A Wind Tunnel Experimental Study of Icing on NACA0012 Aircraft Airfoil with Silicon Compounds Modified Polyurethane Coatings
Abstract
:1. Introduction
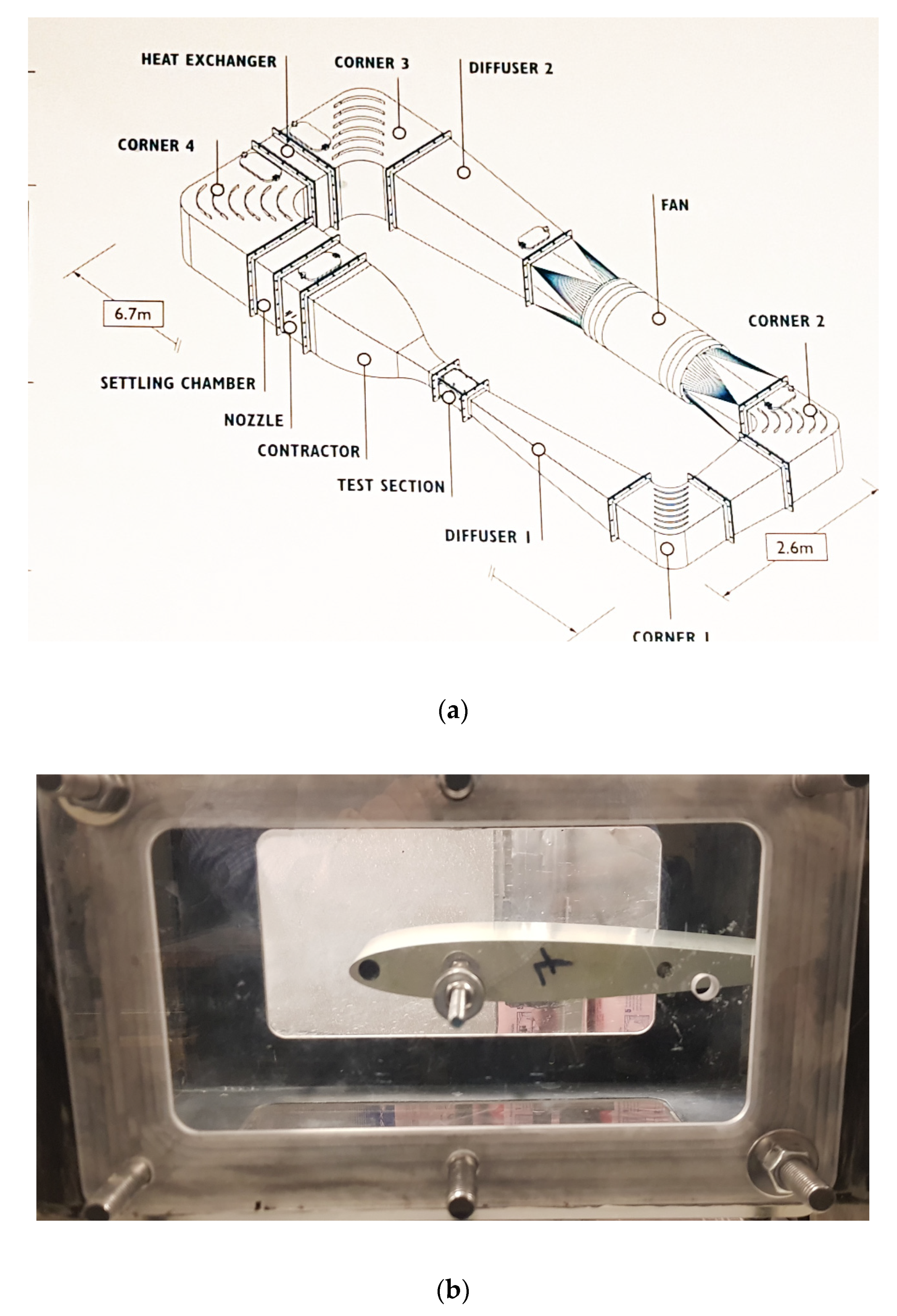
2. Materials and Methods
2.1. Materials
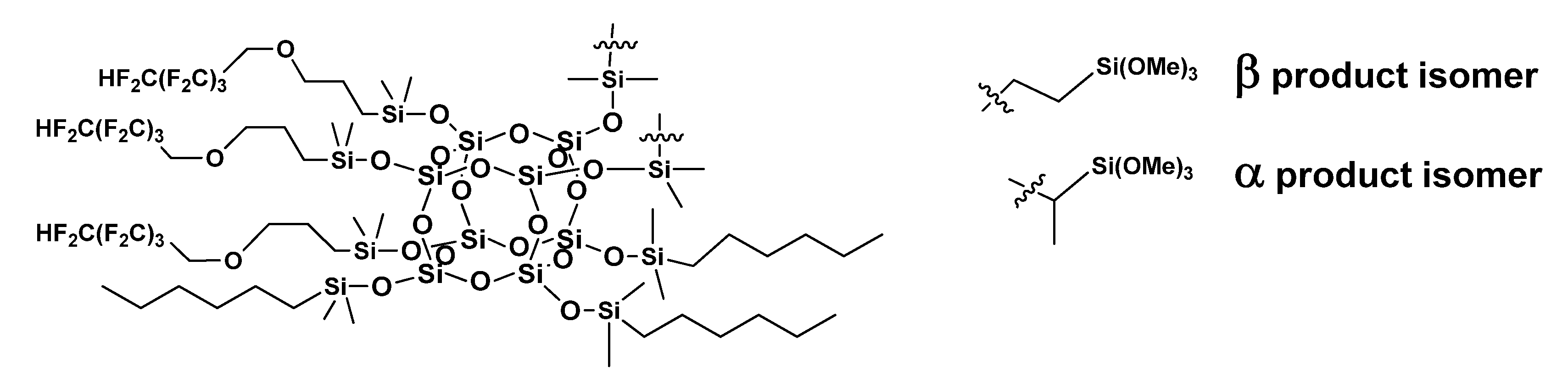
2.1.1. Synthesis of Trifunctional POSS-Based Compounds
2.2. Preparation and Deposition of Silica/POSS14/Polyurethane (PUR) Nanocomposite Coatings
2.3. Characterization
3. Results
3.1. Synthesis of Trifunctional POSS-Based Compounds
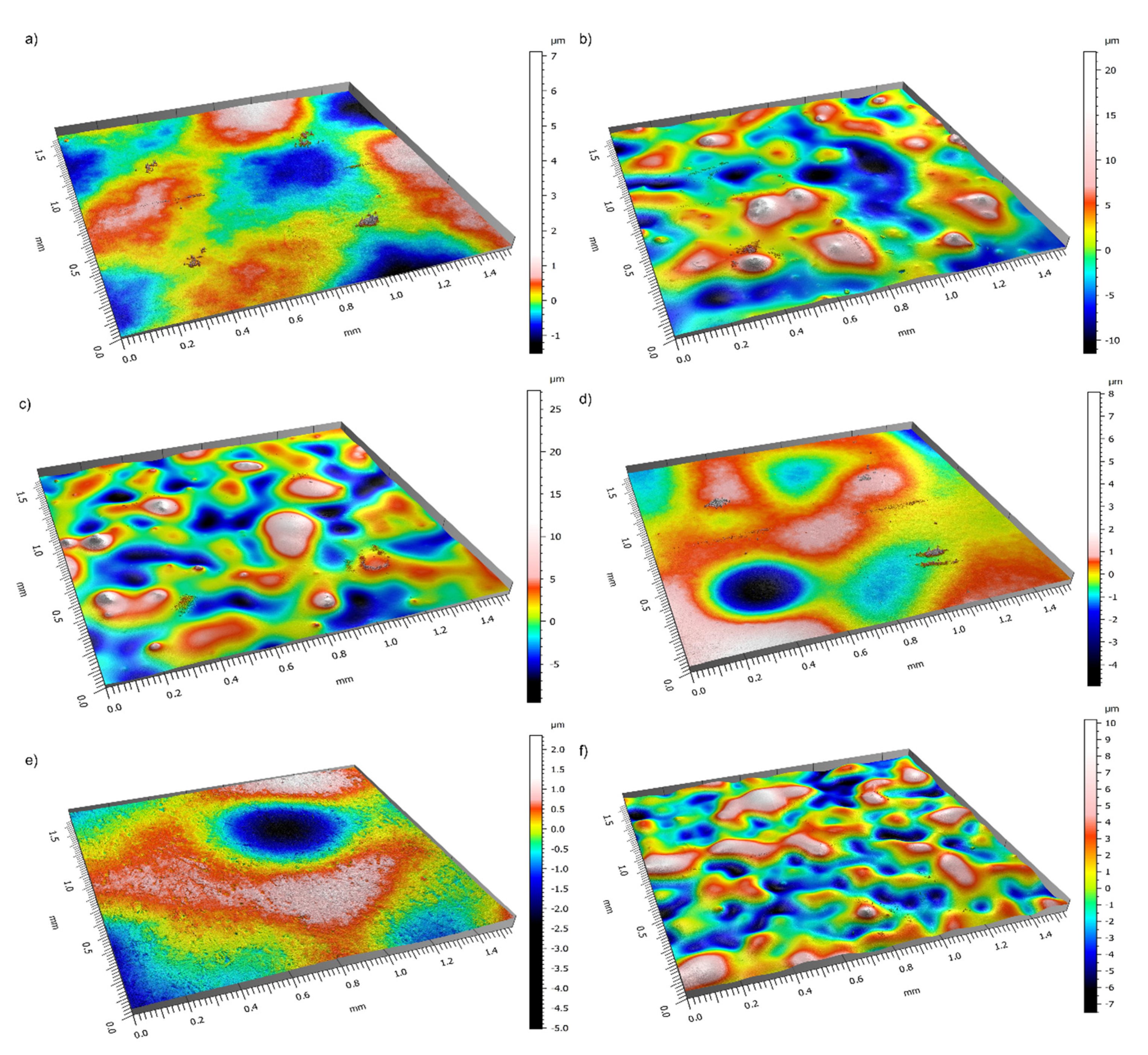
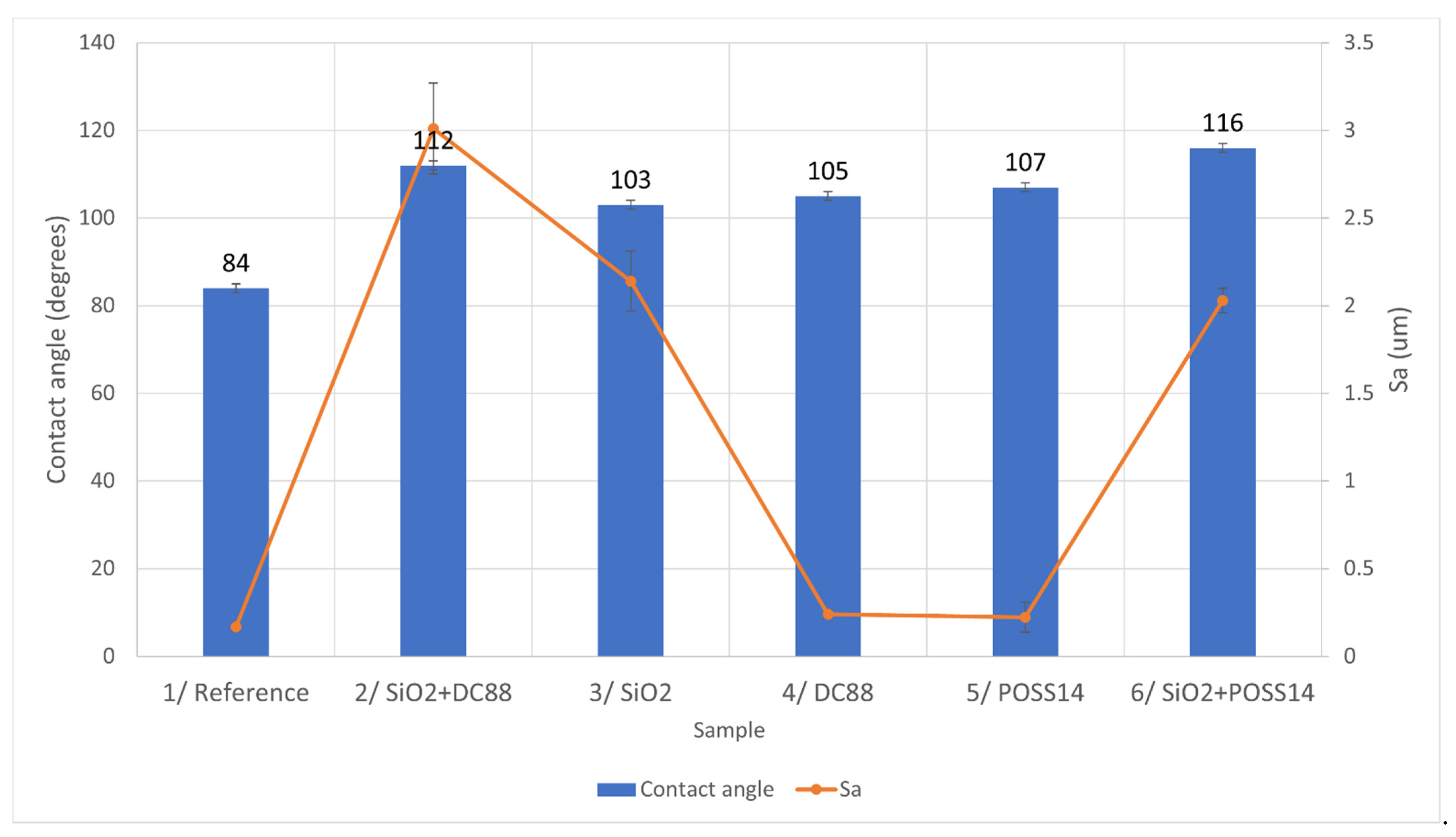
3.2. Coating Thickness and Roughness
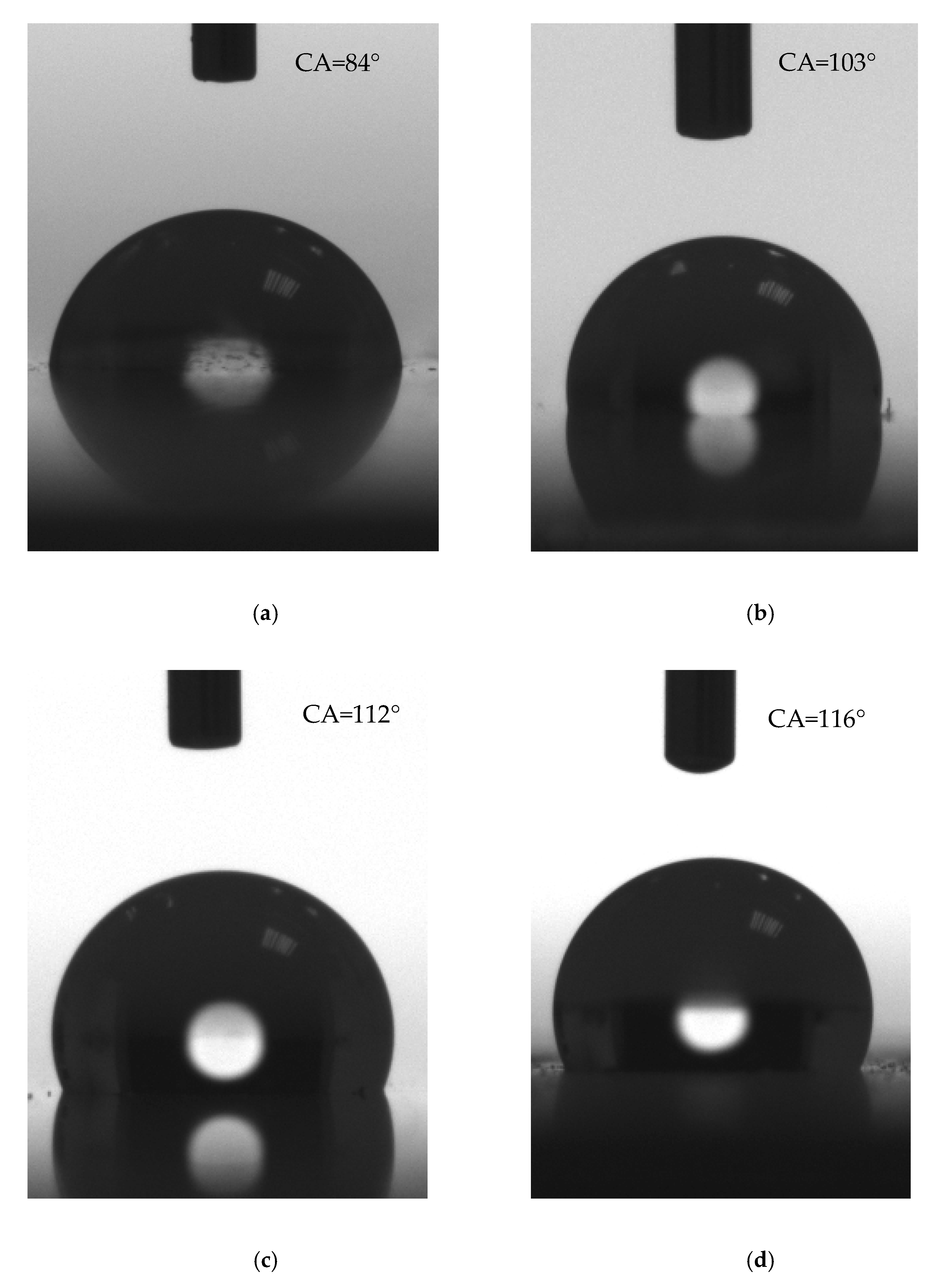
3.3. Wetting Behavior
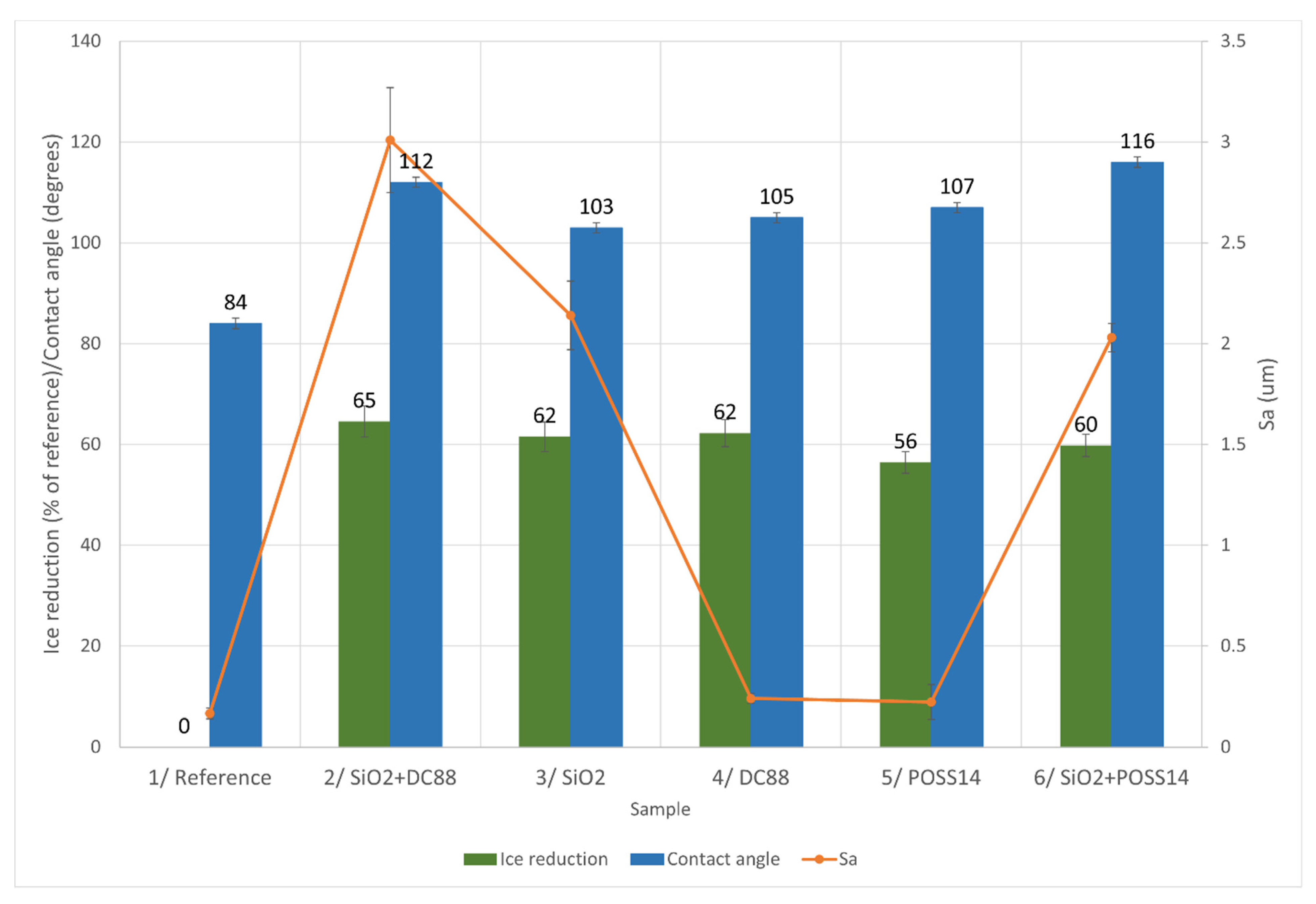
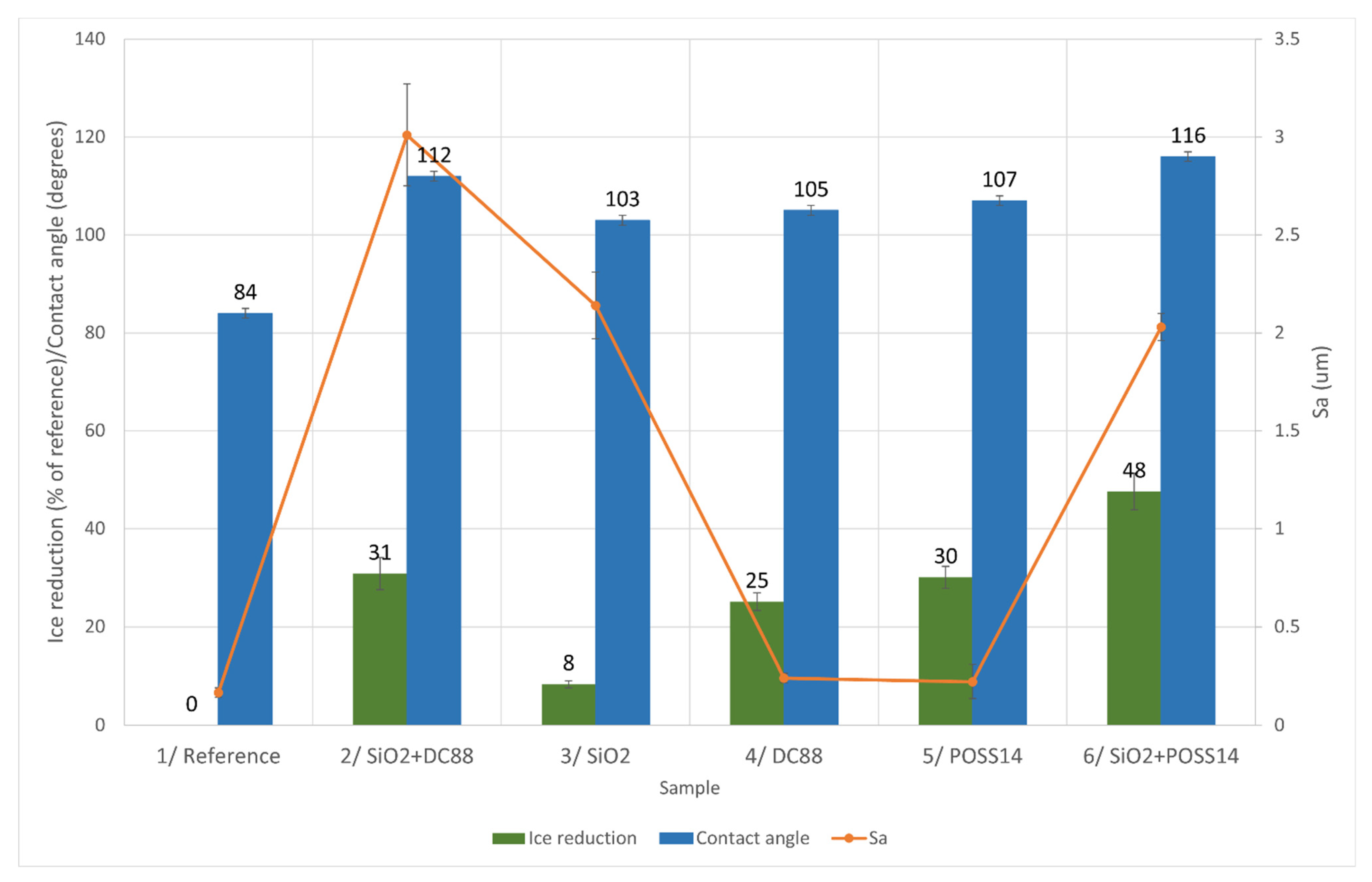
3.4. Ice Accretion
4. Discussion
4.1. Roughness and Wettability
4.2. Relationship between Roughness, Wettability and Ice Reduction
4.2.1. Glaze Ice
4.2.2. Mixed Ice
4.2.3. Rime Ice
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bragg, M.B.; Broeren, A.P.; Blumenthal, L.A. Iced-airfoil aerodynamics. Prog. Aerosp. Sci. 2005, 41, 323–362. [Google Scholar] [CrossRef]
- Diebold, J.M.; Broeren, A.P.; Bragg, M.B. Aerodynamic classification of swept-wing ice accretion. In Proceedings of the 5th AIAA Atmospheric and Space Environments Conference, San Diego, CA, USA, 24–27 June 2013. [Google Scholar] [CrossRef] [Green Version]
- Cooper, W.A.; Sand, W.R.; Politovich, M..; Veal, D.L. Effects of icing on performance of a research airplane. J. Aircr. 1984, 21, 708–715. [Google Scholar] [CrossRef]
- Green, S.D. A study of U.S. inflight icing accidents and incidents, 1978 to 2002. In Proceedings of the 44th AIAA Aerospace Sciences Meeting and Exhibit, Reno, NV, USA, 9–12 January 2006; Volume 2, pp. 1048–1073. [Google Scholar] [CrossRef] [Green Version]
- Politovich, M.K. Aircraft Icing Caused by Large Supercooled Droplets. J. Appl. Meteorol. 1989, 28, 856–868. [Google Scholar] [CrossRef] [Green Version]
- Politovich, M.K. Predicting In-Flight Aircraft Icing Intensity. J. Aircr. 2003, 639–644. [Google Scholar] [CrossRef]
- Schultz, P.; Politovich, M. Toward the Improvement of Aircraft-Icing Forecasts for the Continental United States. Weather Forecast. 1992, 491–500. [Google Scholar] [CrossRef] [Green Version]
- Mingione, G.; Marocco, M.; Denti, E.; Bindi, F.G. Flight in Icing Conditions. Direction Generale de L’aviation Civile: Paris, France, 1997. [Google Scholar]
- Aircraft Deicing and Anti-icing Equipment; AOPA Air Safety Foundation: Frederick, MD, USA, 2004; pp. 1–8.
- Endres, M.; Sommerwerk, H.; Mendig, C.; Sinapius, M.; Horst, P. Experimental study of two electro-mechanical de-icing systems applied on a wing section tested in an icing wind tunnel. CEAS Aeronaut. J. 2017, 8, 429–439. [Google Scholar] [CrossRef]
- Sojoudi, H.; Wang, M.; Boscher, N.D.; McKinley, G.H.; Gleason, K.K. Durable and scalable icephobic surfaces: Similarities and distinctions from superhydrophobic surfaces. Soft Matter 2016, 12, 1938–1963. [Google Scholar] [CrossRef] [Green Version]
- Susoff, M.; Siegmann, K.; Pfaffenroth, C.; Hirayama, M. Evaluation of icephobic coatings—Screening of different coatings and influence of roughness. Appl. Surf. Sci. 2013, 282, 870–879. [Google Scholar] [CrossRef] [Green Version]
- Farhadi, S.; Farzaneh, M.; Kulinich, S.A. Anti-icing performance of superhydrophobic surfaces. Appl. Surf. Sci. 2011, 257, 6264–6269. [Google Scholar] [CrossRef]
- Zheng, S.; Li, C.; Fu, Q.; Hu, W.; Xiang, T.; Wang, Q.; Du, M.; Liu, X.; Chen, Z. Development of stable superhydrophobic coatings on aluminum surface for corrosion-resistant, self-cleaning, and anti-icing applications. Mater. Des. 2016, 93, 261–270. [Google Scholar] [CrossRef]
- Hejazi, I.; Mir Mohamad Sadeghi, G.; Seyfi, J.; Jafari, S.H.; Khonakdar, H.A. Self-cleaning behavior in polyurethane/silica coatings via formation of a hierarchical packed morphology of nanoparticles. Appl. Surf. Sci. 2016, 368, 216–223. [Google Scholar] [CrossRef]
- Seyfi, J.; Jafari, S.H.; Khonakdar, H.A.; Sadeghi, G.M.M.; Zohuri, G.; Hejazi, I.; Simon, F. Fabrication of robust and thermally stable superhydrophobic nanocomposite coatings based on thermoplastic polyurethane and silica nanoparticles. Appl. Surf. Sci. 2015, 347, 224–230. [Google Scholar] [CrossRef]
- Bayer, I.S.; Steele, A.; Martorana, P.J.; Loth, E. Fabrication of superhydrophobic polyurethane/organoclay nano-structured composites from cyclomethicone-in-water emulsions. Appl. Surf. Sci. 2010, 257, 823–826. [Google Scholar] [CrossRef]
- Hejazi, I.; Hajalizadeh, B.; Seyfi, J.; Sadeghi, G.M.M.; Jafari, S.H.; Khonakdar, H.A. Role of nanoparticles in phase separation and final morphology of superhydrophobic polypropylene/zinc oxide nanocomposite surfaces. Appl. Surf. Sci. 2014, 293, 116–123. [Google Scholar] [CrossRef]
- Madbouly, S.A.; Otaigbe, J.U. Recent advances in synthesis, characterization and rheological properties of polyurethanes and POSS/polyurethane nanocomposites dispersions and films. Prog. Polym. Sci. 2009, 34, 1283–1332. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, H.; Tian, X.; Xue, M.; Ding, X.; Ye, X.; Cui, Z. Surface and mechanical properties of hydrophobic silica contained hybrid films of waterborne polyurethane and fluorinated polymethacrylate. Polymer 2014, 55, 187–194. [Google Scholar] [CrossRef]
- Kannan, A.G.; Choudhury, N.R.; Dutta, N. Fluoro-silsesquioxane-urethane hybrid for thin film applications. ACS Appl. Mater. Interfaces 2009, 1, 336–347. [Google Scholar] [CrossRef]
- Seyedmehdi, S.A.; Zhang, H.; Zhu, J. Fabrication of superhydrophobic coatings based on nanoparticles and fluoropolyurethane. J. Appl. Polym. Sci. 2013, 128, 4136–4140. [Google Scholar] [CrossRef]
- Barroso, G.; Li, Q.; Bordia, R.K.; Motz, G. Polymeric and ceramic silicon-based coatings—A review. J. Mater. Chem. A 2019, 7, 1936–1963. [Google Scholar] [CrossRef]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef]
- Tao, C.; Li, X.; Liu, B.; Zhang, K.; Zhao, Y.; Zhu, K.; Yuan, X. Highly icephobic properties on slippery surfaces formed from polysiloxane and fluorinated POSS. Prog. Org. Coat. 2017, 103, 48–59. [Google Scholar] [CrossRef]
- Applications of Polyhedral Oligomeric Silsesquioxanes; Hartmann-Thompson, C. (Ed.) Advances in Silicon Science; Springer: Dordrecht, The Netherlands, 2011; Volume 3, ISBN 978-90-481-3786-2. [Google Scholar]
- Liu, J.; Janjua, Z.A.; Roe, M.; Xu, F.; Turnbull, B.; Choi, K.S.; Hou, X. Super-hydrophobic/icephobic coatings based on silica nanoparticles modified by self-assembled monolayers. Nanomaterials 2016, 6, 232. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, M.; Lv, T.; Wang, Q.; Chen, Q.; Ding, J. Influence of different chemical modifications on the icephobic properties of superhydrophobic surfaces in a condensate environment. J. Mater. Chem. A 2015, 3, 4967–4975. [Google Scholar] [CrossRef]
- Ramirez, S.M.; Diaz, Y.J.; Campos, R.; Stone, R.L.; Haddad, T.S.; Mabry, J.M. Incompletely condensed fluoroalkyl silsesquioxanes and derivatives: Precursors for low surface energy materials. J. Am. Chem. Soc. 2011, 133, 20084–20087. [Google Scholar] [CrossRef]
- Carreño, F.; Gude, M.R.; Calvo, S.; Rodriguez de la Fuente, O.; Carmona, N. Design and development of icephobic coatings based on sol-gel/modified polyurethane paints. Mater. Today Commun. 2020, 25, 101616. [Google Scholar] [CrossRef]
- Lv, L.; Liu, H.; Zhang, W.; Chen, J.; Liu, Z. Facile UV-curable fabrication of robust, anti-icing superhydrophobic coatings based on polyurethane. Mater. Lett. 2020, 258, 126653. [Google Scholar] [CrossRef]
- Rønneberg, S.; Laforte, C.; Volat, C.; He, J.; Zhang, Z. The effect of ice type on ice adhesion. AIP Adv. 2019, 9, 055304. [Google Scholar] [CrossRef] [Green Version]
- Varanasi, K.K.; Deng, T.; Smith, J.D.; Hsu, M.; Bhate, N. Frost formation and ice adhesion on superhydrophobic surfaces. Appl. Phys. Lett. 2010, 97, 234102. [Google Scholar] [CrossRef]
- Kraj, A.G.; Bibeau, E.L. Phases of icing on wind turbine blades characterized by ice accumulation. Renew. Energy 2010, 35, 966–972. [Google Scholar] [CrossRef]
- Cao, Y.; Tan, W.; Wu, Z. Aircraft icing: An ongoing threat to aviation safety. Aerosp. Sci. Technol. 2018, 75, 353–385. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, X.; Min, J. Aircraft icing model considering both rime ice property variability and runback water effect. Int. J. Heat Mass Transf. 2017, 104, 510–516. [Google Scholar] [CrossRef]
- Dai, H.; Zhu, C.; Zhao, H.; Liu, S. A new ice accretion model for aircraft icing based on phase-field method. Appl. Sci. 2021, 11, 5693. [Google Scholar] [CrossRef]
- Boudala, F.; Isaac, G.A.; Wu, D. Aircraft icing study using integrated observations and model data. Weather Forecast. 2019, 34, 485–506. [Google Scholar] [CrossRef]
- Deiler, C.; Kilian, T. Dynamic aircraft simulation model covering local icing effects. CEAS Aeronaut. J. 2018, 9, 429–444. [Google Scholar] [CrossRef]
- Cao, Y.; Huang, J.; Yin, J. Numerical simulation of three-dimensional ice accretion on an aircraft wing. Int. J. Heat Mass Transf. 2016, 92, 34–54. [Google Scholar] [CrossRef]
- Work, A.; Lian, Y. A critical review of the measurement of ice adhesion to solid substrates. Prog. Aerosp. Sci. 2018, 98, 1–26. [Google Scholar] [CrossRef]
- Lynch, F.T.; Khodadoust, A. Effects of ice accretions on aircraft aerodynamics. Prog. Aerosp. Sci. 2001, 37, 669–767. [Google Scholar] [CrossRef]
- Dias Filho, N.L.; Caetano, L.; Do Carmo, D.R.; Rosa, A.H. Preparation of a silica gel modified with 2-amino-1,3,4-thiadiazole for adsorption of metal ions and electroanalytical application. J. Braz. Chem. Soc. 2006, 17, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Il’in, A.A.; Il’in, A.N.; Bakhmutov, Y.L.; Furin, G.G.; Pokrovskii, L.M. Promising prospects for using partially fluorinated alcohols as O-nucleophilic reagents in organofluoric synthesis. Russ. J. Appl. Chem. 2007, 80, 405–418. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, K.; Fu, T.; Wang, J.; Shen, F.; Zhang, X.; Yu, L. Formation of MoSi2 and Si/MoSi2 coatings on TZM (Mo–0.5Ti–0.1Zr–0.02C) alloy by hot dip silicon-plating method. Ceram. Int. 2021, 47, 23053–23065. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, C.; Zhao, N. Experimental study on the effect of different parameters on rotor blade icing in a cold chamber. Appl. Sci. 2020, 10, 5884. [Google Scholar] [CrossRef]
- Przybyszewski, B.; Boczkowska, A.; Kozera, R.; Mora, J.; Garcia, P.; Aguero, A.; Borras, A. Hydrophobic and icephobic behaviour of polyurethane-based nanocomposite coatings. Coatings 2019, 9, 811. [Google Scholar] [CrossRef] [Green Version]

| Sample | SiO2 Content (wt %) | DC88 Content (wt %) | POSS14 Content (wt %) |
|---|---|---|---|
| ref | - | - | - |
| SiO2 | 3 | - | - |
| DC88 | - | 5 | - |
| SiO2 + DC88 | 3 | 5 | - |
| POSS14 | - | - | 5 |
| SiO2 + POSS14 | 3 | - | 5 |
| Sample Number | Chemical Modification | ||
|---|---|---|---|
| 1 | Reference | ||
| 2 | SiO2 + DC88 | ||
| 3 | SiO2 | ||
| 4 | DC88 | ||
| 5 | POSS14 | ||
| 6 | SiO2 + POSS14 |
| Sample Number | Chemical Modification | |||||
|---|---|---|---|---|---|---|
| 1 | Reference | |||||
| 2 | SiO2 + DC88 | |||||
| 3 | SiO2 | |||||
| 4 | DC88 | |||||
| 5 | POSS14 | |||||
| 6 | SiO2 + POSS14 |
| Sample Number | Chemical Modification | Accreted Ice (g) | Ice Reduction (% of Reference) | ||||
|---|---|---|---|---|---|---|---|
| 1 | Reference | 0 | 0 | 0 | |||
| 2 | SiO2 + DC88 | ||||||
| 3 | SiO2 | ||||||
| 4 | DC88 | ||||||
| 5 | POSS14 | 56 | |||||
| 6 | SiO2 + POSS14 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przybyszewski, B.; Kozera, R.; Krawczyk, Z.D.; Boczkowska, A.; Dolatabadi, A.; Amer, A.; Sztorch, B.; Przekop, R.E. A Wind Tunnel Experimental Study of Icing on NACA0012 Aircraft Airfoil with Silicon Compounds Modified Polyurethane Coatings. Materials 2021, 14, 5687. https://doi.org/10.3390/ma14195687
Przybyszewski B, Kozera R, Krawczyk ZD, Boczkowska A, Dolatabadi A, Amer A, Sztorch B, Przekop RE. A Wind Tunnel Experimental Study of Icing on NACA0012 Aircraft Airfoil with Silicon Compounds Modified Polyurethane Coatings. Materials. 2021; 14(19):5687. https://doi.org/10.3390/ma14195687
Chicago/Turabian StylePrzybyszewski, Bartlomiej, Rafal Kozera, Zuzanna D. Krawczyk, Anna Boczkowska, Ali Dolatabadi, Adham Amer, Bogna Sztorch, and Robert E. Przekop. 2021. "A Wind Tunnel Experimental Study of Icing on NACA0012 Aircraft Airfoil with Silicon Compounds Modified Polyurethane Coatings" Materials 14, no. 19: 5687. https://doi.org/10.3390/ma14195687
APA StylePrzybyszewski, B., Kozera, R., Krawczyk, Z. D., Boczkowska, A., Dolatabadi, A., Amer, A., Sztorch, B., & Przekop, R. E. (2021). A Wind Tunnel Experimental Study of Icing on NACA0012 Aircraft Airfoil with Silicon Compounds Modified Polyurethane Coatings. Materials, 14(19), 5687. https://doi.org/10.3390/ma14195687











