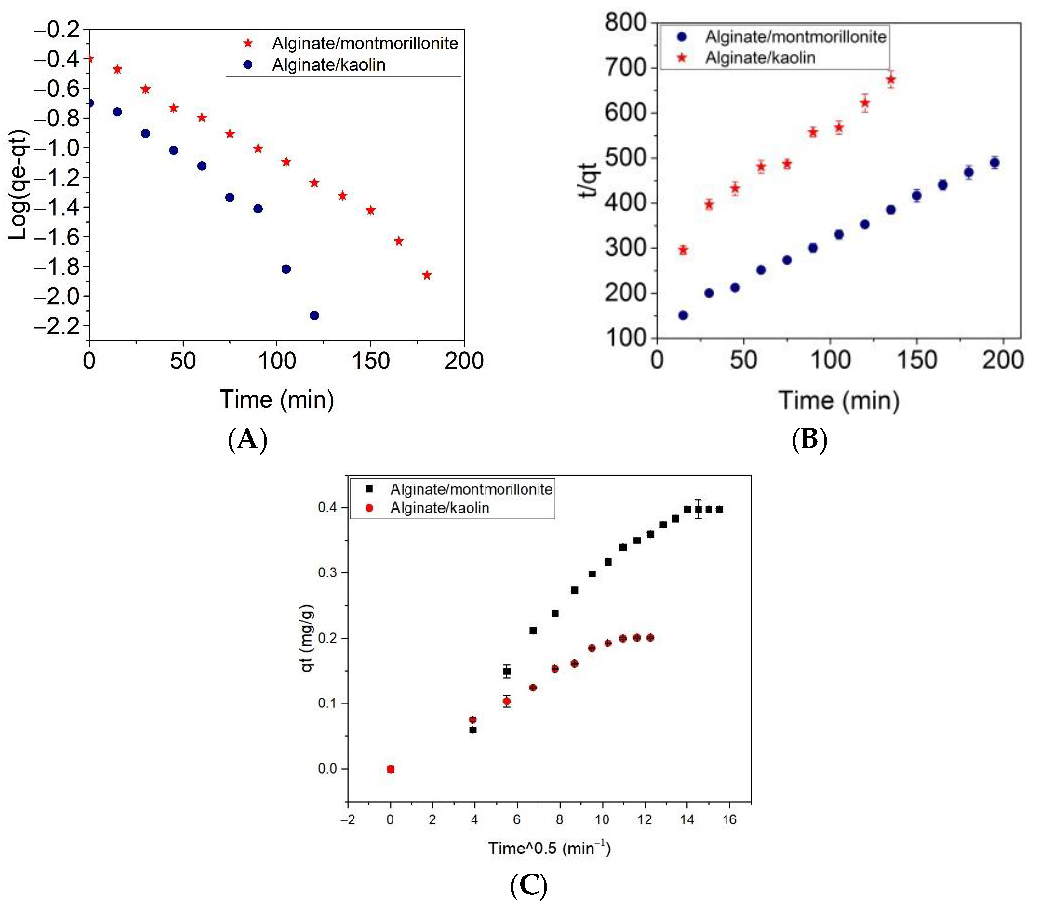
| Adsorbent | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | Intraparticle Diffusion Model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| K1 (L/min) | qmax (mg g−1) | R2 | K2 (g/mg min) | qmax (mg g−1) | R2 | Ki (mg/g min0.5) | Ci | R2 | |
| Alginate/Montmorillonite | 0.0175 | 0.440 ± 0.030 | 0.9892 | 0.0253 | 0.540 ± 0.040 | 0.99435 | 0.027 ± 0.0014 | 0.008 ± 0.016 | 0.95709 |
| Alginate/Kaolin | 0.0230 ± 0.0011 | 0.270 ± 0.080 | 0.9268 | 0.0308 | 0.341 ± 0.023 | 0.95532 | 0.016 ± 0.0006 | 0.02 ± 0.005 | 0.98567 |
| Adsorbent | Langmuir Parameters | Freundlich Parameters | |||||
|---|---|---|---|---|---|---|---|
| qmax (mg g−1) | KL | RL | R2 | KF | 1/n | R2 | |
| Alginate/Montmorillonite | 0.6802 ± 0.02 | 1.225 | 0.7655 | 0.9799 | 2.864 | 0.505 | 0.8593 |
| Alginate/Kaolin | 0.3389 ± 0.03 | 0.665 | 0.8572 | 0.9400 | 7.433 | 0.472 | 0.8426 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Râpă, M.; Ţurcanu, A.A.; Matei, E.; Predescu, A.M.; Pantilimon, M.C.; Coman, G.; Predescu, C. Adsorption of Copper (II) from Aqueous Solutions with Alginate/Clay Hybrid Materials. Materials 2021, 14, 7187. https://doi.org/10.3390/ma14237187
Râpă M, Ţurcanu AA, Matei E, Predescu AM, Pantilimon MC, Coman G, Predescu C. Adsorption of Copper (II) from Aqueous Solutions with Alginate/Clay Hybrid Materials. Materials. 2021; 14(23):7187. https://doi.org/10.3390/ma14237187
Chicago/Turabian StyleRâpă, Maria, Anca Andreea Ţurcanu, Ecaterina Matei, Andra Mihaela Predescu, Mircea Cristian Pantilimon, George Coman, and Cristian Predescu. 2021. "Adsorption of Copper (II) from Aqueous Solutions with Alginate/Clay Hybrid Materials" Materials 14, no. 23: 7187. https://doi.org/10.3390/ma14237187
APA StyleRâpă, M., Ţurcanu, A. A., Matei, E., Predescu, A. M., Pantilimon, M. C., Coman, G., & Predescu, C. (2021). Adsorption of Copper (II) from Aqueous Solutions with Alginate/Clay Hybrid Materials. Materials, 14(23), 7187. https://doi.org/10.3390/ma14237187









